Abstract
Introduction
Myeloid leukaemic blasts can be converted into leukaemia-derived dendritic cells (DC<sub>leu</sub>), characterised by the simultaneous expression of dendritic- and leukaemia-associated antigens, which have the competence to prime and enhance (leukaemia-specific) immune responses with the whole leukaemic antigen repertoire. To display and further specify dendritic cell (DC)- and DC<sub>leu</sub>-mediated immune responses, we analysed the interferon gamma (IFNy) secretion of innate and adaptive immune cells.
Methods
DC/DC<sub>leu</sub> were generated from leukaemic whole blood (WB) with (blast)modulatory Kit-I (granulocyte-macrophage colony-stimulating factor [GM-CSF] + Picibanil [OK-432]) and Kit-M (GM-CSF + prostaglandin E1) and were used to stimulate T cell-enriched immunoreactive cells. Initiated anti-leukaemic cytotoxicity was investigated with a cytotoxicity fluorolysis assay. Initiated IFNy secretion of T, NK, CIK, and iNKT cells was investigated with a cytokine secretion assay (CSA). IFNy positivity was additionally evaluated with an intracellular cytokine assay (ICA). Recent activation of leukaemia-specific cells was verified through addition of leukaemia-associated antigens (LAA; WT-1 and Prame)
Results
We found Kit-I and Kit-M competent to generate mature DC and DC<sub>leu</sub> from leukaemic WB without induction of blast proliferation. Stimulation of immunoreactive cells with DC/DC<sub>leu</sub> regularly resulted in an increased anti-leukaemic cytotoxicity and increased IFNy secretion of T, NK, and CIK cells, pointing to the significant role of DC/DC<sub>leu</sub> in leukaemia-specific alongside anti-leukaemic reactions. Interestingly, an addition of LAA did not further increase IFNy secretion, suggesting an efficient activation of leukaemia-specific cells. Here, both the CSA and ICA yielded comparable frequencies of IFNy-positive cells. Remarkably, the anti-leukaemic cytotoxicity positively correlated with the IFNy secretion in T<sup>CD3+</sup>, T<sup>CD4+</sup>, T<sup>CD8+</sup>, and NK<sup>CD56+</sup> cells.
Conclusion
Ultimately, the IFNy secretion of innate and adaptive immune cells appeared to be a suitable parameter to assess and monitor the efficacy of in vitro and potentially in vivo acute myeloid leukaemia immunotherapy. The CSA in this regard proved to be a convenient and reproducible technique to detect and phenotypically characterise IFNy-secreting cells. In respect to our studies on DC-based immunomodulation, we were able to display the potential of DC/DC<sub>leu</sub> to induce or improve leukaemia-specific and anti-leukaemic activity.
Keywords: Leukaemia-derived dendritic cells, Acute myeloid leukaemia, Anti-leukaemic functionality, Leukaemia-specific cells, Cytokine secretion assay
Introduction
Acute myeloid leukaemia (AML) is a malignant disorder of haematopoietic stem cells characterised by the uncontrolled clonal expansion of abnormally differentiated myeloid blasts. Displacing the physiological haematopoiesis, the accumulation of leukaemic blasts causes typical complications such as erythrocytopenia, thrombocytopenia, and leukocytopenia [1, 2]. Standard therapy for AML consists of chemotherapy with or without allogeneic haematopoietic stem cell transplantation [3, 4]. With overall 5-year survival rates of 28.7%, mainly due to infections and relapses initiated by leukaemic stem cells and residual blasts, the outcome though remains unsatisfactory [5, 6, 7].
In recent years, immunotherapeutic approaches in the treatment of AML have gained attention. Different strategies have been developed with the attempt to redirect the immune system in order to overcome the leukaemic immune escape and enforce a tumour-specific immune response. One of the most auspicious approaches in this regard involves the use of dendritic cell (DC)-based vaccines [8, 9, 10, 11]. DCs are some of the most potent antigen-presenting cells of the immune system. As they link the innate and the adaptive immune system, they are pivotal in initiating and regulating an antigen-specific immune response [12, 13]. As such, DCs can be exploited to present tumour antigens in order to induce a potent anti-tumour immunity.
DCs can be generated ex vivo from leukaemic myeloid blasts resulting in mature leukaemia-derived DC (DCleu) [14, 15, 16], characterised by the simultaneous expression of dendritic cell antigens, leukaemia-specific antigens, and CCR7 as maturation and migration marker [17, 18]. Thus, presenting the whole leukaemic antigen repertoire, mature DCleu have the competence to activate T cells and likely natural killer cells (NK cells), cytokine-induced killer cells (CIK cells), and invariant natural killer cells (iNKT) to (re)gain leukaemia-specific activity [15, 16, 18, 19, 20].
Leukaemia-specific activity or rather cytotoxicity implies a complex synergy of the innate and adaptive cellular immune system as well as the humoral immune system. Cytotoxicity is exerted by various innate and adaptive immune cells via different mechanisms: through the (rather early and fast) release of the cytolytic molecules perforin and granzyme by degranulation, through the (rather late and slow) interaction with the Fas ligand (FasL) and TNF-related apoptosis-including ligand (TRAIL), and/or through the secretion of tumour necrosis factor alpha (TNFa) and interferon gamma (IFNy) [21, 22, 23]. Especially the latter, IFNy, has a wide-ranging role: it is not only essential to regular immunity, promoting innate and adaptive immune responses and thereby licensing immune cells to exert cytotoxicity, but also strongly associated with anti-tumour immunity, promoting tumour surveillance, recognition, and elimination [24]. However, it has been noted before that under certain circumstances IFNy can also stimulate immune-suppressive mechanisms in tumour cells, including upregulation of indoleamine 2,3-dioxygenase and of checkpoint inhibitors such as programmed cell-death ligand 1, leading to immune escape [25, 26].
In the early phase of an immune response, IFNy is mainly secreted by innate immune cells such as NK, iNKT, DC, and macrophages upon activation [27, 28]. It not only stimulates innate immune cells by enhancing their effector mechanisms and IFNy secretion, but also facilitates tumour recognition and elimination. By up-regulation of the major histocompatibility complex (MHC) class I and II antigen- and cross-presentation pathways, as well as the MHC-II expression on classically non-MHC-II-expressing cells, it increases the perceptibility of tumour cells to adaptive immune cells. Moreover, by up-regulation of the expression of FasR, FasL, TRAIL, caspase-1, −3, −7, −8, and BIM and down-regulating the expression of survivin, it increases the susceptibility of tumour cells to extrinsic and intrinsic pathways of apoptosis [25, 26, 29, 30, 31, 32, 33]. Importantly, by promoting the differentiation, proliferation, and activation of helper T cells type 1 (Th1) and cytotoxic T cells (Tc), IFNy links the innate and adaptive immunity, thereby commencing the advanced phase of an immune response. Moreover, it inhibits the differentiation of helper T cells type 2 (Th2) and regulatory T cells [26, 27, 28, 34]. In the advanced phase of an immune response, IFNy is mainly secreted by adaptive immune cells such as Th1 and Tc cells upon primary activation by DC or secondary activation by its specific antigen. IFNy thereupon not only stimulates adaptive immune cells by enhancing their effector mechanisms and IFNy secretion, but also innate immune cells [27, 28, 35]. That way, IFNy creates a positive feedback loop and, together with further cytokines, is able to stimulate and sustain an effective immune response, incorporating both innate and adaptive immune cells.
IFNy is considered as sine qua non for the immune system. It is an important mediator of the innate and adaptive immunity and plays a pivotal role in anti-tumour immunity. A blockade of this critical cytokine restrains an effective immune defence [36, 37]. This inseparable connection between IFNy and cell-based immunity has lead us to hypothesise that IFNy could be a suitable parameter to display leukaemia-specific activity and cytotoxicity. Particularly in respect to future applications, IFNy readouts might have the potential to be a convenient parameter to assess and monitor the efficacy of AML immunotherapy.
The aim of the study was to generate DC/DCleu with immunomodulatory Kit-I and Kit-M (DC/DCleu-Kit-I and DC/DCleu-Kit-M) from leukaemic whole blood (WB) and therewith stimulate autologous T cell-enriched immunoreactive cells. We investigated the resulting anti-leukaemic cytotoxicity with a cytotoxicity fluorolysis assay (CTX) and the resulting IFNy secretion of innate and adaptive immune cells with a cytokine secretion assay (CSA). IFNy production was additionally evaluated with an intracellular cytokine assay (ICA). Ultimately, we correlated the IFNy secretion with the anti-leukaemic cytotoxicity of DC/DCleu-stimulated immunoreactive cells.
Material and Methods
Sample Collection
Sample collection was conducted after obtaining written informed consent of the blood donor and in accordance with the World Medical Association Declaration of Helsinki and the ethic committee of the Ludwig Maximilian University Hospital Munich (vote No. 33905). Samples in form of heparinised peripheral WB were provided by the University Hospitals of Augsburg, Oldenburg, and Munich.
Patients' Characteristics
Blood samples were obtained from AML patients (n = 19) with a mean age of 62.2 years (range 29–98 years) and a female-to-male ratio of 1:1.4, and from healthy volunteers (n = 4) with a mean age of 29.3 (range 16–57 years) and a female-to-male ratio of 1:1. AML patients were characterised by the French-American-British (FAB) classification (M1-M7), the aetiology (primary AML, secondary AML), the stage of disease (first diagnosis, relapse), the blast phenotype, the blast frequency in peripheral blood, and the cyto- and molecular genetics. An overview is given in Table 1.
Table 1.
