Abstract
Background
Pit and fissure sealants are plastic materials that are used to seal deep pits and fissures on the occlusal surfaces of teeth, where decay occurs most often in children and adolescents. Deep pits and fissures can retain food debris and bacteria, making them difficult to clean, thereby causing them to be more susceptible to dental caries. The application of a pit and fissure sealant, a non‐invasive preventive approach, can prevent dental caries by forming a protective barrier that reduces food entrapment and bacterial growth. Though moderate‐certainty evidence shows that sealants are effective in preventing caries in permanent teeth, the effectiveness of applying pit and fissure sealants to primary teeth has yet to be established.
Objectives
To evaluate the effects of sealants compared to no sealant or a different sealant in preventing pit and fissure caries on the occlusal surfaces of primary molars in children and to report the adverse effects and the retention of different types of sealants.
Search methods
An information specialist searched four bibliographic databases up to 11 February 2021 and used additional search methods to identify published, unpublished and ongoing studies. Review authors scanned the reference lists of included studies and relevant systematic reviews for further studies.
Selection criteria
We included parallel‐group and split‐mouth randomised controlled trials (RCTs) that compared a sealant with no sealant, or different types of sealants, for the prevention of caries in primary molars, with no restriction on follow‐up duration. We included studies in which co‐interventions such as oral health preventive measures, oral health education or tooth brushing demonstrations were used, provided that the same adjunct was used with the intervention and comparator. We excluded studies with complex interventions for the prevention of dental caries in primary teeth such as preventive resin restorations, or studies that used sealants in cavitated carious lesions.
Data collection and analysis
Two review authors independently screened search results, extracted data and assessed risk of bias of included studies. We presented outcomes for the development of new carious lesions on occlusal surfaces of primary molars as odds ratios (OR) with 95% confidence intervals (CIs). Where studies were similar in clinical and methodological characteristics, we planned to pool effect estimates using a random‐effects model where appropriate. We used GRADE methodology to assess the certainty of the evidence.
Main results
We included nine studies that randomised 1120 children who ranged in age from 18 months to eight years at the start of the study. One study compared fluoride‐releasing resin‐based sealant with no sealant (139 tooth pairs in 90 children); two studies compared glass ionomer‐based sealant with no sealant (619 children); two studies compared glass ionomer‐based sealant with resin‐based sealant (278 tooth pairs in 200 children); two studies compared fluoride‐releasing resin‐based sealant with resin‐based sealant (113 tooth pairs in 69 children); one study compared composite with fluoride‐releasing resin‐based sealant (40 tooth pairs in 40 children); and one study compared autopolymerised sealant with light polymerised sealant (52 tooth pairs in 52 children).
Three studies evaluated the effects of sealants versus no sealant and provided data for our primary outcome. Due to differences in study design such as age of participants and duration of follow‐up, we elected not to pool the data. At 24 months, there was insufficient evidence of a difference in the development of new caries lesions for the fluoride‐releasing sealants or no treatment groups (Becker Balagtas odds ratio (BB OR) 0.76, 95% CI 0.41 to 1.42; 1 study, 85 children, 255 tooth surfaces). For glass ionomer‐based sealants, the evidence was equivocal; one study found insufficient evidence of a difference at follow‐up between 12 and 30 months (OR 0.97, 95% CI 0.63 to 1.49; 449 children), while another with 12‐month follow‐up found a large, beneficial effect of sealants (OR 0.03, 95% CI 0.01 to 0.15; 107 children). We judged the certainty of the evidence to be low, downgrading two levels in total for study limitations, imprecision and inconsistency.
We included six trials randomising 411 children that directly compared different sealant materials, four of which (221 children) provided data for our primary outcome. Differences in age of the participants and duration of follow‐up precluded pooling of the data. The incidence of development of new caries lesions was typically low across the different sealant types evaluated. We judged the certainty of the evidence to be low or very low for the outcome of caries incidence.
Only one study assessed and reported adverse events, the nature of which was gag reflex while placing the sealant material.
Authors' conclusions
The certainty of the evidence for the comparisons and outcomes in this review was low or very low, reflecting the fragility and uncertainty of the evidence base. The volume of evidence for this review was limited, which typically included small studies where the number of events was low. The majority of studies in this review were of split‐mouth design, an efficient study design for this research question; however, there were often shortcomings in the analysis and reporting of results that made synthesising the evidence difficult. An important omission from the included studies was the reporting of adverse events. Given the importance of prevention for maintaining good oral health, there exists an important evidence gap pertaining to the caries‐preventive effect and retention of sealants in the primary dentition, which should be addressed through robust RCTs.
Keywords: Adolescent; Child; Humans; Dental Caries; Dental Caries/prevention & control; Dentition, Permanent; Fluorides; Pit and Fissure Sealants; Pit and Fissure Sealants/therapeutic use; Tooth, Deciduous
Plain language summary
Sealants for preventing tooth decay in baby teeth
Review question
Can putting sealants over the biting surfaces of baby teeth in the back of the mouth prevent tooth decay forming in them?
Background
Tooth decay is one of the most common diseases of childhood that can affect the overall well‐being of the child. The most commonly affected teeth are the back teeth whose biting surfaces are not flat and have grooves (pits and fissures) that can retain food debris and bacteria, leading to formation of cavities (decay). In addition, the opening of these grooves is so small that a toothbrush bristle cannot enter them completely, making them difficult to clean. Sealing the grooves is one of the ways to prevent decay in back teeth. Sealant acts as a protective barrier to food and bacteria, thus preventing their harmful action on tooth surfaces.
Study characteristics
We included nine studies that involved 1120 children (aged 18 months to eight years). The studies used a variety of dental sealants to prevent tooth decay in baby teeth. We assessed most studies as being at high risk of bias overall, because the dental professionals who were measuring the outcomes could see whether a sealant had been placed, and also differentiated between sealant materials.
Key results
Three studies compared sealants with no sealants, and six studies compared different materials or processes to seal the tooth surface. As there were important differences in the design of the studies in terms of the sealant types, the age of the children at the start of the trial and the length of follow‐up, we were unable to pool the data. Only one trial assessed and reported side effects, the nature of which was gag reflex while placing the sealant material.
Quality of evidence
We found low‐quality evidence regarding the effectiveness of sealants in preventing tooth decay on biting surfaces of back baby teeth in children. Hence, we are unable to draw conclusions about the effectiveness of sealants compared to no sealant or a different sealant in preventing development of decay on baby teeth in children. More well‐conducted studies with long follow‐up times are needed.
How up‐to‐date is the evidence?
The review includes studies available from a search of the literature up to 11 February 2021.
Summary of findings
Summary of findings 1. Fluoride‐releasing resin‐based sealants versus no sealants.
| Fluoride‐releasing resin‐based sealants versus no sealants | |||||
|
Population: children with caries‐free (or non‐cavitated carious lesion) primary molars, aged 3–7 years Settings: paediatric department, dental hospital (France) Intervention: fluoride‐releasing resin‐based sealant Comparison: no treatment | |||||
| Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence | |
| Risk with no sealant | Risk with resin‐based sealant | ||||
|
Development of ≥ 1 new carious lesion (caries incidence) Follow‐up: 12 months |
36 per 1000 |
44 per 1000a (14 to 130) |
BB OR 1.21 (0.37 to 3.94) |
88 children, 274 teeth (1 RCT) |
⨁⨁◯◯ Lowb |
|
Development of ≥ 1 new carious lesion (caries incidence) Follow‐up: 24 months |
205 per 1000 |
164 per 1000c (95 to 268) |
BB OR 0.76 (0.41 to 1.42) |
85 children, 255 teeth (1 RCT) |
⨁⨁◯◯ Lowb |
| Progression of non‐cavitated enamel caries | No studies reported this outcome. | ||||
| Adverse events | No studies reported this outcome. | ||||
| *The basis for the assumed risk is the control group risk in the study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BB OR: Becker Balagtas odds ratio; CI: confidence interval; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aAt 12 months, sealants on 96 (70.1%) teeth were completely retained, 25 (18.3%) were partially retained and 16 (11.65%) were completely lost. bWe downgraded the evidence one level due to study limitations arising from lack of blinding and one level due to imprecision of effect estimates from a single study. The effect estimated included both appreciable benefit and appreciable harm. cAt 24 months, sealants on 58 (45.3%) teeth were completely retained, 29 (22.7%) were partially retained and 41 (32%) were completely lost.
Summary of findings 2. Glass ionomer‐based sealants versus no sealants.
| Glass ionomer‐based sealants versus no sealants | ||||||
|
Population: children with caries‐free primary first molars with or without caries affecting other teeth, aged 1–5 years Settings: paediatric clinic, dental school (India) and community dental setting (UK) Intervention: glass ionomer‐based sealants Comparison: no sealants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with no sealants | Risk with GIC sealants | |||||
|
Development of at ≥ 1 new carious lesion (caries incidence) Follow‐up: 12–30 months |
235 per 1000 | 229 per 1000 (162 to 314)a | OR 0.97 (0.63 to 1.49) | 449 (1 RCT) | ⊕⊕⊝⊝ Lowb | The evidence for this comparison is equivocal. In an additional trial randomising 107 children, the odds of developing a new carious lesion at 6‐ and 12‐month follow‐up were lower for the sealant group than the no‐sealant group at both time points (6 months: OR 0.031, 95% CI 0.002 to 0.601; 12 months: OR 0.033, 95% CI 0.007 to 0.149).c |
| Progression of non‐cavitated enamel caries | No studies reported this outcome. | |||||
| Adverse events | No studies reported this outcome. | |||||
| *The basis for the assumed risk is the control group risk in the study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GIC: glass ionomer‐based sealants; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aOne or more sealants in 69 (31.2%) children were fully or partially retained at follow‐up. bWe downgraded the evidence two levels due to study limitations arising from lack of blinding, imprecision and inconsistency. cAt six months, 82 teeth (49.4%) out of 166 teeth sealed with GIC were completely retained, 54 (32.5%) teeth had partially retained sealants and 30 (18.1%) teeth had completely lost sealants. At 12 months, 75 (43.6%) of sealants were fully retained, 58 (33.7%) were partially retained and 39 (22.7%) were completely lost.
Summary of findings 3. Glass ionomer‐based sealants versus (fluoride‐releasing) resin‐based sealants.
| Glass ionomer‐based sealants versus resin‐based sealants | ||||||
|
Population: 'healthy' children, with caries‐free second primary molars, aged 3–5 years Settings: schools and kindergarten, India and China Intervention: glass ionomer‐based sealants Comparison: fluoride‐releasing or non‐fluoride‐releasing resin‐based sealants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with resin‐based sealants | Risk with glass ionomer‐based sealants | |||||
|
Development of ≥ 1 new carious lesion (caries incidence) Follow‐up: 6–24 months |
N/A Insufficient quantitative information available |
N/A | 200 (2 studies) | ⊕⊝⊝⊝ Very lowa | Due to the methods of data collection, analysis and reporting we were unable to provide any quantitative estimates for this comparison. | |
| Progression of non‐cavitated enamel caries | No studies reported this outcome. | |||||
|
Sealant retention Complete or partial retention of sealant Follow‐up: 24 monthsb |
70 per 1000 | 320 per 1000 (208 to 458) | BB OR 0.20 (0.11 to 0.36) | 100 children, 100 tooth pairs (1 RCT) | ⊕⊝⊝⊝ Very lowa | We were unable to re‐analyse the results from an additional split‐mouth study (several tooth pairs) that failed to consider the split‐mouth nature of the data and the multiple teeth treated. The authors reported that, "At 6 month after pit and fissure seal, detachment rate was lower in the glass ionomer group compared with resin group (P = 0). At 18 months, detachment rate was lower in the glass ionomer group compared with resin group (P = 0.113)." |
| Adverse events | — | — | — | 100 children (1 RCT) | ⊕⊝⊝⊝ Very lowa | 1 study reported adverse events as some discomfort such as nausea among some children. 1 child reported feeling uncomfortable and experienced a strong gag reflex following application of the glass ionomer‐based sealant while 8 children reported feeling uncomfortable after the fluoride resin‐based applications. |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BB OR: Becker Balagtas odds ratio; CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aWe downgraded two levels for study limitations arising from lack of blinding and selective reporting, and one level for imprecision. bThe reported retention percentages for the resin group did not add up to 100% for the six‐ and 12‐month time points and so we were unable to use the reported data. For the 24‐month time point, 32% of sealants were completely or partially retained in the glass ionomer‐based sealant group, and 70% completely or partially retained in the resin‐based sealant group.
Summary of findings 4. Fluoride‐releasing resin‐based sealants versus resin‐based sealants.
| Fluoride‐releasing resin‐based sealants versus resin‐based sealants | ||||||
|
Population: children with caries‐free second primary molars, aged 4–8 years Settings: dental clinic, Turkey and Spain Intervention: fluoride‐releasing resin‐based sealants Comparison: resin‐based sealants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with fluoride‐releasing resin‐based sealants | Risk with resin‐based sealants | |||||
|
Development of ≥ 1 new carious lesion (caries incidence) Follow‐up: 6–24 months |
N/A insufficient quantitative information available | N/A | 69 (2 studies) | ⊕⊕⊝⊝ Lowa | Due to the different sealant materials evaluated, data reporting (split‐mouth studies reported as parallel‐group studies) and the very low number of tooth surfaces developing new carious lesions, we were unable to pool these data in a meta‐analysis. | |
| Progression of non‐cavitated enamel caries | No studies reported this outcome. | |||||
|
Sealant retention Complete or partial retention of sealant Follow‐up: 6–24 months |
— | — | Effect estimate not calculable | 69 (2 studies) | ⊕⊝⊝⊝ Very lowb | Due to the different sealant materials evaluated, data reporting (split‐mouth studies reported as parallel‐group studies) and the very low number of sealants that were lost, we were unable to pool these data in a meta‐analysis. |
| Adverse events | No studies reported this outcome. | |||||
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aWe judged the certainty of the evidence to be low for this comparison, and downgraded two levels for imprecision owing to the small study sample sizes and very low numbers of events. bWe judged the certainty of the evidence to be very low for this outcome, and downgraded two levels for imprecision owing to the small study sample sizes and low numbers of failures, and one level for inconsistency of results.
Summary of findings 5. Flowable resin composite versus resin‐based sealants.
| Flowable resin composite versus resin‐based sealants | ||||||
|
Population: children who were regular dental attenders with caries‐free first or second primary molars Settings: Public Health service clinic in Brazil Intervention: flowable resin composite Comparison: resin‐based sealants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with resin‐based sealants | Risk with flowable resin composite | |||||
| Development of ≥ 1 new carious lesion (caries incidence) | No studies reported this outcome. | |||||
| Progression of non‐cavitated enamel caries | No studies reported this outcome. | |||||
|
Sealant retention Complete or partial retention of sealant Follow‐up: 12 months |
— | — | Effect estimate not calculable. All sealants were completely or partially retained. |
40 (1 RCT) | ⨁⨁◯◯ Lowa | All sealants were retained or partially retained in both groups. |
| Adverse events | No studies reported this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aWe downgraded the evidence two levels due to study limitations arising from lack of blinding and imprecision from a single study with a small number of participants with no failures.
Summary of findings 6. Autopolymerised sealant versus light polymerised sealant.
| Autopolymerised sealant versus light polymerised sealant | |||||
|
Population: children with sound primary molars, aged 2–4 years Settings: municipal dental clinics or hospital paediatric clinics, Denmark Intervention: autopolymerised sealant application Comparison: light polymerised sealant application | |||||
| Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence | |
| Risk with light polymerised sealant | Risk with autopolymerised sealant | ||||
|
Development of ≥ 1 new carious lesion (caries incidence) Follow‐up: 24–36 months |
98 per 1000 |
59 per 1000 (16 to 192) |
OR 0.58 (0.15 to 2.19) |
52 children, 52 tooth pairs (1 RCT) |
⊕⊝⊝⊝ Very lowa |
| Progression of non‐cavitated enamel caries | No studies reported on this outcome. | ||||
|
Sealant retention Complete or partial retention of sealant Follow‐up: 24–36 months |
904 per 1000 |
865 per 1000 (756 to 931) |
OR 0.68 (0.33 to 1.44) |
52 children, 52 tooth pairs (1 RCT) |
⊕⊝⊝⊝ Very lowa |
| Adverse events | No studies reported this outcome. | ||||
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aWe downgraded the evidence three levels to very low due to study limitations arising from lack of blinding, imprecision from a single study and indirectness of comparator sealant.
