Abstract
Background
Oxidative stress is implicated in the neuropathology of bipolar disorder (BD). We investigated the association of single-nucleotide polymorphisms (SNPs) in the antioxidative genes superoxide dismutase 2 (SOD2) and glutathione peroxidase 3 (GPX3) with structural neuroimaging phenotypes in youth BD.
Methods
SOD2 rs4880 and GPX3 rs3792797 SNP genotypes, along with structural magnetic resonance imaging, were obtained from 147 youth (BD = 75; healthy controls = 72). Images were processed using FreeSurfer, yielding surface area, volume, and thickness values for regions of interest (prefrontal cortex [PFC], caudal anterior cingulate cortex, hippocampus) and for vertex-wise whole-brain analysis. Analyses controlled for age, sex, race, and intracranial volume for volume, area, and thickness analyses.
Result
Regions of interest analyses revealed diagnosis-by-SOD2 rs4880 interaction effects for caudal anterior cingulate cortex volume and surface area as well as PFC volume; in each case, there was lower volume/area in the BD GG genotype group vs the healthy controls GG genotype group. There was a significant BD diagnosis × GPX3 rs3793797 interaction effect for PFC surface area, where area was lower in the BD A-allele carrier group vs the other genotype groups. Vertex-wise analyses revealed significant interaction effects in frontal, temporal, and parietal regions related to smaller brain structure in the BD SOD2 rs4880 GG group and BD GPX3 rs3793797 A-allele carrier group.
Conclusion
We found preliminary evidence that SOD2 rs4880 and GPX3 rs3792797 are differentially associated with brain structures in youth with BD in regions that are relevant to BD. Further studies incorporating additional neuroimaging phenotypes and blood levels of oxidative stress markers are warranted.
Keywords: Bipolar disorder, brain structure, rs4880, rs3792797, youth
Significance Statement.
The current study, focused on youth early in their course of bipolar disorder, provides an opportunity to examine genetic effects early in the course of illness. Both the regions-of-interest and whole-brain approaches revealed that the association of antioxidative genes with brain structure in youth differs between those with and without bipolar disorder. This finding addresses a gap in the current literature regarding the intermediate phenotypes related to antioxidative defense system, which is known to be aberrant in bipolar disorder. Future longitudinal studies incorporating peripheral biomarkers are needed to shed light on the long-term impact of these antioxidative genes on brain structure and cognition. Ultimately, this line of research advances knowledge that may in the future inform clinical decisions, such as the use of antioxidative interventions.
Introduction
Bipolar disorder (BD) is a severe psychiatric disorder that affects approximately 2%–5% of the population worldwide (Jann, 2014). BD is polygenic and is thought to result from the interaction of environmental factors with multiple genes of small to moderate effect (Kieseppä, 2004; Purcell, 2009). The disorder has an estimated heritability of approximately 85%, which is among the highest of psychiatric disorders (Uher, 2009). Oxidative stress has been proposed to play an essential role in the neuropathology of BD (Andreazza, 2008; Ng, 2008; Frey, 2013). A recent candidate gene study with 325 adults with BD and 392 healthy control (HC) adults identified that an interaction of 2 antioxidative enzyme single nucleotide polymorphisms (SNPs), superoxide dismutase 2 (SOD2) rs4880 and glutathione peroxidase 3 (GPX3) rs3792797, was associated with an increased risk for BD (Fullerton, 2010). In a prior study, our group found that GPX3 rs3792797 was associated with BD diagnosis in a case-control study of youth (Dimick, 2020).
Oxidative stress is defined as an accumulation of free radicals in the body due to an imbalance between oxidant generation and antioxidant capacity (Ghosh, 2018). Reactive oxygen species (ROS) such as superoxide radical (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (•OH) are oxygen-containing free radicals that are produced as either a by-product of the electron transport chain or as the final products of antioxidative enzyme activity (Ghosh, 2018). Antioxidative enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) are encoded in the human genomes and play an essential in eliminating ROS (Ghosh, 2018). Genetic variation of antioxidative enzymes can affect their expression and function (Shimoda-Matsubayashi, 1996; Forsberg, 2001). The brain is highly susceptible to oxidative stress; as such, deficits in antioxidative capacity are particularly damaging to the brain (Murphy, 2009; Ghosh, 2018).
Previous peripheral biomarker and post-mortem brain studies have demonstrated both an increase in oxidative stress and a decrease in antioxidant capacity in both the peripheral blood and brain of adult BD patients compared with HC (Brown, 2014). Results from adult BD post-mortem brain studies have shown an increase in oxidative stress in the hippocampus and several frontal brain regions, such as the anterior cingulate cortex (ACC) and the prefrontal cortex (PFC) (Wang, 2009; Andreazza, 2010; Che, 2010; Gawryluk, 2011). Importantly, these cortical and subcortical brain regions are known to be relevant to BD (Blumberg, 2003; Bora, 2010; Ellison-Wright and Bullmore, 2010; Hajek, 2012; Janssen, 2014; Hanford, 2016; Ganzola and Duchesne, 2017; Lu, 2019). However, to the extent of our knowledge, no prior study has examined the association of SOD2 rs4880 or GPX3 rs379279 SNPs with neuroimaging phenotypes in any population.
The SOD2 gene, located on chromosome 6q5, encodes functional SOD2 enzyme (also referred to as manganese superoxide dismutase, MnSOD) that mainly functions in the mitochondria (Bresciani, 2015). The rs4880 SNP, a substitution of A to G, results in the switch of valine (Val) to an alanine (Ala) at position 16 (Bag and Bag, 2008). A large multi-site study with 923 schizophrenia inpatients found that SOD2 rs4880 G-allele carriers showed poorer attention in the schizophrenia group compared with the AA homozygous group (Zhang, 2014). In contrast, other studies have reported the SOD2 rs4880 A allele is associated with higher risk of schizophrenia and depression compared with the G allele (Hori, 2000; Cumurcu, 2013; Wigner, 2018). The GPX3 rs3792797 SNP that encodes the extracellular GPX3 enzyme is located on chromosome 5 with A as the minor allele (Pae, 2006). This SNP has not been previously linked to any other psychiatric disorders, but a previous study has suggested a protective effect of GPX3 during brain maturation and aging (Kim, 2009).