Patients' characteristics
| Patient No. | Age | Sex | FAB type | Stage | Blast phenotype (CD) | IC blasts, % | Cyto-, molecular genetics | Source | Conducted experiments |
|---|---|---|---|---|---|---|---|---|---|
| AML | |||||||||
| 1489 | 55 | f | p–M0 | dgn | 13, 33, 34, 65, 117 | 61 | 46, XX; FLT3-ITD mut, NMP1 mut | MNC | DCC, MLC, CTX, CSA |
| 1490 | 65 | m | s–M? | dgn | 15, 33, 56, 65 | 46 | 46, XY; del(20) (q12q13)[12]; NPM1 wt | MNC | DCC, MLC, CTX, CSA |
| 1509 | 60 | m | p–M2 | dgn | 13, 33, 34, 65, 117 | 48 | 46, XY; FLT3-TKD wt, NPM1 mut | WB | DCC, MLC, CTX, CSA, ICA |
| 1511 | 78 | m | p–M4 | rel | 13, 15, 33, 34, 65, 117 | 54 | FLT3-ITD mut, RUNX1 mut | WB | DCC, MLC, CTX, CSA |
| 1514 | 68 | m | s–M? | dgn | 33, 56, 117 | 36 | 46, XY; FLT3-TKD wt, NPM1 mut | WB | DCC, MLC, CSA |
| 1515 | 67 | f | p–M2 | dgn | 33, 34, 65, 117 | 80 | 46, XX; FLT3-TKD wt, NPM1 mut | WB | DCC, MLC, CSA |
| 1518 | 83 | f | p–M5 | dgn | 14, 15, 34, 65 | 72 | 46, XX; FLT3-TKD wt, NPM1 mut | WB | DCC, MLC, CSA, ICA |
| 1521 | 56 | m | p–M4 | dgn | 13, 15, 33, 34, 65, 117 | 72 | 46, XY; FLT3-TKD mut, NPM1 mut | WB | DCC, MLC, CTX, CSA |
| 1525 | 77 | m | p–M1 | dgn | 13, 15, 33, 34, 117 | 78 | 46, XY; FLT3-ITD wt, FLT3-TKD mut, NPM1 mut | WB | DCC, MLC, CSA |
| 1526 | 74 | f | p–M? | dgn | 15, 33, 34, 56, 65, 117 | 59 | 46, XX; FLT3-TKD wt, NPM1 mut | WB | DCC, MLC, CSA |
| 1527 | 42 | m | p–M2 | dgn | 7, 13, 15, 33, 34, 65, 117 | 51 | 46, XY; FLT3-ITD mut, NPM1 mut | WB | DCC, MLC, CTX, CSA, ICA |
| 1531 | 71 | m | p–M4/5 | dgn | 13, 33, 34, 117 | 24 | n.d. | WB | DCC, MLC, CSA, ICA |
| 1536 | 61 | m | p–M5 | dgn | 14, 34, 56 | 73 | 46, XY; NPM1 mut | WB | DCC, MLC, CTX, CSA |
| 1562 | 37 | m | p–M1 | dgn | 2, 7, 13, 34, 117 | 82 | 46, XY; del(2) (q21), der(14) t(2; 14) (q21; q32)[7]/46,XY[3]; FLT3-TKD mut, RUNX1 mut | WB | DCC, MLC, CTX, CSA |
| 1565 | 62 | f | p–M4 | dgn | 13, 15, 34, 65, 117 | 31 | 46, XX | WB | DCC, MLC, CTX, CSA |
| 1567 | 98 | f | p–M? | dgn | 14, 15, 34, 56 | 57 | 46,XX del(5q31), del(5q32-33), MECOM rearrangement inv(3) (q21q26.2)/t(3; 3) (q21;q26.2); FLT3-TKD wt, NPM1 wt | WB | DCC, MLC, CTX, CSA |
| 1568 | 29 | m | p–M? | dgn | 10, 13, 33, 34 | 79 | 46, XY | WB | DCC, MLC, CTX, CSA |
| 1570 | 36 | f | p–M? | dgn | 7, 13, 14, 33, 34, 117 | 33 | 46, XX; NPM1 mut | WB | DCC, MLC, CTX, CSA |
| 1572 | 64 | f | p–M? | dgn | 13, 33, 34, 65, 117 | 50 | 46, XX; RUNX1 mut, EZH2 mut, BCOR mut, U2AF1 mut, ASXL1 mut |
WB | DCC, MLC, CTX, CSA, ICA |
|
| |||||||||
| Healthy | |||||||||
| 1486 | 57 | f | MNC | CSA | |||||
| 1499 | 21 | f | MNC, WB | DCC, MLC, CSA | |||||
| 1505 | 22 | m | MNC, WB | DCC, MLC, CSA | |||||
| 1523 | 17 | m | WB | DCC, MLC, CSA | |||||
AML patients presented in WB/mononuclear cells (MNC) with an average of 57.6/53.6% leukemic blasts (range 23.7–80.4/45.9–61.2%), 11.4/1.4% TCD3+ cells (range 0.9–19.4/0.5–2.2%), 3.3/7.6% BCD19+ cells (range 0.1–3.9/0.7–14.6%), 2.8/0.7% NKCD56+ cells (range 0.3–8.1/0.5–0.9%), and 5.3/2.7% monocytesCD14+ (range 0–16.3/1.6–3.7%). In cases with aberrant expression of T, B, NK, or monocytoid antigens, proportions were not included in the analyses.
Cell Characterisation by Flow Cytometry
Flow cytometric analyses were implemented to evaluate and quantify frequencies, phenotypes, and subsets of leukaemic blasts, DCs, monocytes, B cells, T cells, NK cells, CIK cells, and iNKT cells. Abbreviations of all cell types are given in Table 2.
Table 2.
Cells and cell subsets as evaluated by flow cytometry
| Abbreviation of subgroups | Surface marker | Referred to | Abbreviation | Ref. | |
|---|---|---|---|---|---|
| Blast cells | |||||
| Blasts | BLA | BLA+ (CD15+, CD34+, CD65+, CD117+) | WB or MNC | BLA/WB or/MNC | [17] |
| Proliferating blasts | BLAprol | BLA+DC−CD71+ | BLA | BLAprol/BLA | [39] |
|
| |||||
| Dendritic cells | |||||
| Dendritic cells | DC | DC+ (CD80+, CD206+) | WB or MNC | DC/WB | [17] |
| Leukaemia-derived DC | DCleu | DC+BLA+ | WB or MNC | DCleu/WB or /MNC | [17] |
| DC | DCleu/DC | ||||
| BLA | DCleu/BLA | ||||
| Mature DC | DCmat | DC+CCR7+ | WB or MNC | DCmat/WB or /MNC | [18] |
| DC | DCmat/DC | ||||
| Mature DCleu | DCmat-leu | DC+BLA+CCR7+ | WB or MNC | DCleu-mat/WB or /MNC | |
| DCleu | DCleu-mat/DCleu | ||||
| DCmat | DCleu-mat/DCmat | ||||
|
| |||||
| Monocytoid cells | |||||
| CD14+ monocytes | monocytesCD14+ | CD14+ | WB | monocytesCD14+/WB | [18] |
|
| |||||
| T cells | |||||
| CD3+ pan T cells | TCD3+ | CD3+ | WB or MNC | TCD3+/WB or MNC | [75] |
| CD4+-coexpressing T cells | TCD4+ | CD3+CD4+ | CD3+ | TCD4+/CD3+ | [75] |
| CD8+-coexpressing T cells | TCD8+ | CD3+CD8+ | CD3+ | TCD8+/CD3+ | [75] |
| Naive T cells | Tnaive | CD3+CD45RO− | CD3+ | Tnaive/CD3+ | [41] |
| Non-naive T cells | Tnon-naive | CD3+CD45RO+ | CD3+ | Tnon-naive/CD3 + | [41] |
| Central (memory) T cells | Tcm | CD3+CD45RO+CCR7+ | CD3+ | Tcm/CD3+ | [41] |
| Effector (memory) T cells | Tem | CD3+CD45RO+CCR7− | CD3+ | Tem/CD3+ | [41] |
| Proliferating T cells − early | Tprol-early | CD3+CD69+ | CD3+ | Tprol-early/CD3+ | [75] |
| Proliferating T cells − late | Tprol-late | CD3+CD71+ | CD3+ | Tprol-late/CD3+ | [75] |
|
| |||||
| B cells | |||||
| CD19+ B cells | BCD19+ | CD19+ | WB or MNC | BCD19+/WB or /MNC | [20] |
|
| |||||
| CIK cells | |||||
| CD3+CD56+ CIK cells | CIKCD56+ | CD3+CD56+ | WB or MNC | CIKCD56+/WB or /MNC | [20] |
| CD3+CD161+ CIK cells | CIKCD161+ | CD3+CD161+ | WB or MNC | CikCD161+/WB or /MNC | |
|
| |||||
| NK cells | |||||
| CD3−CD56+ NK cells | NKCD56+ | CD3−CD56+ | WB or MNC | NKCD56+/WB or /MNC | [20] |
| CD3−CD161+ NK cells | NKCD161+ | CD3−CD161+ | WB or MNC | NKCD161+/WB or /MNC | |
|
| |||||
| iNKT cells | |||||
| 6B11+ iNKT cells | iNKT | 6B11+ | WB or MNC | iNKT/WB or /MNC | [20] |
|
| |||||
| IFNy-secreting cells | |||||
| IFNy-secreting CD3+ pan T cells | CD3+IFNy+ | CD3+ | TCD3+IFNy+/TCD3+ | ||
| IFNy-secreting CD4+-coexpressing T cells | CD3+CD4+IFNy+ | CD3+CD4+ | TCD4+IFNy+/TCD4+ | ||
| IFNy-secreting CD8+-coexpressing T cells | CD3+CD8+IFNy+ | CD3+CD8+ | TCD8+IFNy+/TCD8+ | ||
| IFNy-secreting CD3+CD56+ CIK cells | CD3+CD56+IFNy+ | CD3+CD56+ | CIKCD56+IFNy+/CIKCD56+ | ||
| IFNy-secreting CD3+CD161+ CIK cells | CD3+CD161+IFNy+ | CD3+CD161+ | CIKCD161+IFNy+/CIKCD161+ | ||
| IFNy-secreting CD3−CD56+ NK cells | CD3−CD56+IFNy+ | CD3−CD56+ | NKCD56+IFNy+/NKCD56+ | ||
| IFNy-secreting CD3−CD161+ NK cells | CD3−CD161+IFNy+ | CD3−CD161+ | NκCD161+lFNy+/NKCD161+ | ||
| IFNy-secreting 6B11+ iNKT cells | 6B11+IFNy+ | 6B11+ | iNKT+IFNy+/iNKT+ | ||
Surface marker combinations for flow cytometric staining and analysis.
Cells were stained with various monoclonal antibodies (moAbs) labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-cyanine7 tandem conjugate (PE-Cy7), or allophycocyanin (APC). Antibodies were provided by Beckman Coultera (Krefeld, Germany), Becton Dickinsonb (Heidelberg, Germany), Miltenyi Biotecc (Bergisch Gladbach, Germany), BioLegendd (Koblenz, Germany), and Santa Cruz Biotechnologye (Heidelberg, Germany). For analyses FITC-conjugated moAbs CD3b, CD14a, CD15a, CD45ROa, CD65a, CD71a, CD161b, IPO38e; PE-conjugated moAbs CD3a, CD4b, CD34a, CD56a, CD65c, CD80a, 6B11b, IFNyd; PE-Cy7-conjugated moAbs CD3a, CD4a, CD14b, CD33a, CD34a, CD65c, CD117a, CD197b; APC-conjugated moAbs CD3a, CD14a, CD19a, CD34a, CD56a, CD69b, CD117a, CD206b, 6B11d were used. Non-viable cells were detected with 7AADb.
In preparation of staining, erythrocytes in WB samples were lysed using lysing buffer (Becton Dickinson) according to the manufacturer's instructions. Staining was performed by a 15-min incubation of cells with the corresponding moAbs in the dark at room temperature using a staining medium containing 95% PBS (Biochrom, Berlin, Germany) and 5% FCS (Biochrom). Intracellular staining (e.g., IPO38, IFNy) was performed with the FIX&PERM Cell Fixation and Cell Permeabilisation Kit (Thermo Fisher Scientific, Darmstadt, Germany).
Stained cells were analysed with the fluorescence-activated cell sorting flow cytometer FACS Calibur (Becton Dickinson) and the acquisition and analysis software CellQuestPro (Becton Dickinson). Isotype controls were conducted according to the manufacturer's instructions.