Background
Description of the condition
Dental caries is a multifactorial disease of the teeth that results in the localised destruction of tooth structure. Once considered an infectious disease solely, caries is currently defined as "a complex disease caused by an imbalance in physiologic equilibrium between tooth mineral and biofilm fluid" (Fejerskov 2003). Caries is caused by an interplay between the tooth substrate, carbohydrates in the diet and cariogenic bacteria in the dental biofilm. The bacteria metabolise refined carbohydrates (sugars) and produce acid, causing fluctuations in the pH of the biofilm and disturbances in the physiological equilibrium between the tooth and biofilm, resulting in mineral loss (demineralisation) (Herald 2013; Kidd 2011). Under favourable conditions, the mineral loss is reversible (remineralisation); however, if the cariogenic challenge persists, it will lead to the further dissolution of dental hard tissues and possibly visible caries (Figure 1). In the absence of timely treatment, caries can spread through the hard tissues of the tooth to the soft tissue (pulp), leading to pain, inflammation and loss of function (Ten Cate 1999). If left untreated, caries can result in difficulty in chewing, tooth loss, weight loss, changes in behaviour, and poor academic performance and cognitive development in young children (Acs 1992; Abanto 2011; Ayhan 1996; Miller 1992). It can negatively impact the quality of life (Filstrup 2003). Besides personal and public health implications, untreated caries can lead to sizeable economic challenges with huge global cost (Pitts 2021).
1.
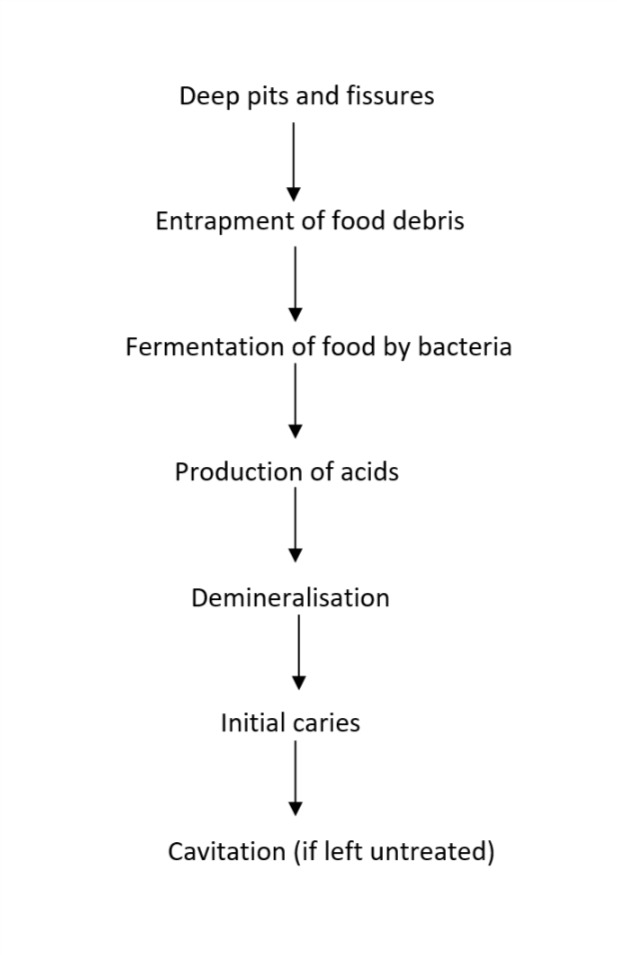
Aetiopathogenesis of pit and fissure caries.
As the most common dental disease affecting people of all ages, caries is a significant health problem in children. Untreated dental caries in primary teeth is considered the 10th most prevalent condition, affecting about 621 million children globally (Kassebaum 2015). One systematic review showed a worldwide caries prevalence of 46.2% in primary teeth and 53.8% in permanent teeth (Kazeminia 2020). The prevalence and burden of caries are higher among children in low‐ and middle‐income countries than among those in high‐income countries (WHO 2014).
Susceptibility to caries is highly variable among individuals and teeth. Teeth are marked with pits and fissures: a pit is a small pinpoint depression located at the junction of developmental grooves or at the terminals of those grooves, whereas a fissure is a deep cleft between adjoining cusps (Tandon 2008). Within the mouth, the risk of caries development is more pronounced on chewing surfaces of back teeth with pits and fissures due to increased plaque retention, permeable immature enamel structure and the reduced effectiveness of fluoride on pits and fissures (Beauchamp 2008). Pit and fissure caries account for 90% of all dental caries in permanent molars even though occlusal surfaces represent only 12.5% of the total surfaces of the teeth (CDC and National Center for Health Statistics 2005). Caries is also prevalent in the primary molars with about 44% of all caries seen in pits and fissures (Dye 2007), even though the occlusal morphology of primary molars is flatter and less fissured than that of permanent molars (Hatrick 2015).
Traditionally, detection of caries has always been at the cavitation stage, with the management focused strongly on operative treatment. However, in recent years, with the changes in patterns of disease presentation, with slower progression of non‐cavitated lesions (Baelum 2006), emphasis is on early detection of non‐cavitated carious lesions, amenable to prevention. The most common method of caries detection is visual‐tactile. Other non‐invasive techniques for detection of early caries include radiographs, quantitative light‐induced fluorescence, DIAGNOdent, fibre‐optic transillumination and electrical conductance (Gomez 2015). Grading the severity of carious lesions is also complex, due in part to a lack of consistency among contemporary assessment criteria. However, the International Caries Detection and Assessment System (ICDAS) has integrated several new criteria into one classification system, which simplifies caries assessment (Ismail 2007). With ICDAS, the assessment codes range from 0 to 6 according to the severity of the carious lesion. A code of 0, 1 or 2 represents a classification ranging from sound tooth surface to caries in enamel without cavitation. At this level of severity, teeth have greater potential for remineralisation than teeth with higher severity caries (ICDAS codes 3 to 6, which represent assessments ranging from cavitated caries in enamel to caries in dentin) (ICDAS II 2008).
Prevention of caries in primary molars is important as the progression of caries is faster here than in permanent molars, owing to thinner enamel and higher porosity (Low 2008; Mortimer 1970).
Description of the intervention
Pit and fissure sealants are applied to the pit and fissure surfaces of teeth that are highly susceptible to dental caries and resistant to other therapeutic approaches such as fluorides and mechanical plaque control (Wright 2016). They can be categorised broadly as resin‐based sealants, glass ionomer‐based sealants and hybrid sealants (Figure 2). The first materials used as pit and fissure sealants were methyl methacrylate or cyanoacrylate cements (Cueto 1967; Herald 2013). With the invention of bisphenol A‐glycidyl methacrylate (BIS‐GMA), resin‐based sealants were introduced (Bowen 1982).
2.

Classification of sealants.
Resin‐based sealants can be classified into four generations based on their content and method of polymerisation. First‐generation sealants were cyanoacrylates activated using an ultraviolet light source of 365 nm. Due to observed degradation in the oral cavity over time, these sealants are no longer available (Pinkham 2005). Second‐generation resin sealants contain BIS‐GMA or urethane dimethacrylate‐based products, which are autopolymerising or chemically cured (Donly 2002; Pinkham 2005). Third‐generation sealants contain a di‐ketone initiator and a reducing agent to initiate polymerisation, and are visible light‐activated (Sanders 2015). Fourth‐generation sealants are fluoride‐releasing resin‐based products, which have an additional potential benefit in terms of caries prevention (Donly 2002).
Glass ionomer‐based sealants are made from glass ionomer cements (GIC) and can bond chemically to the tooth structure. These sealants are used widely due to their fluoride‐releasing properties. They have the advantage of being less sensitive to moisture, making them a potential alternative to resin‐based sealants when moisture control is an issue. However, glass ionomer‐based sealants have poor retention rates on teeth compared with resin‐based sealants (Simonsen 2002). Glass ionomer‐based sealants can be conventional (chemically cured) or resin modified, in which conventional GICs are combined with resin components that are light cured (Anusavice 2013; Arrondo 2009).
Hybrid sealants, such as compomers and giomers, are a combination of resin and GICs. Compomers are polyacid‐modified composite resins and giomers are fluoride‐releasing materials made of urethane resins containing surface prereacted glass ionomer filler particles (Hatrick 2015). These are relatively newer materials and data on their caries‐preventive effects are limited.
How the intervention might work
The anatomy of the pit and fissure surfaces makes them difficult to clean, and they are thus at higher risk for caries development. If the morphology of fissures is deep and complex, it can lead to the entrapment of food debris, which in turn acts as a niche for plaque formation and bacterial growth (Figure 1). Cleaning deep and complex fissures is difficult as a toothbrush bristle cannot reach into the depth of the fissure. Thus, even excellent home care may not be successful in cleaning a deep fissure (Vann 1999).
Sealants applied to sound occlusal teeth surfaces occlude these pits and fissures forming a physical barrier that helps to prevent caries development. The physical barrier may block the carbohydrates from reaching the bacteria at the base of these structures, as well as making the surfaces easier to clean (Herald 2013; Vann 1999). While resin‐based sealants prevent caries by forming a physical barrier (Mertz‐Fairhurst 1984), GIC sealants bond chemically to dental tissues and have anticariogenic effect by releasing fluoride (McLean 1992).
Why it is important to do this review
The use of sealants in preventing caries in permanent teeth in children and adolescents is well established. One Cochrane systematic review found moderate‐certainty evidence that resin‐based sealants were more effective than no sealant for preventing tooth decay in the permanent dentition, reducing it by between 11% and 51% more than in children without sealant when measured two years after sealant application (Ahovuo‐Saloranta 2017). However, results were inconclusive when glass ionomer‐based sealants were compared with no sealant and when one type of sealant material was compared with another. In the four included studies that assessed possible problems from the use of sealants, there were no adverse effects reported. Use of sealants for the prevention of caries in permanent teeth have been recommended in clinical guidelines from professional bodies such as the American Dental Association, the American Association of Pediatric Dentistry and the British Society of Paediatric Dentistry (AAPD 2013; Beauchamp 2008; BSPD 2000; Welbury 2004). When it comes to primary teeth, however, empirical data and systematic reviews on the effectiveness of sealants exclusively in primary molars are lacking. The clinical recommendations for the management of deep pits and fissures on primary teeth have been extrapolated from the findings of sealant effectiveness in permanent teeth (AAPD 2013). The lack of synthesised evidence from trials in the primary dentition is a concern as sealants in primary teeth are increasingly being recommended as part of preventive programmes for young children (AAPD 2013; Gooch 2009).
There is uncertainty regarding the use of sealants in primary molars. Opponents of the placement of sealants in primary molars believe that the flatter fissures of primary molars do not support long‐term sealant retention (Horowitz 1982). Apprehension about sealing over incipient (white spot) and non‐cavitated carious lesions is another concern (Ripa 1976). However, this concern may be unfounded. One report based on a systematic review from the American Dental Association indicated that children with sealed sound or non‐cavitated pit and fissures in primary molars had a 76% lower risk of developing new caries than children without sealants; retention levels in primary molars ranged from 74% to 93% (Beauchamp 2008).
This review intends to provide healthcare policymakers, practitioners and consumers with evidence about the effectiveness of pit and fissure sealants for preventing dental caries in primary teeth. It will complement the existing Cochrane Review on sealant use in permanent teeth (Ahovuo‐Saloranta 2017).
Objectives
To evaluate the effects of sealants compared to no sealant or a different sealant in preventing pit and fissure caries on the occlusal surfaces of primary molars in children and to report the adverse effects and the retention of different types of sealants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel‐group and split‐mouth study designs that investigated the prevention of caries in primary molars. We included studies in which sealants were placed on the occlusal surfaces of primary molar teeth (ICDAS codes 0, 1 and 2 for the purpose of preventing caries (ICDAS II 2008). There were no restrictions on the duration of follow‐up, personnel applying sealants or the unit of randomisation (tooth or teeth, the quadrant, the individual or a cluster, e.g. school, class).
Types of participants
Children up to 12 years of age at the start of the study.
Types of interventions
This review included studies that compared sealants with no sealant, or compared one type of fissure sealant with another sealant, for the prevention of caries in primary molars. There were no restrictions on the type of sealant.
We included studies that used co‐interventions such as oral health preventive measures, oral health education or tooth brushing demonstrations provided that they used the same adjunct with the intervention and comparator (i.e. that the use of sealant was the only systematic difference in interventions between the trial arms).
For studies comparing sealant to no sealant, the comparator group (tooth/teeth) were those that did not have a sealant placed. When comparing the effectiveness of resin‐based sealants to other sealant types, the resin‐based group was used as the comparator. When comparing newer types of sealant materials to more conventional materials, the conventional materials were used as the comparator group.
The sealant application method used in the study was direct application on the tooth surface only. We excluded studies that compared any other caries‐preventive treatments (such as fluoride varnish, acidulated phosphate fluoride gel, laser, etc.) with sealants. We excluded studies of complex interventions for the prevention of dental caries in primary teeth, such as preventive resin restorations, studies that used sealants in cavitated lesions or studies that compared sealants with restorations.
Types of outcome measures
Primary outcomes
Incidence of new dental caries on the treated occlusal surface(s) of sound surfaces of primary molar(s) (dichotomous outcome, presence or absence of a new carious lesion).
Progression of non‐cavitated enamel caries (dichotomous outcome, cavitation into enamel/dentine or no progression).
Mean caries increment, measured as change in decayed, missing and filled primary teeth/surfaces (dmft/s).
Secondary outcomes
Retention of sealant (dichotomous outcome, fully or partially retained/non‐retained).
Adverse events (any type) and safety of sealant.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. Due to the Cochrane Centralised Search project to identify all clinical trials on the database and add them to Cochrane Central Register of Controlled Trials (CENTRAL), we only searched recent years of the Embase database. See the searching page on the Cochrane Oral Health website for more information (oralhealth.cochrane.org/how-search-studies). We placed no other restrictions on the language or date of publication when searching the electronic databases.
Cochrane Oral Health's Trials Register (searched 11 February 2021) (Appendix 1).
CENTRAL (2021, Issue 1) in the Cochrane Library (searched 11 February 2021) (Appendix 2).
MEDLINE Ovid (1946 to 11 February 2021) (Appendix 3).
Embase Ovid (16 September 2017 to 11 February 2021) (Appendix 4).
The subject strategies for databases were modelled on the search strategy designed for MEDLINE Ovid (Appendix 3). Where appropriate, this was combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Box 3c (Lefebvre 2020)).
Searching other resources
We searched the following trial registries for ongoing studies.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 11 February 2021) (Appendix 5).
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 11 February 2021) (Appendix 6).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We checked that none of the included studies in this review were retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (PR, PS) independently selected papers on the basis of title, keywords and abstract, and decided on eligibility. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. We obtained full‐text reports of all studies considered for inclusion. In cases of missing information relevant to the inclusion criteria from the abstract, or in cases of unavailability of abstract where the title was relevant, we obtained the full text of the report. All information gathering and data recording were done independently, and we resolved disagreements by discussion with a third review author (CZ). We contacted trial authors to request additional information where the study seemed to fulfil the review inclusion criteria, but information in the report was incomplete. Only studies with full‐text reports were considered for inclusion in this review. We recorded all studies excluded at the full‐text stage that did not meet the inclusion criteria, along with reasons for exclusion, in the Characteristics of excluded studies tables. A summary of the study selection process has been presented in a PRISMA flow diagram (PRISMA 2009) (Figure 3).
3.
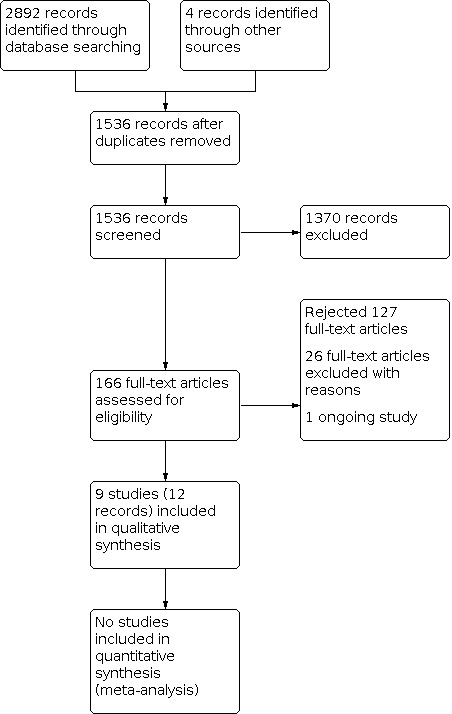
Flow of studies in the review.
Data extraction and management
Two review authors (BF, PS) independently pilot‐tested the data extraction form using a sample of studies to be included. Two review authors (PR, AR) independently extracted data from all included studies in duplicate using the data extraction form. We attempted to contact study authors to request missing information or seek clarification when necessary. We extracted data for the following.