In summary, genetic factors underlying antioxidative capacity, including SOD2 rs4880 and GPX3 rs3792797 SNPs, may be relevant to BD-related neurostructural endophenotypes. Thus, the current study aims to examine SOD2 rs4880 GG genotype and GPX3 rs3792797 in relation to structural neuroimaging phenotypes in youth with and without BD. Examining this relationship in the youth population offers the advantage of providing a glimpse into putative genetic effects early in the course of illness, with fewer years of exposure to the symptoms, lifestyle, medical comorbidity, and treatment of BD (McEwen, 1998; Kapczinski, 2008). Based on the available sample size, we examined SOD2 rs4880 (AA, AG, GG) and GPX3 rs3792797 (AA/AC, CC) separately. We hypothesized interaction such that the association of these genotypes with structural neuroimaging phenotypes would differ for youth with BD vs HC. Pre-specified regions of interest (ROI) analyses, based on prior studies linking these regions with BD and/or oxidative stress, were complemented by whole brain voxel-wise analyses (Blumberg, 2003; Bearden, 2008; Wang, 2009; Andreazza, 2010; Che, 2010; Gawryluk, 2011; Hajek, 2012; Hanford, 2016; Lu, 2019; Wang, 2019).
MATERIALS AND METHODS
Participants
The current study included 147 participants, of which 2 participants were missing GPX3 and 2 participants were missing SOD2 genotype data. As a result, 145 participants were analyzed for each SNP. Of these 147 participants, 91 were also previously included in the aforementioned genetics study that did not include neuroimaging (Dimick, 2020). BD participants with a diagnosis of BD-I (bipolar I disorder), BD-II (bipolar II disorder), or BD-NOS (bipolar-not other specified), were recruited from a tertiary subspecialty clinic at an academic health sciences center. HC were recruited via hospital and community advertisements. All participants were English speaking between the ages of 13 and 20 years old. The study protocol was approved by the Sunnybrook Research Ethics Board, and written informed consent was obtained from all participants and 1 of their guardians prior to participation. Participants were excluded for any of the following reasons: contraindications to magnetic resonance imaging (MRI); pre-existing cardiac, autoimmune and/or inflammatory illness; taking anti-inflammatory, anti-platelet, anti-lipidemic, anti-hypertensive, or hyperglycemic agents; substance dependence in the past 3 months. HC were excluded if they had lifetime mood or psychotic disorders, anxiety disorders, or a family history of BD or psychotic illnesses.
Psychiatric and Anthropometric Measures
Diagnosis and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) version of Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (KSADS-PL) was employed in the current study as KSADS-PL for DSM5 was published in 2016, well after recruitment had begun (participants were enrolled from 2012–2019) (Kaufman, 1997). KSADS-PL is a semi-structured diagnostic interview and was used to evaluate psychiatric diagnoses (including BD and comorbid substance use disorders) for all participants (Kaufman, 1997). The KSADS Depression Rating Scale and the Mania Rating Scale were used to assess related diagnosis and mood symptom severity scores (Chambers, 1985; Axelson, 2003). BD-I and BD-II were defined using DSM-IV. BD-NOS was defined using criteria previously operationalized by the Course and Outcome of Bipolar Illness in Youth study: these include: (1) 2 DSM-IV–confirmed manic symptoms; (2) a minimum of 4 cumulative 24-hour periods of episodes with elevated/irritable mood for at least 4 hours during each episode; and (3) change in functioning (Axelson, 2006). Information regarding ancestry was collected via self-report. Psychotropic medication use and tobacco use were collected during the K-SADS-PL interview. All interviewers in the current study were trained on the KSADS-PL under the supervision of a licensed child-youth psychiatrist (B.I.G.).
Height (cm) and weight (kg) were collected twice from each participant and averaged for accuracy consideration via following the standard procedure (Krebs, 2007). Body mass index (BMI) was computed as weight/height2.
Saliva and DNA Extraction
All participants were instructed to refrain from eating, drinking, smoking, and chewing gum 30 minutes prior to saliva collection. DNA Genotek Oragene-500 (DNA Genotek Inc, Ottawa, Canada) kits were used to collect saliva samples (2 mL) from each participant. DNA extraction was performed in the Neurogenetics Laboratory at the Centre for Addiction and Mental Health (Toronto, Canada) using a chemagic MSM I DNA extractor (Perkin-Elmer, Waltham, MA, USA) per manufacturer instructions. DNA was quantified using Nanodrop 8000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) and diluted to achieve a final concentration of 20 ng/µL.
Genotyping
The SOD2 rs4880 and GPX3 rs3792797 SNPs were genotyped using the TaqMan Format32 method (ThermoFisher Scientific) per manufacturer instructions. A custom assay for the Amelogenin region was included for the purpose of quality control. Briefly, 2 µL of extracted DNA sample was combined with 2 µL of 2X TaqMan Open Array Master Mix. The mixture was loaded onto the Open Array genotyping plates using the AccuFill System (ThermoFisher Scientific). The genotyped data were imported into the TaqMan Genotyper software version 1.3 and manually confirmed by 2 independent researchers. Hardy-Weinberg Equilibrium analysis was performed to examine potential sampling bias using the PLINK software version 1.90, and no violation of the Hardy-Weinberg equilibrium (i.e., all P > .05) was reported (Hosking, 2004; Purcell S, 2007). Technicians were blinded to diagnosis.
MRI Acquisition
Structural images of the brain were collected on a research-dedicated 3 Tesla Philips Achieva MRI scanner (Philips Medical Systems, Best, Netherlands). The acquisition used the body coil for signal transmission and 8-channel head coil for receiving the signal. The T1-weighted high resolution fast-field echo imaging with the following parameters was used: repetition time of 9.5 milliseconds (ms), echo time of 2.3 ms, inversion time of 1400 ms, spatial resolution of 0.94 × 1.17 × 1.2 mm, acquisition matrix of 256 × 164 × 140, field of view of 240 × 191 mm, flip angle of 8°, and scan duration of 8 minutes and 56 seconds.