Sample Preparation
MNC were isolated from WB by density gradient centrifugation using the Ficoll-Hypague technique and a separating solution with a density of 1.077 g/mL (Biocoll, Biochrom). T cells were isolated from MNC using the MACS microbead and column-based immunomagnetic cell separation technology (Miltenyi Biotec) via positive selection of CD3+ cells according to the manufacturer's instructions. Purity of isolated T cells was on average 80.5% (range 57.7–95.3%). MNC and T cells, unless directly used, were frozen with 70% RPMI-1640 medium (Biochrom), 20% human serum (HealthCare Europa GmbH, Vienna, Austria), 10% dimethyl sulfoxide (Sigma Aldrich Chemie GmbH, Steinheim, Germany), stored at −80°C and thawed when required.
Dendritic Cell Culture
The generation of DC/DCleu was performed by the stimulation of MNC or WB with specific response modifiers, including granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sanofi-Aventis, Frankfurt, Germany), interleukin 4 (IL-4) (PeproTech, Berlin, Germany), Picibanil (OK-432) (Chugai Pharmaceutical Co., Kajiwara, Japan), and prostaglandin E1 (PGE1) (PeproTech). Compositions of DC/DCleu-generating protocols are given in Table 3.
Table 3.
DC/DCleu-generating protocols
| DC/DCleu protocol | DC/DCleu source | Composition (total) | Time of addition | Time of culture | Reference |
|---|---|---|---|---|---|
| Pici-PGE1 | MNC | GM-CSF 500 U/mL | d0 | 9 days | [38] |
| IL-4 250 U/mL | d0 | ||||
| OK-432 10 μg/mL | d7 | ||||
| PGE1 1 μg/mL | d7 | ||||
|
| |||||
| Kit-I | WB | GM-CSF 800 U/mL | d0, d2–3 | 7–8 days | [Unpublished data] |
| OK-432 10 μg/mL | d0, d2–3 | European Patent No. 15 801 987.7-1118 | |||
|
| |||||
| Kit-M | WB | GM-CSF 800 U/mL | d0, d2–3 | 7–8 days | [Unpublished data] |
| PGE1 1 μg/mL | d0, d2–3 | European Patent No. 15 801 987.7-1118 | |||
|
| |||||
| Mode of action | GM-CSF | Induction of myeloid and DC differentiation | [16, 38] | ||
| IL-4 | Induction of DC differentiation | ||||
| OK-432 | Danger signalling, stimulation of DC differentiation | ||||
| PGE1 | Danger signalling, stimulation of DC maturation and migration (via CCR7 expression) | ||||
GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-4, interleukin 4; OK-432, Picibanil; PGE1, prostaglandin E1; d day.
DC/DCleu from healthy and leukaemic MNC were generated with the DC/DCleu-generating protocol Pici-PGE1 [38]. For this, 3–4 × 106 MNC were cultured in 12-multiwell culture plates (ThermoFisher Scientific) and diluted with 2 mL x-vivo 15 medium (Lonza, Basel, Switzerland). Response modifiers were added to the cultures (further referred to as MNCDC(P)) according to the protocol. A culture without added response modifiers served as negative control (MNCDC(Control)). A half-medium exchange was carried out every 2–3 days. Cells were harvested after 9 days.
DC/DCleu from healthy and leukaemic WB were generated with the DC/DCleu-generating protocols Kit-I and Kit-M [unpublished data] [38]. For this, 500 μL WB (corresponding to 5.0–30.3 × 106 MNC) were cultured in 24-multiwell culture plates (ThermoFisher Scientific) and diluted with 500 μL x-vivo 15 medium. Response modifiers were added to the cultures (further referred to as WBDC(I), WBDC(M)) according to the protocol. Likewise, a culture without added response modifiers served as negative control (WBDC(Control)). Cells were harvested after 7–8 days.
DC cultures as well as subsequent mixed lymphocyte cultures (MLC) were incubated at 37°C, 21% O2, and 5% CO2. In some cases, additional cultures were incubated simultaneously in hypoxia-like conditions (37°C, 10% O2, 10% CO2).
Flow cytometric analyses of leukaemic blasts, DC, DCleu, and DCmat were performed before and after dendritic cell culture (DCC) using a refined gating strategy [unpublished data] [16, 17, 39]. DCleu were analysed by the co-expression of at least one blast marker (CD15, CD34, CD65, CD117) including lineage-aberrant markers (CD 56) and one DC marker that had not been expressed on naive blasts (CD80, CD206; of which CD80 qualified in 82.4%, CD206 in 94.1%, and both CD80 and CD206 in 76.5% of cases). Maturation of DC/DCleu was analysed by the further co-expression of CCR7 (Fig. 1A, B). Premise for DC subgroup analyses was the presence of ≥5% DCs in the total cell fraction.
Fig. 1.
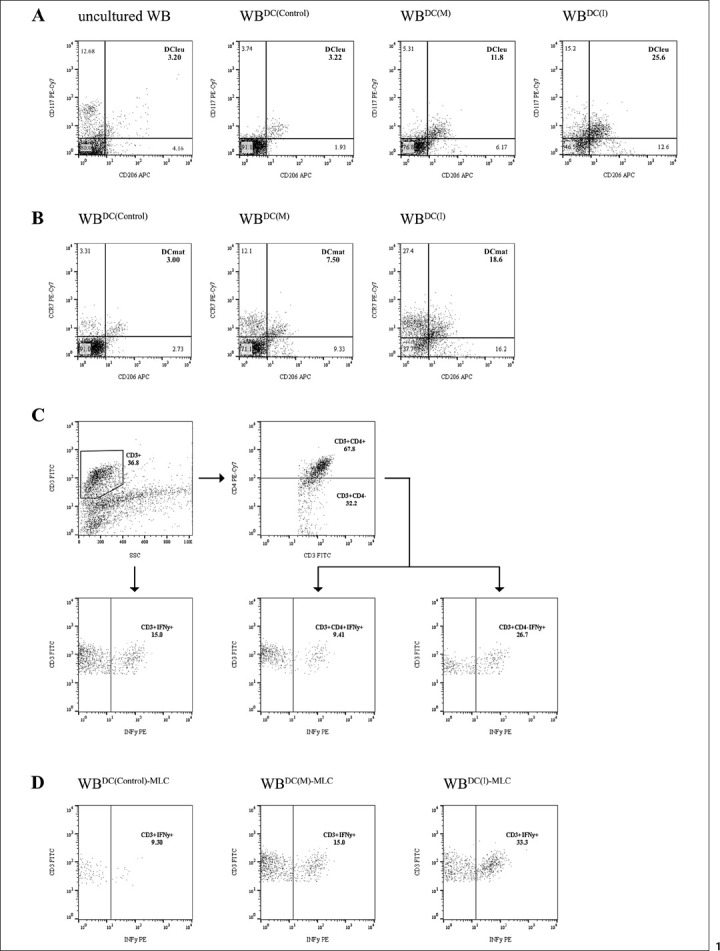
Flow cytometric analyses. A Conversion of myeloid blasts into DCleu through blastmodulatory Kit-I and Kit-M. Exemplary plots show DCleu, characterised by the co-expression of the blast marker CD117 and the DC marker CD206, in uncultured WB, WBDC(Control), WBDC(M), and WBDC(I). B Gain of maturation through Kit-I and -M. Exemplary plots show DCmat, characterised by the co-expression of the DC marker CD206 and the maturation marker CCR7, in WBDC(Control), WBDC(M), and WBDC(I). C Gating of IFNy-secreting TCD3+ (CD3+IFNy+), TCD4+ (CD3+CD4+IFNy+), and TCD8+ (CD3+CD4−IFNy+) cells as detected by CSA. D Comparison of IFNy-secreting TCD3+ (CD3+IFNy+) cells in WBDC(Control)-MLC, WBDC(M)-MLC, and WBDC(I)-MLC. Abbreviations of all cell types are given in Table 2.
We conducted preliminary experiments to assess the feasibility and comparability of DC/DCleu generation in different settings. As these experiments and previous studies [unpublished data] [38, 40] affirmed the feasibility and comparability with Pici-PGE1 in healthy and leukaemic MNC, with Kit-I and Kit-M in healthy and leukaemic WB, as well as under hypoxia-like and normoxia-like conditions (data not shown), further experiments were conducted on leukaemic WB to adapt to more physiological conditions, under normoxia-like conditions.
Mixed Lymphocyte Culture
Consecutive generation of T cell-enriched immunoreactive cells was performed by the stimulation of autologous T cells with DC/DCleu-containing MNCDC or WBDC.
Based on a MNC model, 1 × 106 T cells and a fraction of MNCDC containing 0.25 × 106 DC/DCleu were co-cultured in a 24-multiwell culture plate (total cell count 1.7–4.8 × 106 MNC) and diluted in 1 mL RMPI-1640 medium containing 100 U/mL penicillin (Biochrom) and 15% human serum. 50 U/mL IL-2 were added on day 0 and day 2–3 to all cultures (further referred to as MNCDC(P)-MLC, MNCDC(Control)-MLC). A half-medium exchange was carried out every 2–3 days. Cells were harvested after 7–9 days.
Based on a WB model, 1 × 106 T cells and a fraction of WBDC containing 0.25 × 106 DC/DCleu were co-cultured in a 24-multiwell culture plate (total cell count 1.8–9.4 × 106 MNC) and diluted in 1 mL RMPI-1640 medium containing 100 U/mL penicillin. 50 U/mL IL-2 were added on day 0 and day 2–3 to all cultures (further referred to as WBDC(I)-MLC, WBDC(M)-MLC, WBDC(Control)-MLC). Cells were harvested after 6–7 days.
Flow cytometric analyses of T-cell subsets were performed before and after MLC using a refined gating strategy [18, 19, 41].
We conducted preliminary experiments to assess the feasibility and comparability of DC/DCleu stimulation on immunoreactive cells and its resulting anti-leukaemic cytotoxicity in different settings. As these experiments affirmed the feasibility and comparability in (healthy and) leukaemic MNC and WB (data not shown), further experiments were conducted on leukaemic WB to adapt to more physiological conditions.
Cytotoxity Fluorolysis Assay
A fluorolysis assay was performed to analyse the lytic activity of T cell-enriched immunoreactive cells against leukaemic blasts (further referred to as anti-leukaemic cytotoxicity) of MNCDC-MLC and WBDC-MLC [18]. Therefore, a fraction of MNCDC-MLC and WBDC-MLC containing 1 × 106 T cells (effector cells) and 1 × 106 thawed autologous leukaemic blasts (target cells) were co-cultured (total cell count 2.3–6.0 × 106 MNC) diluted in 1 mL RMPI-1640 medium containing 100 U/mL penicillin and 15% human serum for 3 and 24 h at 37°C, 21% O2, 5% CO2. Target cells were stained with FITC-, PE-, or APC-conjugated blast-specific moAbs before culture, and with 7AAD and a defined number of fluorosphere beads (Beckman Coulter) after culture when harvested. All assays were performed in combination with a control, for which effector and target cells were cultured analogously but separated and only merged prior to flow cytometric analyses.
Flow cytometric analyses were performed using a refined gating strategy [18]. Achieved anti-leukaemic cytotoxicity is described as “blast lysis” defined as the percentual difference of viable target cells between the effector-target cell culture and the control, “cases with blast lysis” defined as the proportion of cases with blast lysis >0%, “improved blast lysis” defined as the percentual difference of the blast lysis of WBDC(I)-MLC or WBDC(M)-MLC and WBDC(Control)MLC, and “cases with improved blast lysis” defined as the proportion of cases with improved blast lysis >0%.
