Abstract
Background
Historical loss in American Indians (AIs) is believed to contribute to high incidence of mental health disorders, yet less is known about the associations between historical loss and physical health.
Purpose
To investigate whether frequency of thought about historical loss predicts risk factors for chronic physical health conditions in an AI community.
Methods
Using Community Based Participatory research (CBPR) and Ecological Momentary Assessment (EMA), we measured frequency of thoughts about historical loss in 100 AI adults residing on the Blackfeet reservation. Participants completed a 1-week monitoring period, during which ambulatory blood pressure and daily levels of psychological stress were measured. At the end of the week, we collected a dried blood spot sample for measurement of C-reactive protein (CRP).
Results
In hierarchical linear regression models controlling for demographics and relevant covariates, greater frequency of thoughts about historical loss predicted higher average daily psychological stress (B = .55, t = 6.47, p < .001, ΔR2 = .30) and higher levels of CRP (B = .33, t = 3.93, p < .001, ΔR2 = .10). Using linear mixed modeling with relevant covariates, we found that greater thoughts about historical loss were associated with higher systolic ambulatory blood pressure (B = .32, 95% CI = .22–.42, t = 6.48, p < .001, ΔR2 = .25; Fig. 1c) and greater diastolic ambulatory blood pressure (B = .19, 95% CI = .11–.27, t = 4.73, p < .001, ΔR2 = .19).
Conclusions
The data suggest that frequency of thought about historical loss may contribute to increased subclinical risk for cardiovascular disease in the Blackfeet community.
Keywords: Historical loss, American Indians, Ambulatory blood pressure, Inflammation, Psychological stress ∙ Ecological Momentary Assessment
In Blackfeet American Indian Adults, more frequent thoughts about historical loss and trauma predicted a psychological and physiological profile linked to increased risk for cardiovascular disease
Introduction
In racial and ethnic minority groups, comprehensive reviews report consistent evidence of adverse health effects of discrimination and stress related to cultural identity across both mental and physical health outcomes [1–3]. As a stressor, discrimination is both unpredictable and uncontrollable, characteristics which are thought to amplify the negative consequences of psychological stress for health [4]. The experience of discrimination as a social stress triggers a pattern of physiological responses. For example, prior work in racial and ethnic minority groups indicates a relationship between perceived racism and ambulatory blood pressure patterns [5–8]. These investigations find that perceived racism or discrimination is associated with higher average ambulatory blood pressure, a pattern which is associated with increased risk for cardiovascular disease (CVD) [9, 10]. To date, the relationship between cultural stressors and health-relevant outcomes have not been investigated in American Indians, a population which is disproportionately affected by CVD compared to Non-Hispanic Whites [11–13].
For over 500 years, American Indians (AIs) have been subjected to various forms of colonization, genocide, and violence imposed by European and American policy [14]. Beginning in 1860, AIs were forcibly estranged from their families and placed in Indian Residential schools, which were designed to impose assimilation and to eradicate the AI way of life [15]. It was not until the passing of the Indian Child Welfare Act in 1978, that AI parents had the legal right to keep their children out of these schools [16]. These traumatic group experiences contribute to historical trauma, which includes cumulative psychological and emotional wounding carried over the lifespan and across generations [14, 17–22]. The Historical Trauma Response (HTR) is described as a collection of responses to these traumas. The HTR can include depression, substance use, suicidal ideation, anger, and difficulties with emotion regulation [21]. As noted previously, historical trauma can be passed across generations [14, 15], and risk factors for poor health may be exacerbated by the HTR [16–18].
Prior research indicates that historical trauma and associated loss of AI culture, language, and land contribute to high incidence of mental health conditions in AI populations [23–26], and while it is posited that historical trauma and these losses may contribute to persistent disparities in AI physical health, the mechanisms which may contribute to this relationship are unknown.
In a previous investigation, narratives from members of a Pacific Northwest tribe indicated that the effects of historical trauma inhibit engagement in protective cardiovascular health behaviors [27]. In line with this, it is possible that the frequency with which AIs think about these losses shapes daily psychological stress, immune system inflammation, and blood pressure, thereby contributing to chronic health disparities.
As noted previously, AIs have disproportionately high incidence of CVD compared to Non-Hispanic Whites [11, 12], and psychological stress, high blood pressure, and chronic immune system inflammation are implicated in risk for CVD [28–30]. As such, these outcomes are important risk factors for AI communities. Based on literature documenting relationships between psychological stress, high blood pressure and immune system inflammation, and heightened risk for CVD [28–30], and building upon documented associations between historical trauma and loss to poor mental health in AIs [14–26], we investigated the relationships between the degree to which AIs think about historical loss and key variables related to CVS risk, including psychological stress, blood pressure and a marker of immune system inflammation.
We investigated these relationships in a sample of AI adults residing on the Blackfeet reservation, located 40 miles south of the Canadian border in Browning, Montana. The Blackfeet tribe is one of the only tribal communities in Montana that remain on their original territory. In 2017, the Blackfeet completed a Community Health Assessment, including incidence of chronic diseases, availability of health services, and measures of substance use. The findings from this assessment indicated that CVD is one of the top health concerns for community members [31], highlighting the importance for an improved understanding of psychosocial factors which may contribute to the observed high incidence of this chronic disease on the reservation. In the present study, we hypothesized that greater frequency of thoughts about historical loss would be associated with risk factors for these chronic diseases including higher perceived psychological stress, greater immune system inflammation, and elevated blood pressure. In order to understand the nature of these relationships in the real-life environments of Blackfeet adults, we utilized Ecological Momentary Assessment (EMA) to capture reports of psychological stress at the end of each day during a week-long-period and measuring blood pressure as Blackfeet adults moved through their daily routines and real-life environments (i.e., ambulatory blood pressure). EMA assesses individual’s experiences as they unfold in real-time in their natural environments [32]. While EMA has not been used in an exclusively AI population previously, it has been used in other racial and ethnic groups to capture daily life measures of CVD risk factors including psychological stress and ambulatory blood pressure [33,34].
