Key Points
Question
Does the use of adjunct intra-arterial thrombolysis following an angiographically successful thrombectomy improve functional outcomes in patients with large vessel occlusion acute ischemic stroke?
Findings
In this randomized clinical trial that included 121 adults, treatment with intra-arterial alteplase compared with placebo resulted in a modified Rankin Scale score of 0 or 1 in 59.0% vs 40.4% of patients at 90 days. This difference was statistically significant.
Meaning
Among patients with large vessel occlusion acute ischemic stroke and successful reperfusion following thrombectomy, the use of adjunct intra-arterial alteplase compared with placebo resulted in a greater likelihood of excellent neurological outcome at 90 days; however, the findings should be considered preliminary until replicated.
Abstract
Importance
It is estimated that only 27% of patients with acute ischemic stroke and large vessel occlusion who undergo successful reperfusion after mechanical thrombectomy are disability free at 90 days. An incomplete microcirculatory reperfusion might contribute to these suboptimal clinical benefits.
Objective
To investigate whether treatment with adjunct intra-arterial alteplase after thrombectomy improves outcomes following reperfusion.
Design, Setting, and Participants
Phase 2b randomized, double-blind, placebo-controlled trial performed from December 2018 through May 2021 in 7 stroke centers in Catalonia, Spain. The study included 121 patients with large vessel occlusion acute ischemic stroke treated with thrombectomy within 24 hours after stroke onset and with an expanded Treatment in Cerebral Ischemia angiographic score of 2b50 to 3.
Interventions
Participants were randomized to receive intra-arterial alteplase (0.225 mg/kg; maximum dose, 22.5 mg) infused over 15 to 30 minutes (n = 61) or placebo (n = 52).
Main Outcomes and Measures
The primary outcome was the difference in proportion of patients achieving a score of 0 or 1 on the 90-day modified Rankin Scale (range, 0 [no symptoms] to 6 [death]) in all patients treated as randomized. Safety outcomes included rate of symptomatic intracranial hemorrhage and death.
Results
The study was terminated early for inability to maintain placebo availability and enrollment rate because of the COVID-19 pandemic. Of 1825 patients with acute ischemic stroke treated with thrombectomy at the 7 study sites, 748 (41%) patients fulfilled the angiographic criteria, 121 (7%) patients were randomized (mean age, 70.6 [SD, 13.7] years; 57 women [47%]), and 113 (6%) were treated as randomized. The proportion of participants with a modified Rankin Scale score of 0 or 1 at 90 days was 59.0% (36/61) with alteplase and 40.4% (21/52) with placebo (adjusted risk difference, 18.4%; 95% CI, 0.3%-36.4%; P = .047). The proportion of patients with symptomatic intracranial hemorrhage within 24 hours was 0% with alteplase and 3.8% with placebo (risk difference, −3.8%; 95% CI, −13.2% to 2.5%). Ninety-day mortality was 8% with alteplase and 15% with placebo (risk difference, −7.2%; 95% CI, −19.2% to 4.8%).
Conclusions and Relevance
Among patients with large vessel occlusion acute ischemic stroke and successful reperfusion following thrombectomy, the use of adjunct intra-arterial alteplase compared with placebo resulted in a greater likelihood of excellent neurological outcome at 90 days. However, because of study limitations, these findings should be interpreted as preliminary and require replication.
Trial Registration
ClinicalTrials.gov Identifier: NCT03876119; EudraCT Number: 2018-002195-40
This randomized clinical trial assesses the effect of intra-arterial alteplase infusion vs placebo on functional outcomes (modified Rankin Scale scores) among patients with large vessel occlusion acute ischemic stroke and successful reperfusion following thrombectomy.
Introduction
Endovascular thrombectomy is the optimal treatment option across a wide range of patients with large vessel occlusion acute ischemic stroke.1 In these patients, the extent of reperfusion is evaluated on digital subtraction angiography using the modified Treatment in Cerebral Ischemia (mTICI) score.2 Reperfusion is typically considered successful if at the end of the procedure there is an antegrade reperfusion of more than 50% to 100% of the initially affected arterial territory, corresponding with an expanded TICI (eTICI) score of 2b50 or greater. Although 71% of patients achieved successful reperfusion scores in previous randomized trials, only 27% of the patients treated were disability free at 90 days.3 It is possible that a substantial volume of brain tissue could have been already irreversibly injured in some patients by the time reperfusion occurred. However, impaired reperfusion of the microcirculation despite complete recanalization could have also contributed to the less than desired clinical outcomes,4 for it has been estimated that nearly every neuron in a human brain has its own capillary, and capillaries account for more than 90% of the total intracerebral vascular volume.5
Digital subtraction angiography remains the gold standard to assess reperfusion during thrombectomy,6 but normal cerebral angiogram findings after thrombectomy are not necessarily indicative of an effective perfusion of the microvascular bed.7 Therefore, it was postulated that thrombi persist within the microcirculation in patients with normal or nearly normal cerebral angiograms at the end of thrombectomy,8 and it was hypothesized that these smaller thrombi would be more suitable to dissolve than more proximal thrombi because the efficacy of thrombolysis is related to the extent of clot burden.9
The Chemical Optimization of Cerebral Embolectomy (CHOICE) trial assessed the preliminary efficacy and safety of adjunct intra-arterial alteplase treatment compared with placebo in patients with large vessel occlusion acute ischemic stroke treated with thrombectomy that resulted in successful reperfusion on digital subtraction angiography.
Methods
Trial Design
The trial was a multicenter, phase 2b, randomized, double-blind, placebo-controlled trial performed in Catalonia, Spain. Intra-arterial alteplase was compared with intra-arterial placebo in patients with acute ischemic stroke secondary to a proximal large vessel occlusion treated with thrombectomy that resulted in angiographic findings of success, defined as an eTICI score of 2b50 or greater.
