This randomized clinical trial evaluates the efficacy and safety of DCVAC/PCa plus chemotherapy followed by DCVAC/PCa maintenance treatment in patients with metastatic castration-resistant prostate cancer.
Key Points
Question
What is the efficacy and safety of DCVAC/PCa, an active cellular immunotherapy, in men with metastatic castration-resistant prostate cancer (mCRPC)?
Findings
In this randomized clinical trial of 1182 patients, there was no difference in overall survival (OS) between the DCVAC/PCa and placebo groups, with median OS of 23.9 and 24.3 months, respectively. Treatment-emergent adverse events related to DCVAC/PCa or placebo occurred in 9.2% and 12.7% of patients, respectively.
Meaning
DCVAC/PCa combined with docetaxel plus prednisone and continued as maintenance treatment did not extend OS and was well tolerated in patients with mCRPC; factors associated with the efficacy of immunotherapy should be identified to better select patients and thus prolong OS.
Abstract
Importance
DCVAC/PCa is an active cellular immunotherapy designed to initiate an immune response against prostate cancer.
Objective
To evaluate the efficacy and safety of DCVAC/PCa plus chemotherapy followed by DCVAC/PCa maintenance treatment in patients with metastatic castration-resistant prostate cancer (mCRPC).
Design, Setting, and Participants
The VIABLE double-blind, parallel-group, placebo-controlled, phase 3 randomized clinical trial enrolled patients with mCRPC among 177 hospital clinics in the US and Europe between June 2014 and November 2017. Data analyses were performed from December 2019 to July 2020.
Interventions
Eligible patients were randomized (2:1) to receive DCVAC/PCa (add-on and maintenance) or placebo, both in combination with chemotherapy (docetaxel plus prednisone). The stratification was applied according to geographical region (US or non-US), prior therapy (abiraterone, enzalutamide, or neither), and Eastern Cooperative Oncology Group performance status (0-1 or 2). DCVAC/PCa or placebo was administered subcutaneously every 3 to 4 weeks (up to 15 doses).
Main Outcomes and Measures
The primary outcome was overall survival (OS), defined as the time from randomization until death due to any cause, in all randomized patients. Survival was compared using 2-sided log-rank test stratified by geographical region, prior therapy with abiraterone and/or enzalutamide, and Eastern Cooperative Oncology Group performance status.
Results
A total of 1182 men with mCRPC (median [range] age, 68 [46-89] years) were randomized to receive DCVAC/PCa (n = 787) or placebo (n = 395). Of these, 610 (81.8%) started DCVAC/PCa, and 376 (98.4%) started placebo. There was no difference in OS between the DCVAC/PCa and placebo groups in all randomized patients (median OS, 23.9 months [95% CI, 21.6-25.3] vs 24.3 months [95% CI, 22.6-26.0]; hazard ratio, 1.04; 95% CI, 0.90-1.21; P = .60). No differences in the secondary efficacy end points (radiological progression-free survival, time to prostate-specific antigen progression, or skeletal-related events) were observed. Treatment-emergent adverse events related to DCVAC/PCa or placebo occurred in 69 of 749 (9.2%) and 48 of 379 (12.7%) patients, respectively. The most common treatment-emergent adverse events (DCVAC/PCa [n = 749] vs placebo [n = 379]) were fatigue (271 [36.2%] vs 152 [40.1%]), alopecia (222 [29.6%] vs 130 [34.3%]), and diarrhea (206 [27.5%] vs 117 [30.9%]).
Conclusions and Relevance
In this phase 3 randomized clinical trial, DCVAC/PCa combined with docetaxel plus prednisone and continued as maintenance treatment did not extend OS in patients with mCRPC and was well tolerated.
Trial Registration
ClinicalTrials.gov Identifier: NCT02111577
Introduction
Prostate cancer is the most frequently diagnosed noncutaneous malignant neoplasm in older adult men and the second leading cause of cancer-related death in Western countries.1 Prostate cancer depends on androgens for growth and progression. Although most patients initially benefit from androgen deprivation therapy, many patients progress to castration-resistant prostate cancer (CRPC), which is invariably fatal.