Methods
This study was approved by the Blackfeet Nation Institutional Review Board and the Montana State University Institutional Review Board. The research utilized community based participatory research (CBPR) methods which emphasizes the importance of equitable involvement of community members and researchers in all stages of the research process [35, 36]. As such, the scope of the research, questionnaires, and measures, were reviewed and approved by a Community Advisory Board (CAB) consisting of four members of the Blackfeet Community. The CAB was also utilized in the interpretation of these findings and decisions about dissemination of these findings to the community. We did not conduct formal a-prior power analysis for this study. Minimum sample size was based on past research using similar methods and linear-mixed models, with general recommendations of 100 participants when collecting three or more time points [37]. Post hoc sensitivity analyses indicate that our sample size was able to detect a minimum effect size of small to medium size (f2 = .08) assuming α = .05 and 1 − β = .80. This effect size is generally smaller than similar studies examining stress, immune functioning, and blood pressure [38, 39].
Using advertisements on various Blackfeet social media sites, we recruited a sample of 100 AI adults ranging from 20 to 78 years of age (M = 42.30, SD = 15.08). To be eligible for participation in this research, individuals had to be 18 years or older, self-identify as American Indian, and currently reside on the Blackfeet reservation. Exclusionary criteria for this research included a clinically diagnosed sleep disorder or chronic health condition. In addition, individuals taking any regular prescription medication were not eligible to participate. Participants visited an office located in a building on the Blackfeet Community College campus. They met with the project coordinator and provided written informed consent. After the informed consent process, participants completed a series of questionnaires including demographics, a measure of symptoms of depression and anxiety, and a measure of the frequency of thoughts about historical loss.
Next, the project coordinator recorded participants’ weight and height and obtained a dried blood spot sample. The project coordinator then set each participant up for a week-long monitoring period. First, the project coordinator installed the Illumivu EMA application on participants’ mobile devices. The project coordinator used the Illumivu EMA software on the office computer to schedule alerts to be delivered to participants at set times to prompt them to complete surveys on their phone and initialize devices. All of the data provided through the app on their mobile device were stored on the app until the participants returned to the office for their final visit. During this final visit, the project coordinator downloaded the data from the participants’ Illumivu app onto a password-protected computer. Subsequently, the data were downloaded into an excel file before being converted to an SPSS file for statistical analyses. The project coordinator also initialized a home blood pressure monitor which participants wore during waking hours for 3 days of the monitoring period. For these 3 days, the Illumivu app was programmed to prompt participants to activate the ambulatory blood pressure cuff four times per day to record a measure of ambulatory systolic and diastolic blood pressure.
Measures
Historical Loss
We used the Historical Loss Scale (HLS), [14] a standardized measure that assesses the frequency with which Indigenous individuals think about the losses to their culture, land and people as a result of European colonization. The HLS includes 12 items. Participants are asked to indicate the frequency with which they think about the described losses. Participants provide their response using a 6-point scale, anchored by (1) several times a day and (6) never. Each response is reverse-scored and a composite score is calculated as the sum of each of these responses. Previous work indicates that the 12 items can be adequately explained by a single latent factor (1). Cronbach’s alpha for the HLS was 0.75.
Psychological Stress
We used the psychological stress subscale from the Depression, Anxiety, and Stress Scale-21 items (DASS-21) [40]. The stress subscale contains seven items and participants were asked to indicate the degree to which the statements applied to them over the course of each day. Example items from the scale are “I found it hard to wind down,” and “I tended to over-react to situations.” At the end of each day of the seven-day monitoring period, participants responded on a scale from 0 to 3 (0: Did not apply to me at all; 1: Applied to me to some degree or some of the time; 2: Applied to me to a considerable degree or a good part of the time; 3: Applied to me very much or most of the time). Responses to each of the seven items were summed and the total score was multiplied by 2 according to the scale instructions. Cronbach’s alpha for the stress subscale of the DASS-21 was 0.78.
Immune System Inflammation
C-reactive protein (CRP) was assessed via dried blood spots (DBS). DBS are collected using a relatively noninvasive procedure that has been well validated against standard methods for whole blood collected via venipuncture [41, 42]. Blood spots were collected at the end of the week-long monitoring period. Briefly, participants’ fingers were cleaned with alcohol and subsequently punctured with a sterile, disposable microlancet commonly used by diabetic patients. Five drops of capillary blood were collected onto standardized filter paper, and blood spot samples were covered and dried overnight. After 24 hr, the DBS were transferred to a −20 °C freezer until later analyses. DBS samples were assayed for CRP using a high-sensitivity enzyme-linked immunosorbent assay [33]. All samples were run in duplicate, and intra- and inter-assay coefficients of variation were 6.7% and 8.9% respectively.
Ambulatory Blood Pressure
Ambulatory blood pressure, which is measured across the day as individuals move through their regular routines, has been shown to be a better predictor of cardiovascular morbidity and mortality risk compared to clinic measures of resting blood pressure [43–45]. Ambulatory blood pressure was recorded using the Qardio Arm Blood pressure device, a home blood pressure monitor. The accuracy of self-measured blood pressure with the Qardio arm device was evaluated using the European Society of Hypertension (ESH) protocol [46] and compared to a criterion device the Omron M3 Intellisense in a sample of 100 participants across two measurement sessions [47]. This work found that the Qardio arm device displayed consistent readings both within and across sessions, and that the measurements corresponded closely to those from the previously validated criterion device, the Omron M3. The Qardio Arm passed all validation standards set by the ESH protocol. We used the Illumivu EMA application to deliver messages to participants to activate the cuff at four evenly spaced intervals across each day of monitoring. Specifically, participants were prompted by the Illumivu app on their phone to initialize the Qardio device to take a reading at 10 am, 1 pm, 4 pm, and 7 pm Participants were instructed to sit down with their legs uncrossed before obtaining each recording during their initial meeting with the project coordinator, and were given these instructions each time the Illumivu application prompted them to obtain a blood pressure recording. If participants successfully recorded all four of the blood pressure readings, this provided a total of 12 measurements of ambulatory blood pressure across the 3-day period. During the initial in-lab visit, the project coordinator provided an extensive training focused on using the Qardio arm device to record blood pressure measurements. Upon returning to the office to meet with the project coordinator 1 week after initial appointment, the project coordinator downloaded all of the recorded blood pressures to a computer. Each recording included a measurement of systolic and diastolic blood pressure.