An independent data and safety monitoring board conducted the study monitoring with the assistance of a clinical research organization.
The original version of the study protocol10 and its modifications were approved by a central medical ethics committee and by the research board at each participating center. A detailed description of the trial protocol is provided in Supplement 1. All patients or their surrogates provided written informed consent before the endovascular procedure was started.
The study was conducted in adherence to Spanish regulations of clinical drug trials, the Declaration of Helsinki,11 and the good clinical practice guidelines of the International Conference on Harmonization.
Participants and Study Sites
In this study, patients were screened at all 7 of the stroke centers that have uninterrupted access to performance of thrombectomy in Catalonia, Spain. Eligible patients had a large vessel occlusion in the anterior, middle, or posterior cerebral artery, were treated with thrombectomy within 24 hours after the point when they were last seen well, and had a postthrombectomy eTICI score of 2b50 or greater as judged by local investigators. Participants were aged 18 years or older and had been able to carry out usual activities in their daily life without support before the stroke. The race and ethnicity of the patients was classified according to their chosen nationality; Hispanic patients were individuals from Mexico/South America/Central America and White individuals were from Europe. The decision to perform thrombectomy followed the current local treatment guidelines, including an Alberta Stroke Program Early CT Score (ASPECTS) of at least 6 on noncontrast computed tomography (NCCT) if symptoms lasted less than 4.5 hours (ASPECTS range, 0-10, with 1 point subtracted for any evidence of early ischemic change in each defined region on the CT scan), or on CT perfusion or diffusion-weighted magnetic resonance imaging (MRI) if symptoms lasted 4.5 hours or longer. Other exclusion criteria to participate in the trial were any contraindication to the use of intravenous alteplase per local and national guidelines (except time to therapy) and a National Institutes of Health Stroke Scale (NIHSS) score on admission of greater than 25, (range, 0-42, with higher values indicating more severe deficit). Complete clinical recovery in the angiography suite during the procedure was also an exclusion criterion. Nonanesthetized patients were evaluated by a neurologist to identify possible full recovery. Detailed clinical and imaging criteria for inclusion and exclusion and the study procedures and their timing are provided in eTable 1 and eTable 2 in Supplement 2.
Randomization
Patients were randomly assigned in the angiography suite using PROC PLAN in SAS software (SAS Institute Inc) with a 1:1 ratio between active treatment with intra-arterial alteplase and placebo, stratifying by center and use of intravenous alteplase before thrombectomy (no or yes), in blocks of 4 elements, once the angiographic eTICI score had been obtained following standard thrombectomy.
Intervention
Endovascular treatment was carried out according to the usual practice of each center. If intravenous alteplase was used before the onset of thrombectomy, local investigators were allowed to decide when to stop the intravenous infusion. Patients were considered having received intravenous alteplase if at least half of the corresponding weight-adjusted dose was administered. Patients randomized to intra-arterial alteplase received a dose of 0.225 mg/kg (maximum dose, 22.5 mg) infused over 30 minutes (at trial onset) or over 15 minutes (after approval of protocol amendment number 3 on November 28, 2019). Patients randomized to intra-arterial placebo received a 15-minute infusion of a lyophilized white powder containing 0.2 mol/L arginine phosphate and 0.01% polysorbate 80, at a pH of 7.4 after reconstitution in sterile water for injection. The active treatment and the placebo solutions were limpid, transparent, and colorless and were injected distally to the origin of the lenticulostriate branches.
Outcome Measures
The primary efficacy outcome measure was the proportion of patients with a score of 0 or 1 on the modified Rankin Scale at 90 days. The modified Rankin Scale is an ordered scale coded from 0 (no symptoms at all) to 5 (severe disability) and 6 (death). A score of 1 or less indicates an excellent (disability-free) outcome.
The secondary efficacy outcomes included the proportion of patients with improved angiographic findings; a shift analysis of the modified Rankin Scale score at day 90 in which severe disability (score of 5) and death (score of 6) were combined into a single “worst” category; the proportion of patients with a modified Rankin Scale score of 0 to 2 at day 90; the infarct expansion ratio of final infarct to initial ischemic tissue volumes; the proportion of patients with an infarct expansion ratio greater than 1; and the infarction volume at 24 to 48 hours after stroke onset.
The tertiary efficacy outcomes included the proportion of patients with a Barthel Scale score of 95 to 100 at day 90; the proportion of ischemic worsening (≥4-point increase on the NIHSS score) within 48 hours to 72 hours of stroke onset; and quality of life as measured with the EuroQol 5-Dimension 3-Level Self-Report Questionnaire (EQ-5D-3L) at 90 days. Scores on the EQ-5D-3L range from −0.33 to 1, with higher scores indicating better quality of life.
The adverse events measured included incidence of symptomatic intracranial hemorrhage, defined as neurological deterioration (≥4-point increase on the NIHSS score) within 24 hours after treatment and evidence of intracranial hemorrhage on imaging studies, and death at 90 days. An independent clinical events committee adjudicated safety outcomes and serious adverse events.
Clinical and Radiologic Assessment
Clinical assessments were performed at baseline, at 24 hours and 48 hours after randomization, at 5 to 7 days (or at discharge if earlier), and at 90 days by certified investigators and included modified Rankin Scale scores for assessing global disability and NIHSS scores for assessing neurological deficit. The primary modified Rankin Scale outcome was assessed by local raters via a face-to-face visit using a validated structured interview.12
Entry and outcome neurovascular images were assessed in a blinded manner by 2 senior neuroradiologists at a central core imaging laboratory and included admission baseline NCCT, admission CT perfusion, 24-hour NCCT, 48-hour brain MRI or NCCT, and pretreatment and posttreatment angiograms using the eTICI score13 (see details in the eAppendix in Supplement 2). At 1 study site, perfusion-weighted MRI and MR spectroscopy were also performed at 48 hours after therapy.
