Abstract
Augustine (AUG) is a blood group system comprising four antigens: AUG1, AUG2 (At<sup>a</sup>), and AUG4 are of very high frequency; AUG3 is of very low frequency. These antigens are located on ENT1, an equilibrative nucleoside transporter encoded by SLC19A1. AUG antibodies are of clinical relevance in blood transfusion and pregnancy: anti-AUG2 have caused haemolytic transfusion reactions; the only anti-AUG3 was associated with severe haemolytic disease of the fetus and newborn. ENT1 is present in almost all human tissues. It facilitates the transfer of purine and pyrimidine nucleosides and is responsible for the majority of adenosine transport across plasma membranes. Adenosine transport appears to be an important factor in the regulation of bone metabolism. The AUG<sub>null</sub> phenotype (AUG:–1,–2,–3,–4) has been found in three siblings, who are homozygous for an inactivating splice-site mutation in SLC29A1. Although ENT1 is very likely to be absent from all cells in these three individuals, they were apparently healthy with normal lifestyles. However, they suffered frequent attacks of pseudogout, a form of arthritis, in various joints with multiple calcifications around their hand joints. Ectopic calcification in the hips, pubic symphysis, and lumbar discs was present in the propositus. The three AUG<sub>null</sub> individuals had misshapen red cells with deregulated protein phosphorylation, but no anaemia or shortening of red cell lifespan. Defective in vitro erythropoiesis in the absence of ENT1 was confirmed by shRNA-mediated knockdown of ENT1 during in vitro erythropoiesis of CD34<sup>+</sup> progenitor cells from individuals with normal ENT1. Nucleoside transporters, such as ENT1, are vital in the uptake of synthetic nucleoside analogue drugs, used in cancer and viral chemotherapy. It is feasible that the efficacy of these drugs would be compromised in patients with the extremely rare AUG<sub>null</sub> phenotype.
Keywords: Augustine blood group system, ENT1, SLC29A1, Arthritis, Erythropoiesis
Introduction
The first example of anti-Ata, an antibody to a blood group antigen of very high frequency, was found in 1967 in an African American woman during her third pregnancy [1]. Other examples were subsequently identified, all in individuals of African origin [2, 3, 4, 5, 6]. Across several surveys, about one in 16,450 African Americans was At(a−) [1, 2, 7, 8, 9]. Family analyses provided strong evidence that the At(a−) phenotype results from homozygosity for a recessive gene [1, 2, 5, 7, 10, 11].
An antibody in an At(a−) pregnant woman with consanguineous parents reacted with all red cells tested, including those of the At(a−) phenotype, except for her own red cells and those of her two At(a−) siblings. This suggested that the three siblings may have a null phenotype in a blood group system containing Ata. In 2015, this antibody was used to purify a protein, which was identified as Equilibrative Nucleoside Transporter 1 (ENT1) encoded by SLC29A1 [12]. Consequently, a new blood group system, Augustine, was recognised by the International Society of Blood Transfusion [13] (Table 1). The Augustine system originally contained two antigens: Ata (AUG2) plus a new antigen, AUG1, defined by the antibody made by the woman with the Augustine-null phenotype (AUGnull). Two more antigens have been added to the system: AUG3 (ATML), an antigen only found in six members of one family [14]; and AUG4 (ATAM), a high-frequency antigen absent from red cells of one person, who had made the corresponding antibody [15]. In this review, the At(a−) phenotype of the original African American type will be notated AUG:1,–2 and the null phenotype unique to one family (AUG:–1,–2) as AUGnull.
Table 1.