Covariates
Biological sex, age, and annual income, and symptoms of depression and anxiety were utilized as covariates in all analyses given known relationships between these variables and each of our outcomes [48–51]. In addition to these covariates, body mass index (BMI) and alcohol use were included as covariates in our models predicting a marker of immune system inflammation and ambulatory blood pressure given their documented relationships with our outcomes as well as with CVD risk [52–55].
Demographic Variables
Participants self-reported their biological sex and age and their annual income. Annual income was measured on a scale from 1 (below US$20,000), 2 (US$20,000–$40,000), 3(US$40,001–$60,000), 4(US$60,001–$80,000), 5(US$80,001–$100,000) and 6 (US$100,001 and above) [56].
Body Mass Index
The project coordinator measured participant weight using a scale and participant height using a stadiometer.
Symptoms of Depression and Anxiety
We used the Hospital Anxiety and Depression Scale (HADS) as a measure of current symptoms of depression and anxiety during the initial office visit [57]. HADS has 14 items, 7 items comprise a depression subscale, and 7 items comprise an anxiety subscale. Individuals respond to each item using a four-point response category (0–3), with possible scores ranging from 0–21 for depression and 0–21 for anxiety. Cronbach’s alpha was 0.85 for the anxiety subscale and 0.88 for the depression subscale.
Alcohol Use
We used the Alcohol Use Disorders Identification Test (AUDIT) [58] to measure alcohol consumption, drinking behaviors and alcohol-related problems. The scale consists of 10 questions. Example questions from this scale include, “How often do you have a drink containing alcohol?” with the response options of never (0), monthly or less (1), two to four times a month (2), two to three times a week (3), and four or more times a week (4) and, “How often during the last year have you found you were not able to stop drinking once you started?” with the response options of never (0), less than monthly (1), monthly (2), weekly (3), and daily or almost daily (4). Cronbach’s alpha for the scale was .63.
Data Analyses
Statistical analyses were conducted using SPSS (version 24; IBM, Armonk, NY) and R (version 4.0.3) [59]. Our analytic sample includes 100 Blackfeet adults. Continuous covariates were centered with z-scores before being used in analyses. Participant sex was coded as male = 1 and female = 2. Initial Pearson product–moment correlation analyses were performed to determine bivariate relationships between historical loss, psychological stress, levels of CRP, and average ambulatory blood pressure. We then examined the distribution of historical loss, and found that it was not highly skewed. Natural log transformations were applied to CRP levels to correct their positively skewed distributions.
We also calculated a measure of ambulatory blood pressure load (i.e., the proportion of blood pressure readings that were above normal ranges). For this measure, we used cutoffs provided by the American Heart Association [60], with systolic blood pressure readings below 120 considered normal, and diastolic blood pressure readings below 80 considered normal.
Next, we used two hierarchical linear regressions to examine i) the relationship between historical loss and psychological stress, and ii) the relationship between historical loss and levels of CRP. Ambulatory blood pressure was measured four times a day for 3 days, resulting in twelve total measurement points. Linear mixed-models using the lme4 package [61] were therefore used to examine the relationship between historical loss and ambulatory blood pressure while accounting for the dependency introduced by repeated measures by specifying participant as a random-effect. Two models were estimated with systolic and diastolic blood pressure specified as dependent variables and historical loss specified as the primary independent variable. All models accounted for age, sex, annual income, alcohol use, BMI, anxiety symptoms, depressive symptoms as covariates based on past research [48–51]. Confidence intervals were calculated using bootstrapping and indicate that the population coefficients will be found in 95% of the 10,000 sampled confidence intervals.
Missing Data
Several efforts were taken to minimize missing data. Participants were provided with extensive training regarding ambulatory blood pressure readings and were sent a message through the Illumivu App at the exact time they were supposed to take the blood pressure reading in order to ensure protocol compliance. Upon completion of the study, three participants were missing one or more ambulatory blood pressure readings. There was no other missing data in this study.
Results
The sample was 53% female. The mean historical loss score was 40.26. (SD = 16.12). Descriptive statistics are listed in Table 1 and bivariate correlations for the main variables of interest and covariates are listed in Table 2. Historical Loss was not significantly related to any of our demographic variables (age, gender, annual income) or alcohol use (r < .09, p > .35).
Table 1.
Means, Standard Deviations, and Correlations
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 42.30 | 15.08 | |||||||||||
| 2. Gender | n/a | n/a | .13 | ||||||||||
| 3. Income | 1.10 | 1.16 | .02 | −.13 | |||||||||
| 4.Dep. Symp. | 9.67 | 4.49 | .01 | .13 | .10 | ||||||||
| 5. Anxiety Symp. | |||||||||||||
| 6. Body Mass Index | 31.37 | 5.94 | .10 | .02 | .04 | .04 | |||||||
| 7. Alcohol use |
|||||||||||||
| 6. Historical Loss | 40.26 | 16.12 | .07 | .10 | .05 | .11 | .09 | ||||||
| 7. Av. Psych. Stress | 21.21 | 7.89 | −.07 | −.04 | −.01 | .18 | .04 | .52** | |||||
| 8. C-Reactive Protein | 2.56 | 1.47 | −.05 | .10 | .15 | .24** | .20* | .40** | .10 | ||||
| 9. Av. Amb. Sys. BP | 129.65 | 8.95 | .09 | .01 | −.11 | −.01 | .12 | .33** | .26** | .02 | |||
| 10. Av. Amb. Dia. BP | 80.17 | 7.54 | .06 | −.01 | −.13 | −.15 | .12 | .31** | .23** | .05 | .64** |
Note. M and SD are used to represent mean and standard deviation, respectively. * p < .05. ** p < .01. Symp. Symptoms; Av. average; Amb. ambulatory; Sys systolic; Dia. diastolic; BP Blood pressure.