Sample Size Calculation
Based on previous data,14 it was calculated that the enrollment of 100 patients per treatment group would provide a power of at least 80% to detect a difference in the primary outcome (modified Rankin Scale score of 0 or 1) assuming a treatment response rate up to approximately 40% in the control group and a 21% absolute benefit in the intervention group for a 0.05 2-sided type I error. No study losses were accounted for since all treated randomized patients were to be included in the analysis.
Statistical Analysis
A detailed description of the analytic approach is provided in the statistical analysis plan (Supplement 3). The main analysis was conducted in all participants treated as randomized. The main efficacy variable, the adjusted risk difference of the proportions of participants with a modified Rankin Scale score of 0 to 1 at 90 days, was estimated using a binomial regression model adjusted for the stratification variable of previous alteplase use, with the link function set to identity.
Secondary outcomes included the shift in the modified Rankin Scale score at day 90, estimated by means of an odds ratio using a proportional odds logistic regression model combining the highest 2 scores of 5 and 6 into a single “worst” rank, with the proportional odds assumption assessed using a score test, which was not statistically significant (P = .08). The adjusted van Elteren test was used as a sensitivity analysis (proportional odds not required). The risk difference for mortality at 90 days was analyzed as described for the primary end point, and exact 95% confidence intervals were calculated for symptomatic intracranial hemorrhage at 24 hours due to lack of model convergence. As predefined, all risk differences, odds ratios, and the van Elteren test were adjusted by the stratification variable of previous alteplase use for all analyses except the subgroup analyses. The rest of the categorical variables were compared using the Fisher exact test and continuous variables by means of the Mann-Whitney test. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary and tertiary end points should be interpreted as exploratory.
Treatment effect modification was explored in prespecified subgroups of patients predicted to have a greater treatment response owing to being at greater risk of poor outcomes, possibly due to microvascular clogging, such as patients not receiving intravenous alteplase before thrombectomy (vs those receiving it), women (vs men),15 patients with admission serum glucose concentrations greater than 100 mg/dL (vs lower values),16 patients with time from stroke onset to groin puncture greater than 7.3 hours (vs lower values).17 Higher study drug concentrations in the microcirculation and, thus, a greater treatment response were predicted in patients with an angiographic eTICI score of 2c/3 (vs an eTICI score of 2b50/2b67).14 Subgroups were analyzed using the same binomial model as for the primary outcome but without the randomization stratum covariate. The subgroup-by-treatment interaction significance was calculated by including that term in an additional model.
Two post hoc sensitivity analyses were conducted for the primary end point. First, study site was added to the primary model as a random effect. Second, a series of models considered imbalances in baseline variables.
No missing data imputation was conducted in this study since there were no missing data for the baseline and primary end points, missing data rates for secondary end points were trivial (<2%), and tertiary end points (<14%) were considered of minor relevance.
The statistical significance of possible differences between subgroups in the treatment effect was tested with interaction terms. No adjustments for multiple tests were made. All analyses were performed using SAS version 9.4, and the level of significance was established at P = .05 (2-sided).
Results
Patients
The inability to maintain placebo availability and a slow enrollment rate, both affected by the COVID-19 pandemic, led to a premature discontinuation of the trial when 60% of the calculated sample size had been enrolled (n = 121 participants). The decision to terminate the trial early was made by the steering committee and the chosen date was that of the expiration of the placebo, without performing an interim analysis.
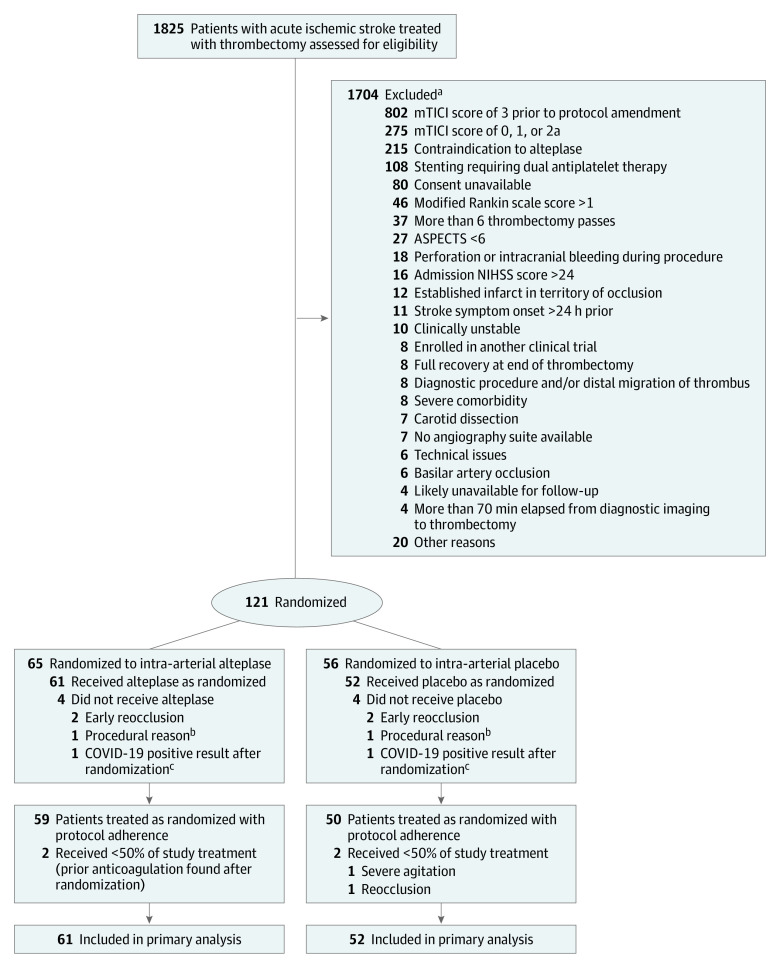
From December 2018 through May 2021, 1825 patients with acute ischemic stroke were treated with thrombectomy at the 7 study sites, including 121 patients (7%) who were randomized in the trial, of whom 113 (6%) received their randomized study treatment. Based on local investigator assessment, 65 (58%) had an eTICI score of 2b50/67 and 48 (42%) had an eTICI score of 2c/3 at the time of randomization. The flow diagram with reasons for exclusion is shown in Figure 1. Among all thrombectomies performed at the study centers during the study period, 802 patients (44%) with an eTICI score of 3 were not eligible for inclusion in the study because they were treated before the amendment of the initial angiographic criteria that restricted the inclusion to patients with an eTICI score of 2b50. All patients had an available evaluation at 90 days for the primary outcome. Eight randomized patients were not included in the treated-as-randomized population because the study treatment was not started for various reasons, including early reocclusion of the treated vessel (n = 4), periprocedural complications (n = 2), and COVID-19 infection diagnosed immediately after randomization (n = 2). The treated-as-randomized analysis included 61 patients in the intra-arterial alteplase group and 52 in the placebo group.