Imaging Processing
Three-dimensional reconstruction of the T1-weighted images was performed using FreeSurfer (V6.0) software. The 3-dimensional images were quality-controlled for head motion or other artifacts prior to further processing. Briefly, the processing steps include automated skull stripping, field inhomogeneity correction, and registration to the Montreal Neurological Institute (MNI305) atlas (Fischl Bruce, 2012). Automated segmentation classified subcortical structures and cortical white and grey matter. This process includes triangular tessellation (triangle-based mesh) generation, smoothing, and topology correction (Fischl B, 2001; Segonne, 2007). Finally, cortical parcellation was completed via registering each participant’s inflated brain to a canonical template, which allowed anatomical alignment of the brain. The registered brain was then mapped to the FreeSurfer default Desikan-Killiany probabilistic atlas to label 34 gyral regions of interest per hemisphere (Fischl Bruce, 2004).
Statistical Analyses
Statistical analyses were performed using the SPSS statistic software (IBM Corp; New York, NY, USA), version 26 for clinical and demographic variables. Shapiro-Wilks test and Levene’s test were used to check normality and equal variance assumptions of all continuous variables. Group differences were evaluated using a 2-way ANOVA for continuous variables and chi-square (χ 2) tests for categorical variables.
Neuroimaging-genetic analyses were completed using MATLAB version R2018b. Main effects for genotype and diagnosis and diagnosis-by-genotype interaction effects, for PFC, caudal ACC (cACC), and hippocampus ROI analyses were tested using a General Linear Model with age, sex, race, and intracranial volume (ICV) as covariates. ICV was not included as a covariate in the model for cortical thickness analysis as ICV does not explain cortical thickness (Liem, 2015). Family-wise Bonferroni correction was used to correct for multiple ROI comparisons (α = .05/3 = .017). For the whole-brain vertex-wise exploratory analyses, a 10-mm kernel of full width at half-maximum was used in brain surface smoothing before mapping volumetric, surface area, and thickness data to the canonical template. Vertex-wise contrasts for genotype main effect and diagnosis-by genotype interaction effect for each brain structure measurement was entered into the General Linear Model together with the aforementioned covariates. The significance level was set at P < .05, and results were corrected for multiple comparisons using Monte-Carlo simulation threshold at a log10 P value of .05. Cluster-wide P values were then calculated as the probability of seeing a cluster of that size and reported for each significant cluster. Post-hoc analyses were conducted for ROI and vertex-wise whole brain analyses in regions that revealed significant SNP main effect and/or diagnosis-by-SNP interaction effect.
RESULTS
Demographics and Clinical Characteristics
Demographic characteristics are summarized in Tables 1 and 2. There were no significant age or sex differences between BD and HC participants. For the SOD2 rs4880 genotype comparison, participants in the BD group were more likely to be of European ancestry and had higher BMI compared with HC participants. For the GPX3 rs3792797 genotype comparison (which differed from SOD2 slightly in terms of subgroup size), BMI was higher for the BD group compared with HC. In addition, there were significant differences for race across the SOD2 genotype groups, explained by a higher proportion of participants of European ancestry in the AG genotype group compared with the AA and GG groups. Clinical characteristics for the BD group are summarized in Table 2. In the current sample, 17 (23%) BD participants had lifetime substance use disorder, of these 17% has current drug/alcohol abuse. No BD participant has current drug/alcohol dependence.
Table 1.
Demographic Characteristics by Diagnosis and Oxidative Defense Genotypes
| BD AA (n = 20) | BD AG (n = 40) | BD GG (n = 14) | HC AA (n = 22) | HC AG (n = 35) | HC GG (n = 14) | ||
|---|---|---|---|---|---|---|---|
|
SOD2
rs4880 |
Age | 17.17 ± 0.36 | 17.51 ± 0.25 | 17.02 ± 0.43 | 17.20 ± 0.34 | 16.99 ± 0.27 | 17.00 ± 0.43 |
| Sex (n, % female) | 11 (55%) | 25 (63%) | 9 (64%) | 11 (50%) | 18 (51%) | 7 (50%) | |
| Race (n, % Caucasian)a,b | 13 (65%) | 32 (80%) | 11 (79%) | 8 (36%) | 25 (71%) | 9 (64%) | |
| BMI (adjusted)a | 23.17 ± 0.85 | 24.73 ± 0.60 | 22.68 ± 1.02 | 21.17 ± 0.81 | 21.80 ± 0.65 | 22.61 ± 1.02 | |
| Tanner Stage | 4.20 ± 0.70 | 4.45 ± 0.64 | 4.57 ± 0.65 | 4.14 ± 0.56 | 4.20 ± 0.68 | 4.31 ± 0.75 | |
| BD CC (n = 53) | BD AA/AC* (n = 22) | HC CC (n = 42) | HC AA/AC (n = 28) | ||||
|
GPX3
rs3792797 |
Age | 17.28 ± 0.21 | 17.49 ± 0.33 | 16.65 ± 0.24 | 17.40 ± 0.29 | ||
| Sex (n, % female) | 36 (68%) | 10 (45%) | 20 (48%) | 15 (54%) | |||
| Race (n, % Caucasian) | 41 (77%) | 16 (72%) | 25 (60%) | 18 (64%) | |||
| BMI (adjusted)a | 24.08 ± 0.53 | 23.36 ± 0.82 | 21.77 ± 0.59 | 21.76 ± 0.72 | |||
| Tanner Stage | 4.27 ± 0.55 | 4.47 ± 0.70 | 4.26 ± 0.71 | 4.14 ± 0.61 |
Abbreviations: BD, bipolar disorder; BMI, body mass index; HC, healthy control.
*AA/AC combined due to limited subgroup size.
Results are reported in mean ± SD unless otherwise specified.
a Indicates significant differences between diagnostic groups.
b Indicates significant difference between genotype groups.
Table 2.
