Abstract
Photoimmunotherapy (PIT) targets and destroys tumor cells through irradiation with 690-nm red light after the administration of cetuximab sarotalocan sodium, which contains IRDye700DX bound to cetuximab. In Japan, PIT is a new treatment that is covered by insurance only for unresectable head and neck cancers. However, this treatment has conditional early approval. There have been no case reports describing the efficacy of this treatment in a real-world setting thus far. We report our experience with PIT for head and neck cancer. A 76-year-old man with laryngeal cancer underwent radiation therapy and surgery. Skin involvement in the right submandibular region was subsequently noted. We diagnosed local recurrence and performed PIT for this lesion. Partial response was achieved after the first PIT session, and progressive disease was diagnosed after the second session. Many aspects of PIT remain unclear and should, therefore, be clarified in further research. Despite this uncertainty, PIT may become an effective treatment strategy for head and neck cancer if the patient selection criteria are delineated.
Keywords: Cetuximab sarotalocan sodium, Head and neck carcinoma, Laryngeal cancer, Photoimmunotherapy, Photosensitivity
Introduction
Photoimmunotherapy (PIT) combines a drug with a laser system. Cetuximab sarotalocan sodium consists of a photosensitive substance complex of cetuximab, a chimeric antihuman epidermal growth factor receptor monoclonal antibody, and a photoactivated dye (IRDye700DX IR700; Rakuten Medical). This treatment specifically destroys tumor cells through irradiation with 690-nm red light after cetuximab sarotalocan sodium administration [1, 2, 3].
However, PIT has only been conditionally approved, and there are currently no clinical reports on this treatment. In Japan, PIT is currently covered by insurance only for the treatment of unresectable head and neck cancers. Here, we present a case of PIT performed at our institution for laryngeal cancer.
Case Report
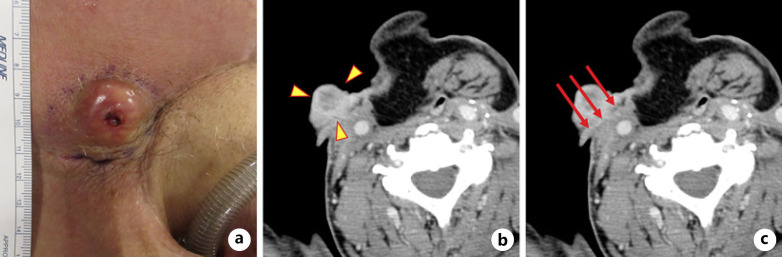
A 76-year-old man underwent radiation therapy (66 Gy) for managing laryngeal cancer (T1aN0M0 stage I squamous cell carcinoma) in November 20xx (date blinded to protect the identity of the patient). In September 20xx + 1, the patient underwent vertical partial laryngectomy owing to local recurrence. In December 20xx + 1, a recurrent lesion developed in the tracheal mucosa, and the patient underwent total laryngectomy followed by reconstruction with a pectoralis major myocutaneous flap. In July 20xx + 2, the patient presented with subsequent lymph node metastasis in the right portion of the neck and underwent neck dissection on the right side. However, cervical computed tomography (CT) performed in November 20xx + 2 revealed lymph node metastasis with skin involvement in the right submandibular region (Fig. 1a, b). The diagnosis of squamous cell carcinoma recurrence was made using fine-needle aspiration cytology. Positron emission tomography − CT showed no obvious accumulation of fluorodeoxyglucose, except for a single recurrent lesion in the right submandibular region. The patient had undergone radiotherapy and surgery three times. Therefore, we decided that PIT was indicated. PIT was approved by the committee of the Japan Society for Head and Neck Surgery after a committee pretreatment discussion meeting. Written consent was obtained from the patient for this report.
Fig. 1.
Target lesion before PIT and treatment.aA tumor measuring 42 × 33 mm present in the right submandibular region.bCT (axial plane) of the target lesion (yellow arrowheads): 35 × 21 mm.cCT (axial plane) showing irradiation direction (red arrows) of the cylindrical diffuser.
Drug Administration 1 Day before Surgery
Cetuximab sarotalocan sodium (640 mg/m2) was administered intravenously over 2 h.
First PIT Session (January 20xx + 3)
For laser treatment, we used a photodynamic therapy (PDT) semiconductor laser (BioBrade® laser; Rakuten Medical) and PDT semiconductor laser probes (BioBrade® cylindrical diffuser, BioBrade® frontal diffuser, and BioBrade® needle catheter; Rakuten Medical). A cylindrical diffuser emitting a cylindrical laser beam with a radius of 10 mm was used for intra-tissue irradiation.