Prior to the development of second-generation antiandrogens, docetaxel in combination with prednisone was considered a standard therapy for patients with metastatic CRPC (mCRPC) progression.2,3,4 Prostate cancer has an immune desert-like phenotype5 with limited response to immune checkpoint inhibition.6
Cancer immunotherapy using active cellular immunotherapy with tumor antigen-loaded dendritic cells (DCs) is an accepted approach in clinical development.7 However, results of the trials to date were not conclusive.7,8,9,10 Considering these findings, it is important to investigate alternative immunotherapies for mCRPC.
DCVAC/PCa (international nonproprietary name/US adopted name: stapuldencel) is an active immunotherapy based on autologous DCs that activates antitumor immunity. Dendritic cells prepared from the patient’s monocytes were collected by leukapheresis and subsequently exposed to a human prostate adenocarcinoma cell line (LNCaP) killed by immunogenic modality. An open-label, single-arm phase 1/2 clinical trial of patients with mCRPC11 revealed that DCVAC/PCa was well tolerated and led to an improved OS compared with the predicted survival using the Memorial Sloan Kettering Cancer Center and Halabi nomograms. In addition, long-term administration of DCVAC/PCa induced a statistically significant increase in prostate-specific antigen (PSA)–specific T cells.11 The VIABLE (Active Immunotherapy Using Dendritic Cell–Based Treatment for Late-Stage Prostate Cancer) study was thus performed to test the hypothesis that combining docetaxel with DCVAC/PCa followed by maintenance therapy with DCVAC/PCa would improve OS in patients with mCRPC.
Because there were no data regarding the use of DCVAC/PCa in patients previously treated with abiraterone and enzalutamide, these patients were excluded during initial protocol development. However, because both compounds were approved in the US and Europe as this study was being activated, the final study protocol allowed the inclusion of patients treated with these compounds to reflect changes in the therapeutic landscape.
Methods
Study Design
VIABLE was a multicenter, double-blind, placebo-controlled, parallel-group, phase 3 randomized clinical trial conducted at 177 hospital clinics. The trial protocol and its amendments, as well as information provided to patients, were reviewed and approved by the appropriate ethical committees. All patients provided written informed consent. The trial protocol is available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients and Randomization
Patients aged 18 years and older with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2 were randomized to receive either DCVAC/PCa plus docetaxel and prednisone (DCVAC/PCa group) or placebo plus docetaxel and prednisone (placebo group). Only patients who demonstrated disease progression despite androgen deprivation therapy were eligible. Stratified block randomization was used to allocate patients to DCVAC/PCa vs placebo at a ratio of 2:1. Patients were stratified according to geographical region (US or non-US); prior therapy (abiraterone, enzalutamide, or neither); and ECOG PS (0-1 or 2). Race and ethnicity were obtained from patients’ medical records. Placebo contained only freezing medium CryoStor CS10, an aqueous, serum-free, animal protein–free balanced electrolyte solution with 10% dimethyl sulfoxide.
Procedures
Within 14 days after randomization, patients underwent a single session of leukapheresis in the course of the study. DCVAC/PCa was manufactured for patients in the DCVAC/PCa group but not for patients in the placebo group (eMethods in Supplement 2). Chemotherapy was started 3 to 7 days after leukapheresis and consisted of docetaxel (75 mg/m2 intravenously at 3-week intervals) and prednisone (5 mg orally twice daily) for up to a total of 10 cycles. Standard supportive care and dose modifications for docetaxel per prescribing information were recommended. Patients received up to 15 doses of DCVAC/PCa or placebo (CryoStor CS10) starting approximately 5 weeks after leukapheresis and more than 7 days after administration of the second dose of docetaxel. On the day of administration, a 1-mL aliquot of DCVAC/PCa or placebo was thawed and diluted with 0.9% saline solution to a total volume of 5 mL. Two 2.5-mL injections were administered subcutaneously into the axillary and contralateral inguinal lymph node areas. The first 9 doses of DCVAC/PCa or placebo were administered concomitantly with chemotherapy at 3-week intervals (±7 days), and the remaining 6 doses as maintenance therapy at 4-week intervals (−7/+14 days). The protocol allowed DCVAC/PCa or placebo treatment until the start of second-line therapy unless refusal, intolerance, or death occurred earlier.