Clinical Characteristics
| BD (n = 75) | |
|---|---|
| BD-I | 26 (35%) |
| BD-II | 23 (31%) |
| BD-NOS | 26 (35%) |
| Age of onset | 14.81 ± 2.66 |
| Lifetime clinical characteristics | |
| Lifetime psychosis | 8 (11%) |
| Lifetime suicide attempts | 12 (16%) |
| Lifetime self-injurious behaviour | 38 (51%) |
| Lifetime suicidal ideation | 47 (63%) |
| Legal history (police contact/arrest) | 19 (25%) |
| Lifetime physical abuse | 2 (3%) |
| Lifetime sexual abuse | 3 (4%) |
| Lifetime any abuse (physical and/or sexual) | 4 (5%) |
| Lifetime psychiatric hospitalization | 37 (49%) |
| Lifetime comorbid diagnoses | |
| ADHD | 37 (49%) |
| Anxiety disorder | 61 (81%) |
| Number of anxiety disorders | 1.63 ± 1.21 |
| Conduct disorder | 3 (4%) |
| Oppositional defiant disorder | 19 (25%) |
| Substance use disorder | 17 (23%) |
| Nicotine use | 11 (15%) |
| Lifetime medications | |
| Second generation antipsychotics | 55 (73%) |
| Lithium | 19 (25%) |
| Non-SSRI antidepressants | 14 (19%) |
| SSRI antidepressant | 25 (33%) |
| Stimulants | 16 (21%) |
| Current medications | |
| Second generation antipsychotics | 44 (59%) |
| Lithium | 14 (19%) |
| Non-SSRI antidepressants | 4 (5%) |
| SSRI antidepressants | 7 (9%) |
| Stimulants | 5 (7%) |
| Family psychiatric history | |
| Mania/hypomania | 36 (48%) |
| Depression | 55 (73%) |
| Anxiety | 46 (61%) |
| ADHD | 25 (33%) |
Abbreviations: ADHD, attention deficit-hyperactivity disorder; BD, bipolar disorder; HC, healthy control; NOS, not otherwise specified; SSRI, selective serotonin reuptake inhibitor.
Results are reported in mean ± SD or percentage (%) yes unless otherwise specified.
SOD2 rs4880 Region of Interest Analyses
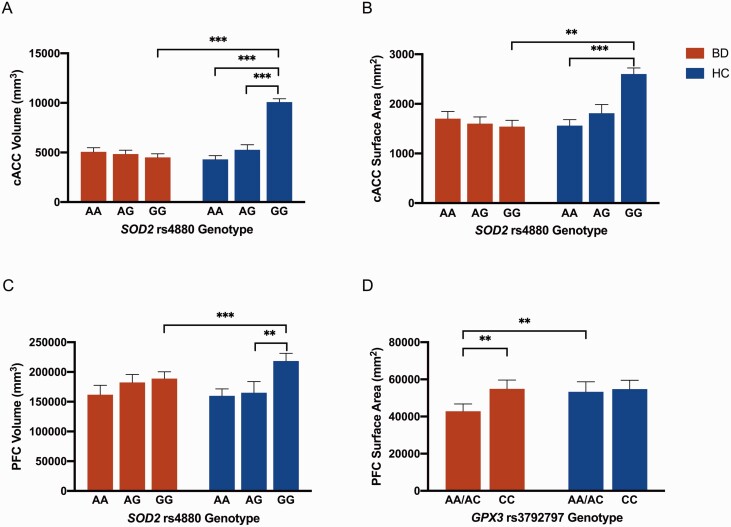
PFC area (F = 4.84; P = .03; η 2 = 0.04) and volume (F = 8.12; P = .01; η 2 = 0.06) were significantly smaller in the BD group compared with the HC. PFC area finding did not remain significant after Bonferroni correction. There were significant gene main effects for cACC (F = 5.28, P = .01; η 2 = 0.04) and PFC (F = 4.91, P = .01; η 2 = 0.04) volume (Table 3). In each case, the AA and AG groups had smaller brain volume than the GG group (Supplementary 1 and 3). There were also significant interaction effects observed for cACC surface area (F = 3.37, P = .04; η 2 = 0.03) and volume (F = 7.50, P < .01; η 2 = 0.06) as well as PFC volume (F = 3.27, P = .04; η 2 = 0.03) (Table 3; Figure 1A–C). Post-hoc analyses for all reported interaction effects revealed significantly smaller brain structures in the BD GG group compared with the HC GG group. cACC volume and surface area were significantly smaller in the HC AA group compared with the HC GG group (Supplementary 1 and 3). In addition, cACC and the PFC volume were significantly smaller in the HC AG group compared with the HC GG group. For cACC surface area and PFC volume, the interaction effect did not remain significant after Bonferroni correction for multiple comparisons.
Table 3.
Results for Region of Interests Analyses
| cACC area | cACC volume | cACC thickness | PFC area | PFC volume | PFC thickness | Hippocampal volume | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| SOD2 rs4880 | Diagnosis main effect | 2.51 | .12 | 0.13 | .72 | 0.03 | .86 | 4.84 | .03 | 8.12 | .01 * | 1.05 | .31 | 1.37 | .24 |
| Gene main effect | 1.89 | .16 | 5.28 | .01 * | 0.78 | .46 | 0.78 | .46 | 4.91 | .01 * | 1.33 | .27 | 1.98 | .14 | |
| Interaction effect | 3.37 | .04 | 7.50 | .001 * | 0.40 | .67 | 1.80 | .17 | 3.27 | .04 | 0.42 | .66 | 1.65 | .20 | |
| GPX3 rs3792797 | Diagnosis main effect | 4.22 | .04 | 0.44 | .51 | 0.03 | .86 | 1.62 | .20 | 1.71 | .19 | 0.59 | .44 | 0.77 | .38 |
| Gene main effect | 2.29 | .13 | 2.67 | .11 | 0.07 | .79 | 7.29 | .008 * | 6.11 | .015 * | 1.10 | .30 | 4.01 | .048 | |
| Interaction effect | 0.12 | .71 | 0.46 | .50 | 0.04 | .83 | 4.44 | .04 | 2.18 | .14 | 0.11 | .75 | 1.10 | .30 |
Abbreviations: cACC, caudal anterior cingulate cortex; PFC, prefrontal cortex.
*Finding remains significant after correction for multiple comparisons.
Significant gene main effect and interaction effect are bolded.
Figure 1.
Post-hoc analyses for regions of interest with significant diagnosis-by-genotype interaction effects. *P < .05, **P < .01, ***P < .001. (A–C) Diagnosis-by-superoxide dismutase 2 (SOD2) rs4880 interaction effect post-hoc results for caudal anterior cingulate cortex (cACC) volume, cACC surface area, and PFC volume respectively. (D) Diagnosis-by-glutathione peroxidase 3 (GPX3) rs3792797 interaction effect post-hoc result for prefrontal cortex (PFC) surface area. Note that only the SOD2 rs4880 cACC volume finding remained significant after correction for multiple comparisons.