Trial characteristics: author; title; source; date of publication; country and language; trial design; location; number of centres; recruitment period; study duration; number of children at the start of the study; method of allocation; inclusion and exclusion criteria; number of children randomised and analysed; blinding of participants, outcome assessors and personnel; exclusion of participants after randomisation; proportion of follow‐up losses.
Participant characteristics: age, sex, dmft/s, stage of caries, comparability of baseline characteristics.
Intervention characteristics: detailed description of the intervention and comparator, including timing and duration, information on compliance with the intervention (type of sealant, type and number of operators, instruments used).
Comparator characteristics: detailed description of the comparator, type of control (placebo, no sealant, different sealant type).
Outcome characteristics: details of the outcomes reported, including method of assessment and time(s) assessed. We extracted data that were presented only in graphs and figures wherever possible.
Other characteristics: adverse events, contact address of authors, declarations or conflicts of interest.
Information related to calibration of examiners and kappa statistics.
Funding source.
Assessment of risk of bias in included studies
Two review authors (PR, AR) independently assessed the risk of bias of included studies using the Cochrane domain‐based, RoB 1 tool as described in Chapter 8 of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011). We contacted study authors for clarification or missing information where necessary and feasible. We resolved any disagreements through discussion, consulting a third review author (CZ or TW) to achieve a consensus when necessary.
We completed a risk of bias table for each included study. We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of operator (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
For each domain, we judged each study at low, high or unclear risk of bias. Unclear risk of bias indicated lack of information or uncertainty about the potential for bias. Detailed criteria used in our assessments can be found in the risk of bias assessments in the Characteristics of included studies table.
Summary assessments of risk of bias
To draw conclusions about the overall risk of bias for caries outcomes within a study, we categorised the overall risk of bias of individual studies at low, high or unclear risk according to the following criteria (Higgins 2011).
Low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at a low risk of bias.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains was at high risk of bias.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains was at unclear risk of bias, but none was at high risk of bias.
We completed a risk of bias assessment for each included study and presented results graphically by domain and study.
Measures of treatment effect
For the primary outcome of caries incidence (presence or absence of at least one new carious lesion) and other dichotomous outcomes, we calculated odds ratios (ORs) and 95% confidence intervals (CI) for the comparisons of sealant with no sealant or of different sealant materials, using Review Manager 5 (Review Manager 2020).
For split‐mouth studies, we calculated ORs using the Becker Balagtas method (BB OR) (Curtin 2002). We chose this method because we intended to pool data from split‐mouth and parallel‐group studies in the same meta‐analyses, and this method facilitated data synthesis (Stedman 2011). If an included study presented paired data by tooth pairs, we calculated the intracluster correlation coefficient (ICC) (required for BB OR calculations) from the paired data. If a split‐mouth study presented data only in marginals (reported as parallel‐group studies, not as 2 × 2 cross‐classification for paired data), we assumed an ICC estimate of 0.05.
For continuous outcomes measured using the same scale, we planned to use the mean difference (MD) and 95% CIs.
Unit of analysis issues
In parallel‐group studies and cluster‐randomised studies, we chose an individual participant to be the unit of analysis. If clustered data were provided (e.g. several measurements per individual (such as more than one tooth or surface, clustering of children at school class level)), we adjusted the standard errors of the estimates to take clustering into account as outlined in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
In split‐mouth studies, we considered the individual participant to be the cluster usually comprising a single tooth pair in which one tooth was considered the intervention and one the comparator, and the tooth to be the unit of analysis. In some split‐mouth studies, more than one pair of tooth surfaces per child could be treated. These tooth pairs are dependent, and this dependency should be taken into account on a per‐child basis. However, we analysed the pairs as independent because otherwise useful information from these studies would have been lost (we are unaware of any widely used methods to correct and account for dependence of multiple tooth pairs of the tooth pairs when, for example, only marginals are reported). This meant that CIs were slightly narrower than they otherwise would have been, and this was taken into consideration when we interpreted the results.
Multi‐arm trials
We included studies with multiple trial arms, combining trial arms where appropriate or selecting only trial arms relevant to the review for meta‐analysis.
Dealing with missing data
We contacted trial authors to obtain missing data when necessary or feasible. We did not consider missing data as a reason to exclude any of the trials from the review.
Assessment of heterogeneity
We assessed clinical heterogeneity in the included studies by examining the similarity between the types of participants, interventions and outcomes. We assessed methodological heterogeneity based on the study characteristics including study design and duration of follow‐up. We also assessed heterogeneity statistically using the Chi2 test, where we considered a P < 0.1 to indicate statistically significant heterogeneity. We quantified heterogeneity using the I2 statistic. A guide to interpretation of the I2 statistic was given in Section 9.5.2 of the Cochrane Handbook for Systemic Reviews of Interventions, as follows: 0% to 40% heterogeneity might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity (Higgins 2011). A value greater than 50% was considered to represent substantial heterogeneity and in such cases, we planned to present results as a narrative only.
Assessment of reporting biases
If at least 10 studies had been included in a meta‐analysis, we had planned to assess publication bias according to the recommendations on testing for funnel plot asymmetry provided in the Cochrane Handbook for Systemic Reviews of Interventions (Sterne 2011). If asymmetry was identified, we would have examined possible causes.
Data synthesis
We grouped and analysed studies according to whether they compared a sealant with placebo or no sealant, or with a different sealant type. We planned to carry out any meta‐analyses using the generic inverse variance method and random‐effects model using Review Manager 5 (Review Manager 2020). For each comparison, we planned to pool the results of studies with similar characteristics in terms of participants, interventions and outcome measures. We carried out analyses at prespecified follow‐up times based on available data. Outcomes for caries were analysed closest to six months for incipient lesions and 12 and 24 months for more severe disease; outcomes for sealant retention were analysed closest to six, 12, and 24 months.
Subgroup analysis and investigation of heterogeneity
If data had been available, we would have performed subgroup analyses based on the following characteristics:
duration of follow‐up (short duration (12 months or less) versus long duration (more than 12 months));
severity of caries (sound tooth versus non‐cavitated/cavitated enamel (ICDAS 0, 1, 2 or 3) or dentinal caries (ICDAS 4, 5 or 6).
Sensitivity analysis
We planned to carry out sensitivity analyses to assess the impact of excluding studies with overall unclear or high risk of bias from the analyses. In a meta‐analyses that included several small studies and a single very large study, we also planned to undertake a sensitivity analysis comparing the effect estimates from both random‐effects and fixed‐effect models. If these were different, we planned to report on both analyses, and consider the possible interpretation of such findings. However, none of the meta‐analyses met these criteria, therefore, we did not carry out a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We produced a summary of findings table for each comparison and for the main outcomes of incidence of new dental caries on the treated occlusal surface(s) of sound surfaces of primary molar(s), progression of non‐cavitated enamel caries, retention of sealants (for studies that compared two different sealants) and adverse events using GRADE methods and software (GRADE 2004; GRADEpro GDT). We assessed the certainty of the body of evidence for each comparison and outcome by considering study design limitations (i.e. the overall risk of bias of the included studies, in particular, which, if any, domains were assessed at high risk of bias), the directness of the evidence, the consistency of the results, the precision of the estimates and publication bias. We categorised the certainty of each body of evidence as high, moderate, low or very low.
Results
Description of studies
Results of the search
We retrieved 2892 records from searches of databases. After removal of duplicates, 1532 records remained. We also identified four potentially relevant reports from searching the reference lists of review articles and identified trial articles (1536 overall). Following assessment of titles and abstracts, we excluded 1370 records that were not relevant and obtained 166 full‐text records. Non‐English language reports were translated for assessment where required. Of these, we excluded 127 records and progressed 38 full‐text reports for eligibility assessment. From these, we excluded 26 articles (Characteristics of excluded studies table), principally for systematic allocation or random sequence generation not reported or indicated. We included nine studies (12 records) in the review (Characteristics of included studies table). There were no studies awaiting classification, but there is one potentially eligible ongoing trial (ChiCTR1800016351; Characteristics of ongoing studies table). See Figure 3.
Included studies
We included nine studies that involved 1120 children (Baca 2007; Chabadel 2021; Chadwick 2005; Corona 2005; Fei 2011; Ganesh 2006; Hotuman 1998; Joshi 2019; Unal 2015), and evaluated 1977 tooth surfaces. Included studies were published between 1998 and 2020, with a follow‐up period that ranged from 12 to 30 months.
Comparisons
Fluoride‐releasing resin‐based sealant versus no sealant – one study with 90 randomised children (Chabadel 2021).
Glass ionomer‐based sealant versus no sealant – two studies with 619 randomised children (Chadwick 2005; Joshi 2019).
Glass ionomer‐based sealant versus resin‐based sealant – two studies with 200 randomised children (Fei 2011; Ganesh 2006).
Fluoride‐releasing resin‐based sealant versus resin‐based sealant – two studies with 69 randomised children (Baca 2007; Unal 2015).
Flowable composite versus fluoride‐releasing resin‐based sealant – one study with 40 randomised children (Corona 2005).
Autopolymerised sealant versus light polymerised sealant – one study with 52 randomised children (Hotuman 1998).
Study designs
One study used a parallel‐group design (Chadwick 2005), and eight studies were split‐mouth studies. Among the split‐mouth studies, one study randomised quadrants (Baca 2007), and seven studies randomised teeth within a tooth pair (Chabadel 2021; Corona 2005; Fei 2011; Ganesh 2006; Hotuman 1998; Joshi 2019; Unal 2015).
Participants and settings
The age of the children ranged from 18 months to eight years, and were representative of the general population, except in two studies where children from high‐caries areas (Chadwick 2005) and children with high risk for caries (Joshi 2019), were specifically included. Most studies treated children at school clinics, paediatric clinics in dental schools and community clinics. However, two studies did not report the study setting (Ganesh 2006; Unal 2015).
Four studies reported the baseline caries prevalence of participants (Baca 2007; Chabadel 2021; Chadwick 2005; Joshi 2019). One study reported that 62.2% of the children were caries free, with a mean DMFT of 0.46 in the permanent dentition and decayed, missing and filled (dft) of 0.63 in the primary dentition (Chabadel 2021). Chadwick 2005 reported that 95.5 % of children were caries free at baseline, and Joshi 2019 reported a mean baseline dmfs score of 8.45 (standard deviation (SD) 6.41). Baca 2007 reported a mean dmft of 1.16 (SD 2.06).
None of the included studies reported on socioeconomic conditions or risk factors such as frequency of sugar intake or oral hygiene habits. However, two studies provided information on the baseline caries risk of the participants as reported above: Chadwick 2005 specifically recruited children from high caries areas, and Joshi 2019, children at high risk for caries.
Two studies were carried out in India (Ganesh 2006; Joshi 2019), and one study each in Brazil (Corona 2005), China (Fei 2011), Denmark (Hotuman 1998), France (Chabadel 2021), Spain (Baca 2007), Turkey (Unal 2015), and the UK (Chadwick 2005).
Interventions
Sealants were applied to the sound occlusal surfaces or to occlusal surfaces with enamel lesion of primary first or second molars. In seven of the nine included studies, sealant was applied to sound occlusal surfaces only (Baca 2007; Chadwick 2005; Corona 2005; Ganesh 2006; Hotuman 1998; Joshi 2019; Unal 2015), and on both sound surfaces and surfaces with non‐cavitated enamel caries in two studies (Chabadel 2021; Fei 2011). Three studies stipulated deep retentive fissures or teeth that required sealant application in their inclusion criteria (Baca 2007; Corona 2005; Ganesh 2006).
The resin‐based sealant materials applied in the studies were autopolymerised resin sealant (Hotuman 1998), light‐cured resin sealant (Baca 2007; Ganesh 2006; Hotuman 1998; Unal 2015), light polymerised fluoride‐releasing resin sealant (Baca 2007; Chabadel 2021; Corona 2005; Fei 2011; Unal 2015), and sealant containing amorphous calcium phosphate (Unal 2015).
The glass ionomers used were high‐viscosity type (Chadwick 2005; Fei 2011; Joshi 2019), except one study that applied a low‐viscosity glass ionomer (Ganesh 2006).
Only one study used flowable resin composite associated with single‐bottle adhesive system as a pit and fissure sealant (Corona 2005).
Co‐interventions
Three studies reported the use of co‐interventions along with the sealants. Chadwick 2005 provided motivation and oral health instruction to study participants; Joshi 2019 instructed participants in both groups to use a low fluoride toothpaste, along with a demonstration on proper tooth brushing technique; Chabadel 2021 gave oral hygiene and dietary recommendations to participants in both groups.
Outcome measures
Seven of the nine included studies reported both caries incidence on occlusal surface and retention of sealants (Baca 2007; Chabadel 2021; Chadwick 2005; Fei 2011; Hotuman 1998; Joshi 2019; Unal 2015). Ganesh 2006 reported caries incidence and sealant retention as a composite outcome measure from which caries incidence could not be isolated. One study reported only retention of sealants (Corona 2005).
The seven studies that reported caries incidence reported the incidence of caries on the occlusal surfaces of the primary molars as dichotomous data (i.e. presence or absence of new carious lesions) (Baca 2007; Chabadel 2021; Chadwick 2005; Fei 2011; Hotuman 1998; Joshi 2019; Unal 2015). Four studies, in addition to the incidence proportion, reported caries increment at follow‐up as mean decayed, missing and filled teeth or surfaces (Baca 2007; Chadwick 2005; Joshi 2019), and one study reported the mean number of new cavitated occlusal lesions (Chabadel 2021). The following studies reported visual‐tactile caries diagnostic methods: x‐rays or visual examination under illumination (or both) (Corona 2005), visual examination (Joshi 2019), and visual and tactile examination (Baca 2007; Chabadel 2021; Fei 2011; Ganesh 2006; Unal 2015); two studies did not mention this (Chadwick 2005; Hotuman 1998). For caries assessment, one study reported the ICDAS classification system (Joshi 2019), two studies used the WHO criteria (Baca 2007; Fei 2011), and one study used the British Association for the Study of Community Dentistry (BASCD) criteria for caries diagnosis (Chadwick 2005).
Six studies reported on training and calibration of examiners (Baca 2007; Chabadel 2021; Chadwick 2005; Fei 2011; Joshi 2019; Unal 2015), but only three of these studies reported the kappa statistic for inter‐examiner agreement (Baca 2007; Fei 2011; Unal 2015), which ranged from 0.82 to 0.92 for caries diagnosis and 0.60 to 0.89 for sealant retention. There was a single examiner for sealants in three studies (Chabadel 2021; Corona 2005; Hotuman 1998).
Only one study reported recording of adverse events (Fei 2011).
All included studies reported the retention of sealants, and all reported this outcome as the proportion of completely retained, partially lost or completely lost sealants at the times of follow‐up examination.
Detailed outcomes for each study are reported in the Characteristics of included studies table.
Funding sources
One study reported that they had received funding to undertake the research (Chadwick 2005). Authors of three studies clarified that they received no funding (Baca 2007; Joshi 2019; Unal 2015). The remaining five studies did not report on funding (Chabadel 2021; Corona 2005; Fei 2011; Ganesh 2006; Hotuman 1998).
Excluded studies
The Characteristics of excluded studies table presents reasons for exclusion of studies. The reasons for exclusion varied, and there was more than one reason for exclusion in some studies. In 16 of the 26 excluded studies, the study design was not appropriate for this review; three studies compared sealants with other preventive treatments; three studies placed sealants on dentinal caries; and four studies had objectives that did not match with objectives of our review.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
One study is ongoing (ChiCTR1800016351; Characteristics of ongoing studies table).
Risk of bias in included studies
We contacted authors of included studies to obtain additional information when the information in the report was insufficient to make a final risk of bias judgement. We requested additional information from authors of seven studies (Baca 2007; Chadwick 2005; Corona 2005; Fei 2011; Ganesh 2006; Joshi 2019; Unal 2015). Additional information was provided for five of the studies (Baca 2007; Chadwick 2005; Corona 2005; Joshi 2019; Unal 2015).
We assessed risk of bias as unclear for most studies for selection bias (the domains of random sequence generation and allocation concealment); high for most studies for performance and detection bias (the domains of blinding of participants, blinding of operator and blinding of outcomes assessor); and low for most studies for attrition bias (incomplete outcome data), reporting bias (selective reporting domain), and other bias. All studies were judged at overall high risk of bias, primarily due to issues around blinding, with the exception of Unal 2015, which we judged at unclear risk of bias overall (Figure 4; Figure 5).
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
5.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Four studies adequately reported the methods used to generate the randomisation sequence, indicating low risk of bias. The randomisation sequence was by computer‐generated random numbers (Baca 2007; Chabadel 2021; Corona 2005), or using a lottery method (Joshi 2019). We classified the other studies as 'unclear' as authors mentioned that the clinical trial was randomised but did not report further details.