Study Assessments
The trial end points and analyses are described in the trial protocol (Supplement 1). The primary end point was OS defined as the time from randomization until death due to any cause. Secondary end points were (1) radiological progression-free survival defined as the time from randomization to the date of the earliest objective evidence of either (i) radiographic progression of bone lesions, (ii) radiographic progression of soft tissue lesions, or (iii) death due to any cause; (2) time to PSA progression defined as the time from randomization to the date of the earliest objective evidence of PSA progression defined as absolute increase of 2 ng/mL or greater and 25% or greater above nadir or baseline; (3) time to the first skeletal-related event (SRE) defined as the time from randomization to the date of the first SRE; (4) time to radiographic progression or SRE; (5) proportion of patients with SREs; and (6) safety. Imaging was performed at screening, every 12 weeks after randomization until disease progression, at the introduction of a subsequent therapy line, or at the end of the trial. Exploratory end points included evaluation of quality of life using the EuroQoL 5-Dimensions questionnaire (collected only in Europe).
Adverse events (AEs) were graded according to the US National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.12 Treatment-emergent AEs (TEAEs) were AEs that started or worsened during trial treatment (docetaxel, prednisone, DCVAC/PCa, or placebo) or within 30 days after the last dose of trial treatment. Hematology and biochemistry laboratory assessments were performed every 3 weeks; urinalysis was performed every 6 weeks during chemotherapy treatment and every 12 weeks afterward. At each contact, open-ended and nonleading verbal questioning of the patient by the investigator was used to inquire about AE occurrences. Causality was assessed by the investigator.
Statistical Analyses
In accordance with the protocol, efficacy data (primary and secondary end points) were analyzed using the efficacy analysis set, which comprised all randomized patients with at least 1 baseline assessment (intent-to-treat analysis [Supplement 1]). All randomized patients were included in the intent-to-treat analysis according to their random allocation. The safety analysis set consisted of all patients who received at least 1 dose of docetaxel and/or at least 1 dose of DCVAC/PCa or placebo.
DCVAC/PCa was compared with placebo for the primary efficacy end point (OS) and for secondary time-to-event variables using a stratified 2-sided log-rank test with α = .05. The strata were geographical region (US or non-US), prior therapy with abiraterone (yes or no), prior therapy with enzalutamide (yes or no), and ECOG PS (0-1 or 2). The effect size was estimated with a Cox proportional hazard model with the same stratification factors (providing hazard ratio [HR] and 95% Wald CIs). Medians and survival curves were estimated with the Kaplan-Meier method. The effect of DCVAC/PCa on OS in subgroups defined by the stratification factors was analyzed in the efficacy analysis set for exploratory purposes.
