This cohort study examines the association between maternal immunization with the BNT162b2 COVID-19 vaccine during pregnancy and neonatal and early infant outcomes.
Key Points
Question
Is prenatal exposure to maternal BNT162b2 messenger RNA COVID-19 vaccine associated with adverse outcomes at birth or in early childhood?
Findings
In a population-based study including 24 288 singleton live births, the risks of preterm birth and small birth weight were similar between newborns prenatally exposed and unexposed to maternal vaccination.
Meaning
Maternal BNT162b2 vaccination in pregnancy was not associated with detrimental outcomes to the offspring.
Abstract
Importance
Pregnant women were excluded from the BNT162b2 messenger RNA (mRNA) COVID-19 vaccine (Pfizer-BioNTech) preauthorization trial. Therefore, observational data on vaccine safety for prenatally exposed newborns are critical to inform recommendations on maternal immunization.
Objective
To examine whether BNT162b2 mRNA vaccination during pregnancy is associated with adverse neonatal and early infant outcomes among the newborns.
Design, Setting, and Participants
Population-based cohort study comprising all singleton live births in March through September 2021, within a large state-mandated health care organization in Israel, followed up until October 31, 2021.
Exposure
Maternal BNT162b2 mRNA vaccination during pregnancy.
Main Outcomes and Measures
Risk ratios (RR) of preterm birth, small birth weight for gestational age (SGA), congenital malformations, all-cause hospitalizations, and infant death. Stabilized inverse probability weighting was used to adjust for maternal age, timing of conception, parity, socioeconomic status, population subgroup, and maternal influenza immunization status.
Results
The cohort included 24 288 eligible newborns (49% female, 96% born at ≥37 weeks’ gestation), of whom 16 697 were exposed (n = 2134 and n = 9364 in the first and second trimesters, respectively) to maternal vaccination in utero. Median (IQR) follow-up after birth was 126 days (76-179) among exposed and 152 days (88-209) among unexposed newborns. No substantial differences were observed in preterm birth rates between exposed and unexposed newborns (RR = 0.95; 95% CI, 0.83-1.10) or SGA (RR = 0.97; 95% CI, 0.87-1.08). No significant differences were observed in the incidence of all-cause neonatal hospitalizations (RR = 0.99; 95% CI, 0.88-1.12), postneonatal hospitalizations after birth (RR = 0.95; 95% CI, 0.84-1.07), congenital anomalies (RR = 0.69; 95% CI, 0.44-1.04), or infant mortality over the study period (RR = 0.84; 95% CI, 0.43-1.72).
Conclusions and Relevance
This large population-based study found no evident differences between newborns of women who received BNT162b2 mRNA vaccination during pregnancy, vs those of women who were not vaccinated, and contributes to current evidence in establishing the safety of prenatal vaccine exposure to the newborns. Interpretation of study findings is limited by the observational design.
Introduction
COVID-19 vaccination during pregnancy plays a crucial role in preventing maternal illness.1 Safety concerns are reported as 1 of the top reasons to decline COVID-19 vaccination during pregnancy.2 The exclusion of pregnant women from the initial COVID-19 vaccine trials led to information gaps regarding maternal and fetal response to vaccination. While several observational studies examined neonatal benefits via antibodies transfer across the placenta,3,4 large-scale assessments of neonatal safety are scarce.
On December 20, 2020, a national campaign for vaccination against COVID-19 was initiated in Israel. Although pregnant women were not excluded from receiving the vaccine, the majority of pregnant women who were vaccinated early were those with maximal occupational exposure risk (mainly medical or educational staff) or underlying comorbidities. On January 19, 2021, during the surge of a third wave in the pandemic, the Israel Ministry of Health released updated recommendations that encouraged all pregnant women to receive the vaccine.5
Two descriptive studies provide preliminary information on neonatal outcomes after COVID-19 vaccination during pregnancy.6,7 Both studies relied on self-reported questionnaires, which may bear selection bias and respondent bias, and were characterized by small sample size, short time frame, and lack of a comparison group of unexposed neonates. One of the studies7 showed lower reactogenicity of the messenger RNA (mRNA) vaccine in pregnant women compared with nonpregnant women. This study aims to examine the association between maternal immunization during pregnancy and neonatal and early infant outcomes using a large population-based cohort.
Methods
Setting
Data were analyzed from the comprehensive database of the Maccabi Healthcare Services, a 2.5-million-member state-mandated health fund in Israel. All Israeli citizens are required by law to choose 1 of 4 nationwide health funds. The Maccabi health fund members represent 26.7% of the population and share similar sociodemographic characteristics with the overall Israeli population. The fund has maintained a computerized database of electronic health records since 1993, containing extensive longitudinal data on a stable population (~1% annual turnover). The study protocol was approved by the Maccabi Healthcare Services institutional review board, and informed consent was waived because only deidentified routinely collected data were used. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The health fund has developed several computerized registries of major clinical conditions. These registries are continuously updated and can detect relevant patients by automated criteria (relying on coded diagnoses, extensive laboratory data, treatments, administrative billing codes, etc), rather than depending on active reporting by physicians. Pregnancy data routinely coded by the patient’s gynecologist on a designated pregnancy-tracking form within the electronic health record were used to construct a pregnancy registry. In 2020, the fund’s pregnancy registry included data on approximately 55 000 new pregnancies, including 40 000 live births, accounting for 24% of all live births in Israel.8
Study Population
The eligible study population included all singleton live births at any time from March 1, 2021, through September 31, 2021. Records with no mother-offspring linkage were excluded because it was not feasible to assess their prenatal exposure status.