Antigens of the Augustine blood group system and their molecular backgrounds
| Antigens |
Molecular basis (SLC29A1)* |
||||
|---|---|---|---|---|---|
| ISBT number | Name | Nucleotides | Exon/intron | Amino acids | |
| AUG 1 | 036001 | c.589+1G (C) | Intron 6 | Splice site mutation | |
| AUG2 | 036002 | Ata | c.1171G (A) | Exon 12 | p.Glu391 (Lys) |
| AUG3 | 036003 | ATML | c.1159O (A) | Exon 12 | p.Pro387 (Thr) |
| AUG4 | 036004 | ATAM | c.242A (G) | Exon 3 | p.Asn81 (Ser) |
Molecular basis of antigen-negative phenotype provided in parentheses.
The discovery of a null phenotype in a blood group system in which the antigens are encoded by the ENT1 gene has provided insight into potential diseases associated with ENT1 deficiency [12].
Clinical Importance of Augustine Antibodies in Transfusion and Pregnancy
Augustine-system antibodies have some clinical relevance to blood transfusion. There are two reports of haemolytic transfusion reactions (HTRs) caused by anti-AUG2: one was an immediate HTR with chills and nausea during a red cell survival study [10]; the other was a severe delayed HTR following transfusion of multiple units of AUG:2 red cells [11]. Anti-AUG2 facilitated rapid destruction of 51Cr-labelled antigen-positive red cells in vivo and gave positive results in in vitro functional assays [5, 10, 16]. Ideally, AUG:−2 red cell units should be selected for transfusion to patients with anti-AUG2, although obtaining these units may prove very difficult.
Despite numerous cases of pregnancies in women with anti-AUG2, there has been no report of severe haemolytic disease of the fetus and newborn (HDFN). One case is described in which mild HDFN was treated with phototherapy [4]. The only known example of anti-AUG1 was probably responsible for a positive direct antiglobulin test on neonatal red cells, but there was no evidence of HDFN [12]. The only example of anti-AUG3, however, was responsible for severe HDFN, requiring two exchange and four top-up transfusions [14].
Molecular Biology of the Augustine Blood Group System
The antibody now called anti-AUG1 was used to purify a red cell membrane protein by immunoprecipitation and polyacrylamide gel electrophoresis [12]. Three tryptic peptides were analyzed by mass spectrometry and the gene encoding their sequences was identified as SLC29A1, the gene for the equilibrative nucleoside transporter 1 (ENT1, or solute carrier 29A1).
SLC29A1 sequencing of DNA from five AUG:1,–2 individuals and DNA from serum samples containing anti-AUG2 (and presumed to be from AUG:1,–2 individuals) showed that all were homozygous for c.1171G>A in exon 12, encoding p.Glu391Lys in the fifth extracellular loop of ENT1 (Table 1; Fig. 1) [12]. The c.1171G>A transition in SLC29A1 corresponds to the minor (A) allele of rs4548701, which has only been detected in people of African ancestry. The US National Institutes of Health, Heart, Lung, and Blood Institute-sponsored Exome Sequencing Project found 49 heterozygotes in 2,203 African Americans, suggesting a frequency of about 1% for the rare allele, but none was found in 4,300 Americans of European origin [12]. From the allele frequency, AUG:1,–2 phenotype would be predicted to be present in about 1 in 10,000 African Americans.
Fig. 1.
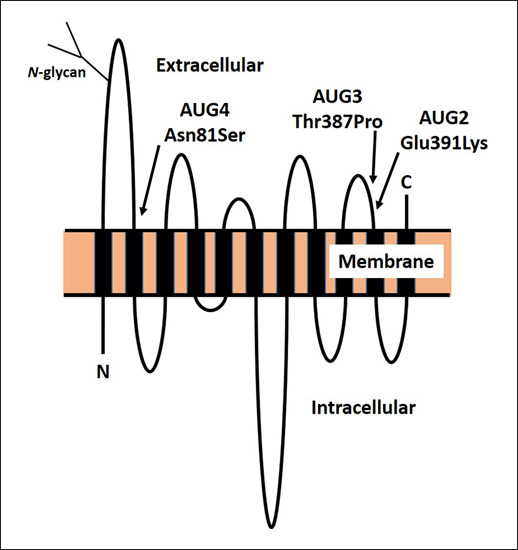
Diagrammatic representation of the conformation of ENT1 in the red cell membrane, with 11 membrane-spanning domains, internal amino-terminal domain (N), external carboxy-terminal domain (C), and showing the approximate positions of the AUG2, AUG3, and AUG4 variants.