Cytokine Secretion Assay
For the detection of IFNy-secreting cells in MNC, MNCDC-MLC, WB and WBDC-MLC, an IFNy secretion assay (Miltenyi Biotec) was performed. According to the manufacturer's instructions, cells were firstly labelled with an IFNy Catch Reagent (Miltenyi Biotec), a bi-specific moAB directed against the pan-leukocytic marker CD45 and IFNy. By connecting to leukocytes during a non-IFNy-secretion period (10 min, on ice), followed by connecting to IFNy during an IFNy-secretion period (45 min, 37°C), IFNy could be bound to the positive secreting cells. For detection by flow cytometry, cells were secondly labelled with an IFNy-specific PE-conjugated IFNy Detection Antibody (Miltenyi Biotec).
In some cases, an additional stimulation of MNC, MNCDC-MLC, WB, and WBDC-MLC was performed prior to the CSA, for which cells were incubated for 4 h with a leukaemia-associated antigen (LAA) suspension containing 50 μg/mL WT-1 (Miltenyi Biotec) and 50 μg/mL PRAME (Miltenyi Biotec) or with 1 μg/mL staphylococcal enterotoxin B (SEB, Sigma Aldrich Chemie GmbH).
For flow cytometric analyses of IFNy-secreting cells, cells were co-stained with FITC-, PE-Cy7-, and APC-conjugated moAbs. Analyses of IFNy-secreting T, NK, CIK, and iNKT cells were performed with a gating strategy described in Figure 1C, D.
We conducted preliminary experiments to assess the feasibility of the CSA in different settings. As these experiments affirmed the feasibility in uncultured and cultured, healthy and leukaemic, MNC and WB, as well as under hypoxia-like and normoxia like conditions, with comparability in MNC and WB and under hypoxia-like and normoxia-like conditions (data not shown), further experiments were conducted on leukaemic WB to adapt to more physiological conditions, under normoxia-like conditions.
Intracellular Cytokine Assay
For the detection of intracellular IFNy in WB and WBDC-MLC, an intracellular cytokine assay was performed. To avoid cytokine secretion during the assay, cells were firstly incubated with brefeldin A (1000X, BioLegend) concentrated at 1:1,000 for 15 h. Intracellular staining of IFNy subsequently was procured using the FIX&PERM Cell Fixation and Cell Permeabilisation Kit according to the manufacturer's instructions. For flow cytometric analysis of IFNy-producing cells, cells were co-stained with FITC-, PE-Cy7-, and APC-conjugated moAbs. Analyses of IFNy-secreting T, NK, CIK, and iNKT cells were performed with the same gating strategy as used for the CSA.
Statistical Methods
Data is presented as mean ± standard deviation (SD). Statistical comparisons for two groups were performed using the two-tailed t test and the Pearson correlation coefficient. Significance was defined as “not significant” (n.s.) with p values >0.10, as “borderline significant” with p values 0.10 to 0.05, and as “significant” with p values <0.05. Correlation was defined as “negligible” with r values 0.00 to 0.30 (−0.00 to −0.30), as “low” with r values 0.30 to 0.50 (−0.30 to −0.50), as “moderate” with r values 0.50 to 0.70 (−0.50 to −0.70), and as “high” with r values 0.70 to 1.00 (−0.70 to −1.00). Statistical analyses and figures were implemented with GraphPad Prism 8 (GraphPad Software, California, USA) and Pages 8.2 (Apple, California, USA).
Results
Generation of Mature DC/DCleu from Leukaemic WB without Induction of Blast Proliferation
We were able to generate significantly higher frequencies of DC/WB and DCleu/WB with Kit-I and Kit-M compared to control, with significantly higher frequencies in WBDC(I) than WBDC(M). Differentiating DCleu in its subgroups showed significantly higher frequencies of DCleu/BLA as well as of DCleu/DC in WBDC(I) and WBDC(M) compared to WBDC(Control). Frequencies of DCleu/BLA and DCleu/DC did not differ significantly in WBDC(I) compared to WBDC(M) (Fig. 1A, 2A, B).
Fig. 2.
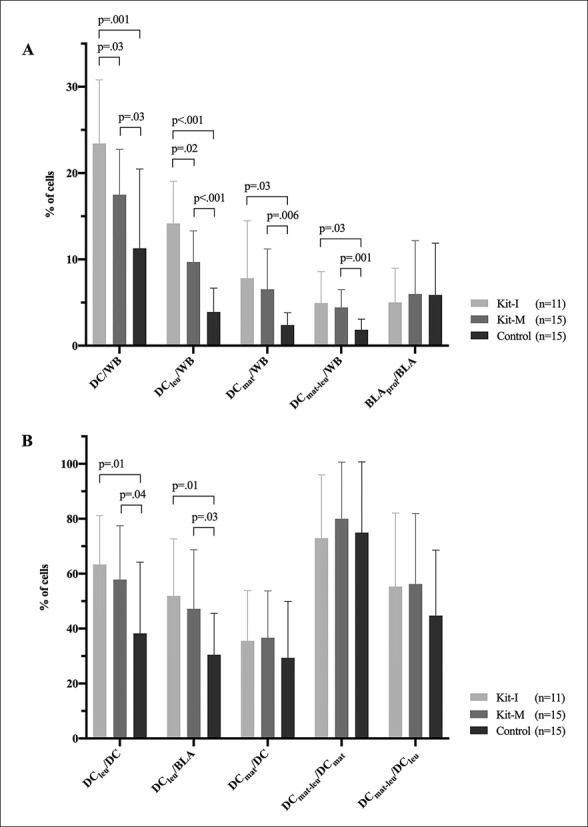
Generation of (mature) DC and DCleu from leukaemic WB with blastmodulatory Kit-I and Kit-M without induction of blast proliferation. Given are the mean ± SD of DC, DCleu, DCmat, and DCmat-leu in the WB fraction and BLAprol in the blast fraction in WBDC(I), WBDC(M), and WBDC(Control) (A), and the mean ± SD of DCleu in the DC and blast fraction, DCmat in the DC fraction, and DCmat-leu in the DCmat and DCleu fraction in WBDC(I), WBDC(M), and WBDC(Control) (B). Statistically significant (p values <0.05) and borderline significant (p values 0.10 to 0.05) differences are given. Abbreviations of all cell types are given in Table 2.
We furthermore evaluated the maturation of DC and found significantly higher frequencies of DCmat/WB and DCmat-leu/WB and no significantly different frequencies of DCmat/DC in WBDC(I) and WBDC(M) compared to WBDC(Control). Differentiating DCmat-leu in its subgroups showed no significantly different frequencies of DCmat-leu/DCmat and no significantly different frequencies of DCmat-leu/DCleu in WBDC(I) and WBDC(M) compared to WBDC(Control). Frequencies of DCmat and DCmat-leu in depicted cell groups did not differ significantly in WBDC(I) compared to WBDC(M) (Fig. 1B, 2A, B).
Reviewing the effect of Kits on the proliferation of non-converted blasts during DCC, we found no significant shift of BLAprol/BLA in WBDC(I) and WBDC(M) compared to WBDC(Control) (Fig. 2A).
Stimulatory Impact of DC/DCleu on T Cell-Enriched Immunoreactive Cells
DC/DCleu and IL-2 Stimulation Increases T-Cell Activation
To assess the potential stimulating effect of generated DC/DCleu on immunoreactive cells in the presence of IL-2, T-cell compositions were compared before (WBDC(Control)) and after (WBDC(I)-MLC, WBDC(M)-MLC, WBDC(Control)-MLC) MLC. Frequencies of TCD4+, TCD8+, Tprol-early, Tprol-late, Tnaive, Tnon-naive, Tcm, and Teff cells were analysed in reference to TCD3+ cells.
We noticed a generally higher activation status of cells in WBDC(I)-MLC, WBDC(M)-MLC as well as in WBDC(Control)-MLC compared to WBDC(Control), characterised by a significant increase of early and late proliferating T cells, a significant shift from naive to non-naive T cells, and a (significant) increase of central and effector memory T cells. TCD4+ and TCD8+ cells did not show any significant transformations. When comparing WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC, no significant differences in T cell compositions could be found (Fig. 3).
Fig. 3.
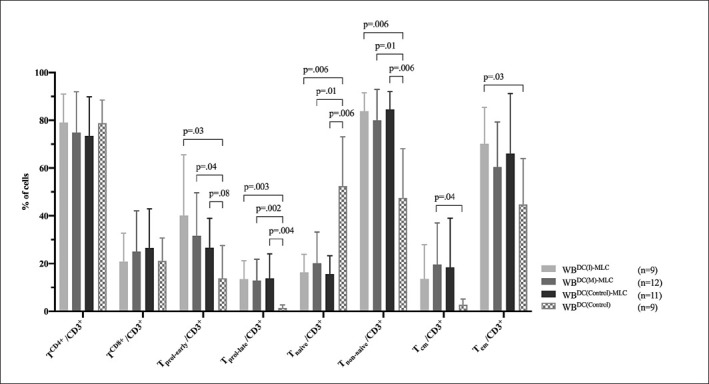
Stimulatory effect of DC/DCleu on the composition of immunoreactive cells. Given are the mean ± SD of T-cell subsets in the CD3+ cell fraction before (WBDC(Control)) and after (WBDC(I)-MLC, WBDC(M)-MLC, WBDC(Control)-MLC) DC/DCleu stimulation. Statistically significant (p values <0.05) and borderline significant (p values 0.10 to 0.05) differences are given. Abbreviations of all cell types are given in Table 2.
DC/DCleu Stimulation Increases Anti-Leukaemic Cytotoxicity
We analysed the lytic activity of WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC through CTX after 3 h and 24 h of incubation of effector and leukaemic target cells, to assess the anti-leukaemic cytotoxicity of DC/DCleu-stimulated immunoreactive cells.
As early as 3 h, we could observe a lysis of target cells in WBDC(I)-MLC as well as WBDC(M)-MLC in about a quarter of the cases, but in WBDC(Control)-MLC in about a half of the cases. Average frequencies of lysed blasts were (n.s.) higher in WBDC(I)-MLC than in WBDC(M)-MLC and WBDC(Control)-MLC (Fig. 4A). After 24 h, more cases of WBDC(I)-MLC and WBDC(M)-MLC, but less cases of WBDC(Control)-MLC attained lysis. Average frequencies of lysed blasts decreased (n.s.) in WBDC(I)-MLC and WBDC(Control)-MLC, but increased (n.s.) in WBDC(M)-MLC, whereby frequencies in WBDC(I)-MLC and WBDC(M)-MLC were (n.s.) higher than in WBDC(Control)-MLC (Fig. 4C). Notably, in cases without lysis, frequencies of blasts showed no significant difference between WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC.
Fig. 4.