Nonsingleton births are closely linked with the outcomes of preterm birth and low infant weight, which would require adjusted or stratified analyses. The relatively small numbers of multiple births preclude such analyses; thus, they were a priori excluded from the study cohort (3.6% excluded in the group prenatally exposed to maternal vaccination, 3.5% in the group unexposed to maternal vaccination).
Vaccine uptake among pregnant women was very low before January 19, 2021 (the date of the local Ministry of Health recommendations5) and surged thereafter (eFigure 1 in the Supplement). Consequently, births during January 2021 and February 2021 with prenatal vaccination were biased toward preterm births and were therefore not included.
For each newborn, follow-up for outcomes after birth lasted from birth to the earliest occurrence of 1 of the following: an outcome of interest, leaving the fund, or the end of the study period, which was October 31, 2021 (ie, minimum follow-up of 1 month).
Baseline Covariates
Parity before the current birth was categorized into nulliparous vs para 1 or more. Population subgroups included secular Jewish, ultra-Orthodox Jewish, and Israeli Arab. Seasonal influenza vaccination during the preceding year was extracted as a proxy for health-seeking behavior.1 Preexisting comorbidities were defined in accordance with previous work1 by automated registries according to carefully validated codes for inclusion and exclusion criteria (including diagnoses, treatments, and laboratory data). Infertility was defined by diagnoses or medications, ovarian stimulation procedures, or receipt of donated egg. High-risk pregnancy was defined by obstetric diagnoses using V23.x codes from the International Classification of Diseases, Ninth Revision (ICD-9). Residential socioeconomic status was a score ranked from 1 (lowest) to 10 derived by the Israel Central Bureau of Statistics.9,10
Primary Outcomes
The prespecified primary outcomes of this study were proportions of preterm births and newborns who were small for gestational age (SGA) among live births. Gestational age and infant weight were extracted via the fund’s pregnancy registry as well as data linkage with the Israeli Ministry of Health database of live-birth certificates.
Gestational age was categorized into extremely or very preterm birth (<32 weeks), late preterm (32-36 weeks), and term birth (37+ weeks) in accordance with the World Health Organization definitions.11 An infant was SGA if body weight at birth was less than the sex-specific 10th percentile for gestational age, accounting for singleton and multiple pregnancies, according to Dolberg et al.12 Birth weight was also examined via binary variables of low birth weight (<2500 g vs ≥2500 g) and very low birth weight (<1500 g vs ≥1500 g).
Exploratory Outcomes
Outcomes after birth were examined as exploratory outcomes and included inpatient hospitalizations, recorded congenital anomalies, jaundice requiring phototherapy, and all-cause death over the study period.
Neonatal hospital admissions included all hospitalization records starting at least 1 day after birth date and until 28 days of age. Postneonatal hospitalizations included all hospitalization records dated at least 28 days after birth.
Congenital malformations (CM) were defined by recorded diagnoses within 1 month from birth either from hospitals or the community. Diagnoses were defined according to the Israeli Ministry of Health dictionary for birth defects, using the following ICD-9 codes: nervous system (740, 741, 741.9, 742, 742.2-4, 742.9), eye (743, 743.1-3, 743.45-46, 743.9), orofacial (744.01, 744.23, 744.29, 749, 749.1-2), cardiovascular (745, 745.1-4, 745.6, 746, 746.1-3, 746.7, 746.81, 746.86, 747.22, 747.3, 747.41), respiratory system (748, 748.5, 748.9), gastrointestinal (750.11, 750.3, 751.1-2, 751.9), genitourinary (752.6-7, 753.1-2, 753.5, 753.9), musculoskeletal (754.51, 755, 755.1, 755.21-22, 755.26, 755.29, 755.31, 755.34-5, 756, 756.4, 756.6-7, 756.9), and chromosomal anomalies (758, 758.1-2, 758.9). In addition, the specific subgroup of cardiovascular malformations was reported separately, including a further drill-down denoting major heart malformations, in which common yet mostly minor anomalies were excluded (ventricular septal defect and patent foramen ovale). Phototherapy was extracted by ICD-9 code 99.38.
