Abstract
Ataxia-telangiectasia (AT) is an autosomal recessive disorder characterized by progressive ataxia, choreoathetosis and immunodeficiency beginning in early childhood. An 8-year-old girl was referred with a diagnosis of AT. She had gait disturbance and dysarthria for 3years. Multiple cutaneous telangiectases were observed on her face, trunk and limbs. Sequence analysis of the ATM gene revealed a homozygous c.7308–15A>G mutation in IVS49. Human Splicing Finder predicted that the mutation could activate an intronic cryptic acceptor site. We designed primers for amplification of related exons (48–50) from cDNA for evaluating splicing pattern. Sequencing of ATM exons 48–50 revealed a 14-nucleotide insertion from intron 49, between exons 49 and 50, resulting in premature termination of translation at codon 2439. To conclude, we report a novel mutation in a classical AT case, which resulted in an alternatively spliced transcript and was predicted to form a truncated protein or null protein due to nonsense-mediated decay.
Keywords: ATM, Ataxia-telengiectasia syndrome, Splicing mutation, Novel mutation, cDNA sequencing
Established Facts
Ataxia-telangiectasia (AT) is an autosomal recessive disorder characterized by progressive ataxia, choreoathetosis and immunodeficiency beginning in early childhood.
The estimated prevalence of the disease ranges from 1/40,000 to 1/300,000 people worldwide, depending on the geographic or ethnic region evaluated.
AT is caused by biallelic mutations in the ataxia telangiectasia mutated (ATM) gene in chromosome 11q22.3.
Novel Insights
A novel homozygous c.7308–15A>G mutation was detected in the intron − 49 of the ATM gene.
We report a novel mutation in a classical AT case, which was showed causing an alternatively spliced transcript and predicted to form a truncated protein or null protein due to nonsense-mediated decay.
Introduction
Ataxia-telangiectasia (AT; MIM #208900) is a rare autosomal recessive neurodegenerative disorder characterized by cerebellar ataxia due to progressive cerebellar degeneration [Anheim et al., 2012]. The other clinical features of AT are telangiectasias, immunodeficiency, sensitivity to ionizing radiation, recurrent sinopulmonary infections, premature aging, poor growth, delayed puberty, diabetes, and increased risk of malignancy, notably of lymphoid origin [Nissenkorn et al., 2016]. Elevated alpha-fetoprotein levels and chromosomal instability are the characteristic laboratory findings of AT cases. The estimated prevalence of the disease ranges from 1 in 40,000 to 1 in 300,000 people worldwide, depending on the geographic or ethnic region evaluated [Renvick et al., 2006; Verhagen et al., 2012].
Different forms of AT have been described in the literature, with those more severe variably categorized as “classic”, “typical”, “early onset” or “childhood onset” AT, while milder forms have been referred to as “variant”, “atypical”, “late onset” or “adult onset” AT [Rothblum-Oviatt et al., 2016]. The classical AT patient phenotype shows progressive cerebellar ataxia, oculomotor apraxia, altered eye movements, cognitive dysfunction, oculocutaneous telangiectasia, dystonia, choreoathetosis, immunodeficiency, and recurrent sinopulmonary infections in early childhood [Ruiz-Botero and Rodríguez-Guerrero, 2017].
AT is caused by biallelic mutations in the ataxia telangiectasia mutated (ATM) gene in chromosome 11q22.3. Monoalelic ATM mutations are responsible for cancer predisposition syndrome including gastric and breast cancer, lymphoma, and other cancer types. The gene contains 64 exons and encodes a protein with 3,050 amino acid residues. The ATM protein belongs to the phosphatidylinositol kinase family, which responds to DNA damage by phosphorylating key substrates involved in DNA repair and/or cell cycle control [Huh et al., 2013]. Truncating variants are the most common variants in the ATM gene, but mutational hotspots have not been identified [Telatar et al., 1998].
Case Report
The proband, an 8-year-old Syrian girl was referred for further evaluation of recurrent upper respiratory tract infections. She was born from a first-cousin consanguineous marriage, by vaginal delivery at normal gestational age. Her birth weight and length were noted as at the 10th and below the 3rd percentile, respectively. She experienced 10–12 episodes of upper respiratory tract infections annually including otitis media and pharyngitis. She had ataxia and dysarthria since 5 years of age and chronic dermatitis on her face since 3 years of age. Her physical examination showed ataxia, abnormal gait, telangiectasia of the eyes, and erythematous rash on the nose and ear. Her weight and height were below the 3rd percentile. She had bilateral crackles in chest auscultation. Laboratory investigations revealed a low lymphocyte count in complete blood count. Her serum α-feto-protein level was high. Serum immunoglobulin analysis showed low IgM, low IgG, and very low IgA levels. Flow cytometry analysis demonstrated that absolute counts of CD3 and CD19 were low and showed a severe combined immune deficiency phenotype (Table 1). Lung CT revealed bilateral bronchiectasis. Brain MRI showed nonspecific mild atrophic changes within the cerebellar hemispheres. She was commenced on regular intravenous immunoglobulin replacement therapy and prophylactic antibiotic (trimethoprim sulfamethoxazole). Infections have been controlled; however, the neurological findings still progressed. She was diagnosed as AT and ATM gene mutations were to be investigated.