Five family members with the rare AUG3 antigen were heterozygous for c.1159A>C in SLC29A1, encoding p.Thr387Pro in the fifth extracellular loop of ENT1, four amino acids from the substitution associated with loss of AUG2 (Table 1; Fig. 1) [14].
Loss of AUG4 is associated with homozygosity for c.242A>G in SLC29A1 encoding p.Asn81Ser [15] (Table 1). The expression level of ENT1 in the red cells of the AUG:–4 propositus was decreased by about 30% of normal, suggesting that p.Asn81Ser affects ENT1 expression at the cell surface [15].
Sequencing of SLC29A1 in the patient who produced anti-AUG1 and who was suspected of having the AUGnull phenotype revealed that she was homozygous for a transversion at the +1 position of intron 6 (c.589+1G>C) (Table 1). This splice-site mutation was absent from the DNA of controls and in public databases. Her two AUGnull siblings were also homozygous for the mutation, and her parents, who were first cousins once removed, were both heterozygous for the mutation. Furthermore, immunoblotting revealed no ENT1 protein in the red cell membranes of the propositus [12].
ENT1
ENT1 (SLC29A1) is a member of the very large family of solute carriers: integral membrane glycoproteins that typically traverse cell membranes several times and transport physiologically important molecules across the membrane. Purine and pyrimidine nucleosides are hydrophilic molecules requiring specialized transport proteins to pass through cell membranes. There are two types of nucleoside transporters: equilibrative bidirectional transporters (ENTs, SLC29), driven by chemical concentration gradients, and concentrative transporters (CNTs, SLC28), driven by the sodium electrochemical gradient [17]. The human ENT1 (hENT1) gene (SLC29A1) was initially isolated from a human placental DNA library in 1997 [18]. It is located on chromosome 6p21.1-21.2. ENT1 is a 456-amino acid glycoprotein that, like other ENTs, spans the membrane 11 times and has a cytosolic N-terminal domain and an external C-terminal domain as shown in Figure 1 [19, 20]. N-linked glycosylation at Asn-48 in the first extracellular loop (Fig. 1) is reported as critical for localization, function, and oligomerization of hENT1 [21]. In addition to its presence on red cells, ENT1 is almost ubiquitously distributed in human tissues. It facilitates the transfer of both purine and pyrimidine nucleosides and is responsible for the majority of adenosine transport across plasma membranes, including both the cell envelope and mitochondrial membranes [22]. Intracellular adenosine metabolism regulates vital biosynthetic pathways, in particular synthesis of adenosine triphosphate (ATP), cyclic adenosine triphosphate (cAMP), and nucleotides for nucleic acids.
Disease Associations
The AUGnull phenotype arises from homozygosity for an inactivating mutation in SLC29A1 [12], so it can be surmised that the AUGnull propositus and her AUGnull siblings are deficient in ENT1 from all tissues and that some resultant morbidity might be expected. When these three individuals were studied, at the ages of 45–50 years, they had no obvious developmental abnormalities and they all had normal lifestyles. Nevertheless, all three had suffered frequent attacks of pseudogout since the age of 18–20 years, mainly in the hands, but also in the feet, elbows, and knees [12]. This was not apparent in their parents or children. Pseudogout or calcium pyrophosphate dihydrate deposition (CPPD) disease is a form of arthritis associated with painful inflammation and swelling of joints, most commonly knees and wrists. It results from the presence of CPPD crystals within the joints. X-ray analysis revealed multiple calcifications around the hand joints of the AUGnull siblings and, in the propositus, at age 47 years, ectopic calcification in the hips, pubic symphysis, and lumbar discs [12]. Serum levels of urea, uric acid, creatinine, and electrolytes were within normal range. From the analysis of just one family, it is not possible to confirm that ENT1 deficiency is responsible for these symptoms, but other evidence suggests that adenosine transport is an important factor in the regulation of bone metabolism. Four adenosine receptors are expressed by bone marrow cells, osteoclasts, and osteoblasts, and this is consistent with adenosine and its receptors playing a role in bone and cartilage homeostasis [23]. CD73, or ecto-5′-nucleotidase, is an enzyme encoded by NT5E, which converts adenosine monophosphate (AMP) to adenosine [24]. In three families, different mutations in NT5E that result in nonfunctional CD73 were responsible for calcification of lower-extremity arteries and hand and foot joints [25].