Statistical Analysis
Descriptive statistics were generated with mean (SD) and median (IQR) for normally and nonnormally distributed continuous covariates, respectively, and percentages for categorical covariates. Stabilized inverse probability of treatment weights (IPTW) were used to balance the groups with regards to baseline covariates,13 based on propensity scores representing the probability of COVID-19 vaccination during pregnancy. The propensity scores were estimated using a logistic regression model that included maternal age, conception timing, parity, seasonal influenza vaccination, population subgroup, and socioeconomic status. Absolute standardized mean differences (SMD) were used to assess baseline balance, where 0.1 or less was considered to indicate a well-balanced covariate.
Comparisons between exposed and nonexposed newborns were analyzed with Wilcoxon rank sum test and χ2 test with Rao and Scott second-order correction for weighted data, for continuous and categorical variables, respectively. As is customary in safety studies, no multiplicity adjustment was performed, to avoid type II error inflation. To avoid a fixed cohort bias, outcomes measured at birth (preterm birth and SGA) were calculated among gestations with a last menstruation date 42 weeks (or more) before the end of the study period.
Risk ratios (RR) for all outcomes were calculated via Poisson regression with the logarithm of person-days from birth as offset. Infant mortality over time from birth was depicted using IPTW Kaplan-Meier survival curves and compared using IPTW log-rank test.
A subgroup analysis was conducted to examine potential risk modification by trimester at vaccination. The subgroup analysis was conducted among gestations exposed in the first trimester or unexposed during pregnancy. Considering the later conception for those exposed during the first trimester, the unexposed group was restricted to gestations with month of conception in the range of the exposed group months of conception (September 2020 and later), and the groups were reweighted to ensure balance. Comparative incidence of congenital anomalies was conducted only among this subgroup because the second- and third-trimester exposure groups are unlikely to be in the risk window for congenital anomaly.14 A further sensitivity analysis was conducted to compare all outcomes among cohort participants without documented SARS-CoV-2 infection before birth.
Assuming a power of 80%, statistical significance level of α = .05, 2-sided tests, a preterm rate of 4 per 100 births, and an SGA rate of 6 per 100 births, this study was powered to detect a risk difference of 1 per 100 for both primary outcomes. All analyses were performed using R version 4.0.2.15
Results
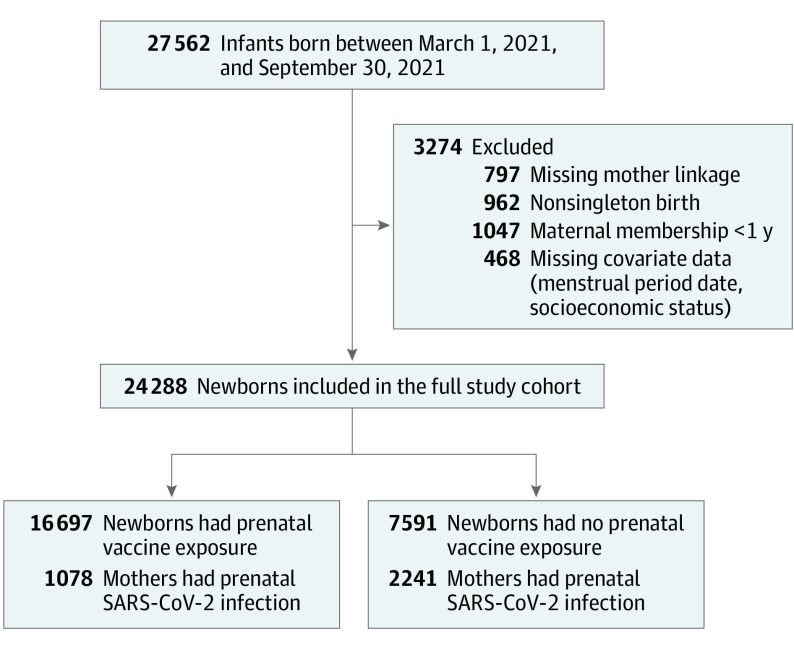
A total of 27 562 eligible infants were identified; 12% were excluded because of missing maternal linkage, multiple births, insufficient prior membership time, or missing covariate data (Figure 1). The excluded newborns included 3 death cases; all were twin births, and all were unexposed to prenatal vaccination.
Figure 1. Flow of Newborn Selection for Cohort Study of Prenatal Exposure to BNT162b2 Vaccination.
After exclusion, a total of 24 288 newborns remained eligible for analysis, of whom 16 697 were prenatally exposed to vaccination. Compared with unexposed infants, exposed infants were characterized by older maternal age at birth, higher seasonal influenza vaccine uptake, lower likelihood of belonging to a population minority subgroup, and greater likelihood of residing in more affluent areas (Table 1).