The PIT plan for this patient is shown in Figure 1c. Preoperative CT confirmed that the target lesion had not invaded the right common carotid artery. We inserted the needle catheter perpendicular to the skin surface and irradiated the skin. Since laser irradiation is performed 20–28 h after the end of cetuximab sarotalocan sodium administration, the start time of the surgery was adjusted such that laser irradiation could be performed within this timeframe.
PIT can only be performed by Head and Neck Illuminox Treatment physicians trained and certified by the Head and Neck Illuminox Committee of the Japan Society for Head and Neck Surgery. At our institution, the first PIT session was performed by our Head and Neck Illuminox Treatment physician under the guidance of a supervisor invited from the Head and Neck Illuminox Committee. Initially, the target lesion was marked and measured as 42 × 33 mm. The distance from the skin surface to the tumor was measured using echosonography. After confirming that the distance from the tumor to the common carotid artery was sufficiently maintained, the puncture points of the needle catheters were determined at five locations (Fig. 2a). The margin was set at 3 mm to maintain the distance from the common carotid artery. Five needle catheters, 50 mm in diameter, and three cylindrical diffusers, 20 mm in diameter, were selected. After calibration, the cylindrical diffuser was inserted into the needle catheters. Three ports were used for the first irradiation, and two ports were used for the second irradiation (Fig. 2b). The duration of irradiation was 8 min and 20 s, and that of surgery was 49 min from the start of marking till the end of the operation.
Fig. 2.
First and second PIT session and course of treatment.aDetermination of the needle catheter puncture points.bLaser irradiation using cylindrical diffusers.cSkin findings on day 13.dCT (axial plane) of the PIT site (yellow arrowheads): 33 × 26 mm.eLaser irradiation using a frontal diffuser.fCT (axial plane) showing a new lymph node metastatic lesion in the right submandibular region (yellow arrowheads): 15 × 6 mm.gCT (axial plane) showing a new lymph node lesion in the left lateral retropharyngeal region (yellow arrowheads).
Course of Treatment
The skin findings after the first PIT session are shown. Epidermal peeling commenced on postoperative day 1 and gradually spread. On postoperative day 7, the epidermis at the center of the lesion became necrotic and sloughed off. The target lesion gradually shrank and disappeared on postoperative day 13 (Fig. 2c). No bleeding or signs of infection were observed in the irradiated area. No surgical procedures such as debridement were required. A grade 2 acne-like skin rash was the only adverse effect that occurred. Nonsteroidal anti-inflammatory drugs were administered to relieve the pain associated with the adverse reaction.
Cervical CT 1 month after PIT is shown in Figure 2d. Most elevated lesions in the right submandibular region were reduced in size, although a 33 × 6-mm-sized lesion with a contrast effect centered on the epidermis remained.
Second PIT Session
The second PIT session was performed 6 weeks after the first. The target lesion was a superficial lesion with a depth of 6 mm. The second treatment plan was to irradiate the lesion horizontally via the cylindrical diffuser and subsequently add a frontal diffuser to the epidermis. We selected two 70-mm needle catheters and two 40-mm cylindrical diffusers and set the diameter of the frontal diffuser to 38 mm (Fig. 2e). The duration of irradiation was 9 min and 43 s, and that of surgery was 37 min from the start of marking till the end of the operation.
Course of Treatment
The skin findings after the second PIT session are shown. On postoperative day 7, the center of the lesion became necrotic, and the epidermis began to slough off. Thereafter, the target lesion remained unchanged; however, on postoperative day 35, swelling of the submandibular lymph node on the medial side of the target lesion was observed. Cervical CT showed a ring-enhanced lymph node metastasis with a maximum diameter of 16 mm in the right submandibular region (Fig. 2f). Additionally, a left retropharyngeal lymph node was present (Fig. 2g). The lesion at the site of laser irradiation was considered stable, but the effect of the second PIT session was determined to be progressive. A third PIT session was considered, although it was thought that the left retropharyngeal lymph node would be difficult to control with PIT. Thus, we decided to change the treatment strategy to pembrolizumab and other pharmacological therapies. The best overall response to PIT in this patient was the partial response.