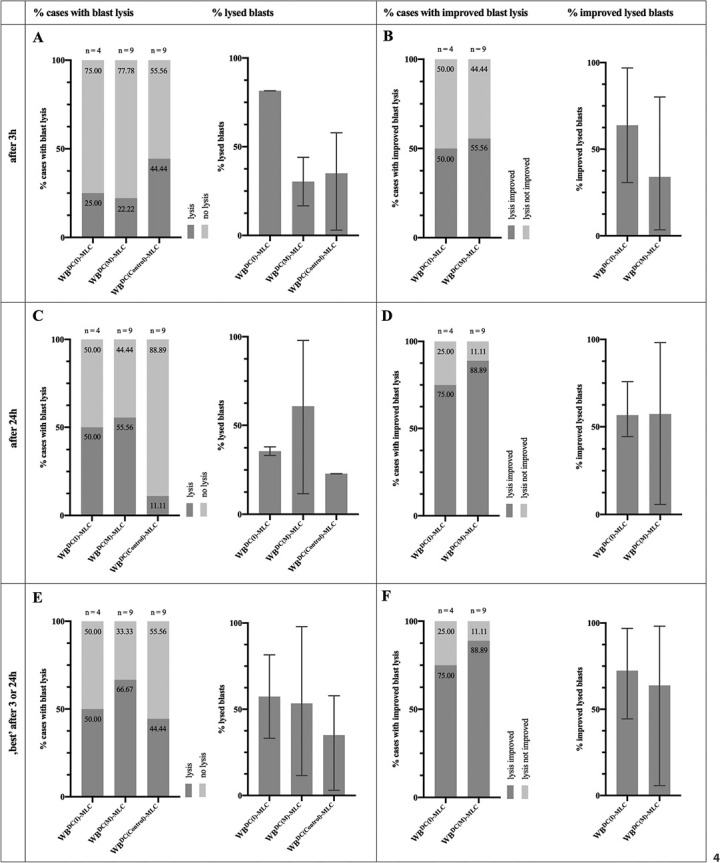
Stimulatory effect of DC/DCleu on the cytotoxic activity of immunoreactive cells as measured by CTX. Given are the proportions of cases with blast lysis (“% cases with blast lysis”) and the mean ± range of lysed blasts (“% lysed blasts”) in WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC after 3 h (A) and 24 h (C), and the “best” achieved blast lysis after 3 h or 24 h (E); the proportions of cases with an improvement in blast lysis (“% cases with improved blast lysis”) and the mean ± range of improved lysed blasts (“% improved lysed blasts”) in WBDC(I)-MLC and WBDC(M)-MLC in relation to WBDC(Control)-MLC after 3 h (B) and 24 h (D), and the “best” achieved improvement in blast lysis after 3 h or 24 h (F).
Concerning the lysis of target cells in WBDC(I)-MLC and WBDC(M)-MLC in relation to WBDC(Control)-MLC, after 3 h, we could observe an improvement in lysis in WBDC(I)-MLC as well as WBDC(M)-MLC in about half of the cases. Average improved lysed blasts were (n.s.) higher in WBDC(I)-MLC than in WBDC(M)-MLC (Fig. 4B). After 24 h, more cases of WBDC(I)-MLC and WBDC(M)-MLC attained an improvement in lysis. Average improved lysed blasts in WBDC(M)-MLC thereby increased (n.s.) to levels of WBDC(I)-MLC (Fig. 4D). Notably, in cases without an improvement in lysis, frequencies of blasts showed no significant difference between WBDC(I)-MLC and WBDC(M)-MLC.
Overall, choosing the best anti-leukaemic cytotoxicity after 3 or 24 h, we found higher numbers of cases with lysis in WBDC(M)-MLC than in WBDC(I)-MLC and WBDC(Control)-MLC. Average frequencies of lysed blasts were (n.s.) higher in WBDC(I)-MLC and WBDC(M)-MLC than in WBDC(Control)-MLC, with equal averages in WBDC(I)-MLC and WBDC(M)-MLC (Fig. 4E). WBDC(I)-MLC and WBDC(M)-MLC attained equal proportions of cases with an improvement in lysis and equal averages of improved lysed blasts (Fig. 4F). There was no significant difference in the frequencies of blasts in cases without lysis between WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC and in cases without an improvement in lysis between WBDC(I)-MLC and WBDC(M)-MLC.
Stimulatory Impact of DC/DCleu on the IFNy Secretion of T Cell-Enriched Immunoreactive Cells Detected via CSA
Analysis of IFNy Secretion
The IFNy secretion of innate and adaptive immune cells was determined through CSA before (uncultured WB) and after (WBDC(I)-MLC, WBDC(M)-MLC, WBDC(Control)-MLC) DC/DCleu stimulation. Frequencies of IFNy-secreting TCD3+, TCD4+, TCD8+, CIKCD56+, NKCD56+, CIKCD161+, NKCD161+, and iNKT cells were analysed.
No Spontaneous Activation of Immunoreactive Cells during DCC and MLC
We compared frequencies of IFNy-secreting immunoreactive cells in uncultured WB to WBDC(Control)-MLC, to assess the effect of cultivation (DCC and MLC). We found low frequencies of IFNy-secreting cells in uncultured WB. Comparing uncultured WB to WBDC(Control)-MLC showed no significant differences, besides significantly lower frequencies of NKCD161+IFNy+/NKCD161+ in WBDC(Control)-MLC (Fig. 5).
Fig. 5.
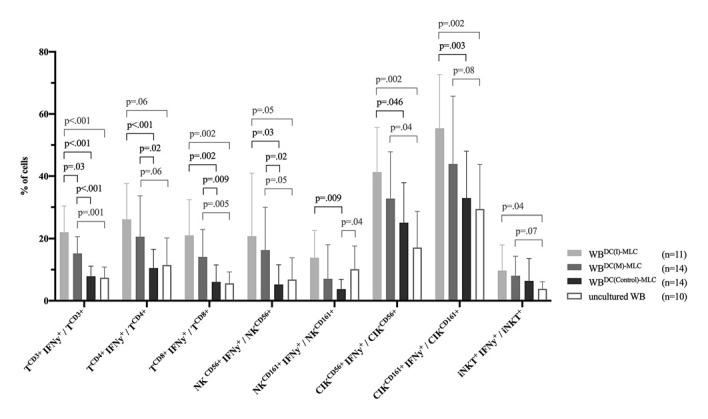
IFNy secretion of immunoreactive cells before (uncultured WB) and after (WBDC(I)-MLC, WBDC(M)-MLC, WBDC(Control)-MLC) DC/DCleu stimulation as measured by CSA. Given are the mean ± SD of frequencies of IFNy-secreting T, NK, CIK, and iNKT cells. Statistically significant (p values <0.05) and borderline significant (p values 0.10 to 0.05) differences are given. Abbreviations of all cell types are given in Table 2.
DC/DCleu Stimulation Increases IFNy Secretion of Adaptive and Innate Immunoreactive Cells
To assess the effect of DC/DCleu stimulation on the secretion of IFNy, we compared frequencies of IFNy-secreting immunoreactive cells in WBDC(I)-MLC, WBDC(M)-MLC to uncultured WB as well as to WBDC(Control)-MLC.
Regarding cells of the adaptive immune system, we found significantly higher frequencies of TCD3+IFNy+/TCD3+ in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB and WBDC(Control)-MLC, with WBDC(I)-MLC holding significantly higher frequencies than WBDC(M)-MLC. Moreover, we could detect significantly higher frequencies of TCD4+IFNy+/TCD4+ and TCD8+IFNy+/TCD8+ in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB and WBDC(Control)-MLC. Frequencies of both cell groups were (n.s.) higher in WBDC(I)-MLC compared to WBDC(M)-MLC (Fig. 1C, 5).
Regarding cells of the innate immune system, we found (significantly) higher frequencies of NKCD56+IFNy+/NKCD56+ and NKCD161+IFNy+/NKCD161+ in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB and WBDC(Control)-MLC, beside NKCD161+IFNy+/NKCD161+ showing no significant difference compared to uncultured WB and WBDC(Control)-MLC, with (n.s.) higher frequencies in WBDC(I)-MLC than WBDC(M)-MLC. Moreover, we could detect (significantly) higher frequencies of CIKCD56+IFNy+/CIKCD56+ and CIKCD161+IFNy+/CIKCD161+ in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB and WBDC(Control)-MLC, with (n.s.) higher frequencies in WBDC(I)-MLC than WBDC(M)-MLC. No significant differences could be found in the frequencies of iNKT+IFNy+/iNKT+ in WBDC(I)-MLC and WBDC(M)-MLC compared to WBDC(Control)-MLC, though compared to uncultured WB (Fig. 5).
No Impact of Age, Sex, and Blast Frequency on Stimulation of IFNy Secretion
Overall, the stimulation of IFNy secretion was possible with both DC/DCleu-Kit-I and -Kit-M and independent from patients' age, sex, or blast frequency (data not shown).
LAA Stimulation Does Not Further Increase IFNy Secretion of DC/DCleu-Stimulated Immunoreactive Cells
To assess whether DC/DCleu-stimulated adaptive immunoreactive cells have been subject to LAA-dependent activation, we added the two LAA WT-1 and PRAME to uncultured WB, WBDC(I)-MLC, WBDC(M)-MLC, and WBDC(Control)-MLC. The addition of LAA did (n.s.) increase frequencies of IFNy-secreting cells in uncultured WB and WBDC(Control)-MLC, whereas it did not further increase frequencies of IFNy-secreting cells in WBDC(I)-MLC and WBDC(M)-MLC (data not shown).
Positive Correlation of IFNy-Positive Immunoreactive Cells Obtained by CSA and ICA
In order to validate frequencies of IFNy-producing immunoreactive cells obtained by CSA, we performed parallel ICAs and correlated the results. There was a significantly high positive correlation (r = 0.793) between frequencies obtained by CSA and frequencies obtained by ICA (Fig. 6). Both the CSA and ICA hereby yielded comparable frequencies of IFNy-positive TCD3+, TCD4+, TCD8+, CIKCD56+, NKCD56+, and iNKT cells.
Fig. 6.
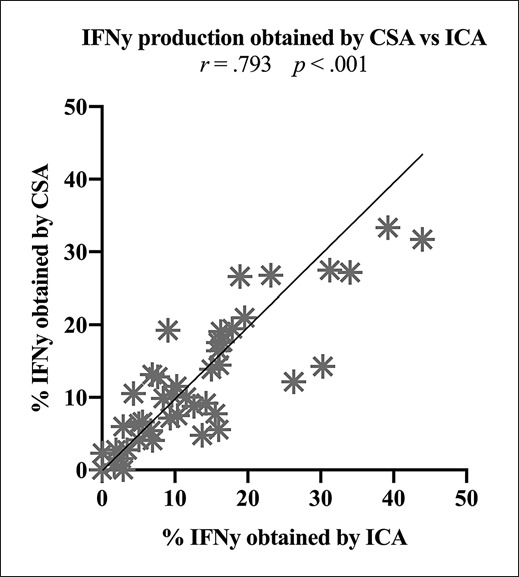
Correlation of the IFNy production of immunoreactive cells (TCD3+, TCD4+, TCD8+, CIKCD56+, NKCD56+, iNKT cells) obtained by CSA and ICA. Statistically significant (p values <0.05) correlations are given. Abbreviations of all cell types are given in Table 2.