Table 2.
Correlations Between Main Variables of Interest and Covariates
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||||
| 2. Gender | .12 | |||||||||||
| 3. Income | .01 | −.15 | ||||||||||
| 4.Dep. Symp. | −.001 | .14 | .13 | |||||||||
| 5. Anxiety Symp. | .12 | .04 | .07 | .59** | ||||||||
| 6. Body Mass Index | −.15 | −.03 | −.03 | −.02 | −.10 | |||||||
| 7. Alcohol use |
.18 | .05 | −.04 | .24** | .13* | .18* | ||||||
| 8. Historical Loss | .10 | .10 | .01 | .11 | .05 | .15 | .10* | |||||
| 9. Av. Psych. Stress | −.07 | −.04 | −.04 | −.19 | .15 | .11 | .16* | .52** | ||||
| 10. C-Reactive Protein | -.05 | .11 | .14 | .24** | .10 | .47** | .02 | .40** | .10 | |||
| 11. Av. Amb. Sys. BP | .04 | .12 | .10 | .12 | .10 | .04 | −.05 | .57** | .23* | .44** | ||
| 12. Av. Amb. Dia. BP | .05 | .08 | .05 | .12 | .11 | .15 | −.06 | .50** | .14 | .39** | .74** |
Note. M and SD are used to represent mean and standard deviation, respectively. * p < .05. ** p < .01. Symp. Symptoms; Av. average; Amb. ambulatory; Sys systolic; Dia.diastolic; BP Blood pressure.
Blood Pressure Load
A total of 17 participants had systolic blood pressure under 120 and 30 participants had diastolic blood pressure under 80. There were a total of 1,200 blood pressure measures across people and time. Of these measurements, n = 1018 (84.83%) were higher than 120 for systolic blood pressure and n = 981 (81.75%) were higher than 80 for diastolic blood pressure.
Historical Loss and Psychological Stress
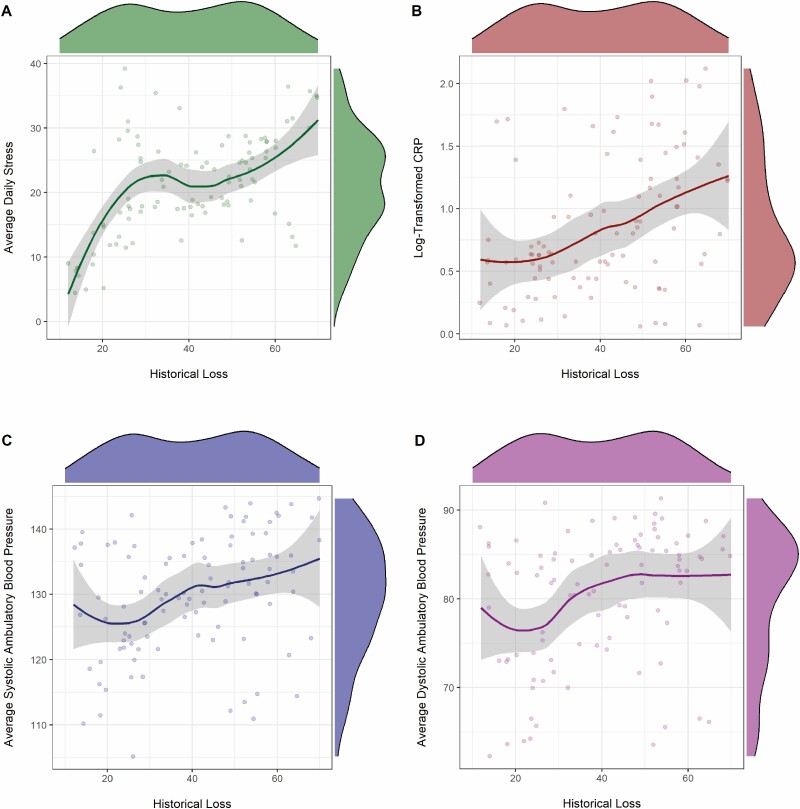
In a hierarchical linear regression controlling for age, sex, income, and depressive symptoms, and symptoms of anxiety, reported frequency of thoughts about historical loss was a significant predictor of average psychological stress over the week-long monitoring period (B = .55, t = 6.47, p < .001, ΔR2 = .30, 95% CI [0.19,0.35]; Fig. 1a), with individuals who reported thinking more about historical loss reporting more psychological stress (see Table 3a for full regression model).
Figure 1.
Associations between historical loss and indicators of stress. (a) Association between historical loss and average daily stress. (b) Association between historical loss and CRP. (c) Association between historical loss and systolic ambulatory blood pressure. (d) Association between historical loss and diastolic ambulatory blood pressure.
