Abstract
Acute toxic leukoencephalopathy (ATL) and delayed post-hypoxic leukoencephalopathy (DPHL) are two possible adverse entities related to opioid intoxication (OI), each having a distinct clinical course. While ATL shows a monophasic course with gradual neurological deterioration, DPHL has a distinct biphasic course. We report a case of ATL along with a case of DPHL happening in young male patients with OI, including their clinical courses as well as imaging characteristics with comparable time intervals. Initially, both leukoencephalopathies typically show magnetic resonance imaging findings with confluent and symmetric white matter (WM) abnormalities in the periventricular regions on T2 and fluid-attenuated inversion recovery images along with restricted diffusion on diffusion-weighted imaging. The DPHL patient however also presented with WM cystic substance loss in the deterioration phase, several weeks after hospital admission, which was previously described in a case of DPHL. Interestingly, similar WM changes have recently been observed in virus-associated necrotizing disseminated acute leukoencephalopathy in patients with coronavirus disease 2019 which may suggest a common pathophysiological mechanism. Knowing the distinct imaging features of ATL and DPHL along with their typical clinical courses can provide a faster and more reliable differentiation between these two entities.
Keywords: Acute toxic leukoencephalopathy, Delayed post-hypoxic leukoencephalopathy, Magnetic resonance imaging, Opioid intoxication
Introduction
Opioid intoxication (OI) may lead to neurotoxic effects with possible long-lasting or even permanent neurological impairments. It has been shown that opioid-related neurological deficits are accompanied by brain magnetic resonance imaging (MRI) abnormalities [1].
Two previously reported adverse OI-associated entities are acute toxic leukoencephalopathy (ATL) and delayed post-hypoxic leukoencephalopathy (DPHL). ATL can be related to various toxic etiologies other than OI and is characterized by an abrupt start, while neurological symptoms may vary, depending on the degree and extent of brain injury [2]. Brain MRI typically shows white matter (WM) abnormalities of the periventricular region and centrum semiovale, which are confluent and symmetric on T2 and fluid-attenuated inversion recovery (FLAIR) images and with restricted diffusion (RD) on diffusion-weighted imaging (DWI) [2, 3, 4]. Due to its extremely variable clinical presentation, as well as potentially reversible nature, ATL is considered underdiagnosed.
DPHL is an infrequent cause of hypoxia-induced WM injury, characterized by a biphasic course where an acute hypoxic episode is followed by a recovery period and subsequent deterioration phase (DP), marked by neuropsychiatric symptoms [5]. The recovery period lasts between 1 and 4 weeks, during which the patient usually reaches the baseline neurological status. As in the case of ATL, a similar imaging pattern on brain MRI is visible on T2, FLAIR, and DWI images in the periventricular WM and centrum semiovale, but with progression of WM involvement in relation to DP development [6, 7]. We present 2 cases of opioid-induced leukoencephalopathies, more specifically a case of ATL and a case of DPHL, together with the corresponding clinical characteristics and MRI findings.