Positive Correlation of IFNy Secretion and Anti-Leukaemic Cytotoxicity of DC/DCleu-Stimulated Immunoreactive Cells
To assess the relationship between the IFNy secretion and anti-leukaemic cytotoxicity of DC/DCleu-stimulated immunoreactive cells, we correlated the absolute improvement of IFNy secretion with the relative improvement of blast lysis (= improved blast lysis) in WBDC(M)-MLC in proportion to WBDC(Control)-MLC. Unfortunately, both groups could not be correlated in WBDC(I)-MLC due to low case numbers.
The IFNy secretion did not correlate with anti-leukaemic cytotoxicity after 3 h, but after 24 h: within the adaptive immune cells, we found a significantly moderate positive correlation between TCD3+IFNy+/TCD3+ and blast lysis (r = 0.600) and between TCD8+IFNy+/TCD8+ and blast lysis (r = 0.596), and a significantly high positive correlation between TCD4+IFNy+/TCD4+ and blast lysis (r = 0.716) (Fig. 7A–C). Moreover, within the innate immune cells, we found a significantly high positive correlation between NKCD56+IFNy+/NKCD56+ and blast lysis (r = 0.976). Other innate immune cells showed no further significant correlations (Fig. 7D).
Fig. 7.
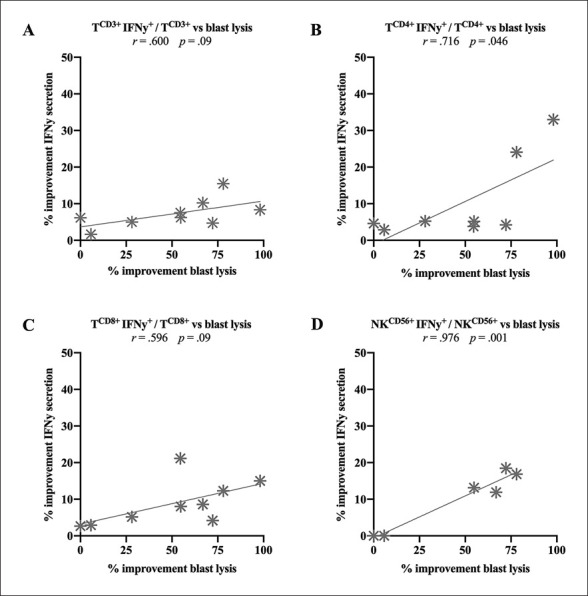
Correlation of the absolute improvement of IFNy secretion with the relative improvement of blast lysis (= improved blast lysis) in WBDC(M)-MLC compared to WBDC(Control)-MLC. Given are the correlation of TCD3+IFNy+/TCD3+ (A), TCD4+IFNy+/TCD4+ (B), TCD8+IFNy+/TCD8+ (C), and NKCD56+IFNy+/NKCD56+ (D) with blast lysis. Statistically significant (p values <0.05) and borderline significant (p values 0.10 to 0.05) correlations are given. Abbreviations of all cell types are given in Table 2.
Discussion
DC-Based Immunotherapy for AML
Based on the realisation that the immune system can be exploited to take control over AML, different immunological strategies have been developed to prompt a potent anti-leukaemic immunity. Here, targeted immunotherapeutic strategies relying on antibodies, engineered T-cell receptors and T cells engineered to express chimeric antigen receptors have shown promising results [42, 43, 44]. Targeted immunotherapy, however, depends on a competent target antigen to assure on-tumour effectivity but prevent off-tumour toxicity. Yet, selecting a proper leukaemic target antigen proves to be difficult due to a pervasive expression pattern overlapping with healthy tissues and haematopoiesis [10, 43, 45, 46]. However, there are strategies which are able to overcome this obstacle, most notably DC-based strategies. DCs generated from myeloid leukaemic blasts (DCleu) are able to simultaneously express dendritic- and leukaemia-specific antigens and thereby prime and enhance leukaemia-specific immune responses with the whole leukaemic antigen repertoire ex and in vivo [11, 47, 48, 49, 50, 51, 52], breaking the burden of finding an appropriate target antigen.
DC/DCleu Generation and Their Stimulatory Impact on the Anti-Leukaemic Activity of T Cell-Enriched Immunoreactive Cells
We generated DC and DCleu from leukaemic WB ex vivo with immunomodulatory Kit-I and Kit-M. Frequencies of DC and DCleu were significantly higher in WBDC(M) and WBDC(I) compared to WBDC(Control), with significantly higher frequencies in WBDC(I) than WBDC(M). Both DC and DCleu showed a significant proportion of mature DC. This stimulation of maturation and CCR7-dependent migration to lymph nodes, as achieved by Kit-I and Kit-M, is essential for DC and DCleu to activate T cells and other immunoreactive cells [53, 54, 55]. Though both Kits are able to generate significant frequencies of DC/DCleu, it appears that the combination of GM-CSF + Picibanil (OK-432) in Kit-I has a stronger danger signalling and stimulatory impact on DC differentiation than the combination of GM-CSF + PGE1 in Kit-M, but a similar stimulatory impact on DC maturation. Importantly, the proliferation of non-converted leukaemic blasts was not induced by Kit-I and Kit-M during DCC. All these findings have already been demonstrated in larger studies [unpublished data] [38, 39]. Comparable results were found under hypoxia-like conditions [40].
Through the stimulation of immunoreactive cells with DC/DCleu we could observe a generally higher activation status in WBDC(I)-MLC, WBDC(M)-MLC as well as WBDC(Control)-MLC compared to WBDC(Control), characterised by a significant increase of proliferating T cells, a significant shift from naive to non-naive T-cell subsets, and a (significant) increase of central and effector memory T cells. This general transformation is most likely caused by the addition of IL-2 to all MLCs, as IL-2 triggers the proliferation and differentiation of T cells as well as the activation and differentiation of other immunoreactive cells [56]. In addition to that, previous larger studies, however, have found an increase of TCD8+ cells and a corresponding decrease of TCD4+ cells in WBDC(I)-MLC and WBDC(M)-MLC compared to WBDC(Control)-MLC and WBDC(Control) [38].
Even though the stimulation of immunoreactive cells with DC/DCleu had no impact on the composition of T-cell subsets, it had an impact on the anti-leukaemic activity. We could demonstrate that the anti-leukemic cytotoxicity of immunoreactive cells could be notably improved through the stimulation with DC/DCleu-Kit-I and -Kit-M in most of the cases. Interestingly, some cases achieved lysis or improved lysis after 3 h and some cases only after 24 h, whereas average lysis was the highest in WBDC(I)-MLC after 3 h and in WBDC(M)-MLC after 24 h. This occurrence might be due to different killing mechanisms of the immunoreactive cells: the early and fast-acting perforin-granzyme pathway and the late and slow-acting Fas/FasL pathway, which can run separately or synergistically [21, 22]. WBDC(I)-MLC hereby appears to perform via the former pathway, while WBDC(M)-MLC appears to operate via the latter pathway. Overall, pooling the best anti-leukaemic cytotoxicity after 3 or 24 h, DC/DCleu-Kit-I and -Kit-M, however, appear to be equally efficient. Taken together, the CTX allows to quantify the acquired anti-leukaemic cytotoxicity, but it does not display participating cells of the immune response, like the CSA does.
Stimulatory Impact of DC/DCleu on the IFNy Secretion of T Cell-Enriched Immunoreactive Cells
DC/DCleu activate adaptive immune cells (TCD4+, TCD8+) by presenting leukaemia-specific antigens over MHC class I and II [35] and presumably innate immune cells (NKCD56+, NKCD161+, CIKCD56+, CIKCD161+, iNKT) by yet unknown MHC-unrestricted mechanisms [57, 58], thereby enhancing their effector mechanisms and IFN secretion, the latter also resulting in cross-stimulation. An increase of IFNy secretion upon DC/DCleu stimulation can hence display functionally active leukaemia-specific cells.
With the CSA we were able to detect and phenotypically characterise IFNy-secreting adaptive and innate immunoreactive cells, and thereby evaluate the effect of DC/DCleu stimulation. Uncultured leukaemic WB, as a starting point, already showed low frequencies of IFNy-secreting cells representing a physiological (and conceivably partially leukaemia-specific) immunological base activity. Through stimulation of immunoreactive cells by DC/DCleu-Kit-I and -Kit-M, we were able not only to increase the anti-leukaemic cytotoxicity, but also to significantly increase the IFNy secretion of adaptive immune cells (TCD3+, TCD4+, TCD8+ cells) in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB as well as WBDC(Control)-MLC. Further, the IFNy secretion of innate immune cells (CIKCD56+, NKCD56+, CIKCD161+, NKCD161+ cells) was (significantly) increased in WBDC(I)-MLC and WBDC(M)-MLC compared to uncultured WB as well as WBDC(Control)-MLC, beside NKCD161+ cells showing no significant difference compared to uncultured WB and iNKT cells showing no significant difference compared to WBDC(Control)-MLC. The IFNy secretion induced with DC/DCleu-Kit-I hereby emerged to be greater than with DC/DCleu-Kit-M. Noteworthy, the stimulation of IFNy secretion was independent from patients' age, sex, or blast frequency. Overall, we found an increased immunological activity of innate and adaptive immunoreactive cells after DC/DCleu stimulation, pointing to an induction of leukaemia-specific cells.
Moreover, we compared frequencies of IFNy-secreting cells in uncultured WB and cultured WBDC(Control)-MLC to assess the effect of cultivation on the secretion of IFNy. We could not find significant differences in any cell types, besides lower levels of IFNy-secreting NKCD161+ cells after cultivation. This might be due to an IL-2-induced expression of lectin-like transcript 1 (LLT1) on various cells, as cross-linking of LLT1 and CD161 on NKCD161+ cells results in an inhibition of IFNy production and cytotoxicity [59, 60]. However, it seems that cells stimulated with DC/DCleu-Kit-I and -Kit-M can slightly compensate this effect. In regard to future clinical application, we suppose the effect of IL-2-induced NKCD161+ inhibition by LLT1 in vitro to be negligible in vivo, as IL-2 was supplemented. Overall, these findings show that no spontaneous activation of immunoreactive cells during DCC and MLC occurs.
We furthermore investigated the effect of LAA (WT-1 and PRAME) stimulation on DC/DCleu-stimulated adaptive immunoreactive cells. We hypothesised, that the addition of LAA can only increase the IFNy secretion of cells that have not been subject to LAA-dependent activation. Conformably, the addition of WT-1 and PRAME did (n.s.) increase frequencies of IFNy-secreting cells in uncultured WB and WBDC(Control)-MLC. In contrast, the addition of LAA did not further increase frequencies of IFNy-secreting cells in WBDC(I)-MLC and WBDC(M)-MLC, affirming that these immunoreactive cells have been subject to activation through leukaemia-specific antigens presented by DCleu-Kit-I and -Kit-M, in this case the known LAAs WT-1 and PRAME, whereby no further effect was possible. Remarkably, WT-1 and PRAME are only two of hundreds of leukaemic blast antigens presented by DCleu, giving DCleu the exceptional potential to initiate a comprehensive leukaemia-specific immune response.