Figure 1. Flow of Patients Through the CHOICE Trial.
ASPECTS indicates Alberta Stroke Program Early CT Score; mTICI, modified Treatment in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale.
aPatients could have more than 1 exclusion.
bMisplacement of balloon-guided catheter.
cExcluded to limit exposure of the interventionalist team.
Baseline Characteristics
Baseline characteristics were similar in the 2 study groups (Table 1; eTable 3 in Supplement 2). The median NIHSS score was 14 (IQR, 9-20), the median ASPECTS was 9 (IQR, 8-10), intravenous alteplase was administered to 69 patients (57%), and the median time from stroke onset to randomization was 306 (IQR, 228-609) minutes.
Table 1. Baseline Characteristics of Patients in the CHOICE Trial of Intra-arterial Alteplase.
| Characteristics | Alteplase (n = 61) | Placebo (n = 52) |
|---|---|---|
| Age, median (IQR), y | 73 (71-76) | 73 (69-67) |
| Race and ethnicity, No. (%) | ||
| White | 59 (97) | 48 (92) |
| Hispanic | 2 (3) | 3 (6) |
| Othera | 0 | 1 (2) |
| Sex, No. (%) | ||
| Female | 28 (46) | 24 (46) |
| Male | 33 (54) | 28 (54) |
| Medical history, No. (%) | ||
| Hypertension | 39 (64) | 34 (65) |
| Diabetes | 19 (31) | 11 (21) |
| Atrial fibrillation | 10 (16) | 9 (17) |
| Ischemic stroke or transient ischemic attack | 5 (8) | 4 (8) |
| NIHSS score at hospital arrival, median (IQR)b | 14 (8-20) | 14 (10-20) |
| Blood pressure at hospital arrival, median (IQR), mm Hg | ||
| Systolic | 139 (121-156) | 136 (113-155) |
| Diastolic | 73 (65-83) | 70 (61-78) |
| Glucose level at hospital arrival, median (IQR), mg/dL | 134 (108-164) | 119 (103-143) |
| Treatment with intravenous alteplase before randomization, No. (%)c | 38 (62) | 31 (60) |
| ASPECTS value, median (IQR)d | 9.0 (9.0-10.0) | 10.0 (8.0-10.0) |
| Baseline modified Rankin Scale score of 1, No. (%) | 9 (15) | 9 (17) |
| Location of intracranial occlusion on angiography, No. (%)e | ||
| Terminal internal carotid artery | 7 (12) | 4 (8) |
| Proximal, middle, or distal M1 | 19 (31) | 20 (39) |
| Proximal or distal M2 | 33 (54) | 28 (54) |
| Ipsilateral cervical carotid occlusion, No. (%) | 6 (10) | 4 (8) |
| Angiographic eTICI scores according to local investigators at randomization, No. (%)f | ||
| 2b50/67: 50%-89% reperfusion | 34 (56) | 31 (60) |
| 2c/3: 90%-100% reperfusion | 27 (44) | 21 (40) |
| Workflow times, median (IQR), min | ||
| Time from stroke onset to randomization | 306.0 (208.0-672.0) | 345.0 (237.0-609.0) |
| Time from stroke onset to start of study treatment | 315.0 (218.0-680.0) | 356.0 (260.5-635.0) |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; eTICI, expanded Treatment in Cerebral Ischemia; NIHSS, National Institutes of Health Stroke Scale; M1, main trunk of the middle cerebral artery; M2, first-order branch of the main trunk of the middle cerebral artery.
SI conversion factor: To convert the values for glucose to mmol/L, multiply by 0.0555.
The “other” category includes Asian and Black.
Scores on the NIHSS range from 0 to 42, with higher scores indicating more severe neurological deficits.
Intravenous alteplase was administered if indicated before randomization. Main contraindications were stroke onset more than 4.5 hours prior and intake of oral anticoagulants. Eleven patients received less than 50% of the estimated weight-adjusted dose (dose range, 5-35 mg).
ASPECTS is an imaging measure of the extent of ischemic stroke. Scores range from 0 to 10, with higher scores indicating a smaller infarct core. Values shown are as recalculated at the central core laboratory.