Discussion
To our knowledge, this is the first real-world case report describing the treatment course and adverse events of PIT for managing head and neck cancer. In this patient, most of the target lesions disappeared following necrosis after the first PIT session, whereas the recurrent lesion was shed following necrosis after the second PIT session. Thus, tumor cell destruction in the target lesion at the laser irradiation site was considered to have occurred. Nonetheless, there was no immune response to immunogenic cell death, such as activation of immune cells [4] and an abscopal effect [5, 6]. This was indicated by the exacerbation of the target lesion and the appearance of multiple new lesions.
Photosensitivity is a common adverse event associated with PIT and PDT. At our institution, we have performed PDT for managing lung and brain tumors. We have also performed PDT in 24 patients with head and neck cancer in a clinical trial [7]. All patients with head and neck cancer had photosensitivity, although none required treatment. Thus, we concluded that photosensitivity is not an issue if appropriate measures are taken [8].
The proper timing of PIT implementation for cancer treatment is difficult to assess. We consider PIT to be the last line of local treatment and the point of immunotherapy initiation. Immune checkpoint inhibitor treatment after PIT has been suggested to be effective [9], and we believe that this treatment can be administered before starting drug therapy. However, patient eligibility and the definite therapeutic effects of the treatment remain unclear.
Our institution has an International Medical Care Department and a system to provide appropriate medical care to foreign visitors. PIT is a treatment that is only covered by insurance in Japan, and we are expecting a substantial response. We intend to study the efficacy of the treatment by treating PIT-eligible patients not just in Japan but worldwide.
Conclusion
We described the progress of PIT based on our treatment experience at our institution. Many aspects of PIT remain unclear and should, therefore, be clarified in further research. Despite this uncertainty, PIT may become an effective treatment strategy for head and neck cancer if the patient selection criteria are delineated.
Statement of Ethics
Because this is a case report, approval by the Ethics Committee of Tokyo Medical University was not required. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Isaku Okamoto designed the case report. Isaku Okamoto wrote the main manuscript text and prepared the figure. Isaku Okamoto, Kiyoaki Tsukahara, and Kunihiko Tokashiki performed the PIT. Isaku Okamoto, Takuro Okada, and Kunihiko Tokashiki were in charge of the treatment of this patient. All authors discussed the results of the case report, made comments on the manuscript, and gave final approval of the version to be published.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the patient and their families for participating in the study. We would also like to thank our medical team and the physicians who participated and collaborated with us for this study.
References
- 1.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011 Nov;17((12)):1685–91. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima K, Takakura H, Shimizu Y, Ogawa M. Changes in plasma membrane damage inducing cell death after treatment with near-infrared photoimmunotherapy. Cancer Sci. 2018 Jun;109((9)):2889–96. doi: 10.1111/cas.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017 Feb;8((6)):10425–36. doi: 10.18632/oncotarget.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaya T, Friedman J, Maruoka Y, Ogata F, Okuyama S, Clavijo PE, et al. Host immunity following near-infrared photoimmunotherapy is enhanced with PD-1 checkpoint blockade to eradicate established antigenic tumors. Cancer Immunol Res. 2019 Mar;7((3)):401–13. doi: 10.1158/2326-6066.CIR-18-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law AW, Mole RH. Direct and abscopal effects of X-radiation on the thymus of the weanling rat. Int J Radiat Biol Relat Stud Phys Chem Med. 1961 May;3:233–48. doi: 10.1080/09553006114551161. [DOI] [PubMed] [Google Scholar]
- 6.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011 May;61((4)):250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Kato H, Okunaka T, Saeki T, Ohashi S, Okudaira T, et al. Photodynamic therapy for head and neck cancer. Diagn Ther Endosc. 1996 Aug;3((1)):41–51. doi: 10.1155/DTE.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida T, Tokashiki R, Ito H, Shimizu A, Nakamura K, Hiramatsu H, et al. Therapeutic effects of a new photosensitizer for photodynamic therapy of early head and neck cancer in relation to tissue concentration. Auris Nasus Larynx. 2008 Dec;35((4)):545–51. doi: 10.1016/j.anl.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Wakiyama H, Furusawa A, Okada R, Inagaki F, Kato T, Maruoka Y, et al. Increased immunogenicity of a minimally immunogenic tumor after cancer-targeting near infrared photoimmunotherapy. Cancers. 2020 Dec;12((12)):3747. doi: 10.3390/cancers12123747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.