GPX3 rs3792797 ROIs Analyses
cACC surface area (F = 4.22; P = .04; η 2 = 0.03) was significantly smaller in the BD group compared with HC, but the finding did not remain significant after Bonferroni correction. There were significant genotype main effects for PFC, with lower surface area (F = 7.29, P < .01; η 2 = 0.05) and volume (F = 6.11, P = .02; η 2 = 0.04) among A-allele carriers compared with CC homozygotes (Table 3). There was also a significant interaction effect for PFC surface area (F = 4.44, P = .04; η 2 = 0.04), whereby the BD A-allele carrier group had lower surface area compared with the HC A-allele carrier group and the BD CC genotype group (Table 3; Figure 1D; Supplementary 2 and 3). The interaction effect did not remain significant after Bonferroni correction.
SOD2 rs4880 Vertex-Wise Whole-Brain Analysis
Results of vertex-wise analyses are summarized in Table 4. In terms of main effect, there were 4 significant clusters. For 3 clusters, located in superior temporal, superior frontal, and caudal middle frontal gyri, there was a lower volume in the AA and AG groups compared with the GG group (Supplementary 4). In addition, for superior frontal gyrus, there was lower volume in the AA group compared with the AG group. In the fourth cluster, in the superior temporal gyrus, the AA and AG groups had reduced thickness compared with the GG group (Supplementary 4).
Table 4.
SOD2 and GPX3 Whole-Brain Analyses
| Cortical measure | Contrast | Peak cluster region | Size of cluster (mm2) | cwp | MNI X | MNI Y | MNI Z | |
|---|---|---|---|---|---|---|---|---|
|
SOD2
rs4880 |
Area | Interaction effect | rh paracentral gyrus | 1815.84 | 0.008 | 11.9 | −12.5 | 48.3 |
| Volume | Gene main effect | lh superior frontal gyrus | 1114.53 | 0.004 | −10.0 | 13.3 | 52.2 | |
| rh caudal middle frontal gyrus | 997.39 | 0.008 | 36.8 | 8.5 | 39.1 | |||
| rh superior temporal gyrus | 774.06 | 0.040 | 56.3 | 3.3 | −11.7 | |||
| Thickness | Gene main effect | rh superior temporal gyrus | 1544.18 | 0.0001 | 52.1 | −7.2 | −13.1 | |
|
GPX3
rs3792797 |
Area | Gene main effect | lh caudal middle frontal gyrus | 1869.75 | 0.042 | −36.5 | 13.5 | 33.7 |
| lh transverse temporal gyrus | 1840.31 | 0.046 | −51.6 | −20.4 | 5.1 | |||
| rh rostral middle frontal gyrus | 3633.70 | 0.0004 | 34.3 | 28.2 | 43.0 | |||
| rh postcentral gyrus | 2417.59 | 0.009 | 47.3 | −18.6 | 57.0 | |||
| Interaction effect | lh superior frontal gyrus | 2839.21 | 0.002 | −8.7 | −7.2 | 57.7 | ||
| rh superior frontal gyrus | 1991.16 | 0.028 | 20.2 | 24.4 | 45.6 | |||
| Volume | Gene main effect | lh postcentral gyrus | 1598.50 | 0.006 | −44.2 | −23.3 | 56.0 | |
| lh caudal middle frontal gyrus | 1202.00 | 0.035 | −36.6 | 17.8 | 49.8 | |||
| rh postcentral gyrus | 1661.38 | 0.001 | 49.2 | −21.1 | 56.6 | |||
| Interaction effect | rh supramarginal gyrus | 1056.16 | 0.043 | 59.8 | −37.8 | 21.8 |
Abbreviations: cwp, cluster wide P value; lh, left hemisphere; MNI, Montreal Neurological Institute; rh, right hemisphere.
In terms of interaction effect, there was 1 significant cluster, for paracentral gyrus surface area (Table 4); post-hoc analyses revealed a significantly lower surface area in the BD GG group compared with BD AA, BD AG, and HC GG groups (Supplementary 5). In addition, surface area was significantly lower in the HC AA group and the HC AG group compared with the HC GG group (Supplementary 5).
GPX3 rs3792797 Vertex-Wise Whole-Brain Analysis
Results of vertex-wise analyses are summarized in Table 4. In terms of main effects, there were 4 significant clusters. For 2 clusters, located in caudal middle frontal and postcentral gyri, there was lower cortical volume and surface area in the A-allele carrier group compared with the CC group. For the third and fourth clusters, in the transverse temporal and rostral middle frontal gyrus, the A-allele carrier group showed lower surface area compared with the CC genotype group.
In terms of interaction effects, there were 2 significant clusters in superior frontal and supramarginal gyri for cortical surface area and volume, respectively (Table 4). For the superior frontal gyrus, the significance was due to a lower surface area for BD A-allele carrier group compared with BD CC group and HC A-allele carrier group (Supplementary 6). For the supramarginal gyrus, post-hoc analyses indicated significantly lower cortical volume in the BD A-allele carrier group and HC CC group compared with BD CC group (Supplementary 6). In addition, there was lower volume in the HC CC group compared with the HC A-allele carrier group.
Discussion
This preliminary study addresses gaps in the literature regarding antioxidative enzyme genes in relation to brain structure in BD and among youth. In ROI analyses, there was a significant interaction effect for SOD2 rs4880 on cACC volume. For the exploratory whole-brain analysis, significant interaction effects were observed in the temporal lobe for SOD2 rs4880, and frontal lobe and parietal lobe for GPX3 rs3792797. Post-hoc analyses revealed smaller regional brain structure in the BD SOD2 rs4880 GG group (compared with HC GG groups) and the BD GPX3 rs3792797 A-allele carrier group (compared with the BD CC and HC A-allele carrier groups). Overall, the results indicated that these antioxidative enzyme SNPs were associated with structural brain differences in regions relevant to BD. Although this is not a mechanistic study, the current findings provide inferences regarding the potential implications of redox balance shifting, a result of altered antioxidative enzyme activity/production, to brain structure in BD.