Table 1. Baseline Characteristics of Mothers With a Singleton Live Birth by Exposure to BNT162b2 Vaccination During Pregnancy.
| Pre-IPTWa | Post-IPTW | |||||
|---|---|---|---|---|---|---|
| Unvaccinated (n = 7591) | Vaccinated (n = 16 697) | Absolute SMDb | Unvaccinated (n = 7452) | Vaccinated (n = 16 738) | Absolute SMDb | |
| Maternal age, mean (SD), y | 30.49 (5.67) | 31.61 (5.22) | 0.21 | 31.1 (5.6) | 31.2 (5.3) | 0.02 |
| Infant sex, No. (%) | 0.01 | 0.01 | ||||
| Female | 3729 (49.1) | 8111 (48.6) | 3684 (49.4) | 8160 (48.8) | ||
| Male | 3862 (50.9) | 8586 (51.4) | 3768 (50.6) | 8578 (51.2) | ||
| Parity, No. (%) | 5107 (67.3) | 11 142 (66.7) | 0.01 | 5013 (67.3) | 11 212 (67.0) | 0.01 |
| Trimester at first vaccine dose (wk), No. (%) | ||||||
| First (<14) | 2134 (12.8) | 1851 (11.1) | ||||
| Second (14-26) | 9364 (56.1) | 8996 (53.7) | ||||
| Third (≥27) | 5199 (31.1) | 5891 (35.2) | ||||
| Socioeconomic status, mean (SD)c | 5.17 (1.98) | 6.13 (2.02) | 0.48 | 5.7 (2.04) | 5.8 (2.07) | 0.04 |
| Population subgroup, No. (%)d | 0.35 | 0.03 | ||||
| Arab | 830 (10.9) | 714 (4.3) | 495 (6.6) | 1072 (6.4) | ||
| Jewish, secular | 4847 (63.9) | 13 145 (78.7) | 5407 (72.6) | 12 328 (73.7) | ||
| Ultra-Orthodox | 1914 (25.2) | 2838 (17.0) | 1550 (20.8) | 3338 (19.9) | ||
| Preexisting maternal comorbidities, No. (%) | ||||||
| Obesity (BMI ≥30) | 862 (11.4) | 1768 (10.6) | 0.03 | 828 (11.1) | 1780 (10.6) | 0.02 |
| Infertility | 84 (1.1) | 304 (1.8) | 0.06 | 129 (1.7) | 260 (1.6) | 0.02 |
| Cancer | 55 (0.7) | 168 (1.0) | 0.03 | 66 (0.9) | 158 (0.9) | 0.01 |
| Hypertension | 76 (1.0) | 159 (1.0) | 0.01 | 74 (1.0) | 158 (0.9) | 0.01 |
| Chronic kidney disease | 67 (0.9) | 118 (0.7) | 0.02 | 75 (1.0) | 114 (0.7) | 0.04 |
| Diabetes | 59 (0.8) | 145 (0.9) | 0.01 | 72 (1.0) | 133 (0.8) | 0.02 |
| Cardiovascular disease | 2 (<0.1) | 8 (<0.1) | 0.01 | 2 (<0.1) | 8 (<0.1) | 0.01 |
| Chronic obstructive pulmonary disease | 2 (<0.1) | 3 (<0.1) | 0.01 | 1 (<0.1) | 3 (<0.1) | 0.00 |
| Smoking | 0.09 | 0.083 | ||||
| Ever | 441 (5.8) | 798 (4.8) | 470 (6.3) | 793 (4.7) | ||
| Never | 7099 (93.5) | 15 865 (95.0) | 6937 (93.1) | 15 909 (95.1) | ||
| Missing | 51 (0.7) | 34 (0.2) | 45 (0.6) | 36 (0.2) | ||
| Maternal SARS-CoV-2 infection | ||||||
| Before birth | 2241 (29.5) | 1078 (6.5) | 0.63 | 2255 (30.3) | 1108 (6.6) | 0.64 |
| Preconception | 383 (5.0) | 354 (2.1) | 0.16 | 448 (6.0) | 358 (2.1) | 0.20 |
| Seasonal influenza vaccine | 1535 (20.2) | 7131 (42.7) | 0.50 | 2469 (33.1) | 5924 (35.4) | 0.05 |
| Pertussis vaccine | 4698 (61.9) | 13 949 (83.5) | 0.50 | 4902 (65.8) | 13 663 (81.6) | 0.37 |
| Gestational diabetes | 156 (2.1) | 314 (1.9) | 0.01 | 159 (2.1) | 315 (1.9) | 0.02 |
| High-risk pregnancy | 842 (11.1) | 1786 (10.7) | 0.01 | 842 (11.3) | 1760 (10.5) | 0.03 |
| Calendar conception median (IQR), mo | 8 (6-10) | 9 (7-11) | 0.29 | 9 (7-11) | 9 (7-11) | 0.06 |
Abbreviations: BMI, body mass index; IPTW, inverse probability of treatment weights; SMD, standardized mean difference.
Propensity adjustment via IPTW was used to balance groups in terms of maternal age, conception timing, parity, seasonal influenza vaccination, population subgroup, and socioeconomic status. Post-IPTW numbers slightly differ from crude pre-IPTW because of propensity weighting.
SMD is the difference between the groups’ means divided by the pooled SD; <0.1 is considered a minor difference.