The importance of ENT1 in regulation of bone metabolism is supported by information obtained from Slc29a1−/− (“knockout”) mice. By 12 months of age, Slc29a1−/− mice developed spinal stiffness, hind limb dysfunction, and calcified lesions resembling symptoms of diffuse idiopathic skeletal hyperostosis in humans [26]. Furthermore, Slc29a1−/− mice have reduced bone density and bone mineral density in the lower thoracic and lumbar spine, leading to severe deficit in motor coordination and locomotor activity. This suggests that ENT1-regulated adenosine signaling plays an essential role in lumbar-spine and femur bone density [27].
In addition to ENT1 being abundant in the membranes of mature red cells [18], SLC29A1 is highly expressed in early erythroid progenitor cells [12]. Nucleosides are essential precursors for nucleotide biosynthesis and the regulation of intracellular adenosine is critical for self-renewal of haemopoietic stem cells (HSCs) and for erythroid commitment [28]. Transport of nucleosides into HSCs is dependent on nucleoside transporters and ENT1 is the major membrane transporter of adenosine in HSCs [29, 30]. GATA transcription factors, which actuate genetic networks in HSCs, erythroid precursor cells, and other erythroid cells [31], regulate many membrane transporters, including ENT1 during erythropoiesis [32]. Slc29a1−/− mice had macrocytosis, anaemia, and reduced numbers of erythroid progenitors in the bone marrow [33]. In an acute anaemia mouse model, an Slc29a1-dependent mechanism promoted erythroblast survival and differentiation and reduced anaemia [32].
Analysis of AUGnull individuals has revealed that ENT1 deficiency results in defective human erythropoiesis, although anaemia was not apparent [33]. Absence of ENT1 in individuals with the AUGnull phenotype is associated with misshapen red cells (macrocytosis, anisopoikilocytosis) and with deregulated protein phosphorylation, but no shortening of red cell lifespan. In addition, defective in vitro erythropoiesis of human CD34+ progenitors derived from individuals with normal ENT1 expression occurred following shRNA-mediated knockdown of ENT1, confirming that reduction of ENT1 in human erythroid progenitors impedes erythropoiesis [33].
ATP-binding cassette sub-family C member 4 (ABCC4), also known as the multidrug resistance-associated protein 4 (MRP4), acts as a regulator of intracellular cyclic nucleotide levels and as a mediator of cAMP-dependent signal transduction to the nucleus [34]. Partial compensation of ENT1 deficiency in two of the three AUGnull siblings resulted from heterozygosity for a loss-of-function variant in ABCC4 (c.559G>T; p.Gly187Trp). The AUGnull sibling without the inactive ABCC4 allele had a greater red cell phenotypic defect. In addition, pharmacological inhibition of ABCC4 rescued erythropoiesis in Slc29a1−/− mice. In summary, inhibition of ABCC4 enhances erythroid differentiation in the absence of ENT1 [33].
The rare AUG:1,–2 phenotype (the original At(a−) phenotype) is not known to be associated with any disease and the p.Glu391Lys ENT1 substitution responsible for AUG:1,–2 does not alter the activity of the ENT1 transporter [35].