Table 3.
a) Estimates from hierarchical linear regression model with historical loss predicting average daily psychological stress. b) Estimates from hierarchical linear regression model with historical Loss predicting C-reactive protein levels. c) Estimates from linear mixed modeling predicting Ambulatory Systolic and Diastolic Blood Pressure
| Average Daily Psychological Stress | ||||||
|---|---|---|---|---|---|---|
| B | 95% CI | p | ||||
| Step 1 | ||||||
| Age | −0.09 | −0.14 to0.04 | 0.293 | |||
| Gender | −0.05 | −3.58 to1.93 | 0.281 | |||
| Income | −.02 | −1.38 to–1.13 | 0.844 | |||
| Depressive Symptoms | −.22 | −0.73 to −0.01 | 0.046 | |||
| Anxiety Symptoms | −.04 | −0.56 to 0.40 | 0.39 | |||
| Step 2 | ||||||
| Historical Loss | 0.55 | 0.19–0.35 | <0.001 | |||
| Ambulatory SBP | Ambulatory DBP | |||||
| B | 95% CI | p | B | 95% CI | p | |
| Step 1 | ||||||
| Age | 0.00 | −0.10 to 0.11 | 0.944 | 0.03 | −0.06 to 0.12 | 0.467 |
| Gender | 2.24 | −0.92 to 5.41 | 0.165 | 0.65 | −1.97 to 3.27 | 0.626 |
| Income | 0.77 | −0.67 to 2.21 | 0.296 | 0.25 | −0.95 to 1.44 | 0.686 |
| Anxiety Symptoms | −0.11 | −0.65 to 0.44 | 0.699 | 0.04 | −0.41 to 0.49 | 0.858 |
| Depressive Symptoms | 0.15 | −0.28 to 0.58 | 0.481 | 0.04 | −0.31 to 0.40 | 0.818 |
| Body Mass Index | −0.06 | −0.36 to 0.24 | 0.707 | 0.14 | −0.11 to 0.39 | 0.268 |
| Alcohol use | −0.15 | −0.49 to 0.20 | 0.409 | −0.16 | −0.45 to 0.12 | 0.259 |
| Step 2 | ||||||
| Historical Loss | 0.32 | 0.22–0.42 | <0.001 | 0.19 | 0.11–0.27 | <0.001 |
Note. SBP systolic blood pressure; DBP diastolic blood pressure.
| C-Reactive Protein | |||
|---|---|---|---|
| B | 95% CI | p | |
| Step 1 | |||
| Age | −0.01 | −0.01 to 0.01 | .930 |
| Gender | 0.08 | −0.09 to 0.24 | .370 |
| Income | 0.10 | −0.03 to 0.12 | .243 |
| Depressive Symptoms | 0.18 | −0.002 to 0.04 | .076 |
| Anxiety Symptoms | −0.02 | −0.03 to 0.03 | .831 |
| Body Mass Index | 0.46 | 0.03–0.06 | .001 |
| Alcohol use | −0.08 | −0.03 to 0.01 | .321 |
| Step 2 | |||
| Historical Loss | 0.33 | 0.01–0.02 | .001 |
Historical Loss and CRP
In a hierarchical linear regression controlling for age, sex, annual income, depressive symptoms, symptoms of anxiety, alcohol use, and body mass index, reported frequency of thoughts about historical loss was a significant predictor of levels of log-transformed CRP (B = .33, t = 3.93, p < .001, ΔR2 = .10, 95 % CI [0.01,0.02]; Fig. 1b), with individuals who reported thinking more about historical loss having higher levels of CRP (see Table 3b for full regression model).
Historical Loss and Ambulatory Blood Pressure
Two linear-mixed models controlling for age, sex, income, depressive symptoms, anxiety symptoms, alcohol use, and body mass index, were estimated to examine associations between frequency of thoughts about historical loss and systolic and diastolic blood pressure. After accounting for covariates, greater thoughts about historical loss were associated with higher systolic ambulatory blood pressure (B = .32, 95% CI = .22–.42, t = 6.48, p < .001, ΔR2 = .25; Fig. 1c) and greater diastolic ambulatory blood pressure (B = .19, 95% CI = .11–.27, t = 4.73, p < .001, ΔR2 = .19; Fig. 1d) (See Table 3c for full regression models).
Discussion
To the best of our knowledge, this is the first investigation to examine the association between historical loss and key psychological and biological risk factors for CVD in an AI community with high incidence of CVD. Our preliminary findings provide initial evidence that frequency of thoughts about historical loss may act as an important predictor of psychological stress, levels of a marker of immune system inflammation, and levels of ambulatory blood pressure, all outcomes which are implicated in CVD. Blackfeet adults who thought more frequently about historical loss (e.g., loss of language, land and traditions), reported higher levels of psychological stress in their daily lives, had higher levels of CRP, and had higher average ambulatory systolic and diastolic blood pressure over a week long period. This psychological and physiological profile is associated with greater risk for CVD [28–30].
It is important to note that this sample was comprised of individuals who were free from clinically diagnosed chronic disease. As a result, the purpose of this preliminary work was to investigate whether frequency of thought about historical trauma associated with subclinical physiological risk for CVD. Prior research indicates that ambulatory blood pressure is a marker of future CVD risk [44, 45]. As such, although the average ambulatory blood pressure observed in this study was in the prehypertensive range, in the current sample individuals who thought more frequently about historical loss had higher ambulatory blood pressure, and thus may be at greater risk for CVD in the future. In a similar manner, high CRP is associated with increased risk of CVD [29]. The average levels of CRP observed in the current research was 2.56 mg/mL. In a previous investigation circulating levels of CRP predicted risk for CVD up to 10 years later. Specifically, adults who had CRP levels over 3 mg/mL had 1.82 times greater risk for developing CVD 10 years later compared to adults who had CRP levels less than 1 mg/mL [62]. In the current sample, 18% of the participants had CRP levels over 3 mg/mL, and the mean CRP levels were only slightly under the cutoff for high levels of CRP used in prior work. As such, the CRP values observed here are relevant given that the observed mean CRP levels were just under what has previously associated with increased risk for subsequent CVD.
In samples comprised of adults from other racial and ethnic groups, psychological stress, levels of immune system inflammation, and blood pressure have predicted risk for future chronic diseases [28, 29]. It is possible that in a similar manner, greater frequency of thoughts about historical loss for Blackfeet adults would predict future risk for CVD. These findings are in line with previous work highlighting the negative impact of historical trauma and associated loss on mental health, well-being, and health-behaviors [14–22], and draw attention to the need for interventions and therapies to focus on resolution of historical loss [18, 20]. While outside of the scope of the data reported here, AI scholars indicate that to be effective, these interventions and therapies should be focused upon a return to the traditional ways of life [23–26], including the incorporation of tribally specific cultural practices such as talking circles and sweat lodges.