Residential area socioeconomic status was a score ranked from 1 (lowest) to 10 derived by the Israel Central Bureau of Statistics census data.
Population subgroup was assessed by enumeration areas (the Israeli census smallest unit of analysis) with a high proportion of Jewish Orthodox and Israeli Arab residents according to voting results, spatial presence of religious schools, religious centers such as synagogues or mosques, and other publicly available databases.
Comparison of propensity scores distribution between the groups indicated substantial overlap before weighting and improved balance after weighting (eFigure 2 in the Supplement). The mean (SD) of the stabilized IPTWs was 1.00 (0.28) in exposed newborns and 0.98 (0.59) in unexposed newborns. The weights varied between a minimum and maximum of 0.70 to 3.66 for exposed newborns and 0.37 to 7.04 for unexposed newborns. A total of 2.3% of the observations were assigned a weight >2.0, and 2.72% had weight <0.5.
After weighting, most baseline maternal characteristics were well balanced (SMD < 0.1, Table 1). Pertussis immunization remained higher (SMD = 0.37) and SARS-CoV-2 infection remained lower in exposed infants (SMD = 0.64) as compared with unexposed.
Primary Outcomes
Table 2 depicts the crude as well as adjusted group-specific proportions of all outcomes. There were no substantial differences between groups in the proportion of newborns with preterm birth (RR = 0.95; 95% CI, 0.83-1.10) or SGA (RR = 0.97; 95% CI, 0.87-1.08).
Table 2. Neonatal and Early Infant Outcomes.
| Pre-IPTW | Post-IPTWa | |||||
|---|---|---|---|---|---|---|
| Unvaccinated (n = 7591) | Vaccinated (n = 16 697) | Risk ratio (95% CI) | Unvaccinated (n = 7452) | Vaccinated (n = 16 738) | Risk ratio (95% CI) | |
| Follow-up time, d | 152 (88-209) | 126 (76-179) | 130 (71-197) | 134 (81-185) | ||
| Gestational age at delivery, No.(%) | ||||||
| <37 wk (Overall preterm) | 315 (4.1) | 730 (4.4) | 1.10 (0.95-1.27) | 358 (4.8) | 699 (4.2) | 0.95 (0.83-1.10) |
| <32 wk (Early preterm) | 48 (0.6) | 60 (0.4) | 0.52 (0.33-0.82) | 62 (0.8) | 60 (0.4) | 0.45 (0.29-0.70) |
| 32-36 wk (Late preterm) | 267 (3.5) | 670 (4.0) | 1.18 (1.02-1.38) | 296 (4.0) | 638 (3.8) | 1.03 (0.89-1.20) |
| Birth weight, No. (%) | ||||||
| SGA | 468 (6.7) | 1040 (6.6) | 0.98 (0.88-1.09) | 473 (6.9) | 1053 (6.7) | 0.97 (0.87-1.08) |
| Low birth weight, <2500 g | 324 (4.6) | 730 (4.6) | 0.98 (0.86-1.13) | 352 (5.1) | 705 (4.5) | 0.89 (0.78-1.01) |
| Very low birth weight, <1500 g | 43 (0.6) | 47 (0.3) | 0.44 (0.27-0.70) | 53 (0.8) | 49 (0.3) | 0.41 (0.26-0.65) |
| Unknown, No. | 619 | 833 | 561 | 932 | ||
| All-cause hospitalizations, No. (%) | ||||||
| Neonatal (1-28 d after birth) | 416 (5.5) | 916 (5.5) | 1.00 (0.89-1.13) | 408 (5.5) | 911 (5.4) | 0.99 (0.88-1.12) |
| Postneonatal (>28 d after birth) | 475 (6.3) | 777 (4.7) | 0.87 (0.78-0.98) | 398 (5.3) | 846 (5.1) | 0.95 (0.84-1.07) |
| Phototherapy | 71 (0.9) | 205 (1.2) | 1.31 (1.01-1.73) | 73 (1.0) | 203 (1.2) | 1.24 (0.95-1.63) |
| Infant death over the study period | 8 (0.1) | 22 (0.1) | 1.43 (0.66-3.43) | 13 (0.2) | 24 (0.1) | 0.84 (0.43-1.72) |
Abbreviations: IPTW, inverse probability of treatment weights; SGA, small for gestational age.
IPTW was used to balance groups in terms of maternal age, conception timing, parity, seasonal influenza vaccination, population subgroup, and socioeconomic status. Post-IPTW numbers slightly differ from crude pre-IPTW because of propensity weighting.
Exploratory Outcomes
The weighted length of follow-up after birth was similar between the groups with median (IQR) of 134 days (81-185) in exposed infants vs 130 days (71-197) in unexposed (P = 0.64). The risk of hospitalizations in exposed as compared with unexposed newborns was similar with RR = 0.99 (95% CI, 0.88-1.12) for neonatal and RR = 0.95 (95% CI, 0.84-1.07) for postneonatal hospitalizations. No significant difference was observed in the rate of phototherapy (RR = 1.24, 95% CI, 0.95-1.63).