The exact physiological mechanisms explaining how SOD2 rs4880 and GPX3 rs3792797 genetic variances influence brain morphology have not yet been fully explored. The smaller brain structure among BD youth with SOD2 rs4880 GG and GPX3 rs3792797 A-allele carrier genotypes may relate in part to abnormal neural pruning in early neurodevelopmental stages (Huttenlocher, 1979; Petanjek, 2011). The reorganization process of the brain during pruning is partially regulated by ROS, underscoring the importance of antioxidative defenses as regulators of this process (Cobley, 2018; Sidlauskaite, 2018). The SOD2 rs4880 SNP is associated with substitution of Val at position 16 to Ala, leading to a conformational change in the secondary structure of SOD2, as the Val allele variant encodes a beta-sheet structure and the Ala allele variant encodes an alpha-helix structure (Shimoda-Matsubayashi, 1996). The switch from a beta-sheet to alpha-helix increases the mobility of this enzyme to move across the mitochondria inner membrane into the matrix, while majority of the Val allele variant encoded SOD2 were imbedded in the inner membrane (Holley, 2012; Bresciani, 2015). Furthermore, the Ala allele encoded SOD2 also has a much higher level of enzymatic activity compared with the Val allele encoded SOD2 (McAtee and Yager, 2010; Bresciani, 2015). Elevated SOD2 level and its enzymatic activity of Ala variant encoded SOD2 in the mitochondria generates increased ROS as a result of SOD2-mediated H2O2 generation (Shimoda-Matsubayashi, 1997; Bag and Bag, 2008; McAtee and Yager, 2010). When conversion of H2O2 to water is inefficient, this results in further generation of ROS, specifically hydroxyl radical species (Ghosh, 2018). Such alternation in the brain ROS level might thus impact the neural pruning processes.
In the current study, the BD SOD2 GG, HC AA, and HC AG groups had significantly lower cACC volume compared with the HC GG group. There is prior evidence of reduced cACC volume in adults with BD (Phillips and Swartz, 2014). The cACC is involved in cognitive control and emotion regulation, which are known to be impaired in BD (Joseph, 2008; Tsitsipa and Fountoulakis, 2015; Heilbronner and Hayden, 2016). The SOD2 rs4880 G allele and higher SOD2 enzyme activity have been linked to impaired attention in adults with schizophrenia (Zhang, 2014). The reduction in cACC volume of the BD GG group may reflect accelerated cortical pruning due to excessive ROS production (Cobley, 2018). Interestingly, the GG genotype effect was divergent for BD (lower volume) compared with HC (greater volume). This finding might support the Differential Susceptibility Model, which posits that the same genotype that acts advantageously under beneficial environmental conditions can act deleteriously under adverse environmental conditions (Jolicoeur-Martineau, 2019). It is well established that individuals with BD have higher rates of early adversity and life stress, and the symptoms and related behaviors of BD comprise additional stressors (Kapczinski, 2008). Thus, this allostatic load of BD might interact with SOD2 rs4880 genotype and present phenotypically as neurostructural changes.
Information regarding the impact of GPX3 rs3792797 SNP on the activity and/or production of its encoded GPX3 enzyme is limited. However, a previous study conducted in healthy individuals across a broad age range reported a positive association of GPX3 gene expression with age in the PFC (r = 0.42, P = .001), indicating the importance of this antioxidative enzyme in brain oxidative stress protection during brain maturation and aging (Kim, 2009). Moreover, a recent study found elevated GPX3 enzyme levels in cerebrospinal fluid among participants with psychiatric disorders (n = 98, including 27 with BD) compared with controls (Maccarrone, 2013). Taken together, these findings highlight the importance of examining neurostructural correlates of GPX3 in a youth population in the midst of major neurodevelopmental changes.
For the vertex-wise whole-brain analyses, the significant interaction effect for SOD2 in the right paracentral gyrus was related to lower surface area in the BD GG group compared with HC GG, BD AG, and BD AA groups. Similar to what has been observed in the ROI analyses, the GG genotype effect was divergent for BD (associated with lower surface area) vs HC (associated with higher surface area). The paracentral gyrus, located in the parietal lobe of the brain, is divided into anterior (part of the primary motor cortex) and posterior (part of the primary somatosensory cortex) regions (Spasojević, 2013). A structural neuroimaging meta-analysis of adults with BD-I reported a higher left paracentral gyrus volume in BD vs HC, while no significant finding was observed for the right paracentral gyrus (McCarthy, 2014). However, no prior studies have examined SOD2 effects or interaction effects with diagnosis in adults.
Whole-brain analyses for GPX3 rs3792797 revealed a significant interaction effect in bilateral superior frontal gyri (SFG) surface area, which was lower in the BD A-allele carrier group than in the BD CC group and HC A-allele carrier group. The SFG is involved in higher cognitive functions such as working memory, which is known to be impaired in BD (Joseph, 2008; Depp, 2012; Li, 2013). Lower SFG volume has been previously reported in individuals with major depressive disorder and in unaffected youth with a family history of BD compared with HC (Cattarinussi, 2019; Kandilarova, 2019). A significant interaction effect was also found for right supramarginal gyrus volume, which was lower in the BD A-allele carrier group and HC CC group compared with the BD CC group. The right supramarginal gyrus plays an essential role in regulating empathy judgment, which is known to be impaired in adults with BD (Shamay-Tsoory, 2009; Silani, 2013; Bodnar and Rybakowski, 2017).
There are several limitations to this study. First, the cross-sectional design precludes inferences regarding the timing of the observed neurostructural differences in relation to the timing of onset of BD. As such, it is unclear whether the observed differences preceded onset of BD or emerged concurrently and/or subsequently to symptom onset. Second, the sample size of the current study, while comparatively large for a neuroimaging study in this field, only allowed for the detection of a medium to large effects while the gene effects are usually small to medium (Kerner, 2014). Moreover, the small sample size limited our ability to conduct subgroup analyses (e.g., BD subtypes, mood states, sex differences, medications) and additive and/or interactive genetic effect analyses. Third, the current study did not include measurement of oxidative stress proteins or neurocognition, which could provide insights about putative mechanisms underlying our observed findings and about whether observed findings are salutary or deleterious.