Allocation concealment
Only one study adequately reported allocation concealment using sealed envelopes (Chabadel 2021), and therefore was at low risk of bias. Though authors of two studies provided additional information that allocation concealment was performed, there was no information on method used, and therefore it was at unclear risk of bias (Joshi 2019; Unal 2015). The remaining six studies did not report the process for allocation concealment and were therefore classified as 'unclear'.
Blinding
Three studies comparing sealants versus no sealant (Chabadel 2021; Chadwick 2005; Joshi 2019), two studies comparing glass ionomer‐based sealants and resin‐based sealants (Fei 2011; Ganesh 2006), and one study comparing autopolymerised sealants with light polymerised sealants (Hotuman 1998), were at high risk of bias for blinding of participants, blinding of operators and blinding of outcome assessors. This is based on our judgement that for the split‐mouth studies, the sealants were placed on one tooth and no sealant or placebo was placed on the contralateral tooth, and similarly for individuals allocated to the intervention and comparator groups in the parallel‐group trial. For studies comparing type of different sealants, the differences in the colour, appearance or texture of sealant materials and the light curing procedure make it difficult to perform blinding.
Two studies comparing fluoride‐releasing resin‐based sealants versus resin‐based sealants (Baca 2007; Unal 2015), and one study comparing flowable composite with fluoride‐releasing resin‐based sealants (Corona 2005) were classed at low risk of bias for the domain of blinding of participants. However, for the domain of blinding of operator, Baca 2007 and Corona 2005 were at high risk and Unal 2015 was at low risk of bias. For assessment bias, Baca 2007 and Unal 2015 were at low risk and Corona 2005 was at high risk of bias.
Incomplete outcome data
Eight studies were at low risk of bias for this domain and one study was at unclear risk (Chadwick 2005). Five trials reported losses to follow‐up (Baca 2007; Chabadel 2021; Chadwick 2005; Hotuman 1998; Joshi 2019), of which only four reported the reasons for attrition (Baca 2007; Chabadel 2021; Fei 2011; Joshi 2019). Where reported, reasons for attrition were typically unrelated to treatment (e.g. moving to another school or city, non‐attendance on the day of clinical examination, illness, tooth exfoliation). As most studies were of a split‐mouth design, attrition was largely equal in both trial arms. And in three studies, there was no attrition (Baca 2007; Corona 2005; Unal 2015).
Selective reporting
Eight studies reported the prespecified outcomes adequately and hence were at low risk of bias (Baca 2007; Chabadel 2021; Chadwick 2005; Corona 2005; Fei 2011; Hotuman 1998; Joshi 2019; Unal 2015). One study was at high risk as the caries were measured but not reported (Ganesh 2006). It provided a composite outcome of sealant retention and presence or absence of caries, coding this outcome on a numeric scale from zero (fully retained sealant, no caries) to four (no retention of sealant and caries present). The caries information could not be extracted from the composite outcome and was not reported separately, so we were unable to use the caries data from this publication.
Other potential sources of bias
All nine studies were at low risk of other bias as no other potential sources of bias were identified.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
, but See: Table 1; Table 2; Table 3; Table 4; Table 5; and Table 6.
Comparison 1: fluoride‐releasing resin‐based sealant versus no sealant
One split‐mouth RCT randomising 139 tooth pairs in 90 children compared fluoride‐releasing resin‐based sealant versus no sealant (Chabadel 2021).
Incidence of new carious lesion
In the 88 children examined at 12 months, 6/137 treated surfaces had developed caries compared to 5/137 untreated surfaces (BB OR 1.21, 95% CI 0.37 to 3.94; Analysis 1.1). In the 85 children examined at 24 months, 21/128 treated surfaces had developed caries compared to 26/127 untreated surfaces (BB OR 0.76, 95% CI 0.41 to 1.42; Analysis 1.2).
1.1. Analysis.

Comparison 1: Resin‐based sealant versus no sealant, Outcome 1: Incidence of caries at 12 months
1.2. Analysis.

Comparison 1: Resin‐based sealant versus no sealant, Outcome 2: Incidence of caries at 24 months
We judged the certainty of evidence as low due to high risk of bias from lack of blinding and imprecision from a single study.
Progression of non‐cavitated enamel caries
The study did not report progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
In the 85 children examined at 24 months' follow‐up, the authors reported that the mean number of new, cavitated occlusal lesions was 0.23 (SD 0.06) in the sealed molars and 0.29 (SD 0.06) in the control molars (Wilcoxon matched pairs signed rank test P = 0.42) (Chabadel 2021). Mean d3ft at baseline, 12 and 24 months was reported overall but not by group.
Retention of sealants
Chabadel 2021 reported that, at 12 months, sealants on 96 (70.1%) occlusal surfaces were completely retained, 25 (18.3%) were partially retained and 16 (11.6%) were completely lost. At 24 months, sealants on 58 (45.3%) occlusal surfaces were completely retained, 29 (22.7%) were partially retained and 41 (32%) were completely lost.
Adverse events
The study did not report adverse events.
Comparison 2: glass ionomer‐based sealant versus no sealant
One parallel RCT (Chadwick 2005), and one parallel‐group/split‐mouth RCT (Joshi 2019), randomising 619 children in total, compared glass ionomer‐based sealant versus no sealant.
Incidence of new carious lesion
Two studies, randomising 619 children in total, reported development of new carious lesion (Chadwick 2005; Joshi 2019).
Chadwick 2005 randomised 508 children with follow‐up between 12 and 30 months, and found similar caries incidence in children allocated to receive glass ionomer‐based sealants to those in the no‐sealant group, with 52 (23.5%) children in the sealant group compared with 55 (24.1%) children in the no‐sealant group developing at least one new carious lesion (OR 0.97, 95% CI 0.63 to 1.49; 449 children; Analysis 2.1).
2.1. Analysis.

Comparison 2: Glass ionomer‐based sealants versus no sealants, Outcome 1: Incidence of caries at different follow‐up
Joshi 2019 randomised 180 tooth pairs in 111 young children. They reported no new carious lesions at six months in the 86 pairs of teeth treated with glass ionomer sealants, but 15 (8.4%) untreated surfaces in the 89 tooth pairs had developed caries (Joshi 2019). This pattern was maintained at 12 months' follow‐up, where caries incidence was lower with glass ionomer‐based sealants (2 teeth in 86 tooth pairs (1.1%)) than with no sealant (47 teeth in 89 tooth pairs (26.4%)). We used the method suggested by Cochrane to inflate the standard error to take into account the clustering of teeth within an individual (Higgins 2021). The odds of developing a carious lesion were lower for the sealant group than the no‐sealant group at six months (OR 0.031, 95% CI 0.002 to 0.601) and 12 months (OR 0.033, 95% CI 0.007 to 0.149) (Analysis 2.1). We used an ICC of 0.05, but there was a favourable effect of sealants with ICCs up to 0.2.
Due to differences in study design (e.g. age of participants, duration of follow‐up), we elected to not pool the data for these studies.
The certainty of the evidence was low, downgraded two levels due to study limitations arising from lack of blinding, inconsistency and imprecision.
Progression of non‐cavitated enamel caries
Neither study reported progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
Joshi 2019 reported caries at various time points from baseline up to 12 months' follow‐up; however, caries increment was not specifically calculated or reported. At 12 months' follow‐up, the authors reported that dmft was lower in the sealants group than the no‐sealant group (8.43 (SD 5.84) with sealant versus 10.05 (SD 6.16) with no sealant), but there was insufficient information to determine the threshold for caries (ICDAS score). Chadwick 2005 reported that, "there was no significant difference between test and control groups in caries increment at the occlusal surfaces of first primary molars or for any other measured variables." There were no summary data provided for this outcome.
Retention of sealants
Two studies randomising 619 children provided data on retention of the glass ionomer‐based sealants (Chadwick 2005; Joshi 2019). Chadwick 2005 reported that one or more sealants in 69 (31.2%) children were fully or partially retained at follow‐up up between 12 and 30 months. Joshi 2019 reported that, at six months, 82/166 (49.4%) teeth sealed with glass ionomer‐based sealants were completely retained, 54/166 (32.5%) teeth had partially retained sealants and 30/166 (18.1%) teeth had completely lost sealants. At 12 months, sealants were completely retained on 75 (43.6%) teeth, partially retained on 58 (33.7%) teeth and completely lost on 39 (22.7%) teeth (Joshi 2019).
Adverse events
Neither study reported adverse events.
Comparison 3: glass ionomer‐based sealant versus resin‐based sealant
Two split‐mouth studies, randomising 200 children in total, reported glass ionomer‐based sealant versus resin‐based sealant (Fei 2011; Ganesh 2006).
Incidence of new carious lesion
Fei 2011 reported a split‐mouth design with multiple sealed teeth where, specifically, molar teeth were randomised to receive the intervention (or comparator) and contralateral molar teeth received the other intervention. The analysis failed to take into account the dependency of the data arising from the split‐mouth study design and the multiple teeth treated within the intervention and comparator groups. The multiple outcomes within each trial arm clustered within an individual meant that we were unable to re‐analyse the data and the authors provided no additional information when requested. With 3/185 (1.6%) surfaces developing caries in the glass ionomer‐based sealant group and 10/168 (5.9%) surfaces developing caries in the resin‐based sealant group, the authors did however state that, "At 6 months, caries incidence was lower in the glass ionomer group compared with resin group (P = 0.029)." Similarly, with 6/188 (3.2%) surfaces developing caries in the glass ionomer‐based sealant group and 10/168 (5.9%) surfaces developing caries in the resin‐based sealant group, the authors reported that, "At 18 months, caries incidence was lower in the glass ionomer group compared with resin group (P = 0.209)".
Ganesh 2006 randomised 100 tooth pairs in 100 children. They measured caries incidence as a composite outcome of the combined presence or absence of caries and sealant retention, graded on a score from zero to four, and where the caries incidence outcome could not be isolated and extracted. We were therefore unable to report on caries incidence in this study. The authors reported that, "results demonstrated that there was no difference in the performance of the materials in primary and permanent teeth."
Due to the inadequate reporting, we were unable to pool the data for this outcome.
We judged the certainty of the evidence to be very low, downgrading two levels for study limitations arising from lack of blinding and selective reporting, and one level for imprecision.
Progression of non‐cavitated enamel caries
Neither study reported progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
Neither study reported mean caries increment.
Retention of sealants
Two studies provided sealant retention data (Fei 2011; Ganesh 2006).
Ganesh 2006 reported complete and partial retention and total loss percentages at six, 12, and 24 months' follow‐up. However, the data reported for the six and 12 month time points were erroneously reported, and so we were unable to use the data. For the 24‐month time point, there was evidence in favour of resin‐based sealants for complete or partial retention of sealants, with 70% of sealants completely or partially retained in the resin‐based sealant group and 32% completely or partially retained in the glass ionomer group (BB OR 0.20, 95% CI 0.11 to 0.36; Analysis 3.1).
3.1. Analysis.

Comparison 3: Glass ionomer sealants versus resin‐based sealants, Outcome 1: Retention of sealants at 24 months
Fei 2011 also reported sealant retention in 89 children with up to eight tooth pairs. However, the analysis failed to take into account the split‐mouth nature of the data and the multiple teeth treated. As indicated above, we were unable to use the data as reported or re‐analyse them. The authors did, however, state that "at 6 month after pit and fissure seal, detachment rate was lower in the glass ionomer group compared with resin group (P = 0). At 18 months, detachment rate was lower in the glass ionomer group compared with resin group (P = 0.113)."
Due to the inadequate reporting of data, we were unable to pool the data for this outcome.
We judged the certainty of the evidence to be very low, downgrading two levels for study limitations arising from lack of blinding and selective reporting, and one level for imprecision.
Adverse events
Only one study reported adverse effects arising from sealant application (Fei 2011). With the glass ionomer‐based sealant application, one child reported feeling uncomfortable and experienced a strong gag reflex. For the fluoride resin‐based application, eight children reported feeling uncomfortable after treatment.
Comparison 4: fluoride releasing resin‐based sealant versus resin‐based sealant
Two split‐mouth studies, randomising 69 children, compared fluoride‐releasing resin‐based sealant versus resin‐based sealant (Baca 2007; Unal 2015).
Incidence of new carious lesion
Two split‐mouth RCTs, randomising 69 children, reported development of new carious lesion (Baca 2007; Unal 2015). The caries incidence was very low in both studies. Unal 2015 reported no new carious lesions in either of the sealant groups at six and 12 months, and 95.5% success rate (only percentages reported, numerator and denominator unclear) for the resin‐based sealants group compared with 100% success for the fluoride‐releasing resin‐based sealants at 24 months. Baca 2007, at 12 months, reported two surfaces treated with unfilled resin‐based sealant had developed caries compared with no surfaces in the fluoride‐releasing resin‐based sealants group.
Due to the different sealant materials evaluated, data reporting (split‐mouth studies reported as parallel‐group studies) and the very low number of tooth surfaces developing new carious lesions, we were unable to pool these data in a meta‐analysis.
We judged the certainty of the evidence to be low, downgrading two levels for imprecision owing to the small study sample sizes and very low numbers of events.
Progression of non‐cavitated enamel caries
Neither study reported progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
Neither study reported mean caries increment.
Retention of sealants
Two studies, randomising 69 children, reported retention of sealants (Baca 2007; Unal 2015).
At six months, all sealants were retained (25 tooth pairs in 25 children) (Unal 2015). Results from the studies at longer follow‐up were equivocal. At 12 months, Unal 2015 reported a higher retention (classed as fully intact sealant or sealant in place with partial loss not involving a susceptible pit or fissure) for the fluoride‐releasing sealants group (100%) than the resin‐based sealant (96.0%), while Baca 2007 reported higher total retention in the resin‐based sealants (97.7%) than the fluoride‐releasing sealants (84.1%). At 24 months, the retention was greatest for the resin‐based sealants (91.8%) than the fluoride‐releasing sealants (87.8%) (Unal 2015).
Due to the different sealant materials evaluated, data reporting (split‐mouth studies reported as parallel‐group studies) and the very low number of sealants that were lost, we were unable to pool this data in a meta‐analysis.
We judged the certainty of the evidence to be very low, downgrading two levels for imprecision owing to the small study sample sizes and low numbers of failures, and for inconsistency of results.
Adverse events
Neither study reported adverse events.
Comparison 5: flowable composite versus fluoride‐releasing resin‐based sealant
One split‐mouth study with 40 randomised children compared flowable composite versus fluoride‐releasing resin‐based sealant (Corona 2005).
Incidence of new carious lesion
The study did not report development of new carious lesion.
Progression of non‐cavitated enamel caries
The study did not report progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
The study did not report mean caries increment.
Retention of sealants
All sealants were retained or partially retained in the flowable composite or resin‐based sealant groups. For the flowable composite sealants, 39/40 sealants placed were completely retained with only one sealant partially lost after six months, and two sealants partially lost after 12 months. For the resin‐based sealants, 33 were completely retained and seven partially retained after six months, and 31 completely retained and nine partially retained after 12 months.
We judged the certainty of the evidence to be low, downgrading due to study limitations arising from lack of blinding and imprecision from a single study with a small number of participants with no failures.
Adverse events
The study did not report adverse events.
Comparison 6: autopolymerised sealant versus light polymerised sealant
One split‐mouth study (Hotuman 1998) randomising 52 tooth pairs in 52 children compared autopolymerised sealant versus light polymerised sealant.
Incidence of new carious lesion
One split‐mouth study randomising 52 tooth pairs in 52 children reported development of new carious lesion (Hotuman 1998). At 24 to 36 months' follow‐up there was insufficient evidence of a difference in the development of new carious lesions with the two different sealant types: 2/51 (5.9%) teeth sealed with autopolymerising sealant developed caries compared to 5/51 (9.8%) teeth sealed with light polymerising sealant (OR 0.58, 95% CI 0.15 to 2.19; Analysis 4.1).
4.1. Analysis.

Comparison 4: Autopolymerised sealant versus light polymerised sealant, Outcome 1: Incidence of caries at 24–36 months
We judged the certainty of the evidence to be very low due to study limitations arising from lack of blinding, imprecision from a single study and indirectness regarding the clinical value of autopolymerised sealants which are no longer typically used.
Progression of non‐cavitated enamel caries
The study did not report progression of non‐cavitated enamel caries.
Mean caries increment, measured as the change in decayed, missing and filled teeth/surfaces
The study did not report mean caries increment.