Prolonged ethanol exposure increased adenosine uptake activity of cells transfected with a variant form of hENT1 (ENT1-216Thr) compared with cells transfected with wild-type protein (ENT1-216Ile). This hENT1 variant, not known to be associated with any blood group change, was also found to be associated with alcohol dependence with withdrawal seizures. In addition, mice lacking ENT1 showed an increased tendency to ethanol withdrawal seizures compared to littermates with ENT1 [36].
Pharmacological Significance
Synthetic nucleoside-analogue drugs are used in cancer and viral chemotherapy, and nucleoside transporters such as ENT1 are vital in the uptake of these drugs [37, 38, 39]. For example, the cancer treatment drug cytarabine is a pyrimidine analogue and is primarily transported into malignant cells by ENT1 [37]. Decreased SLC29A1 expression in tumour cells is correlated with poor prognosis in patients with hepatocellular carcinoma because of reduced drug sensitivity [40]. Patients with pancreatic adenocarcinoma with uniformly detectable ENT1 immunostaining have longer survival after gemcitabine chemotherapy than patients with tumours without detectable ENT1 [41]. Uptake into hepatic cells and red cells of the nucleoside analogue ribavirin, used as front-line treatment for hepatitis C viral infection, appears to be ENT1-dependent [42, 43]. It is very likely that the efficacy of such drugs would be compromised in patients with the extremely rare AUGnull phenotype.
Conclusion
Three siblings with the previously unknown AUGnull blood group phenotype are homozygous for inactivating mutations in SLC29A, the gene encoding the equilibrative nucleoside transporter ENT1. Despite the importance of this molecule as a major transporter of nucleosides across membranes, these three AUGnull individuals were relatively healthy and had normal lifestyles, although further analysis revealed a form of arthritis with calcification around the joints and defective erythropoiesis, but with no symptom of anaemia [12, 33]. Blood group null phenotypes are usually rare and are generally only found through blood group antibodies identified in blood transfusion reference laboratories. AUG joins many other blood group systems that have provided valuable information on the importance and functions of cell surface glycoproteins.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
Funding Sources
There were no funding sources.
References
- 1.Applewhaite F, Ginsberg V, Gerena J, Cunningham CA, Gavin J. A very frequent red cell antigen Ata. Vox Sang. 1967;13:444–5. doi: 10.1111/j.1423-0410.1967.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 2.Gellerman MM, McCreary J, Yedinak E, Stroup M. Six additional examples of anti-Ata. Transfusion. 1973;13:225–30. doi: 10.1111/j.1537-2995.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown A, Harris P, Daniels GL, Frank S, Moore BPL, Berger R. Ata (August) and El (Eldr) are synonymous. Transfusion. 1983;44:123–5. doi: 10.1111/j.1423-0410.1983.tb04114.x. [DOI] [PubMed] [Google Scholar]
- 4.Culver PL, Brubaker DB, Sheldon RE, Martin M, Richter CA. Anti-Ata causing mild hemolytic disease of the newborn. Transfusion. 1987;27:468–70. doi: 10.1046/j.1537-2995.1987.27688071696.x. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney JD, Holme S, McCall L, Huett D, Storry J, Reid M. At(a−) phenotype: description of a family and reduced survival of At(a+) red cells in a proposita with anti-Ata. Transfusion. 1995;35:63–7. doi: 10.1046/j.1537-2995.1995.35195090666.x. [DOI] [PubMed] [Google Scholar]
- 6.McBean R, Liew Y-W, Wilson B, Kupatawintu P, Emthip M, Hyland C, et al. Genotyping confirms inheritance of the rare At(a−) type in a case of haemolytic disease of the newborn. J Pathol Clin Res. 2016;2:53–5. doi: 10.1002/cjp2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank S, Schmidt RP, Baugh M. Three new antibodies to high-incidence antigenic determinants (anti-El, anti-Dp, and anti-So) Transfusion. 1970;10:254–7. doi: 10.1111/j.1537-2995.1970.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Winkler MM, Hamilton JR. Proc 21st Joint Congr Int Soc Blood Transfus Am Ass Blood Banks. 1990. Previously tested donors eliminated to determine rare phenotype frequencies (abstract) p. p. 158. [Google Scholar]