The location of the occlusion was not available for 2 patients in the alteplase group.
eTICI score ranges from 0 (0% reperfusion) to 3 (100% reperfusion). Investigators used the modified TICI (mTICI) scale for clinical decision making because it is easier to use; the core laboratory reanalyzed all mTICI scores and relabeled them according to the eTICI scale. Both scales lead to similar estimations of the degree of perfusion as used in the trial. For example, an mTICI score of 2b to 3 corresponds to an eTICI score of 2b50 to 3.
The median time from symptom onset to randomization was 318 (IQR, 229-641) minutes and from symptom onset to start of the study treatment was 328 (IQR, 240-676) minutes, without significant differences between the groups. General anesthesia was used in 5 patients (9.6%) in the placebo group and in 4 patients (8.0%) in the alteplase group (Table 1; eTable 3 in Supplement 2).
Intra-arterial alteplase was administered in 30 minutes to 19 patients (31%) and placebo to 19 patients (37%). The infusion was interrupted at 20 minutes because of angiographic improvement in 1 patient in each treatment group. Under amendment 3, the study treatment was administered in 15 minutes to 42 patients (69%) in the alteplase group and 33 patients (63%) in the placebo group.
Primary Efficacy Outcome
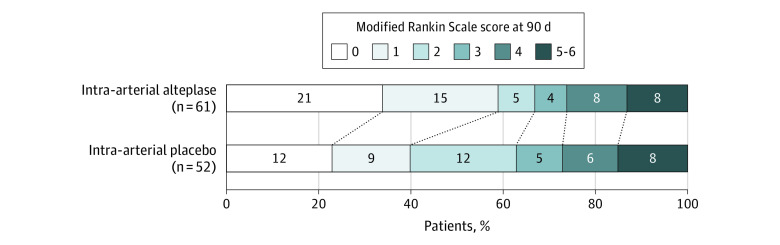
Treatment with intra-arterial alteplase was associated with a favorable outcome (a score of 0 or 1 on the modified Rankin Scale) at 90 days in 36 of 61 patients (59.0%) in the alteplase group and in 21 of 52 patients (40.4%) in the placebo group (adjusted risk difference, 18.4%; 95% CI, 0.3%-36.4%; P = .047) (Table 2 and Figure 2).
Table 2. Primary and Secondary Outcomes of the CHOICE Trial of Intra-arterial Alteplase.
| Outcomes | Alteplase (n = 61) | Placebo (n = 52) | Absolute risk difference, % (95% CI) | P valuea |
|---|---|---|---|---|
| Primary outcome | ||||
| Score of 0 or 1 on modified Rankin Scale at 90 d, No. (%) | 36 (59.0) | 21 (40.4) | 18.4 (0.3 to 36.4) | .047 |
| Secondary outcomes | ||||
| Improved angiographic eTICI score, No. (%) [n = 111]b | 5 (8.5) | 4 (7.7) | 0.6 (−9.5 to 10.7) | .91 |
| Excluding baseline eTICI scores of 3 | 5/44 (11.3) | 4/43 (9.3) | 1.8 (−11.0 to 14.5) | .78 |
| Modified Rankin Scale score at 90 d, No. (%)c | 1.54 (0.79 to 2.94)d | .38e | ||
| 0 | 21 (34.4) | 12 (23.1) | .18f | |
| 1 | 15 (24.6) | 9 (17.3) | ||
| 2 | 5 (8.2) | 12 (23.1) | ||
| 3 | 4 (6.6) | 5 (9.6) | ||
| 4 | 8 (13.1) | 6 (11.5) | ||
| 5-6 | 8 (13.1) | 8 (15.4) | ||
| Infarct expansion ratio, median (IQR) [n = 111]g | 2.0 (0.5-2.03) | 4.2 (0.8-46.1) | .27h | |
| Patients with infarct expansion, No. (%) [n = 110] | 39 (63.9) | 38 (74.5) | −8.9 (−25.6 to 7.9) | .31 |
| Infarct volume at 48 h, median (IQR), mL [n = 110] | 7.7 (3.7-29.3) | 12.7 (3.1-35.5) | .79h | |
| Tertiary outcomes | ||||
| Barthel Index of 95-100 at 90 d, No. (%) [n = 100]i | 38 (67.9) | 27 (61.4) | 6.4 (−12.3 to 25.0) | .60 |
| Ischemic worsening, No. (%) [n = 111]j | 1 (1.6) | 3 (5.8) | −4.9 (−18.0 to 8.3) | .32 |
| EQ-5D-3L score at 90 d, median (IQR) | ||||
| Visual analog scale [n = 97]k | 80 (60-90) | 80 (50-90) | .88h | |
| Overall [n = 97]l | 0.73 (0.49-1.00) | 0.74 (0.51-1.00) | .38h | |
Abbreviations: EQ-5D-3L, EuroQol Group 5-Dimension 3-Level Self-Report Questionnaire; eTICI, expanded Treatment in Cerebral Ischemia.
P values are from binomial regression model unless otherwise specified.
Score ranges from 0 (0% reperfusion) to 3 (100% reperfusion).
The modified Rankin Scale of functional disability ranges from 0 (no symptoms) to 6 (death). The primary analysis was adjusted by previous use of alteplase as predefined.
Odds ratio (95% CI) indicating the odds of a 1-point worsening on the modified Rankin Scale.
Shift analysis. For odds proportionality test, P = .08.
Van Elteren test.
Infarct expansion ratio refers to the ratio of final infarct volume to initial ischemic tissue volume.
Mann-Whitney test.
Scores on the Barthel Index range from 0 to 100, with higher values indicating good performance on activities of daily living. A score between 95 and 100 indicates no disability that interferes with daily activities. Included are patients who were alive at 90 days.
Ischemic worsening was defined as an increment of at least 4 points on the NIHSS at 48 to 72 hours.
The visual analog scale comprises a 100-mm line anchored at the opposing ends of perceived health-related quality of life.