Correlation of IFNy-Positive Cells Obtained by CSA and ICA
In order to validate frequencies of IFNy-producing immunoreactive cells obtained by CSA, we performed parallel ICAs and correlated the results. We found a significantly high correlation of obtained frequencies between both methods. Both the CSA and ICA yielded comparable frequencies of IFNy-positive TCD3+, TCD4+, TCD8+, CIKCD56+, NKCD56+, and iNKT cells.
Correlation of IFNy Secretion and Anti-Leukaemic Cytotoxicity of DC/DCleu-Stimulated Immunoreactive Cells
We conclusively correlated the IFNy secretion with the anti-leukaemic cytotoxicity in WBDC(M)-MLC and found a significantly positive correlation between the IFNy secretion of TCD3+, TCD4+, TCD8+ as well as NKCD56+ cells and the 24-h anti-leukaemic cytotoxicity. Though the other immune cells (NKCD161+, CIKCD56+, CIKCD161+ cells) did not show a direct correlation to cytotoxicity, they showed a (n.s.) increased secretion of IFNy pointing towards an increased activity, which may contribute indirectly to the overall leukaemia-specific activity. Ultimately, cytotoxicity remains dependent on the great interaction of various cells, cytokines, and other factors [12, 35]. Nevertheless, IFNy can reflect the cytotoxic activity of TCD3+, TCD4+, TCD8+, and NKCD56+ cells. With this knowledge, we suppose that IFNy readouts of these specific cell groups are sufficient to assess and monitor the efficacy of AML immunotherapy.
Evaluation of the CSA
There are various techniques to investigate the cytokine production of immune cells on a single cell level, including the CSA, ICA, and Elispot technology. The central technique used in this study was the CSA, which allowed us to detect and phenotypically analyse IFNy-secreting immunoreactive cells on a single cell level and moreover display DC/DCleu-induced leukaemia-specific activity and cytotoxicity. It hereby presented itself as a convenient, valid, and reliable method [61].
The ICA has, like the CSA, the potential to detect and phenotypical characterise IFNy-producing cells, however, potentially with a lower sensitivity than the CSA, especially when dealing with low frequencies of IFNy-positive cells [61, 62, 63, 64]. Overall though, we and others found the IFNy production obtained by the CSA and ICA highly correlating [65]. Notably, the ICA holds the capacity to simultaneously analyse further cellular markers like TNFa, but, contrary to the CSA, is more time consuming and inevitably kills analysed cells [66]. The Elispot technology, an enzyme-linked cytokine capture assay, enables the detection of IFNy-producing cells with a high sensitivity, but lacks their phenotypical characterisation if cells are not previously sorted into cellular subsets of interest [67]. Noteworthily, the CSA, contrary to the ICA and the Elispot, holds the potential to isolate viable IFNy-expressing cells, if the interest lies in expansion, further functional analyses (e.g., MHC/peptide-tetramer staining), or cell therapy (e.g., adoptive T-cell transfer) [68, 69].
With IFNy assays being able to display leukaemia-specific cells as well as anti-leukaemic cytotoxicity, they are in advantage of regular cytotoxicity assays. Assays like fluorochrome-labelled assays, 51CR-labelled assays, degranulation assays, and LDH assays only allow the evaluation of achieved cytotoxicity but lack the characterisation of overall participating (leukaemia-specific) cells [70, 71, 72]. However, they best depict functionally active Tc, as they directly measure the lysis of target cells [73]. Nonetheless, these assays are often very labour-intensive and unsuited for upscaling as needed in clinical applications. A more specific identification of Tc can only be accomplished by MHC/peptide-tetramer staining, yet this method requires a specific target antigen, in contrary to IFNy and cytotoxicity assays, and does not assure the functionality of the specific T-cell receptor [73, 74].
All in all, the CSA technology holds multiple advantages compared to other cytokine and cytotoxicity assays, especially by combining characterising and functional data.
Conclusion
We were able to describe the potential of DC/DCleu to induce or improve leukaemia-specific and anti-leukaemic activity through the detection of IFNy-secreting innate and adaptive immune cells ex vivo. The CSA in this regard proved to be a convenient and reproducible technique to detect and phenotypically characterise IFNy-secreting cells. As such, we believe that IFNy could become a very valuable parameter to assess and monitor the efficacy of AML immunotherapy in future clinical applications.
Statement of Ethics
Sample collection was conducted after obtaining written informed consent of the blood donor and in accordance with the World Medical Association Declaration of Helsinki and the ethic committee of the Ludwig Maximilian University Hospital Munich (vote No. 33905).
Conflict of Interest Statement
Modiblast Pharma GmbH (Oberhaching, Germany) holds the European Patent 15 801 987.7-1118 and US Patent 15-517627 “Use of immunomodulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias”, in which H.M.S. is involved.
Author Contributions
L.K.K conducted DCC, MLC, CTX, and CSA experiments and all flow cytometric and statistical analyses. O.S., S.U., F.D.-G., and N.R. performed additional DCC, MLC, CTX, and CSA experiments, which were analysed by L.K.K. O.S. conducted ICA experiments, which were analysed by L.K.K. D.K., A.R., and C.S. provided leukaemic whole blood samples and corresponding diagnostic reports. D.C.A. and B.E.-V. supported functionality assays. H.M.S. designed the study. L.K.K and H.M.S. drafted the manuscript.
Acknowledgement
The authors thank patients, nurses, and physicians for their support with sample materials and diagnostic reports. The results presented in this article are part of the doctoral thesis of Lara Kristina Klauer at the University Hospital of the Ludwig Maximilian University Munich.
References
- 1.Sawyers CL, Denny CT, Witte ON. Leukemia and the disruption of normal hematopoiesis. Cell. 1991;64((2)):337–50. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Barnett M, Bassan R, Gatta G, Tondini C, Kern W. Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004;50((3)):197–222. doi: 10.1016/j.critrevonc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129((4)):424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392((10147)):593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Acute Leukemias. Springer; 2008. pp. p. 47–56. [DOI] [PubMed] [Google Scholar]
- 6.Hokland P, Woll PS, Hansen MC, Bill M. The concept of leukaemic stem cells in acute myeloid leukaemia 25 years on: hitting a moving target. Br J Haematol. 2019 Oct;187((2)):144–56. doi: 10.1111/bjh.16104. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Cancer Stat Facts: Leukaemia − Acute Myeloid Leukaemia (AML) [Internet] 2020. [cited 11 February 2020]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html.
- 8.Pyzer AR, Avigan DE, Rosenblatt J. Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum Vaccin Immunother. 2014;10((11)):3125–31. doi: 10.4161/21645515.2014.982993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantino J, Gomes C, Falcão A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res. 2017;65((4)):798–810. doi: 10.1007/s12026-017-8931-1. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenegger FS, Krupka C, Haubner S, Köhnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol. 2017;10((1)):142. doi: 10.1186/s13045-017-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansprenger C, Amberger DC, Schmetzer HM. Potential of immunotherapies in the mediation of antileukemic responses for patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS)-With a focus on Dendritic cells of leukemic origin (DCleu) Clinical Immunology. 2020:108467. doi: 10.1016/j.clim.2020.108467. [DOI] [PubMed] [Google Scholar]
- 12.Chaplin DD. Overview of the immune response. Journal of Allergy and Clinical Immunology. 2010;125((2)):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1((3)):145–9. doi: 10.1158/2326-6066.CIR-13-0102. [DOI] [PubMed] [Google Scholar]
- 14.Narita M, Takahashi M, Liu A, Ayres F, Satoh N, Abe T, et al. Generation of dendritic cells from leukaemia cells of a patient with acute promyelocytic leukaemia by culture with GM-CSF, IL-4 and TNF-alpha. Acta Haematol. 2001;106((3)):89–94. doi: 10.1159/000046595. [DOI] [PubMed] [Google Scholar]
- 15.Cignetti A, Vallario A, Roato I, Circosta P, Allione B, Casorzo L, et al. Leukemia-derived immature dendritic cells differentiate into functionally competent mature dendritic cells that efficiently stimulate T cell responses. J Immunol. 2004;173((4)):2855–65. doi: 10.4049/jimmunol.173.4.2855. [DOI] [PubMed] [Google Scholar]
- 16.Kremser A, Dressig J, Grabrucker C, Liepert A, Kroell T, Scholl N, et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods. J Immunother. 2010;33((2)):185–99. doi: 10.1097/CJI.0b013e3181b8f4ce. [DOI] [PubMed] [Google Scholar]
- 17.Schmetzer HM, Kremser A, Loibl J, Kroell T, Kolb HJ. Quantification of ex vivo generated dendritic cells (DC) and leukemia-derived DC contributes to estimate the quality of DC, to detect optimal DC-generating methods or to optimize DC-mediated T-cell-activation-procedures ex vivo or in vivo. Leukemia. 2007;21((6)):1338–41. doi: 10.1038/sj.leu.2404639. [DOI] [PubMed] [Google Scholar]
- 18.Grabrucker C, Liepert A, Dreyig J, Kremser A, Kroell T, Freudenreich M, et al. The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive factor for the lytic potential of dendritic cells-primed leukemia-specific T cells. J Immunother. 2010;33((5)):523–37. doi: 10.1097/CJI.0b013e3181d87ffd. [DOI] [PubMed] [Google Scholar]
- 19.Liepert A, Grabrucker C, Kremser A, Dreyssig J, Ansprenger C, Freudenreich M, et al. Quality of T-cells after stimulation with leukemia-derived dendritic cells (DC) from patients with acute myeloid leukemia (AML) or myeloid dysplastic syndrome (MDS) is predictive for their leukemia cytotoxic potential. Cell Immunol. 2010;265((1)):23–30. doi: 10.1016/j.cellimm.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Boeck CL, Amberger DC, Doraneh-Gard F, Sutanto W, Guenther T, Schmohl J, et al. Significance of frequencies, compositions, and/or antileukemic activity of (DC-stimulated) invariant NKT, NK and CIK cells on the outcome of patients with AML, ALL and CLL. J Immunother. 2017;40((6)):224–48. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 21.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370((6491)):650–2. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 22.Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology. 2011;133((2)):190–6. doi: 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Lostao L, de Miguel D, Al-Wasaby S, Gallego-Lleyda A, Anel A. Death ligands and granulysin: mechanisms of tumor cell death induction and therapeutic opportunities. Immunotherapy. 2015;7((8)):883–2. doi: 10.2217/imt.15.56. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13((2)):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 25.Kursunel MA, Esendagli G. Corrigendum to “The untold story of IFN-γ in cancer biology” [Cytokine Growth Factor Rev. 31 (2016) 73–81] Cytokine Growth Factor Rev. 2017 2017/06;35((97)) doi: 10.1016/j.cytogfr.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol. 2018 2018/05/04;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2003 2003/10/02;75((2)):163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 28.Schoenborn JR, Wilson CB. Regulation of Interferon-γ During Innate and Adaptive Immune Responses. Advances in Immunology Elsevier. 2007:p. 41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998 Jul 1;58((13)):2832–7. [PubMed] [Google Scholar]