To investigate whether the number of DCVAC/PCa doses influences OS, we performed post hoc analyses of OS in patients who received 10 or more, 12 or more, or 15 doses of DCVAC/PCa or placebo. This analysis was performed using patients who (1) had at least 1 postbaseline efficacy assessment, (2) did not have any major protocol violation that would affect the end points being assessed, and (3) received at least 10 doses of DCVAC/PCa or placebo. Quality-of-life data were analyzed descriptively. Additional details on statistical analyses are provided in the statistical analysis plan (Supplement 1). Analyses were conducted using SAS, version 9.2 or newer (SAS Institute).
Results
Patients
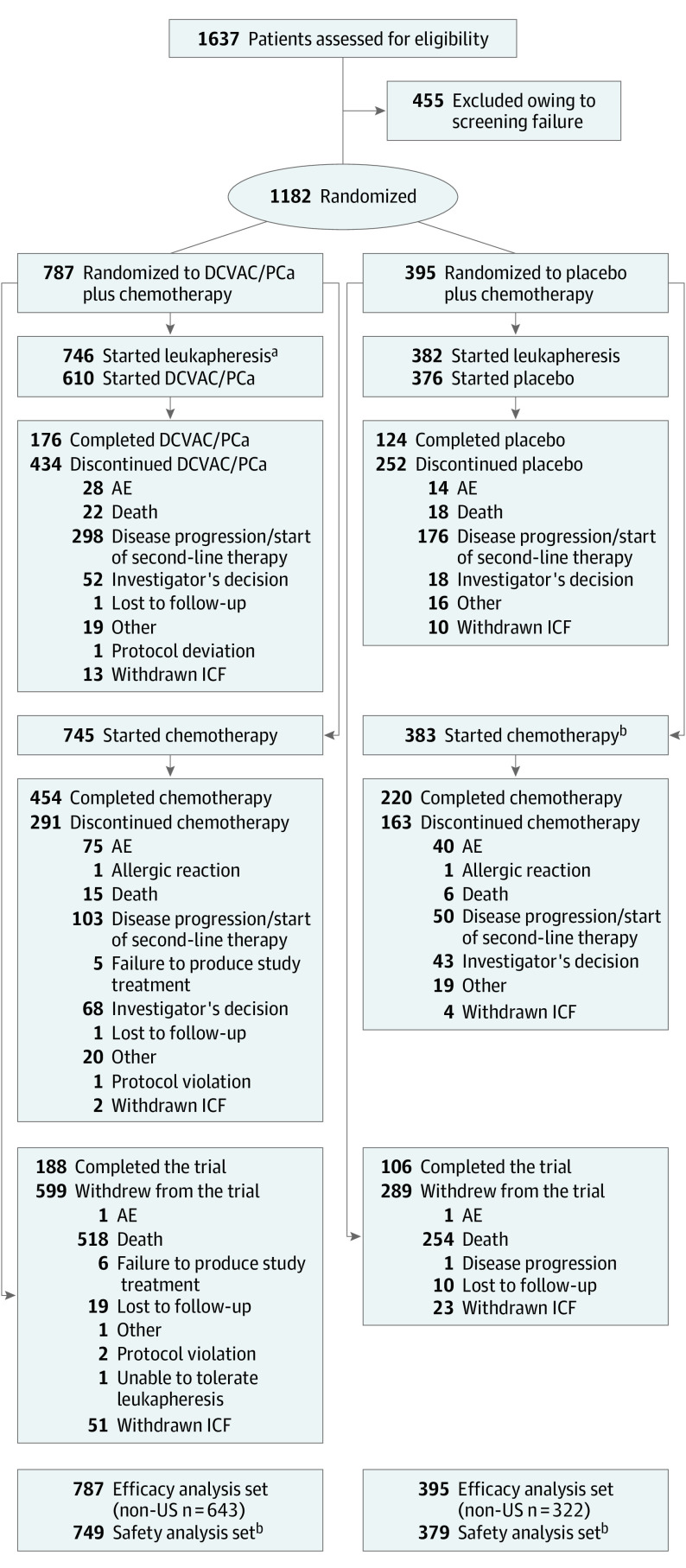
A total of 1182 patients were randomized (at 169 of 177 participating sites) (Figure 1); 787 (66.6%) were assigned to DCVAC/PCa group and 395 (33.4%) to placebo groups. All of these patients were included in the efficacy analysis set.
Figure 1. CONSORT Diagram.
aA total of 136 patients did not start DCVAC/PCa (119 because of production failures and 19 for other reasons).
bBecause of a protocol violation, 4 patients in the placebo group received DCVAC/PCa; these 4 patients were included in the DCVAC/PCa group in the safety analysis set.
AE indicates adverse event; ICF, informed consent form.
A total of 1128 (95.4%) randomized patients underwent leukapheresis (DCVAC/PCa vs placebo: 746 [94.8%] vs 382 [96.7%]). Docetaxel and prednisone chemotherapy was started in 1128 patients (745 [94.7%] vs 383 [97.0%]). Of the patients who underwent leukapheresis, 610 (81.8%) started DCVAC/PCa and 376 (98.4%) started placebo. DCVAC/PCa could not be produced for 119 patients. The most frequent reasons for production failure were leukapheresis product quality and DCVAC/PCa potency. A total of 888 (75.1%) randomized patients were withdrawn from the trial (599 [76.1%] vs 289 [73.2%]), mostly due to death (518 [86.5%] vs 254 [87.9%]). The safety analysis set comprised 749 and 379 patients in the DCVAC/PCa and placebo groups, respectively, who received at least 1 dose of DCVAC/PCa or placebo and/or at least 1 dose of chemotherapy.
Patients’ demographic characteristics and medical history at baseline were well balanced between the trial groups (Table 1; eTable 1 in Supplement 2). Of the 1182 patients, most were White (1091 [92.3%]), aged 65 years and older (803 [67.9%]), and had an ECOG PS of 0 or 1 (ECOG PS 0: 716 [60.6%]; ECOG PS 1: 450 [38.1%]; ECOG PS 2: 16 [1.4%]). The predominant stage at diagnosis was stage IV, and 542 (45.9%) patients had measurable disease at screening.