The findings reported here build upon a significant literature indicating that historical trauma and associated loss of AI culture, traditions, language, and land have contributed to enduring disparities in health. As noted previously, this body of work largely focuses on mental health (i.e., chronic mental health disorders including depression and anxiety disorders) and health behaviors [14–22]. To the best of our knowledge, this is the first research to indicate that historical loss may also contribute to psychological (i.e., psychological stress) and biological risk factors (i.e., immune system inflammation and ambulatory blood pressure) for CVD. After discussion with the CAB, we plan to share the findings of this research with the community at the North American Indian Day Event held on the Blackfeet reservation in the coming year. Future work should extend upon these findings to elucidate additional health behaviors and processes (e.g., dietary intake, sleep-wake cycles), and social factors (e.g., social relationships and environments) which may be responsible for the observed relationship between thoughts about historical loss and risk factors for CVD in the Blackfeet community. In order to better understand mechanistic pathways which may account for the observed relationship between frequency of thought about historical trauma and risk factors for CVD, future work should include simultaneous measurement of psychological and physiological responses to experiences as they unfold in the real-life environment. For example, a measurement of the physical and social environment the person is at during the time of the ambulatory blood pressure reading, along with the measurement of the psychological stress the person is experiencing in that moment, will allow us to have a better understanding of whether psychological stress accounts for the observed relationship between historical trauma and ambulatory blood pressure.
It will also be important to identify resilience factors. As a community, the Blackfeet people have demonstrated tremendous resilience in spite of the high levels of adversity and trauma they have faced. In our previous work, we found that sense of belonging to the community offset the physiological risk typically associated with childhood trauma [63]. It is possible that similar psychosocial factors may reduce the degree to which historical loss affects risk for chronic diseases. As with the current research, future work in this area should utilize CBPR in order to ensure that the aims, methods, and measures are in line with the community needs and values and that the developed interventions are culturally congruent [64–66].
This work comes with significant limitations which must be acknowledged. First, the relationships observed here are specific to the Blackfeet community and cannot be generalized to other AI populations. Furthermore, the sample of community members who participated in this project do not necessarily reflect the larger Blackfeet community. Second, we were unable to obtain multiple dried blood spot samples, which would have allowed us to track changes in levels of CRP to better understand dynamic correspondence between psychological stress related to historical loss and subsequent changes in levels of immune system inflammation. Relatedly, we were only able to measure one marker of immune system inflammation. Future work should aim to obtain a more comprehensive measurement of immune system profiles including multiple pro-inflammatory cytokines (e.g., interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)) as well as anti-inflammatory cytokines (e.g., interleukin-4 (IL-4) and interleukin-10 (IL-10)). While there is limited work on the relationship between proinflammatory cytokines and CVD risk in AIs, there is some evidence from an AI sample that levels of proinflammatory cytokines associate with increased risk of heart failure [67].
In addition, while our collection of ambulatory blood pressure across three full days provides a strong reflection of average levels of blood pressure as community members moved through their daily lives, we were unable to investigate full 24-hr blood pressure profiles. This may be warranted in future work given that the absence of nocturnal dipping of blood pressure has been shown to be a predictor of future risk for CVD [68, 69]. Finally, in future investigations, it will be important to better disentangle one’s tendency to think about historical trauma with general rumination tendencies. While including depressive symptoms as a covariate in our analyses may address this issue to some degree, future research should include a more precise measure of rumination tendencies.
Finally, more research is needed to better understand how thoughts about historical trauma may change physiological outcomes linked to risk for CVD. For example, it is possible that thinking about historical trauma may contribute to a sedentary lifestyle or poor diet, both of which might contribute to higher ambulatory blood pressure and high levels of immune system inflammation. Separately, previous work indicates that AIs have worse sleep compared to other racial and ethnic groups [70, 71]. It is possible that thoughts about historical trauma may contribute to poor sleep in this community, and poor sleep is known to affect levels of immune system inflammation and blood pressure [72]. Future work should investigate whether in AI communities, patterns of sleep contribute to observed relationships between historical trauma and physiological outcomes linked to CVD risk.