In summary, the current study showed that the association of SOD2 rs4880 and GPX3 rs3792797 SNPs with brain structure in youth differs between those with vs without BD. This finding adds to the current literature regarding the potential role of anomalous antioxidant defense mechanisms in the early stage of disease development, highlighting specific regions that may be particularly susceptible to oxidative stress. Future longitudinal studies are warranted to explore the long-term impact of these 2 antioxidative defense genes on neurostructural changes, ideally integrating protein levels of antioxidative enzymes and neurocognitive testing. Such studies hold the potential of identifying individuals for whom antioxidant therapeutic approaches may be beneficial and identifying intermediate phenotypes for target engagement.
Supplementary Material
Acknowledgments
Dr Goldstein acknowledges research support from the Brain & Behavior Research Foundation, Brain Canada, Canadian Institutes of Health Research, the Heart and Stroke Foundation, the National Institute of Mental Health, and the Departments of Psychiatry of Sunnybrook Health Sciences Centre and the University of Toronto. The authors thank the study participants, staff and students at the Center for Youth Bipolar Disorder, and the study MRI technologists.
This study was funded by the Ontario Mental Health Foundation and the Canadian Institutes of Health Research (CIHR MOP 136947).
Interest Statement
The authors have declared no potential or actual conflicts of interest.
References
- Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN (2008) Oxidative stress markers in bipolar disorder: a meta-analysis. j Affect Disord 111:135–144. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Shao L, Wang JF, Young LT (2010) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67:360–368. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N (2003) A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. j Child Adolesc Psychopharmacol 13:463–470. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M (2006) Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:1139–1148. [DOI] [PubMed] [Google Scholar]
- Bag A, Bag N (2008) Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev 17:3298–3305. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Soares JC, Klunder AD, Nicoletti M, Dierschke N, Hayashi KM, Narr KL, Brambilla P, Sassi RB, Axelson D, Ryan N, Birmaher B, Thompson PM (2008) Three-dimensional mapping of hippocampal anatomy in adolescents with bipolar disorder. j Am Acad Child Adolesc Psychiatry 47:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS (2003) Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 60:1201–1208. [DOI] [PubMed] [Google Scholar]
- Bodnar A, Rybakowski JK (2017) Increased affective empathy in bipolar patients during a manic episode. Braz j Psychiatry 39:342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C (2010) Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry 67:1097–1105. [DOI] [PubMed] [Google Scholar]
- Bresciani G, da Cruz IB, González-Gallego J (2015) Manganese superoxide dismutase and oxidative stress modulation. Adv Clin Chem 68:87–130. [DOI] [PubMed] [Google Scholar]
- Brown NC, Andreazza AC, Young LT (2014) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 218:61–68. [DOI] [PubMed] [Google Scholar]
- Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F (2019) Neural signatures of the risk for bipolar disorder: a meta-analysis of structural and functional neuroimaging studies. Bipolar Disord 21:215–227. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M (1985) The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry 42:696–702. [DOI] [PubMed] [Google Scholar]
- Che Y, Wang JF, Shao L, Young T (2010) Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. j Psychiatry Neurosci 35:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley JN (2018) Synapse pruning: mitochondrial ROS with their hands on the shears. Bioessays 40:e1800031. [DOI] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Ates O, Gul IG, Demir S, Karlıdag R (2013) Analysis of manganese superoxide dismutase (MnSOD: Ala-9Val) and glutathione peroxidase (GSH-Px: Pro 197 Leu) gene polymorphisms in mood disorders. Bosn j Basic Med Sci 13:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL (2012) Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord 14:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimick MK, Cazes J, Fiksenbaum LM, Zai CC, Tampakeras M, Freeman N, Youngstrom EA, Kennedy JL, Goldstein BI (2020) Proof-of-concept study of a multi-gene risk score in adolescent bipolar disorder. j Affect Disord 262:211–222. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E (2010) Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- Fischl B (2012) FreeSurfer. Neuroimage 62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. ieee Trans Med Imaging 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Forsberg L, de Faire U, Morgenstern R (2001) Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys 389:84–93. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant’Anna M, Yatham LN, Kapczinski F, Young LT (2013) Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust n z j Psychiatry 47:321–332. [DOI] [PubMed] [Google Scholar]
- Fullerton JM, Tiwari Y, Agahi G, Heath A, Berk M, Mitchell PB, Schofield PR (2010) Assessing oxidative pathway genes as risk factors for bipolar disorder. Bipolar Disord 12:550–556. [DOI] [PubMed] [Google Scholar]
- Ganzola R, Duchesne S (2017) Voxel-based morphometry meta-analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disord 19:74–83. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int j Neuropsychopharmacol 14:123–130. [DOI] [PubMed] [Google Scholar]
- Ghosh N, Das A, Chaffee S, Roy S, Sen CK (2018) Reactive oxygen species, oxidative damage and cell death. In: Emerging roles of nutraceuticals and functional foods in immune support, pp 45–55. Cambridge, MA: Academic Press. [Google Scholar]
- Hajek T, Kopecek M, Höschl C, Alda M (2012) Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. j Psychiatry Neurosci 37:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford LC, Nazarov A, Hall GB, Sassi RB (2016) Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord 18:4–18. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Hayden BY (2016) Dorsal anterior cingulate cortex: a bottom-up view. Annu Rev Neurosci 39:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley AK, Dhar SK, Xu Y, St Clair DK (2012) Manganese superoxide dismutase: beyond life and death. Amino Acids 42:139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, Ohmori O, Shinkai T, Kojima H, Okano C, Suzuki T, Nakamura J (2000) Manganese superoxide dismutase gene polymorphism and schizophrenia: relation to tardive dyskinesia. Neuropsychopharmacology 23:170–177. [DOI] [PubMed] [Google Scholar]
- Hosking L, Lumsden S, Lewis K, Yeo A, McCarthy L, Bansal A, Riley J, Purvis I, Xu CF (2004) Detection of genotyping errors by Hardy-Weinberg equilibrium testing. Eur j Hum Genet 12:395–399. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR (1979) Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res 163:195–205. [DOI] [PubMed] [Google Scholar]