- 9.Vengelen-Tyler V. Efficient use of scarce sera in screening thousands of donors (abstract) Transfusion. 1985;25:476. [Google Scholar]
- 10.Ramsey G, Sherman LA, Zimmer AM, Spies WG, Sharma L, Arndt P, et al. Clinical significance of anti-Ata. Vox Sang. 1995;69:135–7. doi: 10.1111/j.1423-0410.1995.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 11.Cash KL, Brown T, Sausais L, Uehlinger J, Reed LJ. Severe delayed hemolytic transfusion reaction secondary to anti-Ata. Transfusion. 1999;39:834–7. doi: 10.1046/j.1537-2995.1999.39080834.x. [DOI] [PubMed] [Google Scholar]
- 12.Daniels G, Bailif BA, Helias V, Saison C, Grimsley S, Mannessier L, et al. Lack of nucleoside transporter ENT1 results in the Augustine-null blood type and ectopic mineralization. Blood. 2015;125:3651–4. doi: 10.1182/blood-2015-03-631598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storry JR, Castilho L, Chen Q, Daniels G, Denomme G, Flegel WA, et al. International Society of Blood Transfusion Working Party on Red Cell Immunogenetics and Terminology: Report of the Seoul and London meetings. ISBT Sci Ser. 2016;11:118–22. doi: 10.1111/voxs.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millard GM, McGowan EC, Wilson B, Martin JR, Spooner M, Morris S, et al. A proposed new low-frequency antigen in the Augustine blood group system associated with a severe case of hemolytic disease of the fetus and newborn. Transfusion. 2018;58:1320–2. doi: 10.1111/trf.14562. [DOI] [PubMed] [Google Scholar]
- 15.Vrignaud C, Mikdar M, Raneri A, Hermine O, Colin Y, Le Van Kim C, et al. Characterization of a novel high-prevalence red blood cell antigen in the Augustine blood group system (abstract) Vox Sang. 2018;113((Suppl 1)):64–5. [Google Scholar]
- 16.Arndt PA, Garratty G. A retrospective analysis of the value of monocyte monolayer assay results for predicting the clinical significance of blood group alloantibodies. Transfusion. 2004;44:1273–81. doi: 10.1111/j.1537-2995.2004.03427.x. [DOI] [PubMed] [Google Scholar]
- 17.Cass CE, Young JD, Baldwin SA. Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem Cell Biol. 1998;76:761–70. doi: 10.1139/bcb-76-5-761. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, et al. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med. 1997;3:89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 20.Wright NJ, Lee SY. Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat Struct Mol Biol. 2019;26:599–606. doi: 10.1038/s41594-019-0245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bicket A, Coe IR. N-linked glycosylation of N48 is required for equilibrative nucleoside transporter 1 (ENT1) function. Biosci Rep. 2016;36:e00376. doi: 10.1042/BSR20160063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters-A review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30. doi: 10.1080/15257770.2016.1210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends Endocrinol Metab. 2013;24:290–300. doi: 10.1016/j.tem.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allard B, Cousineau I, Spring K, Stagg J. Measurement of CD73 enzymatic activity using luminescence-based and colorimetric assays. Methods Enzymol. 2019;629:269–89. doi: 10.1016/bs.mie.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 25.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, et al. NT5E mutations and arterial calcifications. New Engl J Med. 2011;364:432–42. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warraich S, Bone DB, Quinonez D, Ii H, Choi DS, Holdsworth DW, et al. Loss of equilibrative nucleoside transporter 1 in mice leads to progressive ectopic mineralization of spinal tissues resembling diffuse idiopathic skeletal hyperostosis in humans. J Bone Miner Res. 2013;28:1135–49. doi: 10.1002/jbmr.1826. [DOI] [PubMed] [Google Scholar]