Scores on the EQ-5D-3L have 3 levels of self-reported quality of life corresponding to no problems, some problems, and extreme problems.
Figure 2. Distribution of Functional Scores at 90 Days in the CHOICE Trial of Intra-arterial Alteplase.
Scores on the modified Rankin Scale for patients in the intra-arterial alteplase group (n = 61) and the placebo group (n = 52) who were evaluated by local investigators via face-to-face interview. Scores range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability (able to handle own affairs without assistance but unable to carry out all previous activities); 3, moderate disability (requiring some help, but able to walk unassisted); 4, moderately severe disability (unable to attend body needs and unable to walk); 5, severe disability (requiring constant nursing care and attention); and 6, death. Scores of 5 and 6 were combined for the analysis. Treatment with intra-arterial alteplase was associated with a favorable outcome (a score of 0 or 1 on the modified Rankin Scale) at 90 days, with an adjusted risk difference of 18.4% (95% CI, 0.3%-36.4%; P = .047). The difference between the intra-arterial alteplase group and the placebo group in the overall distribution of scores was not statistically significant (shift analysis, adjusted common odds ratio for worsening of 1 point on the modified Rankin Scale, 1.54; 95% CI, 0.79-2.94).
Results of analyses of randomized patients regardless of treatment and analyses regardless of protocol adherence are presented in eTable 4 in Supplement 2. The findings of the additional analyses were consistent with the results of the primary analysis, showing a more favorable outcome in the alteplase group.
Secondary and Tertiary Outcomes
Predefined secondary and tertiary clinical efficacy outcomes are shown in Table 2. According to core laboratory assessments, the overall proportion of participants with angiographic improvement in the eTICI score was 8.5% with alteplase and 7.7% with placebo (risk difference, 0.6%; 95% CI, −9.5% to 10.7%) and was 11.4% with alteplase and 9.3% with placebo (risk difference, 1.8%; 95% CI, −11.0% to 14.5%) after exclusion of patients with a pretreatment eTICI score of 3. There was no significant difference between treatment with intra-arterial alteplase vs placebo in the shift in the distribution of global disability scores on the modified Rankin Scale at 90 days (odds ratio, 1.54; 95% CI, 0.79-2.94), the infarct expansion ratio, the proportion of patients with infarct expansion, or the infarction volume at 48 hours after stroke onset. Also, there was no significant difference between treatment with intra-arterial alteplase vs placebo in the tertiary outcomes: the proportion of patients with a Barthel Scale score of 95 to 100 at day 90 (risk difference, 6.4%; 95% CI, −12.3% to 25.0%); the proportion with ischemic worsening (≥4-point increase in NIHSS score) within 48 to 72 hours of stroke onset (risk difference, −4.9%; 95% CI, −18.0% to 8.3%); and quality of life as measured with the EQ-5D-3L at 90 days (P = .88 and P = .38 for the visual analog scale score and the overall score, respectively).
The treatment effects on secondary brain imaging and clinical outcomes are shown in Table 2.
Subgroup and Sensitivity Analyses
Subgroup analyses are shown in the eFigure in Supplement 2. There were no statistically significant interactions in any of the tested subgroups.
The first sensitivity analysis (post hoc) for the primary outcome using other study populations and including center as a random effect and unadjusted models (eTable 4 in Supplement 2) showed that treatment with intra-arterial alteplase was associated with a favorable outcome compared with placebo, although some of the models did not reach statistical significance. The second post hoc sensitivity analysis using a series of models considering baseline covariates that by chance might have some unbalance (eTable 5 in Supplement 2) showed similar results and conclusions.
Adverse Events
At 90 days, death (modified Rankin Scale score of 6) occurred in 5 of 61 patients (8.2%) in the alteplase group and in 8 of 52 patients (15.4%) in the placebo group (risk difference, −7.3%; 95% CI, −19.3% to 4.6%) (Table 3).
Table 3. Adverse Events in the CHOICE Trial of Intra-arterial Alteplase.
| Outcomes | No. (%) of participants | |
|---|---|---|
| Alteplase (n = 61) | Placebo (n = 52) | |
| Primary safety outcomes | ||
| Symptomatic intracranial hemorrhage at 24 h | 0 | 2 (3.8) |
| Death at 90 d | 5 (8.2) | 8 (15.4) |
| Additional safety outcomes | ||
| Any serious adverse eventsa | 10 (16.4) | 15 (28.8) |
| Any cerebral hemorrhage | 19 (31.1) | 18 (34.6) |
| Hemorrhagic infarction | ||
| Type 1b | 11 (18.0) | 8 (15.4) |
| Type 2c | 1 (1.6) | 0 |
| Parenchymal hematoma | ||
| Type 1d | 0 | 0 |
| Type 2e | 2 (3.2) | 4 (7.7) |
| Remote | 1 (1.6) | 0 |
| Subarachnoid hemorrhage | 4 (6.6) | 6 (11.5) |
A serious adverse event was defined as an adverse event that leads to death or permanent impairment, is life-threatening, or causes or prolongs hospitalization.
Hemorrhagic infarction type 1: petechial hemorrhages at the infarct margins.
Hemorrhagic infarction type 2: petechial hemorrhages throughout the infarct and no mass effect.
Parenchymal hematoma type 1: 30% or less of the infarcted area and minor mass effect.
Parenchymal hematoma type 2: more than 30% of infarct zone and substantial mass effect.
Overall, any type of cerebral hemorrhage occurred in 19 of 61 patients (31.1%) in the alteplase group and in 18 of 52 patients (34.6%) in the placebo group (risk difference, −3.9%; 95% CI, −21.2% to 13.4%) (Table 3). Symptomatic intracranial hemorrhage (≥4-point increase in the NIHSS score) occurred in 0 of 61 patients (0%) in the alteplase group and in 2 of 52 patients (3.8%) in the placebo group (risk difference, −3.8%; 95% CI, −13.2% to 2.5%) (Table 3).