Table 1.
Immunological evaluation of the patient
| Parameters | Patient | Reference values |
|---|---|---|
| Absolute lymphocyte/mm3 | 1,200 | 2,600–10,400 |
| Immunoglobulins, mg/dL | ||
| IgG | 601 | 764–2,134 |
| IgM | 22 | 69–387 |
| IgA | 6.6 | 78–383 |
| IgE, IU/L | 9.8 | 2–97 |
| Immunophenotyping | ||
| CD3+ cells %, (n) | 54 (648) | 55–88 (1,213–4,128) |
| CD3+CD4+ %, (n) | 26 (312) | 24–50 (647–1,111) |
| CD3+CD8+ %, (n) | 19 (228) | 17.5–42 (379–2083) |
| CD4+/CD8+ ratio | 1.3 | 1.3–3.9 |
| CD3+CD4+CD45RA+, % | 1 | 41–81 |
| CD3+CD4+CD45RO+, % | 99 | 26–58 |
| CD3+CD8+CD45RA+, % | 24 | 39–93 |
| CD3+CD8+CD45RO+, % | 77 | 11–79 |
| CD19+ cells %, (n) | 5.4 (64) | 7.4–22 (197–867) |
| Naïve CD19+CD27−IgD+, % | 51 | 5.5–90 |
| Memory unswitched CD19+CD27+IgD+, % | 27 | 5.9–28 |
| Memory switched CD19+CD27−IgD−, % | 11 | 6.7–31 |
| CD16+/CD56+ cells, % | 33 | 4–29 |
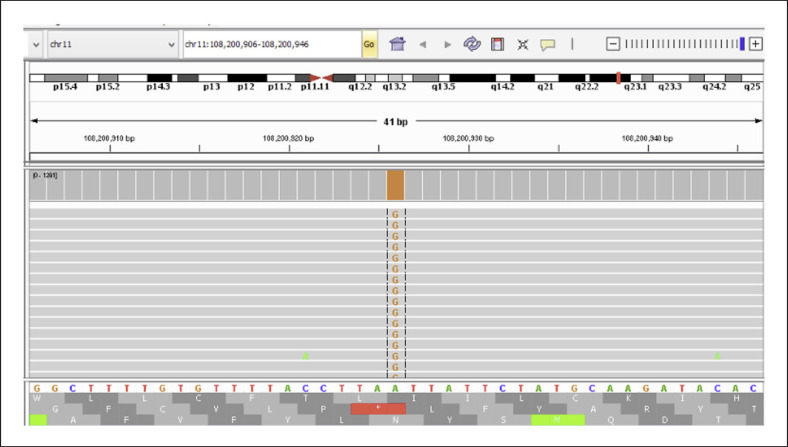
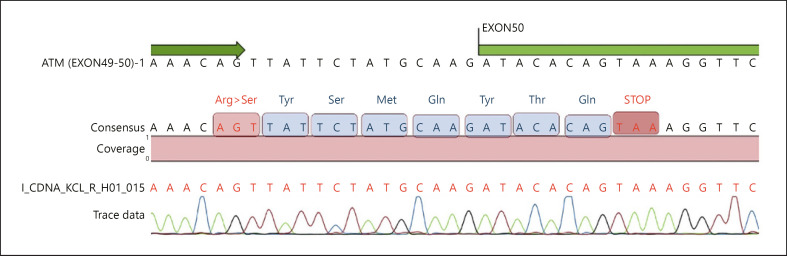
With a diagnosis of AT, all coding exons and exon-intron boundaries of ATM (NM_000051) were sequenced via next-generation sequencing after DNA extraction from peripheral blood lymphocytes. A homozygous c.7308–15A>G mutation was detected in intron 49 (Fig. 1). Mutation was not present in population studies and in-house database. Insilico analysis using Human Splicing Finder of the mutation revealed that the mutation could activate an intronic cryptic acceptor site [Desmet et al., 2009]. To evaluate splicing pattern, mRNA was isolated and via revers transcription cDNA obtained from peripheral blood lymphocytes. ATM exons 49–50 were amplified using primers that were designed to bind to exons 48 (forward) and 50 (reverse). Sanger sequencing of cDNA revealed a 14-nucleotide insertion from intron 49, between exons 49 and 50, resulting in premature termination of translation at codon 2439 (Fig. 2). The patient's healthy parents were heterozygous for the mutation. They were informed about cancer predisposition and consultated to the oncology clinic for follow-up care.