- 30.Detjen KM, Farwig K, Welzel M, Wiedenmann B, Rosewicz S. Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut. 2001 2001/08/01;49((2)):251–62. doi: 10.1136/gut.49.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Ruiz C, LÓPez-Rivas A. Mitochondria-dependent and -independent mechanisms in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis are both regulated by interferon-γ in human breast tumour cells. Biochemical Journal. 2002 2002/08/01;365((3)):825–32. doi: 10.1042/BJ20020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzoferrato E, Liu Y, Gambotto A, Armstrong MJ, Stang MT, Gooding WE, et al. Ectopic Expression of Interferon Regulatory Factor-1 Promotes Human Breast Cancer Cell Death and Results in Reduced Expression of Survivin. Cancer Res. 2004 2004/11/15;64((22)):8381–8. doi: 10.1158/0008-5472.CAN-04-2223. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Ruiz C, Ruiz de Almodóvar C, Rodríguez A, Ortiz-Ferrón G, Redondo JM, López-Rivas A. The Up-regulation of Human Caspase-8 by Interferon-γ in Breast Tumor Cells Requires the Induction and Action of the Transcription Factor Interferon Regulatory Factor-1. Journal of Biological Chemistry. 2004 2004/03/01;279((19)):19712–20. doi: 10.1074/jbc.M313023200. [DOI] [PubMed] [Google Scholar]
- 34.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008 2008/03;123((3)):326–38. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010 2010/02;125((2)):S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995 1995/10;58((4)):373–81. [PubMed] [Google Scholar]
- 37.Shtrichman R, Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001 2001/06;4((3)):251–9. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 38.Amberger DC, Doraneh-Gard F, Gunsilius C, Weinmann M, Möbius S, Kugler C, et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int J Mol Sci. 2019 2019/09/17;20((18)):4590. doi: 10.3390/ijms20184590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plett CA, Christoph D, Rabe A, Deen D, Stankova Z, Hirn A, et al. Kits do not induce AML-blasts' proliferation ex vivo. IPO-38 is an appropriate and reliable marker to detect and quantify proliferating blasts. Eur J Cancer. 2017;5((1)):3–4. [Google Scholar]
- 40.Doraneh gard F, Amberger D, Weinmann M, Boeck C, Gunsilius C, Kugler C, et al. Standard normoxic versus physiological hypoxic culture of AML patients' (pts) whole blood (WB) samples with immune modulatory kits yields comparable proportions of dendritic cells and functional results. European Journal of Cancer. 2018 2018/03;92:S10–1. [Google Scholar]
- 41.Vogt V, Schick J, Ansprenger C, Braeu M, Kroell T, Kraemer D, et al. Profiles of activation, differentiation-markers, or β-integrins on T cells contribute to predict T cells' antileukemic responses after stimulation with leukemia-derived dendritic cells. J Immunother. 2014;37((6)):331–47. doi: 10.1097/CJI.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 42.Geiger TL, Rubnitz JE. New approaches for the immunotherapy of acute myeloid leukemia. Discov Med. 2015 Apr;19((105)):275–84. [PMC free article] [PubMed] [Google Scholar]
- 43.Holzinger A, Barden M, Abken H. The growing world of CAR T cell trials: a systematic review. Cancer Immunol Immunother. Immunotherapy2016. 2016/09/09;65((12)):1433–50. doi: 10.1007/s00262-016-1895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biernacki MA, Brault M, Bleakley M. T-Cell Receptor-Based Immunotherapy for Hematologic Malignancies. Cancer J. 2019;25((3)):179–90. doi: 10.1097/PPO.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012 2012/06/01;26((10)):2186–96. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 46.Goswami M, Hensel N, Smith BD, Prince GT, Qin L, Levitsky HI, et al. Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia. 2014 2014/01/10;28((5)):1167–70. doi: 10.1038/leu.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Giannopoulos K, Reinhardt P, Tabarkiewicz J, Schmitt A, Greiner J, et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int J Oncol. 2006 Apr;28((4)):855–61. [PubMed] [Google Scholar]
- 48.Roddie H, Klammer M, Thomas C, Thomson R, Atkinson A, Sproul A, et al. Phase I/II study of vaccination with dendritic-like leukaemia cells for the immunotherapy of acute myeloid leukaemia. Br J Haematol. 2006 Apr;133((2)):152–7. doi: 10.1111/j.1365-2141.2006.05997.x. [DOI] [PubMed] [Google Scholar]
- 49.Anguille S, Lion E, Smits E, Berneman ZN, van Tendeloo VF. Dendritic cell vaccine therapy for acute myeloid leukemia: Questions and answers. Hum Vaccin. 2011 2011/05;7((5)):579–84. doi: 10.4161/hv.7.5.14652. [DOI] [PubMed] [Google Scholar]
- 50.Dong M, Liang D, Li Y, Kong D, Kang P, Li K, et al. Autologous dendritic cells combined with cytokine-induced killer cells synergize low-dose chemotherapy in elderly patients with acute myeloid leukaemia. J Int Med Res. 2012;40((4)):1265–74. doi: 10.1177/147323001204000405. [DOI] [PubMed] [Google Scholar]
- 51.Amberger DC, Schmetzer HM. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia. Transfusion Medicine and Hemotherapy. 2020 doi: 10.1159/000512452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eiz-Vesper B, Schmetzer HM. Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfusion Medicine and Hemotherapy. 2020 doi: 10.1159/000512729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogihara T, Iinuma H, Okinaga K. Usefulness of immunomodulators for maturation of dendritic cells. International Journal of Oncology. 2004 2004/08/01; [PubMed] [Google Scholar]
- 54.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008 2008/05;8((5)):362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 55.Platt AM, Randolph GJ. Development and Function of Myeloid Subsets. Elsevier; 2013. Dendritic Cell Migration Through the Lymphatic Vasculature to Lymph Nodes; pp. p. 51–68. [DOI] [PubMed] [Google Scholar]
- 56.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012 2012/02/17;12((3)):180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 57.Osada T, Clay T, Hobeika A, Lyerly HK, Morse MA. NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol ImmunotherImmunotherapy. 2005 2005/11/05;55((9)):1122–31. doi: 10.1007/s00262-005-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Introna M, Correnti F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int J Mol Sci. 2018 2018/01/25;19((2)):358. doi: 10.3390/ijms19020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldemir H, Prod'homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, et al. Cutting Edge: Lectin-Like Transcript 1 Is a Ligand for the CD161 Receptor. J Immunol. 2005 2005/12/08;175((12)):7791–5. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- 60.Llibre A, Klenerman P, Willberg CB. Multi-functional lectin-like transcript-1: A new player in human immune regulation. Immunol Lett. 2016 2016/09;177:62–9. doi: 10.1016/j.imlet.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Desombere I, Meuleman P, Rigole H, Willems A, Irsch J, Leroux-Roels G. The interferon gamma secretion assay: a reliable tool to study interferon gamma production at the single cell level. J Immunol Methods. 2004 2004/03;286((1–2)):167–85. doi: 10.1016/j.jim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Nomura LE, Walker JM, Maecker HT. Optimization of whole blood antigen-specific cytokine assays for CD4(+) T cells. Cytometry. 2000 2000/05/01;40((1)):60–8. doi: 10.1002/(sici)1097-0320(20000501)40:1<60::aid-cyto8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 63.Koehne G, Smith KM, Ferguson TL, Williams RY, Heller G, Pamer EG, et al. Quantitation, selection, and functional characterization of Epstein-Barr virus-specific and alloreactive T cells detected by intracellular interferon-gamma production and growth of cytotoxic precursors. Blood. 2002 2002/03/01;99((5)):1730–40. doi: 10.1182/blood.v99.5.1730. [DOI] [PubMed] [Google Scholar]
- 64.Jaimes MC, Maecker HT, Yan M, Maino VC, Hanley MB, Greer A, et al. Quality assurance of intracellular cytokine staining assays: Analysis of multiple rounds of proficiency testing. J Immunol Methods. 2011 2011/01;363((2)):143–57. doi: 10.1016/j.jim.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oelke M, Kurokawa T, Hentrich I, Behringer D, Cerundolo V, Lindemann A, et al. Functional Characterization of CD8+ Antigen-Specific Cytotoxic T Lymphocytes after Enrichment Based on Cytokine Secretion: Comparison with the MHC-Tetramer Technology. Scandinavian Journal of Immunology. 2000 2000/12;52((6)):544–9. doi: 10.1046/j.1365-3083.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- 66.Mair F, Tosevski V. Methods in Molecular Biology. New York: Springer; 2014. Intracellular Staining for Cytokines and Transcription Factors; pp. p. 39–49. [DOI] [PubMed] [Google Scholar]
- 67.Calarota SA, Baldanti F. Enumeration and Characterization of Human Memory T Cells by Enzyme-Linked Immunospot Assays. Clinical and Developmental Immunology. 2013;2013:1–8. doi: 10.1155/2013/637649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oelke M, Moehrle U, Chen JL, Behringer D, Cerundolo V, Lindemann A, et al. Generation and purification of CD8+ melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin Cancer Res. 2000 May;6((5)):1997–2005. [PubMed] [Google Scholar]
- 69.Van Rhijn I, Iwany SK, Fodran P, Cheng TY, Gapin L, Minnaard AJ, et al. CD1b-mycolic acid tetramers demonstrate T-cell fine specificity for mycobacterial lipid tails. Eur J Immunol. 2017 2017/07/31;47((9)):1525–34. doi: 10.1002/eji.201747062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kienzle N, Olver S, Buttigieg K, Kelso A. The fluorolysis assay, a highly sensitive method for measuring the cytolytic activity of T cells at very low numbers. J Immunol Methods. 2002 2002/09;267((2)):99–108. doi: 10.1016/s0022-1759(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 71.Lorenzo-Herrero S, Sordo-Bahamonde C, Gonzalez S, López-Soto A. Methods in Molecular Biology. New York: Springer; 2018. CD107a Degranulation Assay to Evaluate Immune Cell Antitumor Activity; pp. p. 119–30. [DOI] [PubMed] [Google Scholar]
- 72.Lemonnier FA. Evaluating CD8+ T Cell Responses In VitroAntigen Processing. New York: Springer; 2019. pp. p.199–215. [Google Scholar]
- 73.Michel N, Öhlschläger P, Osen W, Freyschmidt E-J, Guthöhrlein H, Kaufmann AM, et al. T Cell Response to Human Papillomavirus 16 E7 in Mice: Comparison of Cr Release Assay, Intracellular IFN-γ Production, ELISPOT and Tetramer Staining. Intervirology. 2002;45((4–6)):290–9. doi: 10.1159/000067923. [DOI] [PubMed] [Google Scholar]
- 74.Dolton G, Tungatt K, Lloyd A, Bianchi V, Theaker SM, Trimby A, et al. More tricks with tetramers: a practical guide to staining T cells with peptide-MHC multimers. Immunology. 2015 2015/08/04;146((1)):11–22. doi: 10.1111/imm.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schick J, Vogt V, Zerwes M, Kroell T, Kraemer D, Köhne CH, et al. Antileukemic T-cell Responses Can Be Predicted by the Composition of Specific Regulatory T-cell Subpopulations. J Immunother. 2013 2013/05;36((4)):223–37. doi: 10.1097/CJI.0b013e31829180e7. [DOI] [PubMed] [Google Scholar]