Serious adverse events during the 90-day follow-up period were numerically more frequent in the alteplase group than in the placebo group (Table 3). A complete list of adverse events is provided in eTables 6 and 7 in Supplement 2.
Discussion
In this trial of patients with large vessel occlusion acute ischemic stroke and successful reperfusion following thrombectomy, the use of adjunct intra-arterial alteplase resulted in a greater likelihood of excellent neurological outcome at 90 days compared with placebo. However, because of study limitations, these findings should be interpreted as preliminary and require replication.
Adjunct administration of intra-arterial alteplase at the end of the endovascular procedure resulted in improved clinical outcome despite only minor differences between the treatment groups in angiographic scores or in other surrogate imaging. This suggests that the improved functional outcome may be explained by an amelioration in the microcirculatory reperfusion.18 Recently, perfusion imaging studies performed in patients with large vessel occlusion acute ischemic stroke at the end of successful thrombectomies showed a high prevalence of around 40% of areas of hypoperfusion in patients with normal angiogram findings,19,20 highlighting the limitations of conventional angiography to ensure the adequacy of perfusion in the microcirculation. In these studies, the identification of areas of hypoperfusion was clinically relevant,20 as patients with areas of hypoperfusion had a more limited functional recovery than patients with normal perfusion imaging findings at the end of thrombectomy.
Possible explanations for the findings in the current trial include easier access of the drug and more effective thrombolytic activity within the microcirculation, not impeded by the coexistence of more proximal occlusions, as would be expected to occur in patients with worse angiographic scores after thrombectomy. The results of an ancillary study with perfusion-weighted MRI and MR spectroscopy at 48 hours after therapy may provide insights into the effects of alteplase on the microcirculation and on surrogate imaging biomarkers.
The study supports the safety of intra-arterial alteplase infusion for 15 to 30 minutes at a dose of 0.225 mg/kg (maximum dose, 22.5 mg) and including patients who, unlike in previous observational nonrandomized studies,21,22 could also have received preceding intravenous thrombolysis. There are no clear predictors for postthrombectomy symptomatic intracranial hemorrhage,23 and the low rate of bleeding complications in both treatment groups precluded analysis. The finding of a low bleeding rate was reassuring and consistent with recent randomized clinical trials24,25 and case series26,27 supporting that pharmacological thrombolysis did not increase the risk of hemorrhagic complications after thrombectomy.
Current guidelines recommend that all eligible patients receive intravenous alteplase before thrombectomy,1 and the results of this trial do not contradict this recommendation. The study results support the safety of adjunct intra-arterial alteplase in patients with successful reperfusion at the end of thrombectomy, including in patients treated previously with intravenous alteplase, although the findings on effectiveness should be interpreted as preliminary, requiring replication before any recommendations for practice change.
Limitations
This trial has several limitations. First, the study was terminated early because the COVID-19 pandemic reduced the admission of eligible patients at the study sites,28 and the placebo could not be restocked after its shelf-life expiration. Because of this, the sample size was only 60% of what had been planned. This likely led to a loss of power to assess secondary and tertiary outcomes as well as interactions in the subgroup analyses. It rendered the study more vulnerable to the potential imbalance of prognostic factors. Randomized clinical trials stopped early for benefit systematically overestimate treatment effects29; while that is possible in the current study, the trial was not stopped early for clinical benefit but instead for logistic reasons.
Second, any conclusions about benefit should not be based solely on the point estimate for the effect size, but need to consider the very wide confidence interval, with a lower confidence limit for the adjusted absolute risk difference of 0.3%.
Third, the population included in the study represented only 7% of the total population treated with thrombectomy in Catalonia during the study period, raising a concern about generalizability of the findings. A major reason for exclusion from the study was contraindication to use of alteplase, usually due to recent intake of oral anticoagulants. Fourth, although it has been suggested that shift analysis improves the overall study power compared with a dichotomized analysis, this is true when treatment effects are small and, in particular, uniformly distributed over all respective ranges of stroke severity.30 Dichotomization at excellent outcome levels was a more powerful approach in early recanalization trials.30 A large effect size concentrated on the milder range of the scale (score of 0 or 1) was consistent with the hypothesis of restoring microvascular patency to induce secondary parenchymal neuroprotection, unlike a major neurological improvement (Lazarus effect), which would be associated with recanalization of large proximal vessels. Larger studies are needed to refine the true effect of adjunct intra-arterial thrombolysis and also to assess efficacy in secondary clinical outcomes and surrogate imaging end points.
Conclusions
Among patients with large vessel occlusion acute ischemic stroke and successful reperfusion following thrombectomy, the use of adjunct intra-arterial alteplase compared with placebo resulted in a greater likelihood of excellent neurological outcome at 90 days. However, because of study limitations, these findings should be interpreted as preliminary and require replication.