Case Report/Case Presentation
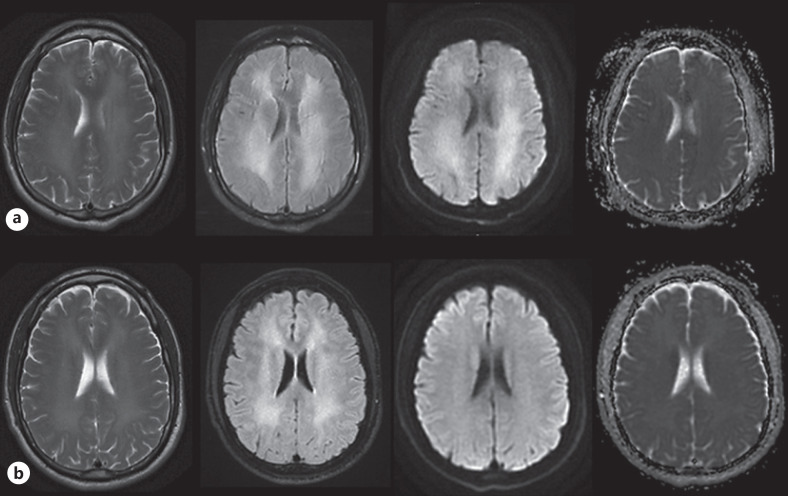
The first patient, a 30-year-old male sought acute medical assistance at the psychiatric department following 3 days of altered behavior. The patient had a prior history of paranoia, symptoms of depression and anxiety, as well as a known drug addiction, involving amphetamine, cocaine, fentanyl, ecstasy, fantasy, benzodiazepine, and cannabis, according to previous medical records. The patient and his partner informed that the patient consumed ecstasy, amphetamine, cannabis, and alcohol 3 days prior to admission, whereafter he started to behave gradually more unusual over the following days. Agitation, growing confusion, as well as impaired concentration and short-term memory, were reported. The patient did not agree to have a drug-test at admission. Neurological examination was normal as well as routine blood test. One day after admission, due to newly appearing signs of apraxia, the patient was transferred to the neurology department (ND), and a head computed tomography was performed which was unremarkable. Cerebrospinal fluid analysis showed normal results. Antipsychotic treatment had no effect, and apraxia along with agitation and memory impairment persisted. Brain MRI obtained 8 days after admission (AA), showed significant bilateral confluent and symmetric supratentorial WM abnormalities of high signal on T2, FLAIR, and DWI images, but without corresponding low apparent diffusion coefficient values (shown in Fig. 1a). A diagnosis of ATL was postulated, and no further treatment was offered at the ND, therefore the patient was moved to the neurorehabilitation unit. During the following months the patient showed gradual clinical improvement, but still with a degree of impaired short-term memory, concentration, and abstract thinking. On the follow-up brain MRI 7 weeks AA there was minimal regression of T2 and FLAIR WM changes, without DWI abnormalities (shown in Fig. 1b).
Fig. 1.
Serial MRI of the brain with T2, FLAIR, and DWI (b) 1,000 images as well as ADC maps presented from left to right columns performed on 1 week (a) and 7 weeks (b) AA. AA, after admission; ADC, apparent diffusion coefficient.
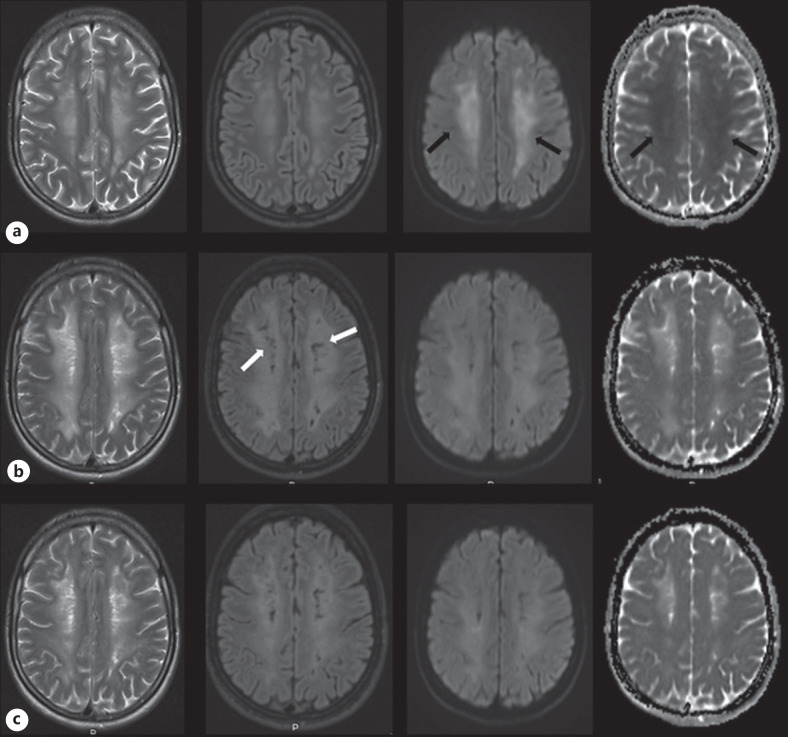
The second patient, a 24-year-old male was admitted to the emergency department after being discovered unconscious with bradypnea, signs of poor circulation and Glasgow Coma Scale 7–8. The patient had a history of substance abuse the past 5 years and traces of methadone, morphine, cocaine, benzodiazepines, amphetamine, and cannabis were detected in an urine drug-test. Head computed tomography showed no abnormalities, and routine blood test, as well as cerebrospinal fluid analyses, were normal. The patient was taken to the intensive care unit and sedated. Sedation stopped on day 4 AA, and upon awakening, the patient showed significant cognitive and physical impairments and was transferred to the ND. Brain MRI done 10 days AA showed extensive bilateral symmetric changes in the centrum semiovale, deep and periventricular WM of increased signal on T2, FLAIR, and DWI images and low apparent diffusion coefficient values denoting RD (shown in Fig. 2a). Over the next few weeks, the patient gradually improved, consequently being moved to the neurorehabilitation unit. Five weeks AA, the patient became