Retention of sealants
One study randomising 52 tooth pairs in 52 children provided data for retention of sealants (Hotuman 1998). At 24 to 36 months' follow‐up, the sealants on 28 teeth sealed with autopolymerising sealant were completely retained, and seven teeth retained the sealant partially, compared with 28 completely retained sealants and eight partially retained sealants in teeth sealed with light polymerising sealant (OR 0.68, 95% CI 0.33 to 1.44; Analysis 4.2).
4.2. Analysis.

Comparison 4: Autopolymerised sealant versus light polymerised sealant, Outcome 2: Retention of sealants at 24–36 months
We judged the certainty of the evidence to be very low due to study limitations arising from lack of blinding, imprecision from a single study and indirectness regarding the clinical value of autopolymerised sealants which are no longer typically used.
Adverse events
The study did not report adverse events.
Discussion
Summary of main results
Sealant versus no sealant
We are unable to draw any firm conclusions regarding the effects of sealant as compared to no sealant for reducing caries on the occlusal surface of primary molars in children due to low‐certainty evidence. This was based on data from three studies with differences in study design such as age of participants and duration of follow‐up.
One study randomising 90 children provided data for the comparison of fluoride‐releasing resin‐based sealant versus no sealant (Chabadel 2021). At 24 months, there was insufficient evidence of a difference in the development of new caries lesions for the fluoride‐releasing sealants or no‐sealant groups (BB OR 0.76, 95% CI 0.41 to 1.42; 1 study, 85 children with 255 tooth surfaces; low‐certainty evidence). Some clinical studies have reported that resin‐based sealants control the initiation of occlusal caries by formation of a physical barrier, which prevents the metabolic exchange between the fissure cariogenic micro‐organisms and the oral environment, unlike glass ionomer‐ based sealants, which can prevent caries initiation due to additional factors such as fluoride release. Hence, the effectiveness of resin sealants inherently relies on their retention and integrity over time (Corona 2005). In this study, sealants on 58 (45.3%) teeth were completely retained, 29 (22.7%) were partially retained and 41 (32%) were completely lost at 24 months.
Two studies randomising 619 children considered to be of high caries risk provided data for the comparison of glass ionomer‐based sealant with no sealant (Chadwick 2005; Joshi 2019). For glass ionomer‐based sealants, the evidence was equivocal; one study found insufficient evidence of a difference at follow‐up between 12 and 30 months (OR 0.97, 95% CI 0.63 to 1.49; 449 children; Chadwick 2005), while another with 12 months' follow‐up found a large, beneficial effect of sealants (OR 0.033, 95% CI 0.007 to 0.149; 107 children; Joshi 2019). We judged the certainty of the evidence to be low for this outcome. Although the relationship between caries incidence and sealant retention is more relevant to resin‐based sealants, in Chadwick 2005, one or more sealants in 69 (31.2%) children were fully or partially retained at follow‐up, and in Joshi 2019, sealants were completely retained on 75 (43.6%) teeth, partially retained on 58 (33.7%) teeth and completely lost on 39 (22.7%) teeth.
One sealant material versus another sealant material
We included six trials randomising 411 children that directly compared different sealant materials or sealant processes, four of which randomising 221 children provided data for our primary outcome of caries incidence. Studies within and between the comparisons varied in terms of types of sealants assessed, age of participants and duration of follow‐up, precluding pooling of data. The incidence of development of new caries lesions was typically low across the different sealant types evaluated. We judged the certainty of the evidence to be low or very low for the outcome of caries incidence across the different sealant comparisons. The main reasons for downgrading were lack of blinding, and imprecision from small sample sizes and low numbers of events.
Only one study that compared glass ionomer‐based sealant versus resin‐based sealant assessed and reported adverse events, the nature of which was gag reflex while placing the sealant material (Fei 2011). Eight children reported being uncomfortable after the procedure.
Overall completeness and applicability of evidence
Sealant materials and settings
Of the included studies, those comparing sealants versus no sealants were mostly recently conducted. Two of the three studies with a no sealant comparator group were conducted within the last three years (Chabadel 2021; Joshi 2019) and one was conducted in early 2000 (Chadwick 2005). As the studies were quite recent, the contemporaneous findings are directly applicable to current clinical practice. One study that compared resin‐based sealant to no sealant was conducted in France. And out of two studies that compared glass ionomer‐based sealants to no sealant, one was performed in the UK and the other in India. Six studies compared different sealant materials and were published between 1998 and 2015 (Baca 2007; Corona 2005; Fei 2011; Ganesh 2006; Hotuman 1998; Unal 2015). One study each was conducted in Denmark, Brazil, India, Spain, China and Turkey. Of these studies, the one conducted in 1998 compared autopolymerising sealant versus light polymerising sealant (Hotuman 1998). This may not be relevant to present times as autopolymerising sealants are no longer used in clinical practice. Most studies recruited children from a general population, except for two studies that recruited children with high caries risk (Chadwick 2005; Joshi 2019). Four studies were carried out at paediatric dental clinics, three at community health centres and two studies did not report the settings.
Variation in caries risk levels
Caries risk of a child can be based on many factors. Baseline caries experience of a child has been considered a crucial predictor for future caries development (Mejàre 2014). Apart from this, a variety of other factors has also been associated with caries risk assessment including sugar intake, dietary habits, oral hygiene habits, oral environment, salivary factors, sociodemographic characteristics, etc. (Carvalho 2014; Mejàre 2014). The majority of the included studies did not provide any information on baseline caries risk, except for the two studies referred to above, but four studies reported on baseline dmft (Baca 2007; Chabadel 2021; Chadwick 2005; Joshi 2019). One study reported the fluoride content in the drinking water (Baca 2007), but none of the nine included studies reported socioeconomic status, dietary habits or oral hygiene practices.
Diagnosis
The main objective of our review was to assess the effect of sealants in preventing dental caries on occlusal surfaces of primary molars that were either sound or had non‐cavitated enamel caries, and hence, studies in which sealants were used for treatment of cavitated dental caries were outside the remit of this review. Seven out of nine included studies reported placing sealants on sound occlusal surfaces, while two studies reported placing sealants on either sound occlusal surfaces or occlusal surfaces with non‐cavitated enamel caries (Chabadel 2021; Fei 2011). For caries diagnosis, most included studies used visual and tactile methods; however, the index used varied between studies including Pitts and Evans criteria, WHO criteria and ICDAS. In addition to visual‐tactile caries diagnostic methods, one study reported the use of bitewing and apical radiographs (Corona 2005).
Quality of the evidence
The largest body of evidence in the review compared sealants with no sealants in three studies with 709 randomised children, of which one compared resin‐based sealant to no sealant and two compared glass ionomer‐based sealants to no sealant. We judged the certainty of evidence to be low for these two comparisons for the outcome of caries incidence, indicating that "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate"(GRADE 2004). Even though the studies are recent, owing to the low certainty of evidence, considerable uncertainty as to the effects of sealants remains for this outcome and for the additional primary outcomes of this review. Reasons for downgrading were study limitations (lack of blinding), imprecision (low numbers of events, narrative synthesis) and inconsistency of results for the glass ionomer‐based sealant comparison.
We included six trials randomising 411 children that directly compared different sealant materials or sealant processes, four of which randomising 221 children provided data for our primary outcome of caries incidence. Studies within and between the comparisons varied in terms of types of sealants assessed, age of children, and duration of follow‐up, precluding pooling of data within a comparison. Additionally, the analysis and reporting of several of the split‐mouth studies was inappropriate, requiring assumptions to be made in the re‐analysis of the data provided, or presenting the authors' conclusions in a narrative synthesis. We judged the certainty of the evidence to be low or very low for the outcome of caries incidence across the different sealant comparisons. The main reasons for downgrading were lack of blinding (only one of the studies was judged at low risk of bias for all three components of the blinding domain), imprecision from small sample sizes, low numbers of events or narrative synthesis.
Potential biases in the review process
Though the main objective of the review was to examine the effectiveness of sealants in caries prevention, our secondary objective was to report on retention of sealants as studies have shown that sealant retention is an important factor in caries prevention (Mascarenhas 2008). This is especially true with resin‐based sealants. This decision was supported by findings from one systematic review (Mickenautsch 2013). Hence, we included studies that reported either caries prevention with sealants, or sealant retention, or both.
Our aim was to assess the effectiveness of sealants in caries prevention, and hence we included only those trials in which a sealant was placed on a sound occlusal surface or non‐cavitated enamel caries, and excluded all those trials where sealants were placed on cavitated caries or as part of complex intervention such as preventive resin restoration. This study eligibility criterion potentially restricts the clinical applicability of the review.
We included studies that used a variety of sealants such as glass ionomer‐based sealants and all generations of resin‐based sealants. We included studies comparing resin‐based sealants to flowable composites as their retention is similar to that of conventional resin‐based sealants. We also included studies in which there were co‐interventions such as oral health education or tooth brushing provided that the adjunct was common to all trial arms and that the sealant was the only systematic difference in the intervention and comparator.
We included all studies that fulfilled the inclusion criteria, whether the analysis in the trial report was deemed to be appropriate or otherwise. If a split‐mouth study presented data only in marginals (as parallel‐group studies, not as 2 × 2 cross‐classification for paired data), then we used the marginal Becker Balagtas method for calculating ORs with appropriate standard errors taking the clustering of the teeth within a tooth pair into account. We used the conservative ICC of 0.05 in calculations. The Becker Balagtas method as proposed by Curtin 2002 and later by Elbourne 2002 (correction in Stedman 2011) is recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021)
Our review included only full‐text reports of published studies. Studies reported only as abstracts were excluded if the full text was not available, to avoid any inconsistent data reporting occurring between the abstracts and published full reports. It has also been found that information on trial quality indicators is often lacking (Hopewell 2006). To minimise risk of publication bias, we contacted authors of potentially eligible abstracts to seek the availability of full‐text study reports, published or unpublished. We also contacted all the authors of included studies to obtain any additional information for assessment of study bias. Articles in native languages were translated to English to assess its eligibility for inclusion.
Agreements and disagreements with other studies or reviews
Our review findings agree with one systematic review of RCTs and quasi‐RCTs of the effectiveness of pit and fissure sealants in preventing and arresting caries on the occlusal surfaces of primary molars (Lam 2020). Four electronic databases were searched until March 2018. Despite differences in eligibility criteria with the current Cochrane Review, Lam 2020 concluded that the certainty of the evidence for caries prevention and arrest was low or very low, mainly due to overall high risk of bias in the studies and imprecision. The authors concluded that there were currently insufficient well‐controlled RCTs to determine whether sealants are beneficial in preventing or arresting non‐cavitated occlusal caries in the primary molars. Even given the restriction of the Cochrane Review to RCTs, the most robust study design to answer the research question, along with an updated search to include the most current evidence, the conclusions are the same, there is a substantial evidence gap regarding the effects of pit and fissure sealants in the primary dentition.
However, the findings of our review are not in agreement with another review conducted on sealants for preventing and arresting pit‐and‐fissure occlusal caries in primary and permanent molars (Wright 2016). The resulting evidence profile comparing the use of sealants with non‐sealants in pit and fissure occlusal surfaces of children and adolescents indicating moderate certainty of evidence that participants who received sealants had a reduced risk of developing carious lesions in occlusal surfaces of permanent molars compared with those who did not receive sealants after seven or more years of follow‐up (OR 0.15, 95% CI 0.08 to 0.27). However, it should be noted that there were no eligible RCTs evaluating caries incidence in the primary dentition to inform the evidence profile for this outcome at different time points. The authors also concluded, in line with our findings, that further research is needed to provide information about the relative merits of the different types of sealant materials.
Authors' conclusions
Implications for practice.
The effectiveness of pit and fissure sealants and the relative effectiveness of different types of sealants in preventing caries on the occlusal surfaces of primary teeth has yet to be established.
Implications for research.
Primary dentition plays a paramount role in a child's well‐being and quality of life. As studies have shown a correlation between early childhood caries and health of the permanent dentition, the early establishment of preventive measures for primary dentition is of critical importance. The beneficial role of sealants is well established in permanent teeth. However, the evidence is lacking for primary teeth. There are insufficient randomised controlled trials conducted in primary teeth for assessing the effectiveness of sealants in preventing caries, unlike in the permanent teeth. Most of the trials included in this review were of inadequate duration, with typical follow‐up of 12 months. Though studies followed experimental design, most did not have adequate random sequence generation and did not take any measures for blinding the participants and assessors, especially the studies that compared different types of sealants where blinding could have been used. Many studies did not report sample size determination, with some studies having small sample size. Several studies were published prior to the publication of the CONSORT checklist, with incomplete reporting of study conduct and results, which made evidence synthesis challenging. Therefore, there is a need for well‐designed long‐term trials on the effectiveness of sealants in preventing caries in primary teeth, initially to establish whether effective compared to no sealant. Important information on participants' characteristics such as demographics, socioeconomic status and caries risk, and also protective factors such as exposure to sources of fluoride or other preventive measures, should be reported for better comparison and generalisability of the results.
History
Protocol first published: Issue 3, 2018
Acknowledgements
We would like to thank Anne Littlewood (Information Specialist) and Janet Lear (administrator) for their help in searching the literature. Our heartfelt thanks goes to Laura MacDonald (Managing Editor) of Cochrane Oral Health, for her suggestions and help during the review preparation. We also thank Professor Jan E Clarkson, Dr Jo C Dumville and Dr Joanne Elliott, and peer reviewers Siobhan Barry and Carly Dixon for their comments. We thank Gillian Gummer and Anne Lawson for copy editing. Our sincere thanks to Dr Melissa Wong for helping us with translation of trial reports. We would also like to thank the following authors of the studies for providing us with additional information: Dr Pilar Baca, Dr BL Chadwick, Dr SAM Corona, Dr Sakshi Joshi and Dr Murat Unal. We would like to express our heartfelt appreciation to Jo Weldon (Research Co‐ordinator, Cochrane Oral Health) and Professor Jacqueline Ho of Penang Medical College, Malaysia, for their valuable guidance and support. We thank Dr Datuk Khairiyah Muttalib for work on the protocol. We thank SEGi University, Malaysia for supporting this work.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
Cochrane Oral Health's Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see oralhealth.cochrane.org/trials.
MESH DESCRIPTOR Pit and Fissure Sealants
((resin* or fissure* or dental or compomer or tooth or composite* or "glass ionomer" or glassionomer or cyanoacrylate* or methacrylate or BIS‐GMA* or dimethacrylate* or "light activat*" or fluorid* or "chemical* cure*" or "light cure*" or GIC* or Giomer*) and seal*)
#1 or #2
MESH DESCRIPTOR Child EXPLODE ALL
(child* or adolescen* or teen* or pediatric or baby or babies or toddler* or pre‐school or "pre school" or infant* or paediatric or minor* or (immature NEAR5 teeth))
MESH DESCRIPTOR Tooth, Deciduous
((tooth or teeth) NEAR2 (primary or deciduous or milk*))
#4 or #5 or #6 or #7
#3 and #8
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
MESH DESCRIPTOR Pit and Fissure Sealants
((resin* or fissure* or dental or compomer or tooth or composite* or "glass ionomer" or glassionomer or cyanoacrylate* or methacrylate or BIS‐GMA* or dimethacrylate* or "light activat*" or fluorid* or "chemical* cure*" or "light cure*" or GIC* or Giomer*) and seal*)
#1 or #2
MESH DESCRIPTOR Child EXPLODE ALL
(child* or adolescen* or teen* or pediatric or baby or babies or toddler* or pre‐school or "pre school" or infant* or paediatric or minor* or (immature NEAR5 teeth))
MESH DESCRIPTOR Tooth, Deciduous
((tooth or teeth) NEAR2 (primary or deciduous or milk*))
#4 or #5 or #6 or #7
#3 and #8
Appendix 3. MEDLINE Ovid search strategy
1. "Pit and Fissure Sealants"/ 2. ((resin$ or fissure$ or dental or compomer or tooth or composite$ or "glass ionomer" or glassionomer or cyanoacrylate$ or methacrylate or BIS‐GMA$ or dimethacrylate$ or "light activat$" or fluorid$ or "chemical$ cure$" or "light cure$" or GIC$ or Giomer$) adj seal$).mp. 3. 1 or 2 4. exp Child/ 5. (child$ or adolescen$ or teen$ or pediatric or baby or babies or toddler$ or pre‐school or "pre school" or infant$ or paediatric or minor$ or (immature adj5 teeth)).mp. 6. Tooth, deciduous/ 7. ((tooth or teeth) adj2 (primary or deciduous or milk$)).mp. 8. or/4‐7 9. 3 and 8
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in MEDLINE (as described in Lefebvre 2020, box 3c).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
“Fissure sealant”/
((resin$ or fissure$ or dental or compomer or tooth or composite$ or "glass ionomer" or glassionomer or cyanoacrylate$ or methacrylate or BIS‐GMA$ or dimethacrylate$ or "light activat$" or fluorid$ or "chemical$ cure$" or "light cure$" or GIC$ or Giomer$) adj seal$).mp.