Trial Protocol
List of Sites, Investigators, and Administrative Staff
CHOICE Trial Data and Safety Monitoring Board (DSMB) Charter
eAppendix. Supplementary Methods
eTable 1. Inclusion and Exclusion Criteria and Summary of Protocol Amendments
eTable 2. Timing of Study Procedures
eTable 3. Additional Baseline Characteristics of the 121 Patients
eTable 4. First Post-Hoc Sensitivity Analysis for the Primary Outcome (Score of 0 to 1 in the Modified Rankin Scale at 90 Days): Analyses Using Other Populations and Statistical Strategies, Including Center as a Random Effect and Unadjusted Models
eTable 5. Second Post-Hoc Sensitivity Analysis for the Primary Outcome (Score of 0 to 1 in the Modified Rankin Scale at 90 Days): Series of Models Considered Imbalances in Baseline Variables
eTable 6. Other Adjudicated Serious Adverse Events by Treatment Groups
eTable 7. Other Adverse Events Reported by Local Investigators by Treatment Groups
eFigure. Subgroup Analyses
Statistical Analysis Plan
Nonauthor Collaborators. CHOICE Investigators
Data Sharing Statement
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading Collaborators; STIR Revascularization Working Group; STIR Thrombolysis in Cerebral Infarction Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4.Ames A III, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia, II: the no-reflow phenomenon. Am J Pathol. 1968;52(2):437-453. [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178-201. doi: 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Scalzo F, Liebeskind DS. Perfusion angiography in acute ischemic stroke. Comput Math Methods Med. 2016;2016:2478324. doi: 10.1155/2016/2478324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab. 2021;41(2):253-266. doi: 10.1177/0271678X20954929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desilles JP, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46(11):3241-3248. doi: 10.1161/STROKEAHA.115.010721 [DOI] [PubMed] [Google Scholar]
- 9.Mattle HP, Arnold M, Georgiadis D, et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke. 2008;39(2):379-383. doi: 10.1161/STROKEAHA.107.492348 [DOI] [PubMed] [Google Scholar]
- 10.Renú A, Blasco J, Millán M, et al. ; CHOICE Investigators . The Chemical Optimization of Cerebral Embolectomy trial: study protocol. Int J Stroke. 2021;16(1):110-116. doi: 10.1177/1747493019895656 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-2246. doi: 10.1161/01.STR.0000027437.22450.BD [DOI] [PubMed] [Google Scholar]
- 13.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 14.Chamorro Á, Blasco J, López A, et al. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci Rep. 2017;7(1):11636. doi: 10.1038/s41598-017-11946-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheth SA, Lee S, Warach SJ, et al. Sex differences in outcome after endovascular stroke therapy for acute ischemic stroke. Stroke. 2019;50(9):2420-2427. doi: 10.1161/STROKEAHA.118.023867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamorro Á, Brown S, Amaro S, et al. ; HERMES Collaborators . Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke. 2019;50(3):690-696. doi: 10.1161/STROKEAHA.118.023769 [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 18.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. 2012;32(12):2091-2099. doi: 10.1038/jcbfm.2012.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosior JC, Buck B, Wannamaker R, et al. Exploring reperfusion following endovascular thrombectomy. Stroke. 2019;50(9):2389-2395. doi: 10.1161/STROKEAHA.119.025537 [DOI] [PubMed] [Google Scholar]
- 20.Rubiera M, Garcia-Tornel A, Olivé-Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke. 2020;51(6):1736-1742. doi: 10.1161/STROKEAHA.120.029212 [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AI, Suri MF, Shatla AA, et al. Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen. Neurosurgery. 2000;47(2):473-476. [PubMed] [Google Scholar]
- 22.Broderick JP, Palesch YY, Demchuk AM, et al. ; Interventional Management of Stroke III Investigators . Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893-903. doi: 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueira RG, Gupta R, Jovin TG, et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: a multicenter retrospective analysis of 1122 patients. J Neurointerv Surg. 2015;7(1):16-21. doi: 10.1136/neurintsurg-2013-010743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian B, Tian X, Shi Z, et al. ; DIRECT-MT Investigators . Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular thrombectomy. Stroke. Published online December 7, 2021. doi: 10.1161/STROKEAHA.121.035425 [DOI] [PubMed] [Google Scholar]
- 25.Tomsick T, Broderick J, Carrozella J, et al. ; Interventional Management of Stroke II Investigators . Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29(3):582-587. doi: 10.3174/ajnr.A0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon W, Park MS, Cho KH. Low-dose intra-arterial urokinase and aggressive mechanical clot disruption for acute ischemic stroke after failure of intravenous thrombolysis. AJNR Am J Neuroradiol. 2010;31(1):161-164. doi: 10.3174/ajnr.A1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery. 2002;51(5):1319-1327. doi: 10.1097/00006123-200211000-00040 [DOI] [PubMed] [Google Scholar]
- 28.Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke. 2020;51(7):1991-1995. doi: 10.1161/STROKEAHA.120.030329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassler D, Briel M, Montori VM, et al. ; STOPIT-2 Study Group . Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303(12):1180-1187. doi: 10.1001/jama.2010.310 [DOI] [PubMed] [Google Scholar]
- 30.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72(15):1310-1315. doi: 10.1212/01.wnl.0000341308.73506.b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
List of Sites, Investigators, and Administrative Staff
CHOICE Trial Data and Safety Monitoring Board (DSMB) Charter
eAppendix. Supplementary Methods
eTable 1. Inclusion and Exclusion Criteria and Summary of Protocol Amendments
eTable 2. Timing of Study Procedures
eTable 3. Additional Baseline Characteristics of the 121 Patients
eTable 4. First Post-Hoc Sensitivity Analysis for the Primary Outcome (Score of 0 to 1 in the Modified Rankin Scale at 90 Days): Analyses Using Other Populations and Statistical Strategies, Including Center as a Random Effect and Unadjusted Models
eTable 5. Second Post-Hoc Sensitivity Analysis for the Primary Outcome (Score of 0 to 1 in the Modified Rankin Scale at 90 Days): Series of Models Considered Imbalances in Baseline Variables
eTable 6. Other Adjudicated Serious Adverse Events by Treatment Groups
eTable 7. Other Adverse Events Reported by Local Investigators by Treatment Groups
eFigure. Subgroup Analyses
Statistical Analysis Plan
Nonauthor Collaborators. CHOICE Investigators
Data Sharing Statement