agitated along with signs of impaired short-term memory and extrapyramidal symptoms. A follow-up brain MRI made 7 weeks AA showed progression of WM involvement on T2 and FLAIR images with newly appearing small areas of WM cystic substance loss (CSL) in relation to a part of the changes that showed RD on the previous brain MRI, while RD was not detectable anymore (shown in Fig. 2b). The patient was readmitted to the ND, where a gradual clinical improvement occurred over the following weeks. The last follow-up brain MRI done 10 weeks AA, presented moderate regression of WM lesions without CSL progression (shown in Fig. 2c). At the time of discharge, 4 months AA, the patient almost returned to his near habitual level of function. The clinical course along with MRI findings led to a conclusive diagnosis of DPHL.
Fig. 2.
Serial MRI with T2, FLAIR, and DWI (b) 1,000 images along with ADC maps shown from left to right columns acquired on 1 week (a), 7 weeks (b), and 10 weeks (c) AA. Areas of WM high signal on DWI images with corresponding low ADC values indicating RD are detectable on the brain MRI made 1 week AA (black arrows). The T2 and FLAIR images done 7 weeks AA, during the period when the patient developed new neuropsychiatric symptoms, show progression in extent of WM involvement as well as new small areas of CSL (white arrows). ADC, apparent diffusion coefficient; CSL, cystic substance loss.
Discussion/Conclusion
In seldom cases, OI related to heroin, morphine or methadone abuse leads to leukoencephalopathy, which can manifest as ATL or DPHL [1]. However, both entities are not exclusively related to OI. Exposure to different toxic substances can induce ATL, and DPHL is also related to overdoses of benzodiazepines and/or barbiturates, carbon monoxide poisoning, strangulation, and cardiopulmonary arrest [2, 8]. While the initial imaging features of ATL and DPHL appear similar, showing confluent changes throughout the supratentorial WM with U-fibers preservation, a different evolution of WM changes was noted when comparing follow-up brain MRI examinations of our 2 patients done at almost the same time points [9]. This is most likely related to the proposed distinct pathophysiological mechanisms of ATL and DPHL. ATL has a monophasic course related to toxin exposure, where a pathophysiological mechanism of direct toxin injury to the myelin sheath has been suggested [2]. Our ATL patient had gradual clinical improvement, while there were minimal changes in appearances of WM abnormalities on T2 and FLAIR images on the follow-up brain MRI, which is in concordance with previously described partial regression and persistence of WM abnormalities over a longer period of time in OI ATL [3, 10]. Our DPHL patient reached the DP in week 5 AA, which was accompanied by progression of WM changes on T2 and FLAIR images on the follow-up brain MRI done in week 7 AA, a previously described imaging feature of DPHL. The proposed underlying mechanisms of the DP in DPHL might be hypoxia-related myelin-sheath damage due to inappropriate myelin secretion or impaired oligodendroglial function [8]. The expected turnover rate of myelin-related proteins is between 19 and 23 days, and the DP onset was previously reported to be between 2 and 5 weeks, which would be in line with the appearance of the DP in our DPHL patient [5].