Fig. 1.
Homozygous c.7308–15A>G mutation in the ATM (NM_000051) gene.
Fig. 2.
Sanger sequencing of the ATM gene exons 49–50.
Discussion
AT is an autosomal recessive disorder with multisystem involvement. In classical form, neurological deficits starts in the early years of life, and children become wheelchair dependent around the age of 10. Immunodeficiency is seen in two-thirds and pulmonary disease is relatively common in classical AT than in the mild form of AT which presents with milder neurological findings and involuntary movements appearing before ataxia [Rothblum-Oviatt et al., 2016]. Our patient's presentation was compatible with the classical form of AT with more severe symptoms starting in the first decade. The girl had gait ataxia and recurrent sinopulmonary infections at the age 8. It is known that AT patients are at risk of malignacy especially lymphoid malignancies at younger ages in classical AT [Rothblum-Oviatt et al., 2016]. However, there was no evidence for malignancy at the age of 8.
Bialellic ATM gene mutations are responsible for AT cases. However monoalleic mutations cause distinct types of cancer susceptibility such as breast cancer [Milne, 2009]. Therefore, molecular genetic diagnosis is important for preimplantation, prenatal genetic diagnosis and also prediction of hereditary cancer predisposition for patients, parents, and other family members.
Splicing substitions constitute a significant proportion with a frequency of 18% in all mutations in the ATM gene [Teraoka et al., 1999]. Most common splice site mutations form new exon/intron boundaries or activate new cryptic exons as a result of alterations at donor or acceptor sites [Lewandowska et al., 2013]. In our case, a novel intronic mutation resulting in missplicing of mRNA was detected. The mutation was detected 15 nucleotide from exon activating a cryptic acceptor splice site in IVS49. Analysis of cDNA revealed a 14-nucleotide insertion from intron 49, between exons 49 and 50, resulting in stop codon at codon 2439, which was predicted to lead to a truncated ATM protein or no detectable ATM protein as a result of nonsense-mediated decay. However, we were not able to reveal these hypothesis at protein level in the cells.
To conclude we report a novel mutation in a classical AT case, which was showed to cause an alternatively spliced transcript and predicted to form a truncated protein or null protein due to nonsense-mediated decay.
Statement of Ethics
All experimental procedures were conducted in accordance with the recommendations of the principles of the Declaration of Helsinki, and the Ethics Committee of Marmara University, İstanbul, Turkey. Informed written consent was obtained from parents.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No specific funding was received for this study.
Author Contributions
E.A.A. conceived and designed the study. E.A.A. and A.T. performed the ATM gene sequencing analysis as well as the segregation study, and drafted the manuscript. S.B.E. collected the clinical data. S.B., and A.I.G. critically reviewed the manuscript. All authors discussed the results and contributed to the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366:636–46. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh HJ, Cho KH, Lee JE, Kwon MJ, Ki CS, Lee PH. Identification of ATM mutations in Korean siblings with ataxia-telangiectasia. Ann Lab Med. 2013;33:217–20. doi: 10.3343/alm.2013.33.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska MA. The missing puzzle piece: splicing mutations. Int J Clin Exp Pathol. 2013;6:2675–82. [PMC free article] [PubMed] [Google Scholar]
- Milne RL. Variants in the ATM gene and breast cancer susceptibility. Genome Med. 2009;1((1)):12. doi: 10.1186/gm12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissenkorn A, Levy-Shraga Y, Banet-Levi Y, Lahad A, Sarouk I, Modan-Moses D. Endocrine abnormalities in ataxia telangiectasia: findings from a national cohort. Pediatr Res. 2016;79:889–94. doi: 10.1038/pr.2016.19. [DOI] [PubMed] [Google Scholar]
- Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–5. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11:159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Botero F, Rodríguez-Guerrero JT. [New mutation in ATM gene in patient whith Ataxia Telangiectasia: Clinical case] Rev Chil Pediatr. 2017;88:524–8. doi: 10.4067/S0370-41062017000400013. [DOI] [PubMed] [Google Scholar]
- Telatar M, Teraoka S, Wang Z, Chun HH, Liang T, Castellvi-Bel S, et al. Ataxia-telangiectasia: identification and detection of founder-effect mutations in the ATM gene in ethnic populations. Am J Hum Genet. 1998;62:86–97. doi: 10.1086/301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka SN, Telatar M, Becker-Catania S, Liang T, Onengüt S, Tolun A, et al. Splicing defects in the ataxia-telangiectasia gene, ATM: underlying mutations and consequences. Am J Hum Genet. 1999;64:1617–31. doi: 10.1086/302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen MM, Last JI, Hogervorst FB, Smeets DF, Roeleveld N, Verheijen F, et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia-telangiectasia: a genotype-phenotype study. Hum Mutat. 2012;33:561–71. doi: 10.1002/humu.22016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.