1 or 2
exp Child/
(child$ or adolescen$ or teen$ or pediatric or baby or babies or toddler$ or pre‐school or "pre school" or infant$ or paediatric or minor$ or (immature adj5 teeth)).mp.
“deciduous tooth”/
((tooth or teeth) adj2 (primary or deciduous or milk$)).mp.
or/4‐7
3 and 8
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in Embase (as described in Lefebvre 2020, box 3e).
Randomized controlled trial/
Controlled clinical study/
random$.ti,ab.
randomization/
intermethod comparison/
placebo.ti,ab.
(compare or compared or comparison).ti.
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
(open adj label).ti,ab.
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
double blind procedure/
parallel group$1.ti,ab.
(crossover or cross over).ti,ab.
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
(assigned or allocated).ti,ab.
(controlled adj7 (study or design or trial)).ti,ab.
(volunteer or volunteers).ti,ab.
human experiment/
trial.ti.
or/1‐19
random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
(((case adj control$) and random$) not randomi?ed controlled).ti,ab.
(Systematic review not (trial or study)).ti.
(nonrandom$ not random$).ti,ab.
"Random field$".ti,ab.
(random cluster adj3 sampl$).ti,ab.
(review.ab. and review.pt.) not trial.ti.
"we searched".ab. and (review.ti. or review.pt.)
"update review".ab.
(databases adj4 searched).ab.
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
Animal experiment/ not (human experiment/ or human/)
or/21‐33
20 not 34
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
Expert search:
(tooth AND (resin OR fissure OR dental OR compomer OR composite OR "glass ionomer" OR glassionomer OR cyanoacrylate OR methacrylate OR BIS‐GMA* OR dimethacrylate OR "light activated" OR fluoridated OR "chemical cure" OR "light cure" OR GIC* OR Giomer) AND (sealant OR seal))
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
(tooth AND resin AND seal* OR tooth AND fissure AND seal* OR tooth AND dental AND seal* OR tooth AND compomer AND seal* OR tooth AND composite AND seal* OR tooth AND "glass ionomer" AND seal* OR tooth AND glassionomer AND seal* OR tooth AND cyanoacrylate AND seal* OR tooth AND methacrylate AND seal* OR tooth AND BIS‐GMA* AND seal* OR tooth AND dimethacrylate AND seal* OR tooth AND "light activated" AND seal* OR tooth AND fluoridated AND seal* OR tooth AND "chemical cure" AND seal* OR tooth AND "light cure" AND seal* OR tooth AND GIC* AND seal* OR tooth AND Giomer AND seal*)
Data and analyses
Comparison 1. Resin‐based sealant versus no sealant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of caries at 12 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Incidence of caries at 24 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Glass ionomer‐based sealants versus no sealants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of caries at different follow‐up | 2 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 6‐month follow‐up | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 12‐ to 30‐month follow‐up | 2 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Glass ionomer sealants versus resin‐based sealants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Retention of sealants at 24 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 24 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Autopolymerised sealant versus light polymerised sealant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incidence of caries at 24–36 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Retention of sealants at 24–36 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baca 2007.
| Study characteristics | ||
| Methods |
Design: RCT with split‐mouth design Number of participants: 67 Setting: children were recruited from 5 primary schools and sealants were placed at the dental clinic of school of dentistry Country: Spain Unit of randomisation: quadrant Unit of analysis: tooth Follow‐up: 12 months Dropout: 11 children (16.41%) |
|
| Participants |
Number randomised: not mentioned clearly, assumed to be 67 children that met the inclusion criteria Number analysed: 44 children providing data for the primary dentition (176 teeth) Age: mean 7.32 years, SD 0.47 (range 7–8 years) Sex: 27 boys and 29 girls (among 56 present for final follow‐up) Mean dmft score at baseline: dft was 1.16, SD 2.06 Inclusion criteria: children with 4 healthy deciduous second molar teeth that required sealing Exclusion criteria: no information provided Baseline caries risk of participants: not mentioned |
|
| Interventions | 4 quadrants in each mouth were randomised to receive 1 of the 4 interventions.
Co‐intervention: none |
|
| Outcomes |
Study primary outcome
Study secondary outcome
Diagnostic criteria for caries: visual and tactile examination using WHO criteria, 1997 |
|
| Notes |
Funding: none (additional information provided by the author) Trial register: not registered (additional information provided by the author) Inter‐evaluator consistency: examiners were calibrated and kappa scores were > 0.60. Sample size: not calculated Personal communication: Pilar 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "every child received a different sealing material in each quadrant on a random basis." Comments: additional information provided by the author that computer‐assisted method was used for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided (information provided in response to request was unclear). |
| Blinding of participants and personnel (performance bias)‐Participants | Low risk | Comment: additional information provided by the author that participants were blinded to the group allocation. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: additional information provided by the author that operators were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "coloured glasses were worn by clinician to minimize sealant colour differences, guaranteeing a blind examination." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "11 participants (16.4%) had been lost to follow up because of changing school, illness or absenteeism." Comment: reasons for drop‐out unrelated to treatment. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | Comment: no evidence of any other bias. |
Chabadel 2021.
| Study characteristics | ||
| Methods |
Design: RCT with split‐mouth design Number of participants: 90 Setting: paediatric dental department of Montpellier hospital Country: France Unit of randomisation: teeth within a tooth pair Unit of analysis: tooth Follow‐up: 24 months Dropout: 5 children (19 teeth), 5.6% |
|
| Participants |
Number randomised: 90 children (278 teeth) Number analysed: 85 children (255 teeth) Age: range 3–7 years Sex: 49 boys and 41 girls Mean dmft score at baseline: d3ft was 1.63, SD 2.04 and D3MFT was 0.46, SD 0.86 Inclusion criteria: children covered by health insurance, having 1 or 2 pairs of contralateral first or second (or both) primary molars Exclusion criteria: presence of a sealant or a restoration and abnormal development like hypoplasia Baseline caries risk of participants: not reported. However, caries risk of the included children was assessed using AAPD caries risk assessment form. |
|
| Interventions | 139 tooth pairs (278 teeth) were randomised into 1 of 2 groups of 139 teeth each.
Co‐intervention: oral hygiene and dietary recommendations were given to all. |
|
| Outcomes |
Study primary outcomes
Diagnostic criteria for caries: visual and tactile examination. If the explorer caught on the tooth, the surface was coded as decayed. |
|
| Notes |
Funding: not reported Trial register: registered with Clinical Trials Registry (NCT02896088) Inter‐evaluator consistency: examiner was calibrated. Kappa value was not reported. Sample size: calculated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence was generated on a computer. The side was randomly allocated at each new inclusion." |
| Allocation concealment (selection bias) | Low risk | Quote: "An envelope was provided to the clinician in charge of the sealant placement." |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Comment: blinding was not possible as participants could see the material placed and control group had no treatment. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: the operator could not be blinded as sealant was placed on 1 tooth and contralateral tooth served as control. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the same operator who placed the sealant conducted all examinations at baseline and follow up." Comment: blinding of outcome assessor could not be performed in such studies. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all missing data mentioned. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | No other bias found. |
Chadwick 2005.
| Study characteristics | ||
| Methods |
Design: RCT with parallel design Number of participants: 508 Setting: children recruited from dental planning areas with high levels of caries and sealants were placed in community clinics, health centres and some in patient homes Country: UK Unit of randomisation: child Unit of analysis: child Follow‐up: mean (range): 1.34 years, SD 0.50 (12–30 months) Dropout: 11.6% |
|
| Participants |
Number randomised: 508 children Number analysed: 449 children Age: mean 2.02 years, SD 0.29 (range 1–3 years) Sex: 251 (49%) boys and 257 (51%) girls Mean dmft score at baseline: d‐0.72, m‐0.49, f‐0.08 Inclusion criteria: children aged 18–30 months, with caries‐free primary first molars, with or without caries elsewhere in the mouth, at high risk of developing caries Exclusion criteria: unerupted primary molars and primary molars with dentinal caries (additional information provided by the author) Baseline caries risk of participants: high caries risk |
|
| Interventions | 2 arms
Co‐intervention: standard package of dental health education on feeding and healthy eating was delivered; child‐sized toothbrushes and toothpaste were given for brushing for the participants in both groups. |
|
| Outcomes |
Study primary outcomes
Study secondary outcome
Diagnostic criteria for caries: visual and tactile using BASCD criteria for caries by Pitts and Evans 1997 |
|
| Notes |
Funding: NHS Research and Development Programme in Primary Dental Care Trial register: registered (ISRCTN98615437) (additional information provided by the author) Inter‐evaluator consistency: no information provided Sample size: calculated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomly allocated to active and control groups." Comment: no additional information was provided by the author. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. No additional information provided by the author. |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Comment: blinding of participants usually not possible in this study design for sealants as they can see the material on tooth. No information provided on 'placebo.' |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: the operator could not be blinded as sealant was placed on 1 tooth and contralateral tooth served as control. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor could not be performed in such studies. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The overall dropout rate was 11.6%. At follow up, number of subjects in the test group was 221 (drop out rate 8.3%) and control group was 228 (drop rate 10.6%)." Comment: drop‐out similar in intervention and comparator groups but reasons for attrition not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | Comments: no evidence of other bias. |
Corona 2005.
| Study characteristics | ||
| Methods |
Design: RCT with split‐mouth design Number of participants: 40 Setting: children were recruited from the ones seeking regular dental care from Public Health service Country: Brazil Unit of randomisation: teeth within a tooth pair Unit of analysis: tooth Follow‐up: 12 months Dropout: none |
|
| Participants |
Number randomised: 40 children; 40 pairs of primary and 40 pairs of permanent teeth (total 160 teeth) Number analysed: 40 children; 160 teeth Age: range 4–7 years Sex: 40% boys and 60% girls (additional information provided by the author) Mean dmft score: no information provided Inclusion criteria: children aged 4–7 years with ≥ 1 homologous pair of intact, caries‐free, fully erupted first or second primary molars or first permanent molars (or both), with deep and retentive pits and fissures Exclusion criteria: children without homologous primary and permanent molars, occlusal caries in molars and children with systemic diseases (additional information provided by the author) Baseline caries risk of participants: no information provided |
|
| Interventions | 2 treatment arms
Both materials sealants were placed under isolation with a rubber dam and saliva ejector. The occlusal surfaces were etched with 37% phosphoric acid gel (Gel Etchant, Kerr Corporation, Orange, California, USA) for 30 seconds Co‐intervention: none Diagnostic criteria for caries: visual inspection and bitewing radiograph |
|
| Outcomes |
Study primary outcome
|
|
| Notes |
Funding: none (additional information provided by the author) Trial register: not registered (additional information provided by the author) Inter‐evaluator consistency: examiner was calibrated and kappa scores were 0.86 (additional information provided by the author) Sample size: was calculated (additional information provided by the author) Personal communication: Regina 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a half mouth design, a filled resin based pit and fissure sealant was applied on randomly assigned upper/lower primary and permanent molars on one side of the mouth and a single bottled adhesive system used in association with a flowable resin composite was applied to the contralateral side." Comment: additional information provided by the author that random numbers were generated using Excel. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias)‐Participants | Low risk | Comment: additional information provided by the author that participants were blinded to group allocation. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: blinding was not possible as operator could visualise the difference in material. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor was not possible due to difference in material. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comments: all participants assessed at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | No evidence of any other bias. |
Fei 2011.
| Study characteristics | ||
| Methods |
Design: double‐blind RCT with split‐mouth design Number of participants: 100 Setting: children were recruited from kindergartens and sealants also placed in kindergarten Country: Guangzhou city, China Unit of randomisation: teeth within a tooth pair Unit of analysis: tooth Follow‐up: 18 months Dropout: 11% |
|
| Participants |
Number randomised: 100 children (200 teeth pairs) Number analysed: 89 children; 178 teeth pairs (168 teeth in resin‐based sealant group and 188 teeth in GIC sealant group) Age: mean 3 years Sex: no information provided Mean dmft score: no information provided Inclusion criteria: healthy 3‐year‐old children whose parents consented, with deep fossa or enamel caries not involving dentin, with or without radiographic evidence of caries Exclusion criteria: no information provided |
|
| Interventions | 2 treatment arms
Both materials sealants were placed under isolation with cotton rolls. There is no mention on etchant used. GIC was mixed in 1:1 ratio, filled in fossa and pressed with a vaseline‐coated gloved forefinger. Co‐intervention: none |
|
| Outcomes |
Study primary outcomes
Study secondary outcomes
Diagnostic criteria for caries: visual and tactile as per criteria in Oral Health Surveys basic methods 4th edition recommended by WHO 1997, recorded as dmft. |
|
| Notes |
Funding: no information provided Trial register: no information provided Inter‐evaluator consistency: for dental caries, kappa values were 0.85, 0.82, 0.90 and for sealants were 0.80 and 0.89 Sample size: not calculated There were flaws in reporting the data. There was variation in the number of resin sealants placed in the first and second follow‐up. Number of resin sealants placed is 168 in 6 months' follow‐up and 172 in 18 months' follow‐up. No explanation is found in the results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eight left or right unilateral deciduous molar teeth were treated with ART glass ionomer sealant. One week later contralateral deciduous molar teeth were treated with Resin sealant." Comment: no reply to request for information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Comment: no information provided. Comment: we consider it as high risk as GIC sealants and light‐cured resin sealants were placed at 2 separate points in time. Hence, the participants would know. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: same operator performed both ART sealants and resin sealants. Also there would be a difference in the sealant materials. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Examiner was blinded." Comment: blinding not possible – assessor could visualise the difference in sealant materials as GIC sealants are more opaque than resin‐based sealants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Children who did not participate in the test twice consecutively due to sick leave or transfer to another school were excluded. Finally 89 children were included with the loss rate of 11%." Comment: dropout not related to treatment. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | Comment: no evidence of any other bias. |
Ganesh 2006.
| Study characteristics | ||
| Methods |
Design: RCT with bilateral study design Number of participants: 100 Setting: children were recruited from 7 different schools Country: India Unit of randomisation: tooth pair Unit of analysis: group Follow‐up: 24 months Dropout: no dropout |
|
| Participants |
Number randomised: 100 children; 100 tooth pairs (100 resin‐based sealant group and 100 glass ionomer sealant group) Number analysed: 100 children; 100 teeth pairs Age: range 3–5 years Sex: no information provided Mean dmft score: no information provided Inclusion criteria: teeth erupted < 4 years ago, healthy, non‐hypoplastic, caries‐free second primary molars, with complete intact tooth structure Exclusion criteria: hypoplastic, unhealthy, lost tooth structure |
|
| Interventions | 2 treatment arms
Both the sealants were placed under isolation with cotton rolls and suction and also rubber dam wherever feasible. Occlusal surface was etched with 37% phosphoric acid, light‐cured for 20 seconds. GIC was mixed in 1.8:1 ratio, filled in fossa and light cured for 20 seconds. Fuji varnish was applied. Co‐intervention: none |
|
| Outcomes |
Study primary outcome
|
|
| Notes |
Funding: no information provided Trial register: no information provided Inter‐evaluator consistency: no information provided Sample size: not calculated Notes: few teeth were isolated using cotton rolls and few teeth with rubber dam. Paired data not considered for analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized study with bilateral study design was followed in which both sealant materials were applied in the same mouth on contra‐lateral teeth for direct comparison. For each of these patients, Fuji Vii was placed on one side while concise was used on contra lateral tooth." Comment: method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Quote: "Fuji VH (glass ionomer pink sealant, GC Corporation – Tokyo, Japan) was placed on one side while Concise (unfilled white resin sealant, 3M ESPE Dental Products, St.Paul, Minn) was used on the contra‐lateral tooth." Comment: difference in colour of the sealant material would make blinding impossible. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Blinding not possible – operator could visualise the material difference in sealants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding not possible – assessor could visualise the material difference in sealants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants assessed at follow‐up. |
| Selective reporting (reporting bias) | High risk | Comment: all prespecified outcomes reported, but a composite outcome of caries incidence and retention was used. The caries information could not be extracted from the composite outcome and was not reported separately, therefore, we were unable to use the caries data from this study. |
| Other bias | Low risk | No evidence of any other bias. |
Hotuman 1998.