Detection of areas with WM CSL on follow-up brain MRI in a DPHL patient, done in relation to the DP, has been described before [6]. Therefore, we suggest that the appearance of symmetric and bilateral CSL in the periventricular WM and centrum semiovale, in patients with a history of drug abuse and a clinical course typical for DPHL, represent a hallmark of late DPHL-related WM changes. Interestingly, presence of WM CSL on follow-up brain MRI appearing in areas with RD on the initial brain MRI, was also recently described in patients affected by coronavirus disease 2019 that developed virus-associated necrotizing disseminated acute leukoencephalopathy (VANDAL) [11]. As CSL changes in DPHL and VANDAL are visible in the same areas that are affected by internal watershed infarcts, a common underlying mechanism that involves endothelial injury or disfunction as well as thrombotic microangiopathy could be postulated. On the other hand, our DPHL patient nearly fully recovered at discharge, while patients with VANDAL showed poor functional outcomes.
In conclusion, distinct imaging features in relation to the clinical course are crucial in the differentiation between ATL and DPHL, as well as when ATL and DPHL are compared to other metabolic or toxic leukoencephalopathies. The development of CSL in patients with VANDAL, as in DPHL, indicates a possible shared pathophysiological mechanism of WM injury.
Statement of Ethics
The research in this article was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from the patients for publication of this case report and the accompanying figures. This case report did not require an institutional review board approval.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
G.H.K. and R.A. wrote the manuscript and carried out the literature review. N.K. and C.H.K.-J. edited the manuscript. All authors revised the manuscript for intellectual content and approved the final manuscript.
Data Availability Statement
No data from this article are openly available. Further inquiries can be directed to the corresponding author.
References
- 1.Haghighi-Morad M, Naseri Z, Jamshidi N, Hassanian-Moghaddam H, Zamani N, Ahmad-Molaei L. Methadone-induced encephalopathy: a case series and literature review. BMC Med Imaging. 2020 Jan 17;20((1)):6. doi: 10.1186/s12880-020-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozutemiz C, Roshan SK, Kroll NJ, Benson JC, Rykken JB, Oswood MC, et al. Acute toxic leukoencephalopathy: etiologies, imaging findings, and outcomes in 101 patients. Am J Neuroradiol. 2019 Feb;40((2)):267–75. doi: 10.3174/ajnr.A5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrot S, Poretti A, Tucker EW, Soares BP, Huisman TA. Acute brain injury following illicit drug abuse in adolescent and young adult patients: spectrum of neuroimaging findings. Neuroradiol J. 2017 Apr;30((2)):144–50. doi: 10.1177/1971400917691994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinicu AI, Chaudhari A, Kayyal S. Diffuse subcortical white matter injury and bilateral basal ganglia neuronal loss after acute opioid overdose. Neuroradiol J. 2020 Jun;33((3)):267–70. doi: 10.1177/1971400920927878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamora CA, Nauen D, Hynecek R, Ilica AT, Izbudak I, Sair HI, et al. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav. 2015 Aug;5((8)):e00364. doi: 10.1002/brb3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bileviciute-Ljungar I, Häglund V, Carlsson J, von Heijne A. Clinical and radiological findings in methadone-induced delayed leukoencephalopathy. J Rehabil Med. 2014 Sep;46((8)):828–30. doi: 10.2340/16501977-1820. [DOI] [PubMed] [Google Scholar]
- 7.Phan MN, Nakawah MO. Longitudinal magnetic resonance findings in delayed posthypoxic leukoencephalopathy. Proc. 2020 Jul 16;33((4)):679–80. doi: 10.1080/08998280.2020.1778423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26((1)):65–72. [PMC free article] [PubMed] [Google Scholar]
- 9.Riley KJ, O'Neill DP, Kralik SF. Subcortical U-fibers: signposts to the diagnosis of white matter disease. Neurograph. 2018;8((4)):234–43. [Google Scholar]
- 10.Koksel Y, Ozutemiz C, Rykken J, Ott F, Cayci Z, Oswood M, et al. CHOICES: an acronym to aid in delineating potential causes of non-metabolic, non-infectious acute toxic leukoencephalopathy. Eur J Radiol Open. 2019;6:243–57. doi: 10.1016/j.ejro.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal S, Conway J, Nguyen V, Dogra S, Krieger P, Zagzag D, et al. Serial imaging of virus-associated necrotizing disseminated acute leukoencephalopathy (VANDAL) in COVID-19. Am J Neuroradiol. 2021 Jan;42((2)):279–84. doi: 10.3174/ajnr.A6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data from this article are openly available. Further inquiries can be directed to the corresponding author.