- 27.Hinton DJ, McGee-Lawrence ME, Lee MR, Kwong HK, Westendorf JJ, Choi DS. Aberrant bone density in aging mice lacking the adenosine transporter ENT1. PLoS One. 2014;9:e88818. doi: 10.1371/journal.pone.0088818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oburoglu L, Tardito S, Fritz V, de Barros SC, Merida P, Craveiro M, et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15:169–84. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 29.An X, Schulz VP, Li J, Wu K, Liu J, Xue F, et al. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood. 2014;123:3466–77. doi: 10.1182/blood-2014-01-548305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier E, Ducamp S, Leduc M, Salnot V, Guillonneau F, Dussiot M, et al. Comprehensive proteomic analysis of human erythropoiesis. Cell Rep. 2016;16:1470–84. doi: 10.1016/j.celrep.2016.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsumura KR, Bresnick EH, GATA Factor Mechanisms Group The GATA factor revolution in hematology. Blood. 2017;129((15)):2092–102. doi: 10.1182/blood-2016-09-687871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwifelhofer NM, Cai X, Liao R, Mao B, Conn DJ, Mehta C, et al. GATA factor-regulated solute carrier ensemble revels a nucleoside transporter-dependent differentiation mechanism. PLoS Genet. 2020;16:e1009286. doi: 10.1371/journal.pgen.1009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikdar M, Gonzalez-Menedez P, Cai X, Zhang Y, Serra M, Dembélé AK, et al. The equilibrative nucleoside transporter 1 (ENT1) is critical for nucleotide homeostasis and optimal erythropoiesis. Blood. 2021;137:3548–62. doi: 10.1182/blood.2020007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Aubart FC, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–57. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osata DH, Huang CC, Kawamoto M, Johns SJ, Stryke D, Wang J, et al. Functional characterization in yeast of genetic variants in the human equilibrative nucleoside transporter. ENT1. Pharmacogenetics. 2003;13:297–301. doi: 10.1097/00008571-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kim J-H, Karpyak VM, Biernacka JM, Nam HW, Lee MR, Preuss UW, et al. Functional role of the polymorphic 647 T/C variant of ENT1 (SLC29A1) and its association with alcohol withdrawal seizures. PLoS ONE. 2011;6:e16331. doi: 10.1371/journal.pone.0016331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26:85–110. doi: 10.1007/s10555-007-9044-4. [DOI] [PubMed] [Google Scholar]
- 38.Rahn JJ, Kieller DM, Tyrrell DL, Gati WP. Modulation of the metabolism of beta-L-(-)-2′,3′-dideoxy-3′-thiacytidine by thymidine, fludarabine, and nitrobenzylthioinosine. Antimicrob Agents Chemother. 1997;41:918–23. doi: 10.1128/aac.41.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 40.Gao P-T, Cheng J-W, Gong Z-J, Hu B, Sun Y-F, Cao Y, et al. Low SLC29A1 expression is associated with poor prognosis in patients with hepatocellular carcinoma. Am J Cancer Res. 2017;7:2465–77. [PMC free article] [PubMed] [Google Scholar]
- 41.Spratlin J, Randeep S, Glubrecht D, Dabbagh L, Young JD, Dumontet C, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with Gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–61. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 42.Endres CJ, Moss AM, Ke B, Govindarajan R, Choi DS, Messing RO, et al. The role of the equilibrative nucleoside transporter 1 (ENT1) in transport and metabolism of ribavirin by human and wild-type or Ent1–/− mouse erythrocytes. J Pharmacol Exp Ther. 2009;329:387–98. doi: 10.1124/jpet.108.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibarra KD, Pfeiffer JK. Reduced ribavirin antiviral efficacy via nucleoside transporter-mediated drug resistance. J Virol. 2009;83:4538–47. doi: 10.1128/JVI.02280-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