- Jann MW (2014) Diagnosis and treatment of bipolar disorders in adults: a review of the evidence on pharmacologic treatments. Am Health Drug Benefits 7:489–499. [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Alemán-Gómez Y, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Castro-Fornieles J, Otero S, Baeza I, Moreno D, Bargalló N, Parellada M, Arango C, Desco M (2014) Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res 158:91–99. [DOI] [PubMed] [Google Scholar]
- Jolicoeur-Martineau A, Belsky J, Szekely E, Widaman KF, Pluess M, Greenwood C, Wazana A (2019) Distinguishing differential susceptibility, diathesis-stress, and vantage sensitivity: beyond the single gene and environment model. Dev Psychopathol 32:73–83. [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, Soares JC (2008) A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. j Child Adolesc Psychopharmacol 18:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandilarova S, Stoyanov D, Sirakov N, Maes M, Specht K (2019) Reduced grey matter volume in frontal and temporal areas in depression: contributions from voxel-based morphometry study. Acta Neuropsychiatr 31:252–257. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, Kauer-Sant’anna M, Grassi-Oliveira R, Post RM (2008) Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev 32:675–692. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. j Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kerner B (2014) Genetics of bipolar disorder. Appl Clin Genet 7:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseppä T, Partonen T, Haukka J, Kaprio J, Lönnqvist J (2004) High concordance of bipolar I disorder in a nationwide sample of twins. Am j Psychiatry 161:1814–1821. [DOI] [PubMed] [Google Scholar]
- Kim WS, Wong J, Weickert CS, Webster MJ, Bahn S, Garner B (2009) Apolipoprotein-D expression is increased during development and maturation of the human prefrontal cortex. j Neurochem 109:1053–1066. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D (2007) Assessment of child and adolescent overweight and obesity. Pediatrics 120 (Suppl 4):S193–S228. [DOI] [PubMed] [Google Scholar]
- Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C (2013) Subregions of the human superior frontal gyrus and their connections. Neuroimage 78:46–58. [DOI] [PubMed] [Google Scholar]
- Liem F, Mérillat S, Bezzola L, Hirsiger S, Philipp M, Madhyastha T, Jäncke L (2015) Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. Neuroimage 108:95–109. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhong Y, Ma Z, Wu Y, Fox PT, Zhang N, Wang C (2019) Structural imaging biomarkers for bipolar disorder: meta-analyses of whole-brain voxel-based morphometry studies. Depress Anxiety 36:353–364. [DOI] [PubMed] [Google Scholar]
- Maccarrone G, Ditzen C, Yassouridis A, Rewerts C, Uhr M, Uhlen M, Holsboer F, Turck CW (2013) Psychiatric patient stratification using biosignatures based on cerebrospinal fluid protein expression clusters. j Psychiatr Res 47:1572–1580. [DOI] [PubMed] [Google Scholar]
- McAtee BL, Yager JD (2010) Manganese superoxide dismutase: effect of the ala16val polymorphism on protein, activity, and mRNA levels in human breast cancer cell lines and stably transfected mouse embryonic fibroblasts. Mol Cell Biochem 335:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Liang S, Spadoni AD, Kelsoe JR, Simmons AN (2014) Whole brain expression of bipolar disorder associated genes: structural and genetic analyses. PLoS One 9:e100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998) Protective and damaging effects of stress mediators. n Engl j Med 338:171–179. [DOI] [PubMed] [Google Scholar]
- Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem j 417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int j Neuropsychopharmacol 11:851–876. [DOI] [PubMed] [Google Scholar]
- Pae CU, Yoon SJ, Patkar A, Kim JJ, Jun TY, Lee C, Paik IH (2006) Manganese superoxide dismutase (MnSOD: Ala-9Val) gene polymorphism and mood disorders: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry 30:1326–1329. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci u s a 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA (2014) A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am j Psychiatry 171:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am j Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Wray N, Stone J, Visscher P, O’Donovan M, Sullivan P, Sklar P (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B (2007) Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. ieee Trans Med Imaging 26:518–529. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Harari H, Szepsenwol O, Levkovitz Y (2009) Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. j Neuropsychiatry Clin Neurosci 21:59–67. [DOI] [PubMed] [Google Scholar]
- Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y (1996) Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem Biophys Res Commun 226:561–565. [DOI] [PubMed] [Google Scholar]
- Shimoda-Matsubayashi S, Hattori T, Matsumine H, Shinohara A, Yoritaka A, Mori H, Kondo T, Chiba M, Mizuno Y (1997) Mn SOD activity and protein in a patient with chromosome 6-linked autosomal recessive parkinsonism in comparison with Parkinson’s disease and control. Neurology 49:1257–1262. [DOI] [PubMed] [Google Scholar]
- Sidlauskaite E, Gibson JW, Megson IL, Whitfield PD, Tovmasyan A, Batinic-Haberle I, Murphy MP, Moult PR, Cobley JN (2018) Mitochondrial ROS cause motor deficits induced by synaptic inactivity: implications for synapse pruning. Redox Biol 16:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff CC, Singer T (2013) Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. j Neurosci 33:15466–15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević G, Malobabic S, Pilipović-Spasojević O, Djukić-Macut N, Maliković A (2013) Morphology and digitally aided morphometry of the human paracentral lobule. Folia Morphol 72:10–16. [DOI] [PubMed] [Google Scholar]
- Tsitsipa E, Fountoulakis KN (2015) The neurocognitive functioning in bipolar disorder: a systematic review of data. Ann Gen Psychiatry 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R (2009) The role of genetic variation in the causation of mental illness: an evolution-informed framework. Mol Psychiatry 14:1072–1082. [DOI] [PubMed] [Google Scholar]
- Wang JF, Shao L, Sun X, Young LT (2009) Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord 11:523–529. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo Q, Tian F, Cheng B, Qiu L, Wang S, He M, Wang H, Duan M, Jia Z (2019) Brain grey-matter volume alteration in adult patients with bipolar disorder under different conditions: a voxel-based meta-analysis. j Psychiatry Neurosci 44:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigner P, Czarny P, Synowiec E, Bijak M, Białek K, Talarowska M, Galecki P, Szemraj J, Sliwinski T (2018) Variation of genes involved in oxidative and nitrosative stresses in depression. Eur Psychiatry 48:38–48. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Xiu MH, Yang FD, Tan Y, Luo X, Zuo L, Kosten TA, Kosten TR (2014) Cognitive function, plasma MnSOD activity, and MnSOD Ala-9Val polymorphism in patients with schizophrenia and normal controls. Schizophr Bull 40:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.