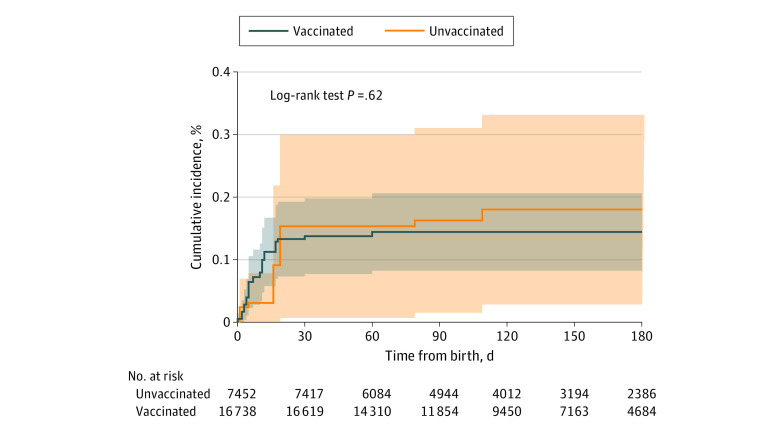
Overall infant mortality rates were low in both groups, with crude counts of n = 8 death cases (0.1%) among unexposed newborns and n = 22 (0.1%) in the exposed group. Among them, n = 4, n = 14, and n = 4 were exposed in the first, second, and third trimester, respectively. Among the death cases, 5 of 8 were preterm births in the unexposed group, and 12 of 22 were preterm births in the exposed group. Cumulative incidence over time is shown in Figure 2.
Figure 2. Cumulative Incidence of Mortality by Exposure to BNT162b2 Vaccination During Pregnancy.
Kaplan-Meier cumulative probability of mortality among infants exposed and unexposed to BNT162b2 vaccination during pregnancy. Follow-up initiated on birth. Number at risk is depicted at each month since initiation of follow-up. Shading indicates 95% CIs.
Subgroup Analysis Among First Trimester Vaccination vs No Vaccination During Pregnancy
After IPTW, there were n = 2021 newborns exposed in the first trimester and n = 3580 unexposed newborns (Table 3). No difference was observed in the proportion of preterm births (RR = 0.87; 95% CI, 0.67-1.12), with early preterm rates of 0.8% vs 1.3% and moderate/late preterm rates of 5.4% vs 5.3% in the exposed and unexposed groups, respectively. No difference was observed between the groups in the proportion of SGA (7.9% vs 6.9%; RR = 1.14; 95% CI, 0.92-1.40).
Table 3. Neonatal and Early Infant Outcomes for First Trimester Vaccinationa.
| Pre IPTW | Post IPTWb | |||||
|---|---|---|---|---|---|---|
| Unvaccinated (n = 3584) | Vaccinated (n = 2134) | Risk ratio (95% CI) | Unvaccinated (n = 3570) | Vaccinated (n = 2032) | Risk ratio (95% CI) | |
| Follow-up time, d | 86 (57-116) | 55 (42-71) | 73 (48-105) | 63 (48-83) | ||
| Gestational age at delivery, No. (%) | ||||||
| <37 wk (Overall preterm) | 218 (6.1) | 158 (7.9) | 1.15 (0.91-1.46) | 236 (6.6) | 126 (6.2) | 0.87 (0.67-1.12) |
| <32 wk (Early preterm) | 40 (1.1) | 19 (0.9) | 0.39 (0.15-0.88) | 47 (1.3) | 16 (0.8) | 0.24 (0.07-0.61) |
| 32-36 wk (Late preterm) | 178 (5.0) | 149 (7.0) | 1.30 (1.01-1.67) | 189 (5.3) | 110 (5.4) | 1.00 (0.76-1.30) |
| Birth weight, No. (%) | ||||||
| SGA (small for gestational age) | 223 (6.8) | 150 (7.5) | 1.10 (0.89-1.36) | 226 (6.9) | 145 (7.9) | 1.14 (0.92-1.40) |
| Low birth weight, <2500 g | 188 (5.8) | 153 (7.6) | 1.12 (0.88-1.43) | 206 (6.3) | 125 (6.8) | 0.95 (0.74-1.22) |
| Very low birth weight, <1500 g | 33 (1.0) | 16 (0.8) | 0.41 (0.15-0.93) | 40 (1.2) | 12 (0.6) | 0.23 (0.07-0.61) |
| Unknown, No. | 327 | 124 | 314 | 198 | ||
| Congenital anomalies, No. (%) | ||||||
| Heart malformations | 49 (1.4) | 27 (1.3) | 0.93 (0.57-1.47) | 44 (1.2) | 19 (0.9) | 0.75 (0.43-1.26) |
| Major heart malformationsc | 51 (1.4) | 16 (0.7) | 0.53 (0.29-0.90) | 49 (1.4) | 13 (0.6) | 0.46 (0.24-0.82) |
| Any congenital anomalies | 87 (2.4) | 43 (2.0) | 0.83 (0.57-1.19) | 76 (2.1) | 30 (1.5) | 0.69 (0.44-1.04) |
| All-cause hospitalizations | ||||||
| Neonatal (1-28 d after birth) | 210 (5.9) | 115 (5.4) | 0.92 (0.73-1.15) | 201 (5.6) | 99 (4.9) | 0.86 (0.67-1.09) |
| Postneonatal (>28 d after birth) | 151 (4.2) | 34 (1.6) | 0.72 (0.49-1.03) | 121 (3.4) | 43 (2.1) | 0.78 (0.54-1.09) |
| Phototherapy | 33 (0.9) | 42 (2.0) | 2.14 (1.36-3.40) | 36 (1.0) | 34 (1.7) | 1.71 (1.06-2.73) |
| Infant death over the study period | 6 (0.2) | 4 (0.2) | 1.68 (0.43-5.88) | 8 (0.2) | 3 (0.1) | 0.69 (0.14-2.41) |
Abbreviations: IPTW, inverse probability of treatment weights; SGA, small for gestational age.