Table 1. Characteristics of the Patients at Baseline, Efficacy Analysis Seta.
| Characteristic | No. (%) | |
|---|---|---|
| DCVAC/PCa group (n = 787) | Placebo group (n = 395) | |
| Age, median (range) [No.], y | 68 (46-89) [787] | 69 (46-89) [395] |
| Raceb | ||
| Asian | 5 (0.6) | 4 (1.0) |
| Black or African American | 26 (3.3) | 19 (4.8) |
| Native Hawaiian or Other Pacific Islander | 1 (0.1) | 0 |
| White | 733 (93.1) | 358 (90.6) |
| Otherc | 4 (0.5) | 3 (0.8) |
| Unknown | 18 (2.3) | 11 (2.8) |
| Ethnicity | ||
| Hispanic or Latino | 22 (2.8) | 6 (1.5) |
| Not Hispanic or Latino | 763 (97.0) | 388 (98.2) |
| Unknown | 2 (0.3) | 1 (0.3) |
| Region | ||
| US | 144 (18.3) | 73 (18.5) |
| Non-US | 643 (81.7) | 322 (81.5) |
| ECOG PS | ||
| 0 | 477 (60.6) | 239 (60.5) |
| 1 | 300 (38.1) | 150 (38.0) |
| 2 | 10 (1.3) | 6 (1.5) |
| Gleason score at diagnosis | ||
| 8-10 | 431 (54.8) | 223 (56.5) |
| <8 | 341 (43.3) | 158 (40.0) |
| Unknown | 15 (1.9) | 14 (3.5) |
| Time from prostate adenocarcinoma diagnosis, median (range) [No.], y | 4.0 (0-25) [786] | 3.7 (0-32) [395] |
| Patients with measurable disease at screening | 359 (45.6) | 183 (46.3) |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
See eTable 1 in Supplement 2 for additional baseline characteristics.
Information was obtained from the patients’ medical records.
Races other than those listed were recorded as “Other.”
All 15 scheduled doses of the DC-based immunotherapy or placebo were administered to 176 of 787 (22.4%) and 123 of 395 (31.1%) patients in the DCVAC/PCa and placebo groups, respectively (eTable 2 in Supplement 2). This imbalance was mainly caused by patients with leukapheresis or manufacturing product failure (Figure 1). Of patients who received at least 1 dose of DCVAC/PCa or placebo, 176 of 610 (28.9%) and 123 of 376 (32.7%) patients, respectively, received all 15 doses. The mean duration of exposure was 7.7 and 7.9 months in the DCVAC/PCa and placebo groups, respectively (eTable 2 in Supplement 2). Both groups were comparable with regard to exposure to docetaxel and prednisone. The median number of doses was 8 for the DCVAC/PCa group and 10 for the placebo group. The median duration of docetaxel treatment was 5.6 and 6.2 months, respectively.
Efficacy
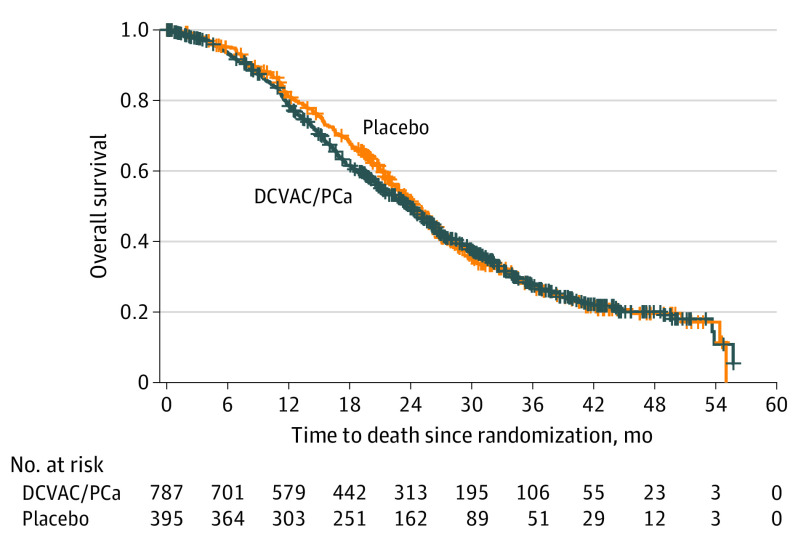
At the primary analysis, 505 of 787 patients (64.2%) in the DCVAC/PCa group and 254 of 395 patients (64.3%) in the placebo group had died. There was no difference in OS between the 2 groups, with median OS of 23.9 months (95% CI, 21.6-25.3) in the DCVAC/PCa group vs 24.3 months (95% CI, 22.6-26.0) in the placebo group (HR, 1.04 [95% CI, 0.90-1.21]; P = .60; Figure 2).