In conclusion, our preliminary findings demonstrate a significant association between frequency of thoughts about historical loss and several key psychological and physiological variables that contribute to cardiovascular risk, including perceived psychological stress, immune system inflammation and blood pressure. Based on our findings, it is possible that community-driven interventions which are designed to help community members cope with historical loss, or which work to restore the traditional practices and values of the Blackfeet people, could reduce psychological stress, immune system inflammation, and blood pressure levels, and in doing so positively impact the health and well-being of the community.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P20GM104417, P20GM103474 and U54GM115371. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to thank Emily Salois and Community Advisory Board Members Brad Hall, Lester Johnson, Melveena Malatare, and Mary Ellen Laframboise for their help with the development of this project as well as the Blackfeet Nation IRB for their time reviewing the research proposal and research products.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Neha A. John-Henderson, Benjamin Oosterhoff, Taylor D. Kampf, Brad Hall, Lester R. Johnson III, Mary Ellen Laframboise, Melveena Malatare, Emily Salois, Jason R. Carter, Alexandra K. Adams declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Authors’ Contributions
N.A.J.-H. designed the study, analysed and interpreted the data, and wrote the manuscript. B.O. conducted statistical analyses, created figures and tables, and participated in writing and reviewing manuscript. T.D.K. conducted literature review and helped with data cleaning. B.H., L.R.J., M.E.L., M.M., and E.S., are members of the community advisory board which developed the project. They also helped with data interpretation and manuscript preparation. J.R. and A.K.A. helped with data interpretation and writing and reviewing of manuscript.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Paradies Y. A systematic review of empirical research on self-reported racism and health. Int J Epidemiol. 2006;35: 888–901. [DOI] [PubMed] [Google Scholar]
- 2. Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135: 531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams DR. Racial/ethnic variations in women’s health: the social embeddedness of health. Am J Public Health. 2008;98:S38–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beatty Moody DL, Waldstein SR, Tobin JN, Cassells A, Schwartz JC, Brondolo E. Lifetime racial/ethnic discrimination and ambulatory blood pressure: The moderating effect of age. Health Psychol. 2016;35:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brondolo E, Libby DJ, Denton EG, et al. . Racism and ambulatory blood pressure in a community sample. Psychosom Med. 2008;70:49–56. [DOI] [PubMed] [Google Scholar]
- 7. Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosom Med. 2003;65:746–750. [DOI] [PubMed] [Google Scholar]
- 9. Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26:1290–1299. [DOI] [PubMed] [Google Scholar]
- 10. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–250. [DOI] [PubMed] [Google Scholar]
- 11. Shalala DE, Trujillo MH, Hartz GJ, D’Angelo AJ.. Regional differences in Indian health: 1998–1999. Rockville, MD: Indian Health Service; 1999. [Google Scholar]
- 12. Espey DK, Jim MA, Cobb N, et al. . Leading causes of death and all-cause mortality in American Indians and Alaska Natives. Am J Public Health. 2014;104(Suppl 3):S303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhoades DA, Welty TK, Wang W, et al. . Aging and the prevalence of cardiovascular disease risk factors in older American Indians: the Strong Heart Study. J Am Geriatr Soc. 2007;55:87–94. [DOI] [PubMed] [Google Scholar]
- 14. Whitbeck LB, Adams GA, Hoyt DR, Chen X. Conceptualizing and measuring historical trauma among American Indian people. Am J Comm Psychol. 2004;33:119–130. [DOI] [PubMed] [Google Scholar]
- 15. Bombay A, Matheson K, Anisman H. The intergenerational effects of Indian Residential Schools: Implications for the concept of historical trauma. Transcult Psychiatr. 2014;51(3):320–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher MLM, Singel WT, Fort KE.. Facing the Future (American Indian studies series). East Lansing: Michigan State University Press; 2009. [Google Scholar]
- 17. Brave Heart MY. The historical trauma response among natives and its relationship with substance abuse: a Lakota illustration. J Psychoactive Drugs. 2003;35:7–13. [DOI] [PubMed] [Google Scholar]
- 18. Brave Heart MY, DeBruyn LM. The American Indian Holocaust: healing historical unresolved grief. Am Indian Alsk Native Ment Health Res. 1998;8:56–78. [PubMed] [Google Scholar]
- 19. Brave Heart MY. Gender differences in the historical trauma response among the Lakota. J Health Soc Policy. 1999;10:1–21. [DOI] [PubMed] [Google Scholar]
- 20. Grant H. American Indians: working with American Indians and historical trauma. Illness Crisis Loss. 2008;16(2):125–136. [Google Scholar]
- 21. Evans-Campbell T. Historical trauma in American Indian/Native Alaska communities: A multilevel framework for exploring impacts on individuals, families, and communities. J Interpers Violence. 2008;23(3):316–338. [DOI] [PubMed] [Google Scholar]
- 22. Robin RW, Chester B, Goldman D. Cumulative trauma and PTSD in American Indian communities. In Marsella A. J., Friedman M. J., Gerrity E. T., & Scurfield R. M. (Eds.), Ethnocultural aspects of posttraumatic stress disorder: Issues, research, and clinical applications (p. 239–253). American Psychological Association; 1996. doi: 10.1037/10555-009 [DOI] [Google Scholar]
- 23. BigFoot DS, Schmidt SR. Honoring children, mending the circle: cultural adaptation of trauma-focused cognitive-behavioral therapy for American Indian and Alaska native children. J Clin Psychol. 2010;66:847–856. [DOI] [PubMed] [Google Scholar]
- 24. Gray JS, Rose WJ. Cultural adaptation for therapy with American Indians and Alaska natives. J Multicult Couns Devel. 2012;40(2):82 [Google Scholar]
- 25. Gone JP, Alcántara C. Identifying effective mental health interventions for American Indians and Alaska Natives: a review of the literature. Cultur Divers Ethnic Minor Psychol. 2007;13:356–363. [DOI] [PubMed] [Google Scholar]
- 26. Walker SC, Whitener R, Trupin EW, Migliarini N. American Indian perspectives on evidence-based practice implementation: results from a statewide Tribal Mental Health Gathering. Adm Policy Ment Health. 2015;42:29–39. [DOI] [PubMed] [Google Scholar]
- 27. Beltrán R, Schultz K, Fernandez AR, Walters KL, Duran B, Evans-Campbell T. From ambivalence to revitalization: negotiating cardiovascular health behaviors related to environmental and historical trauma in a Northwest American Indian Community. Am Indian Alsk Native Ment Health Res. 2018;25:103–128. [DOI] [PubMed] [Google Scholar]
- 28. Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: the third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol. 2008;18:302–309. [DOI] [PubMed] [Google Scholar]