This subgroup analysis included gestations exposed in the first trimester and unexposed during pregnancy with conception timing within the range of the exposed group conception (September 2020 and later).
IPTW was used to balance groups in terms of maternal age, conception timing, parity, seasonal influenza vaccination, population subgroup, and socioeconomic status. Post-IPTW numbers slightly differ from crude pre-IPTW because of propensity weighting.
Major heart malformations were congenital heart malformations other than ventricular septal defect and patent foramen ovale.
The risk of any congenital malformations was nonsignificantly different with RR = 0.69 (95% CI, 0.44-1.04), as well as for heart malformations with RR = 0.75 (95% CI, 0.43-1.26). The risk for major heart malformations was lower among the exposed group with RR = 0.46 (95% CI, 0.24-0.82). No difference was observed in the risk of inpatient hospitalizations, with RR = 0.86 (95% CI, 0.67-1.09) for neonatal hospitalizations and RR = 0.78 (95% CI, 0.54-1.09) for postneonatal hospitalizations. No difference was observed in mortality with RR = 0.69 (95% CI, 0.14-2.41) for exposed as compared with unexposed newborns.
Sensitivity Analysis Excluding SARS-CoV-2 Infection Prebirth
The findings of the sensitivity analysis when excluding mothers who had SARS-CoV-2 infection before they gave birth were generally similar to the primary analysis (eTable in the Supplement), other than an increased risk of jaundice requiring phototherapy (RR = 1.46; 95% CI, 1.06-2.06) associated with exposure.
Discussion
This large, nationally representative study examined various congenital outcomes and detected no increased risk for early infant morbidity or mortality among live-born infants prenatally exposed to BNT162b2 vaccination as compared with unexposed infants. Gestational age and birth weight were in a similar range among infants prenatally exposed to BNT162b2 vaccine as compared with unexposed infants. In addition, there were no apparent differences in gestational age and birth weight between infants born to mothers vaccinated in the first vs second trimester. Furthermore, the rate of congenital malformations in the exposed population was not higher than in the unexposed population and was similar to reports from prior years (prepandemic).16
All-cause infant mortality rate was similar among exposed as compared with unexposed newborns. The low overall number of events precludes definitive conclusions. An increased risk of jaundice requiring phototherapy was observed only in a sensitivity analysis among newborns without maternal infection prebirth. Exposed gestations were characterized by higher smoking rate, which is considered negatively associated with jaundice. Although smoking status was generally balanced, we cannot rule out some residual confounding. The absolute risk of phototherapy was generally low in both groups (~1%).
Vaccination during pregnancy is common to prevent morbidity from other infectious diseases. Specifically, vaccines to prevent influenza and pertussis are recommended during pregnancy.17 The clinical data on safety and efficacy of influenza vaccination are abundant and cover both early and late gestation,18 as well as longer-term implications into early childhood.19 However, COVID-19 vaccines were not expressly studied in pregnant women and offspring prior to their availability in the United States under Emergency Use Authorization, leaving an unmet need for safety data.
Strengths and Limitations
The main study strengths are the longitudinal follow-up over a large, stable cohort of pregnancies linked to offspring, coverage of first trimester exposure, and the population-based setting, which reduce the risk of patient selection or loss to follow-up. In addition, events of preterm births and birth defects were collected via active surveillance, rather than self-report and passive surveillance.
The following study limitations should be noted. Inference regarding rare outcomes in this study is primarily focused on exposure during the second or third trimesters, because the number of newborns exposed in the first trimester is underpowered to detect rare safety events at birth or after birth. This stems from the timing of this analysis was (November 2021) in relation to vaccination campaign initiation and Ministry of Health recommendations5 for pregnant women (January 19, 2021). Further accrual of follow-up time is required to facilitate examination of outcomes among newborns exposed during the embryogenesis stage.