| Study characteristics | ||
| Methods |
Design: RCT with split‐mouth design Number of participants: 52 Setting: children were recruited from paediatric dentistry section, municipal dental clinics around municipality of Arhus Country: Denmark Unit of randomisation: teeth within each tooth pair Unit of analysis: tooth pairs Follow‐up: 2–2.3 years Dropout: 1 pair of teeth |
|
| Participants |
Number randomised: 52 pairs of teeth in 52 children Number analysed: 51 pairs of teeth Age: mean 3.7 years (range 2.11–4.11 years) Sex: 25 boys and 27 girls Mean dmft score at baseline: not mentioned Inclusion criteria: children with pairs of sound primary molars Exclusion criteria: no information provided Baseline caries risk of participants: no information provided |
|
| Interventions | 2 treatment arms
Co‐intervention: none |
|
| Outcomes |
Study primary outcomes
Diagnostic criteria for caries: caries was diagnosed at cavitation level. |
|
| Notes |
Funding: no information provided Trial register: no information provided Inter‐evaluator consistency: no information provided Sample size: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The teeth within each tooth pair were randomly assigned to sealing with Delton1 or Prisma‐Shield1." Comment: method of random sequence generation not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Comment: no information provided, but difference in material may not allow for blinding of participants. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Quote: "All children were examined by the same dentist, who also placed all the sealants." Comment: blinding of operator not possible as 1 was a light‐cured resin and the other was autopolymerising resin. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessor was not possible due to material difference in sealants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One pair was omitted because the control tooth had been extracted." |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | No evidence of any other bias. |
Joshi 2019.
| Study characteristics | ||
| Methods |
Design: hybrid RCT with split‐mouth design (for comparison of retention of different sealant types) Number of participants: 111 Setting: paediatric dental department of a dental college Country: India Unit of randomisation: 2‐stage randomisation. At first, tooth pairs were randomised into study and control groups (sealant vs no sealant). Next, in study group alone, each tooth in a tooth pair was randomised to receive additional light curing or not Unit of analysis: tooth Follow‐up mean: 1 year Dropout: 10 teeth (8 teeth in study group and 2 teeth in control group) |
|
| Participants |
Number randomised: 180 pairs of primary second molars Number analysed: 175 pairs of primary molars Age: mean 4.19 years (range 3–5 years) Sex: 64 boys and 47 girls Mean dmft score: 8.45 SD 6.4 in study group and 8.35 SD 5.4 in control Inclusion criteria: fully erupted primary teeth with ≥ 1 pair of bilateral maxillary/mandibular caries‐free primary second molars, no history of preventive treatment in preceding 6 months, high risk of developing caries Exclusion criteria: permanent molars, medically compromised, children with physical limitation Baseline caries risk of participants: high caries risk |
|
| Interventions | 2 treatment arms
Co‐intervention: demonstration using videos and models for proper tooth brushing; all participants brushed twice daily with low‐fluoride toothpaste in all groups. |
|
| Outcomes |
Study primary outcome
Study secondary outcomes
Caries diagnostic criteria: visual using ICDAS |
|
| Notes |
Funding: none (additional information provided by the author) Trial register: registered (CTRI/2017/10/010248) Inter‐evaluator consistency: kappa values were 0.6 for dental caries and 0.7 for sealants Sample size: not calculated Personal communication: Sakshi 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "lottery method, even numbers for the study group and odd numbers for the control group, another round of randomisation was also done by asking the child to pick up the chit with right or left written on it." |
| Allocation concealment (selection bias) | Unclear risk | Comment: additional information provided by the author as allocation concealment done. However, no information provided on how it was done. |
| Blinding of participants and personnel (performance bias)‐Participants | High risk | Comment: children would be aware that they were receiving sealant or no sealant or an additional light‐curing technique. |
| Blinding of participants and personnel (performance bias)‐ Operator | High risk | Comment: additional information provided by the author that operator was not blinded as single operator performed all the intervention. Comment: blinding operator was not possible as 1 required light curing and the other did not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Assessors were blinded." Comment: blinding outcome assessor not possible to compare sealant with no sealant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At the first follow up a total dropout of 8 teeth in study group and 2 teeth in control group was recorded. No further dropouts occurred." Comment: drop‐out low and similar across groups. Reasons for drop‐out due to patient non‐attendance at clinical examination. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes were reported. |
| Other bias | Low risk | No evidence of any other bias. |
Unal 2015.
| Study characteristics | ||
| Methods |
Design: RCT with split‐mouth design Number of participants: 75 Setting: university clinic (additional info provided by the author) Country: Turkey (additional info provided by the author) Unit of randomisation: tooth Unit of analysis: child and tooth surface Follow‐up: 24 months Dropout: no dropouts (additional info provided by the author) |
|
| Participants |
Number randomised: 75 children, 150 teeth (25 children helioseal + Aegis, 25 children helioseal F + helioseal and 25 children Aegis + helioseal F) Number analysed: 75 children, 150 teeth Age: mean 4.88 years (range 4–7 years) Sex: 36 boys and 39 girls Mean dmft score: no information provided Inclusion criteria: occlusal surfaces of fully erupted teeth with deep and retentive fissures, without pre‐existence of caries, sealants, fillings and developmental defects, in healthy co‐operative children aged 4–7 years Exclusion criteria: no information provided Baseline caries risk: no information provided |
|
| Interventions | 3 treatment arms
All materials sealants were placed under isolation with cotton rolls and saliva ejector. There is no mention on etchant used. All were cured with LED curing light. Co‐intervention: all children and parents were informed about satisfactory oral hygiene procedures and dietary advice was also given. |
|
| Outcomes |
Primary outcomes
Caries diagnostic criteria: visual and tactile |
|
| Notes |
Funding: none (additional information provided by the author) Trial register: not registered (additional information provided by the author) Inter‐evaluator consistency: examiners were calibrated. Kappa scores were 0.87 for retention, 0.92 for marginal discolouration and 0.92 for marginal adaptation Sample size: calculated (additional information provided by the author) Notes: sealants were placed by different operators who were students Personal communication: Murat 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "The 75 children were randomly divided into three group (n‐25)." "Aegis and Helioseal were randomly applied in a split mouth design on mandibular second primary molars." Comment: additional information provided by author was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: additional information provided by the author that allocation concealment was done, but no information on how it was done. |
| Blinding of participants and personnel (performance bias)‐Participants | Low risk | Comment: additional information provided by author – participants were blinded. |
| Blinding of participants and personnel (performance bias)‐ Operator | Low risk | Comment: additional information provided by author – operator was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: additional information provided by author – assessor was blinded. We classified this as low risk of bias as Helioseal and Helioseal F are similar in appearance and difficult to be differentiated clinically. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: additional information provided by author – no dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported. |
| Other bias | Low risk | No evidence of any other bias. |
AAPD: American Association of Pediatric Dentistry; ART: atraumatic restorative treatment; BASCD: British Association for the Study of Community Dentistry; dft: decayed filled primary teeth; dmft: decayed missing filled primary teeth; DMFT: decayed missing filled permanent teeth; GIC: glass ionomer; ICDAS: International Caries Detection and Assessment System; LED: light‐emitting diode; RCT: randomised controlled trial; SD: standard deviation; World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvesalo 1975 | Not a randomised controlled trial. |
| Bakhshandeh 2015 | Sealant was placed on cavitated caries in dentin on occlusal surface of primary molars. |
| Borges 2011 | The study compared sealants with composite restoration on primary teeth on non‐cavitated dentinal caries. |
| Buonocore 1970 | Not a randomised controlled trial. |
| Buonocore 1971 | Not a randomised controlled trial. |
| Cline 1979 | Not a randomised controlled trial. Study did not have a control group. |
| Cogo 2009 | Not a randomised controlled trial. |
| Dias 2018 | Flowable resin was used as sealant to seal cavitated dentinal caries. |
| Duggal 1997 | The study investigated the effects of different etching times on the retention of sealants on primary and permanent molars and did not have a control group. |
| Going 1976 | Not a randomised controlled trial. |
| Hesse 2014 | Sealant was placed on cavitated caries in dentin on occlusal surface of primary molars. |
| Honkala 2015 | The study compared sealants with fluoride varnish. |
| Jing 2019 | The study compared the effect of fluorine protective paint used with sealant. |
| Luoma 1973 | Not a randomised controlled trial. |
| Maher 2013 | This study compared the effectiveness of different types of etchant on retention of sealants. |
| Poulsen 1979 | This study compared the isolation method on retention of a single fissure sealant. |
| Provenzano 2010 | Not a randomised controlled trial. |
| Rajic 2000 | Not a randomised controlled trial. |
| Raucci‐Neto 2015 | Randomisation was systematic. |
| Richardson 1977 | Not a randomised controlled trial. |
| Siripokkapat 2018 | Randomisation was systematic. |
| Tang 2018 | Not a randomised controlled trial. Study did not have a control group. |
| Vrbic 1983 | Not a randomised controlled trial. |
| Vrbic 1986 | Not a randomised controlled trial. |
| Vrbic 1999 | Not a randomised controlled trial. The study compared the sealant retention between primary and permanent molars. |
| Zhang 2008 | This study compared effectiveness of different types of etchant on retention of sealant. |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800016351.
| Study name | Four sealing materials combined with self‐etched adhesive system used as pit and fissure |
| Methods | RCT with parallel design, follow‐up 12 months |
| Participants | Health children aged 3–5 years with 4 second deciduous molars with deep fissures or fissure with signs of early caries |
| Interventions | Resin‐based sealant, flowable composite resin, glass‐ionomer cement and glass‐ionomer protective film |
| Outcomes | Survival rate and caries prevention rate |
| Starting date | September 2018 |
| Contact information | Dr Luo Yu, Stomatological Hospital of Kunming Medical University, China |
| Notes |
Differences between protocol and review
We had planned to conduct subgroup analyses based on sealant type. Instead we opted to present different sealant types as separate comparisons (Ramamurthy 2018).
Contributions of authors
Drafting of the protocol: PR, AR, PS, BF, SN, KM, PF, CZ, TW.
Screening trials: PR, AR, PS, BF.
Study selection: PR, AR, BF, PS, CZ, PF.
Data extraction: PR, AR, PS, BF.
Assessment of risk of bias: PR, CZ, TW.
Data analysis: CZ, TW.
Drafting of the review: PR, AR, PS, BF, PF, CZ, TW.
Sources of support
Internal sources
-
SEGi University, Malaysia
Provided fund for training in 'Writing a Protocol for Cochrane Systematic Reviews'
-
The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC) and NIHR Manchester Biomedical Research Centre, UK
Support to Cochrane Oral Health
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the National Health Service, or the Department of Health and Social Care.
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships-alliances). Contributors over recent years have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; Swiss Society for Endodontology, Switzerland.
Declarations of interest
PR: none.
AR: none.
PS: none.
BF: none.
SN: none.
PF: none. Dr Fee is a clinical advisor with Cochrane Oral Health.
CZ: none.
TW: none. Professor Walsh is a Statistical Editor with Cochrane Oral Health.
New
References
References to studies included in this review
Baca 2007 {published data only}
- Baca P, Bravo M, Baca AP, Jimenez A, Gonzalez-Rodriguez MP.Retention of three fissure sealants and a dentin bonding system used as fissure sealant in caries prevention: 12-month follow-up results. Medicina Oral Pathologia Oral Y Cirugia Bucal 2007;12:E459-63. [PubMed] [Google Scholar]
- Pilar B.Information required for Cochrane Review (personal communication). Email to: P Ramamurthy 16 September 2019.
Chabadel 2021 {published data only}
- Chabadel C, Veronneau J, Montal S, Tramini P, Moulis E.Effectiveness of pit and fissure sealants on primary molars: a 2-yr split-mouth randomized clinical trial. European Journal of Oral Sciences 2021;129(1):e12758. [DOI] [PubMed] [Google Scholar]
- NCT02896088.Effectiveness of sealants on molars (sealants). clinicaltrials.gov/ct2/show/NCT02896088 (first received 12 September 2016).
Chadwick 2005 {published data only}
- Barbara C.Additional information required for Cochrane Review (personal communication). Email to: P Ramamurthy 17 September 2019.
- Chadwick BL, Treasure ET, Playle RA.A randomised controlled trial to determine the effectiveness of glass ionomer sealants in pre-school children. Caries Research 2005;39(1):34-40. [DOI] [PubMed] [Google Scholar]
- ISRCTN98615437.A randomised controlled trial to determine the effectiveness of glass ionomer sealants in pre-school children. www.isrctn.com/ISRCTN98615437 (first received 23 January 2004).
Corona 2005 {published data only}
- Corona SA, Borsappo MC, Garcia L, Ramos RP, Palma-Dibb RG.Randomized, controlled trial comparing the retention of a flowable restorative system with a conventional resin sealant: one-year follow up. International Journal of Paediatric Dentistry 2005;15:44-50. [DOI] [PubMed] [Google Scholar]
- Regina P.Additional information required for Cochrane Review. Email to: P Ramamurthy 10 November 2019.
Fei 2011 {published data only}
- Fei R, Jian-Ping L, Shao-Hong H, Yan-Rong L, Wei-Hua F, Xiao-Chun C.Application of glass ionomer and light-cured resin sealant to the pit and fissure of deciduous teeth. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(38):7165-69. [Google Scholar]
Ganesh 2006 {published data only}
- CTRI/2017/10/010248.To compare retention, marginal discoloration and caries incidence between ART high viscosity GIC sealant applied with and without additional light curing. trialsearch.who.int/?TrialID=CTRI/2017/10/010248 (first received 30 October 2017).
- Ganesh M, Tandon S.Clinical evaluation of FUJI VII sealant material. Journal of Clinical Pediatric Dentistry 2006;31(1):52-7. [DOI] [PubMed] [Google Scholar]
Hotuman 1998 {published data only}
- Hotuman E, Rolling I, Poulsen S.Fissure sealants in a group of 3-4-year-old children. Internal Journal of Paediatric Dentistry 1998;8:159-60. [DOI] [PubMed] [Google Scholar]
Joshi 2019 {published data only}
- Joshi S, Sandhu M, Sogi S, Garg S, Dhindsa A.Split-mouth randomised clinical trial on the efficacy of GIC sealants on occlusal surfaces of primary second molar. Oral Health and Preventive Dentistry 2019;17:17-24. [DOI] [PubMed] [Google Scholar]
- Sakshi J.Information required for Cochrane Review. Email to: P Ramamurthy 2 November 2019.
Unal 2015 {published data only}
- Murat U.Information required for Cochrane Review. Email to: P Ramamurthy 9 September 2019.