- 29. Pepys MB, Berger A. The renaissance of C reactive protein. BMJ. 2001;322:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackfeet Community Health Assessment. Center for Health Equity, Education, & Research website. Updated January, 2017. Available at https://www.cheerequity.org/blackfeet-community-health-assessment.html. Accessibility verified March, 2020. [Google Scholar]
- 32. Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavorial medicine. Ann Behav Med. 1994;16(3):199–202. doi: 10.1093/abm/16.3.199 [DOI] [Google Scholar]
- 33. Mendez DD, Sanders SA, Lai YH, et al. . Ecological momentary assessment of stress, racism and other forms of discrimination during pregnancy using smartphone technology. Paediatr Perinat Epidemiol. 2020;34:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dunton G, Dzubur E, Li M, Huh J, Intille S, McConnell R. Momentary assessment of psychosocial stressors, context, and asthma symptoms in hispanic adolescents. Behav Modif. 2016;40:257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holkup PA, Tripp-Reimer T, Salois EM, Weinert C. Community-based participatory research: an approach to intervention research with a Native American community. ANS Adv Nurs Sci. 2004;27:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. John-Henderson NA, Henderson-Matthews B, Ollinger SR, et al. . Development of a biomedical program of research in the blackfeet community: challenges and rewards. Am J Community Psychol. 2019;64:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smart Richman L, Pek J, Pascoe E, Bauer DJ. The effects of perceived discrimination on ambulatory blood pressure and affective responses to interpersonal stress modeled over 24 hours. Health Psychol. 2010;29:403–411. [DOI] [PubMed] [Google Scholar]
- 39. Van Dyke ME, Vaccarino V, Dunbar SB, et al. . Socioeconomic status discrimination and C-reactive protein in African-American and White adults. Psychoneuroendocrinology. 2017;82:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–343. [DOI] [PubMed] [Google Scholar]
- 41. Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography Soc Biol. 2014;60(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–654. [DOI] [PubMed] [Google Scholar]
- 43. Schwartz JE, Burg MM, Shimbo D, et al. . Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer-based US population: results from the masked hypertension study. Circulation. 2016;134:1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang YY, Li Y, Wang JG. Ambulatory blood pressure monitoring in the prediction and prevention of coronary heart disease. Curr Hypertens Rep. 2013;15:167–174. [DOI] [PubMed] [Google Scholar]
- 45. Kikuya M, Ohkubo T, Asayama K, et al. . Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. [DOI] [PubMed] [Google Scholar]
- 46. O’Brien E, Pickering T, Asmar R, et al. ; Working Group on Blood Pressure Monitoring of the European Society of Hypertension . Working group on blood pressure monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17. [DOI] [PubMed] [Google Scholar]
- 47. Mazoteras Pardo V, Losa Iglesias ME, López Chicharro J, Becerro de Bengoa Vallejo R. The qardioarm app in the assessment of blood pressure and heart rate: reliability and validity study. JMIR Mhealth Uhealth. 2017;5:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hampton T. Chronic stress and depression. JAMA. 2012;308(5):444. [Google Scholar]
- 49. Fawzy M, Hamed SA. Prevalence of psychological stress, depression and anxiety among medical students in Egypt. Psychiatry Res. 2017;255:186–194. [DOI] [PubMed] [Google Scholar]
- 50. Hildrum B, Mykletun A, Holmen J, Dahl AA. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. Br J Psychiatry. 2008;193:108–113. [DOI] [PubMed] [Google Scholar]
- 51. Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology. 2013;38:1573–1585. [DOI] [PubMed] [Google Scholar]
- 52. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270–278. [DOI] [PubMed] [Google Scholar]
- 54. Santana NMT, Mill JG, Velasquez-Melendez G, et al. . Consumption of alcohol and blood pressure: results of the ELSA-Brasil study. PLOS ONE. 2018;13:e0190239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Husain K, Ansari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J Cardiol. 2014;6:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. John-Henderson NA, Stellar JE, Mendoza-Denton R, Francis DD. Socioeconomic status and social support: social support reduces inflammatory reactivity for individuals whose early-life socioeconomic status was low. Psychol Sci. 2015;26:1620–1629. [DOI] [PubMed] [Google Scholar]
- 57. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 59. R Core Team 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. URL https://www.R-project.org/. [Google Scholar]
- 60. High Blood Pressure. http://www.heart.org. https://www.heart.org/en/health-topics/high-blood-pressure. Accessibility verified November 23, 2020.
- 61. Bates D, Sarkar D, Bates MD, Matrix L. The lme4 package. R package version. 2007;2(1):74. [Google Scholar]
- 62. Cushman M, Arnold AM, Psaty BM, et al. . C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. [DOI] [PubMed] [Google Scholar]
- 63. John-Henderson NA, Henderson-Matthews B, Ollinger SR, et al. . Adverse childhood experiences and immune system inflammation in adults residing on the blackfeet reservation: the moderating role of sense of belonging to the community. Ann Behav Med. 2020;54:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caldwell JY, Davis JD, Du Bois B, et al. . Culturally competent research with American Indians and Alaska Natives: findings and recommendations of the first symposium of the work group on American Indian Research and Program Evaluation Methodology. Am Indian Alsk Native Ment Health Res. 2005;12:1–21. [DOI] [PubMed] [Google Scholar]
- 65. Kelley MN, Lowe JR. A culture-based talking circle intervention for native American youth at risk for obesity. J Community Health Nurs. 2018;35:102–117. [DOI] [PubMed] [Google Scholar]
- 66. Walters KL, Johnson-Jennings M, Stroud S, et al. . Growing from our roots: strategies for developing culturally grounded health promotion interventions in American Indian, Alaska native, and native Hawaiian communities. Prev Sci. 2020;21:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barac A, Wang H, Shara NM, et al. . Markers of inflammation, metabolic risk factors, and incident heart failure in American Indians: the Strong Heart Study. J Clin Hypertens (Greenwich). 2012;14:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shivpuri S, Allison MA, Macera CA, Lindsay S, Gallo LC. Associations between nocturnal blood pressure dipping and the metabolic syndrome in high- vs. low-acculturated Mexican American women. Am J Hypertens. 2013;26:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peixoto AJ, White WB. Circadian blood pressure: clinical implications based on the pathophysiology of its variability. Kidney Int. 2007;71:855–860. [DOI] [PubMed] [Google Scholar]
- 70. Chapman DP, Croft JB, Liu Y, Perry GS, Presley-Cantrell LR, Ford ES. Excess frequent insufficient sleep in American Indians/Alaska natives. J Environ Public Health. 2013;2013:259645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ehlers CL, Wills DN, Lau P, Gilder DA. Sleep quality in an adult American Indian community sample. J Clin Sleep Med. 2017;13:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42:141–152. [DOI] [PubMed] [Google Scholar]