Moreover, with a focus on newborn health, only live births were included in our cohort. Fetal safety was not assessed because of potential live-born bias,20 although current evidence suggests the risk of miscarriage or stillbirth among vaccinated mothers is similar to that among nonvaccinated.6,21,22
The study population was limited to newborns registered in the health fund. Cases of very early infant mortality (occurring during the first 24 hours of life) may not be fully captured if the newborn was unassigned to a health fund. Any information, if it exists, is likely nondifferential by exposure. Moreover, the study population was restricted to singleton births because the small number of multiples precluded their inclusion in the analysis.
The interpretation of the study findings is limited by the observational design. However, the alternative of an experimental study may no longer be ethical considering increasing evidence for the high effectiveness of the vaccine in preventing maternal SARS-CoV-2 infections.1 The weighting by likely confounders applied in this study to derive balanced groups reduces potential confounding bias.
Despite limitations, these findings contribute to current evidence in establishing the safety of BNT162b2 to offspring and can be used to inform pregnant patients, couples planning pregnancy, and counseling physicians. Robust assessments of maternal and offspring safety are important to reduce vaccine hesitancy and increase confidence among pregnant women.
Conclusions
This large population-based study found no evident differences between newborns of women who received BNT162b2 mRNA vaccination during pregnancy, vs those of women who were not vaccinated, and contributes to current evidence in establishing the safety of prenatal vaccine exposure to the newborns.
eFigure 1. Vaccination among pregnant women in Maccabi Healthcare Services by calendar month
eFigure 2. Distribution of propensity scores stratified by exposure to vaccination, before and after IPTW
eTable. Neonatal and early infant outcomes among the sub-cohort with no maternal SARS-CoV-2 infection pre-birth
References
- 1.Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728-735. doi: 10.1001/jama.2021.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197-211. doi: 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beharier O, Mayo RP, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(13):e154834. doi: 10.1172/JCI154834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israel Ministry of Health . Recommendation for pregnant women who are highly exposed to the public or with background diseases to get vaccinated. Published January 19, 2021. Accessed February 19,2021.https://www.gov.il/he/departments/news/19012021-05
- 6.Shimabukuro TT, Kim SY, Myers TR, et al. ; CDC v-safe COVID-19 Pregnancy Registry Team . Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273-2282. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450-456. doi: 10.1002/uog.23729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Insurance Institute of Israel . Capitation tables. Article in Hebrew. Published February 1, 2021. Accessed March 1, 2021. https://www.btl.gov.il/Mediniyut/Situation/haveruth1/2021/Pages/capitatia_022021.aspx
- 9.Characterization and classification of geographical units by the socio-economic level of the population 2015. Published August 2019. Accessed October 1, 2021. https://www.cbs.gov.il/he/publications/DocLib/2019/1765_socio_economic_2015/e_print.pdf.
- 10.Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. COVID-19 dynamics after a national immunization program in Israel. Nat Med. 2021;27(6):1055-1061. doi: 10.1038/s41591-021-01337-2 [DOI] [PubMed] [Google Scholar]
- 11.Quinn J-A, Munoz FM, Gonik B, et al. ; Brighton Collaboration Preterm Birth Working Group . Preterm birth: Case definition and guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047-6056. doi: 10.1016/j.vaccine.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon E-S. Birth weight standards in the live-born population in Israel. Isr Med Assoc J. 2005;7(5):311-314. [PubMed] [Google Scholar]
- 13.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSilva M, Munoz FM, Mcmillan M, et al. ; Brighton Collaboration Congenital Anomalies Working Group . Congenital anomalies: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2016;34(49):6015-6026. doi: 10.1016/j.vaccine.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2014.
- 16.Israel Ministry of Health . Congenital anomalies in Israel 2000-2014 trends over time. Article in Hebrew. 2017. https://www.health.gov.il/publicationsfiles/birth_2000_2017.pdf
- 17.Israel Ministry of Health . Vaccines for women before pregnancy, during pregnancy and after childbirth. Accessed October 3, 2021. https://www.health.gov.il/English/Topics/Pregnancy/during/Pages/vaccine_pregnant.aspx
- 18.Steinhoff MC, Katz J, Englund JA, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981-989. doi: 10.1016/S1473-3099(17)30252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrabadi A, Dodds L, MacDonald NE, et al. Association of maternal influenza vaccination during pregnancy with early childhood health outcomes. JAMA. 2021;325(22):2285-2293. doi: 10.1001/jama.2021.6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015;44(1):345-354. doi: 10.1093/ije/dyu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629-1631. doi: 10.1001/jama.2021.15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008-2010. doi: 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Vaccination among pregnant women in Maccabi Healthcare Services by calendar month
eFigure 2. Distribution of propensity scores stratified by exposure to vaccination, before and after IPTW
eTable. Neonatal and early infant outcomes among the sub-cohort with no maternal SARS-CoV-2 infection pre-birth