- Unal M, Oznurhan F, Kapdan A, Durer A.A comparative clinical study of three fissure sealants on primary teeth: 24-month results. Journal of Clinical Pediatric Dentistry 2015;39:113-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvesalo 1975 {published data only}
- Alvesalo L, Brummer R, Bell YL.On the use of fissure sealants in caries prevention. Acta Odologia Scandinavica 1975;33(5):155-9. [DOI] [PubMed] [Google Scholar]
Bakhshandeh 2015 {published data only}
- Bakhshandeh A, Ekstrand K.Infiltration and sealing versus fluoride treatment of occlusal caries lesions in primary molar teeth. 2-3 years results. International Journal of Paediatric Dentistry 2015;25(1):43-50. [DOI] [PubMed] [Google Scholar]
Borges 2011 {published data only}
- Borges BC, Araujo RF, Dantas RF, Lucena A, Pinheiro IV.Efficacy of a non-drilling approach to manage non-cavitated dentin occlusal caries in primary molars: a 12-month randomized controlled clinical trial. International Journal of Paediatric Dentistry. 2011;22:44-51. [DOI] [PubMed] [Google Scholar]
Buonocore 1970 {published data only}
- Buonocore M.Adhesive sealing of pits and fissures for caries prevention with use of ultraviolet light. Journal of American Dental Association 1970;80:324. [DOI] [PubMed] [Google Scholar]
Buonocore 1971 {published data only}
- Buonocore MG.Caries prevention in pits and fissures sealed with an adhesive resin polymerised by ultraviolet light: a two year study of a single adhesive application. Journal of the American Dental Association 1971;82:1090-4. [DOI] [PubMed] [Google Scholar]
Cline 1979 {published data only}
- Cline JT, Messer LB.Long term retention of sealants applied by inexperienced operators in Minneapolis. Community Dentistry and Oral Epidemiology 1979;7(4):206-12. [DOI] [PubMed] [Google Scholar]
Cogo 2009 {published data only}
- Cogo E, Calura G.Clinical evaluation of two materials used as pit and fissure sealants: 2-year follow-up. International Journal of Clinical Dentistry 2009;2(4):241-7. [Google Scholar]
Dias 2018 {published data only}
- Dias KR, Andrade CB, Wait TT, Chamon R, Ammari MM, Soviero VM, et al.Efficacy of sealing occlusal caries with a flowable composite in primary molars: a 2-year randomized controlled clinical trial. Journal of Dentistry 2018;74:49-55. [DOI] [PubMed] [Google Scholar]
Duggal 1997 {published data only}
- Duggal MS, Tahnassebi JF, Toumba KJ, Mavromati C.The effect of different etching times on the retention of fissure sealants in second primary and first permanent molars. International Journal of Pediatric Dentistry 1997;7:81-6. [DOI] [PubMed] [Google Scholar]
Going 1976 {published data only}
- Going RE, Conti AJ, Haugh LD, Grainger DA.Two-year clinical evaluation of a pit and fissure sealant. Part-II: caries initiation and progression. Journal of American Dental Association 1976;92:578-85. [DOI] [PubMed] [Google Scholar]
Hesse 2014 {published data only}
- Hesse D, Bonifacio CC, Mendes FM, Braga MM, Imparato JC, Raggio DP.Sealing versus partial caries removal in primary molars: a randomised clinical trial. BMC Oral Health 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Honkala 2015 {published data only}
- Honkala S, ElSalhy M, Shyama M, Al-Mutawa SA, Boodai H, Honkala E.Sealant versus fluoride in primary molars of kindergarten children regularly receiving fluoride varnish: one-year randomized clinical trial follow-up. Caries Research 2015;49:458-66. [DOI] [PubMed] [Google Scholar]
Jing 2019 {published data only}
- Jing L, Hua Y.Effects of pit and fissure sealant combined with fluorine protective paint on prevention of children caries aged 5-8 years old. Shanghai Journal of Stomatology 2019;28(4):384-7. [PubMed] [Google Scholar]
Luoma 1973 {published data only}
- Luoma H, Meurman J, Helminen S, Heikkila H.Retention of fissure sealant with caries reduction in Finnish children after six months. Scandinavian Journal of Dental Research 1973;81:510-2. [DOI] [PubMed] [Google Scholar]
Maher 2013 {published data only}
- Maher MM, Elkshlan HI, El-Housseiny AA.Effectiveness of a self-etching adhesive on sealant retention in primary teeth. Pediatric Dentistry 2013;35:351-4. [PubMed] [Google Scholar]
Poulsen 1979 {published data only}
- Poulsen S, Peltoniemi AL.Retention of fissure sealant in primary second molars after 6 months. Scandinavian Journal of Dental Research 1979;87(4):328-30. [DOI] [PubMed] [Google Scholar]
Provenzano 2010 {published data only}
- Provenzano MG, Rios D, Fracasso ML, Marchesi A, Honorio HM.Clinical evaluation of a resin-modified glass ionomer cement (Vitremer®) used as pit-and-fissure sealant in primary molars [Avaliação Clínica dos Selantes Realizados com Cimento de Ionômero de Vidro Modifi cado por Resina (Vitremer®) em Molares Decíduos]. Brazilian Research in Pediatric Dentistry and Integrated Clinic 2010;10(2):233-40. [Google Scholar]
Rajic 2000 {published data only}
- Rajic Z, Gvozdanovic Z, Rajic-Mestrovic S, Bagic I.Preventive sealing of dental fissures with Heliosil: a two year follow up. Collegium Antropologicum 2000;24(1):151-5. [PubMed] [Google Scholar]
Raucci‐Neto 2015 {published data only}
- Raucci-Neto W, Castro-Raucci LM, Lepri CP, Faraoni-Romano JJ, da Silva JM, Palma-Dibb RG.Nd:YAG laser in occlusal caries prevention of primary teeth: a randomized clinical trial. Lasers in Medical Science 2015;30(2):761-8. [DOI] [PubMed] [Google Scholar]
Richardson 1977 {published data only}
- Richardson BA, Smith DC, Hargreaves JA.Study of a fissure sealant in mentally retarded Canadian children. Community Dentistry and Oral Epidemiology 1977;5:220-6. [DOI] [PubMed] [Google Scholar]
Siripokkapat 2018 {published data only}
- Siripokkapat K, Nakornchai S, Vichayanrat T.Comparison of giomer and fluoride releasing resin sealants in caries prevention among primary molars. South East Asian Journal of Tropical Medicine and Public Health 2018;49(3):527-36. [Google Scholar]
Tang 2018 {published data only}
- Tang YX, Wu J, Xu WT, Chen Y, Yu SX.Clinical efficacy of the glass ionomer cement used as pit and fissure sealant with and without acid etching in primary teeth. West China Journal of Stomatology 2018;36(6):646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrbic 1983 {published data only}
- Vrbic V.Retention of fissure sealant and caries reduction. Quintessence International 1983;4:421-4. [Google Scholar]
Vrbic 1986 {published data only}
- Vrbic V.Five-year experience with fissure sealing. Quintessence International 1986;17(6):371-2. [Google Scholar]
Vrbic 1999 {published data only}
- Vrbic V.Retention of a fluoride-containing sealant on primary and permanent teeth 3 years after placement. Quintessence International 1999;30(12):825-8. [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang S, Qin M, Li J.A comparison study on the effect of self-etching adhesive and phosphoric acid fissure sealant in children. West China Journal of Stomatology 2008;26(6):630-2. [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800016351 {published data only}
- ChiCTRI1800016351.Four sealing materials combined with self-etched adhesive system used as pit and fissure. chictr.org.cn/searchprojen.aspx/ChiCTR1800016351 (first received 9 March 2018).
Additional references
AAPD 2013
- American Academy of Pediatric Dentistry.Guideline on caries risk assessment and management for infants, children and adolescents. Pediatric Dentistry 2013;35(5):157-64. [PubMed]
Abanto 2011
- Abanto J, Carvalho TS, Mendes FM, Wanderley MT, Bonecker M, Raggio DP.Impact of oral diseases and disorders on oral health related quality of life in preschool children. Community Dentistry and Oral Epidemiology 2011;39(2):105-14. [DOI] [PubMed] [Google Scholar]
Acs 1992
- Acs G, Lodolini G, Kaminski S, Cisneros GJ.Effect of nursing caries on body weight in a paediatric population. Pediatric Dentistry 1992;14:302-5. [PubMed] [Google Scholar]
Ahovuo‐Saloranta 2017
- Ahovuo-Saloranta A, Forss H, Walsh T, Nordblad A, Mäkelä M, Worthington HV.Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD001830. [DOI: 10.1002/14651858.CD001830.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anusavice 2013
- Anusavice KJ, Shen C, Rawls HR.Phillips' Science of Dental Materials. 12th edition. St Louis (MO): Elsevier/Saunders, 2013. [Google Scholar]
Arrondo 2009
- Arrondo JL, Collado MI, Soler I, Triana R, Ellacuria J.The setting reaction of polyacid modified composite resins or compomers. Open Dentistry Journal 2009;3:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayhan 1996
- Ayhan H, Suskan E, Yildirim S.The effect of nursing or rampant caries on height, body weight and head circumference. Journal of Clinical Pediatric Dentistry 1996;20:209-12. [PubMed] [Google Scholar]
Baelum 2006
- Baelum V, Heidmann J, Nyvad B.Dental caries paradigms in diagnosis and diagnostic research. European Journal of Oral Sciences 2006;114(4):263-77. [DOI] [PubMed] [Google Scholar]
Beauchamp 2008
- Beauchamp J, Caufield PW, Crall JJ, Donly K, Feigal R, Gooch B, et al.Evidence-based clinical recommendations for the use of pit-and-fissure sealants: a report of the American Dental Association Council on Scientific Affairs. Journal of American Dental Association 2008;139(3):257-68. [DOI] [PubMed] [Google Scholar]
Bowen 1982
- Bowen RL.Composite and sealant resins past, present, and future. Pediatric Dentistry 1982;4(1):10-5. [PubMed] [Google Scholar]
BSPD 2000
- British Society of Paediatric Dentistry.British Society of Paediatric Dentistry: a policy document on fissure sealants in paediatric dentistry. International Journal of Paediatric Dentistry 2000;10:174-7. [DOI] [PubMed] [Google Scholar]
Carvalho 2014
- Carvalho JC.Caries process on occlusal surfaces: evolving evidence and understanding. Caries Research 2014;48(4):339-46. [DOI] [PubMed] [Google Scholar]
CDC and National Center for Health Statistics 2005
- Centers for Disease Control and Prevention, National Center for Health Statistics.National Health and Nutrition Examination Surveys 1999–200. www.cdc.gov/nchs/nhanes.htm (accessed 15 July 2017).
Cueto 1967
- Cueto EI, Buonocore MG.Sealing of pits and fissures with an adhesive resin: its use in caries prevention. Journal of the American Dental Association 1967;75(1):121-8. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG.Meta-analysis combining parallel and cross-over clinical trials. II: binary outcomes. Statistics in Medicine 2002;21(15):2145-59. [DOI] [PubMed] [Google Scholar]
Donly 2002
- Donly KJ, García-Godoy F.The use of resin-based composite in children. Pediatric Dentistry 2002;24:480-8. [PubMed] [Google Scholar]
Dye 2007
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al.Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Statistics 2007;248:1-92. [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A.Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Fejerskov 2003
- Fejerskov O, Nyvad B.Is dental caries an infectious disease? Diagnostic and treatment consequences for the practitioner. In: Schou L, editors(s). Nordic Dentistry. 2003 edition. Copenhagen (Denmark): Quintessence Publishing Company, 2003:141-51. [Google Scholar]
Filstrup 2003
- Filstrup SL, Briskie D, da Fonseca M, Lawrence L, Wandera A, Inglehart MR.Early childhood caries and quality of life: child and parent perspectives. Pediatric Dentistry 2003;25(5):431-40. [PubMed] [Google Scholar]
Gomez 2015
- Gomez J.Detection and diagnosis of the early caries lesion. BMC Oral Health 2015;15(S1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gooch 2009
- Gooch BF, Griffin SO, Gray SK, Kohn WG, Rozier RG, Siegal M, et al.Preventing dental caries through school-based sealant programs. Journal of the American Dental Association 2009;140(11):1356-65. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group.Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 15 August 2021. Available at gradepro.org.
Hatrick 2015
- Hatrick CD, Eakle WS.Dental Materials Clinical Applications for Dental Assistants and Dental Hygienists. 3rd edition. New York (NY): Elsevier, 2015. [Google Scholar]
Herald 2013
- Herald H, Edward SJ, Andre VR, Clifford S.Sturdevant's Art and Science of Operative Dentistry. 6th edition. St Louis (MO): Elsevier, 2013. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hopewell 2006
- Hopewell S, Clarke M, Askie L.Reporting of trials presented in conference abstracts needs to be improved. Journal of Clinical Epidemiology 2006;59(7):681-84. [DOI] [PubMed] [Google Scholar]
Horowitz 1982
- Horowitz AM, Frazier PJ.Issues in the widespread adoption of pit and fissure sealants. Journal of Public Health Dentistry 1982;42(4):312-23. [DOI] [PubMed] [Google Scholar]
ICDAS II 2008
- International Caries Detection and Assessment System (ICDAS) Coordinating Committee.ICDAS II International Caries Assessment and Detection System. www.icdas.org/assets/downloads/Appendix.pdf (accessed 1 August 2017).
Ismail 2007
- Ismail AI, Sohn W, Tellez M, Amaya A, Hasson H, Pitts NB.The International Caries Detection and Assessment System for measuring dental caries. Community Dentistry and Oral Epidemiology 2007;35:170-8. [DOI] [PubMed] [Google Scholar]
Kassebaum 2015
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W.Global burden of untreated caries: a systematic review and metaregression. Journal of Dental Research 2015;94(5):650-8. [DOI] [PubMed] [Google Scholar]
Kazeminia 2020
- Kazeminia M, Abdi A, Shohaimi S, Jalali R, Vaisi-Raygani A, Salari N, et al.Dental caries in primary and permanent teeth in children's worldwide, 1995 to 2019: a systematic review and meta-analysis. Head & Face Medicine 2020;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidd 2011
- Kidd E.The implications of the new paradigm of dental caries. Journal of Dentistry 2011;Suppl 2(39):S3-8. [DOI] [PubMed] [Google Scholar]
Lam 2020
- Lam PV, Sardana D, Ekambaram M, Lee GH, Yiu CK.Effectiveness of pit and fissure sealants for preventing and arresting occlusal caries in primary molars: a systematic review and meta-analysis. Journal of Evidence-Based Dental Practice 2020;20(2):101404. [DOI] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al.Technical supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Low 2008
- Low LM, Duraman N, Mahmood U.Mapping the structure, composition and mechanical properties of human teeth material. Science and Engineering 2008;28:243-7. [Google Scholar]
Mascarenhas 2008
- Mascarenhas AK, Nazar H, Al-Mutawaa S, Soparkar P.Effectiveness of primer and bond in sealant retention and caries prevention. Pediatric Dentistry 2008;30(1):25-8. [PubMed] [Google Scholar]
McLean 1992
- McLean JW.Clinical applications of glass ionomer cements. Operative Dentistry 1992;Suppl 5:184-90. [PubMed] [Google Scholar]
Mejàre 2014
- Mejàre I, Axelsson S, Dahlén G, Espelid I, Norlund A, Tranæus S, et al.Caries risk assessment: a systematic review. Acta Odontologica Scandinavica 2014;72(2):81-91. [DOI] [PubMed] [Google Scholar]
Mertz‐Fairhurst 1984
- Mertz-Fairhurst EJ.Current status of sealant retention and caries prevention. Journal of Dental Education 1984;48(Suppl 2):18-26. [PubMed] [Google Scholar]
Mickenautsch 2013
- Mickenautsch S, Yengopal V.Validity of sealant retention as surrogate for caries prevention – a systematic review. PLoS One 2013;8(10):e77103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1992
- Miller J, Vaughan-Williams SE, Furlong R, Harrison L.Dental caries and children's weights. Journal of Epidemiology and Community Health 1982;36:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortimer 1970
- Mortimer KV.The relationship of deciduous enamel structure of dental disease. Caries Research 1970;4:206-23. [DOI] [PubMed] [Google Scholar]
Pinkham 2005
- Pinkham J, Casamassimo P.Pediatric Dentistry. Infancy through Adolescence. 4th edition. New Delhi (India): Saunders, 2005. [Google Scholar]
Pitts 2021
- Pitts N, Mayne C.A global consensus for achieving a dental cavity-free future. www.acffglobal.org (accessed prior to 8 February 2022):1-36.
PRISMA 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group.Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ripa 1976
- Ripa LW.The current status of occlusal sealants. Journal of Preventive Dentistry 1976;3:6-14. [PubMed] [Google Scholar]
Sanders 2015
- Sanders BJ.Pit and fissure sealants and preventive resin restorations. In: McDonald and Avery's Dentistry for the Child and Adolescent. 10th edition. New York (NY): Elsevier, 2015:177-84. [Google Scholar]
Simonsen 2002
- Simonsen RJ.Pit and fissure sealant: review of the literature. Pediatric Dentistry 2002;24:393-414. [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA.Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732-4. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D.Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Tandon 2008
- Tandon S.Textbook of Pedodontics. Second edition. Hyderabad (India): Paras, 2008. [Google Scholar]
Ten Cate 1999
- Ten Cate JM.Current concepts on the theories of the mechanism of action of fluoride. Acta Odontologica Scandinavica 1999;57(6):325-9. [DOI] [PubMed] [Google Scholar]
Vann 1999
- Vann WF, Mclver FT.Pit and fissure sealants: an overview of issues related to diagnosis and treatment decisions. www.mchoralhealth.org/PDFs/Pit_fissureMonograph.PDF (accessed 14 February 2018).
Welbury 2004
- Welbury R, Raadal M, Lygidakies NA.EAPD guidelines for the use of pit and fissure sealants. European Journal of Pediatric Dentistry 2004;5(3):179-84. [PubMed] [Google Scholar]
WHO 2014
- World Health Organization.Global health estimates 2014 summary tables – World Bank regions, year 2012: DALYs by age, sex and cause. who.int/entity/healthinfo/global_burden_disease/GHE_DALY_WBR_2000_2012.xls (accessed prior to 8 February 2022).
Wright 2016
- Wright JT, Tampi MP, Graham L.Sealants for preventing and arresting pit and fissure occlusal caries in primary and permanent molars. A systematic review of randomized control trials – a report of the American Dental Association and the American Academy of Pediatric Dentistry. Pediatric Dentistry 2016;38(4):282-308. [PubMed] [Google Scholar]
References to other published versions of this review
Ramamurthy 2018
- Ramamurthy P, Rath A, Sidhu P, Fernandes B, Nettem S, Muttalib K, et al.Sealants for preventing dental caries in primary teeth. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD012981. [DOI: 10.1002/14651858.CD012981] [DOI] [PMC free article] [PubMed] [Google Scholar]