Figure 2. Kaplan-Meier Estimates of Overall Survival, Efficacy Analysis Set.
Median overall survival (95% CI): DCVAC/PCa, 23.9 (21.6-25.3) months; placebo, 24.3 (22.6-26.0) months. Stratified log-rank test: hazard ratio, 1.04 (95% CI, 0.90-1.21). Tick marks indicate censored data.
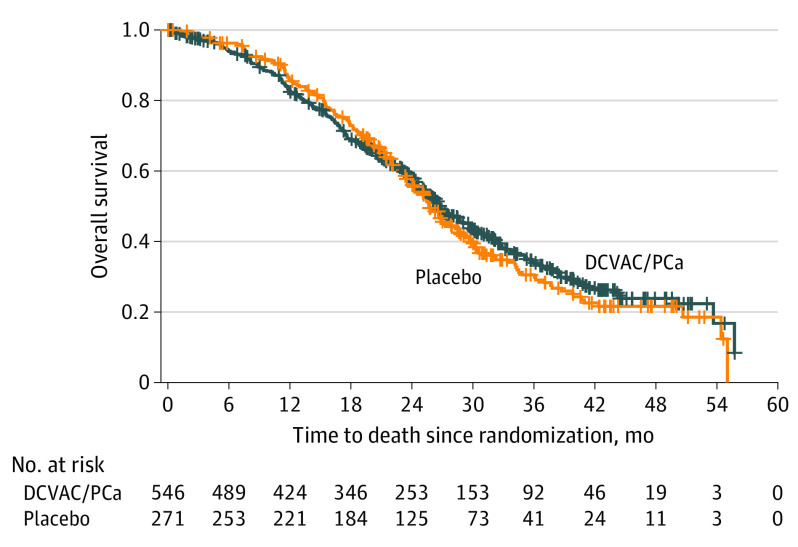
Because nearly 70% of patients had not received abiraterone or enzalutamide prior to the trial (Table 1), prior treatment status may act as an effect modifier. Thus, we also determined OS according to prior treatment, using the efficacy analysis set. Among abiraterone- and enzalutamide-naive patients (n = 817), we observed no difference in median OS between the DCVAC/PCa and placebo groups (26.7 months [95% CI, 25.2-28.8] vs 25.7 months [95% CI, 23.8-28.3]; HR, 0.94 [95% CI, 0.78-1.13], P = .50; Figure 3). Among patients pretreated with abiraterone and/or enzalutamide (n = 365), the median OS was shorter in the DCVAC/PCa group (16 months [95% CI, 14.7-18.3] vs 21.0 months [95% CI, 17.0-24.1]; HR, 1.28 [95% CI, 0.98-1.67]; P = .07). No effect of DCVAC/PCa was demonstrated (HR, 0.99 [95% CI, 0.84-1.16]) in sensitivity analysis where patients with leukapheresis/manufacturing failure were censored on study day 1.
Figure 3. Kaplan-Meier Estimates of Overall Survival in Abiraterone and/or Enzalutamide-Naive Patients, Efficacy Analysis Set.
Median overall survival (95% CI): DCVAC/PCa, 26.7 (25.2-28.8) months; placebo, 25.7 (23.8-28.3) months. Stratified log-rank test: hazard ratio, 0.94 (95% CI, 0.78-1.13). Tick marks indicate censored data.
Post hoc analyses in subgroups of patients who received 10 or more, 12 or more, and 15 doses of DCVAC/PCa or placebo revealed a trend in median OS suggestive of a treatment effect with increasing exposure (≥10 doses: 31.5 vs 27.0 months; HR, 0.92 [95% CI, 0.74-1.15]; P = .48; ≥12 doses: 35.9 vs 29.8 months; HR, 0.77 [95% CI, 0.60-1.00]; P = .05; 15 doses: 41.2 vs 38.7 months; HR, 0.72 [95% CI, 0.49-1.06]; P = .09; eFigure 1 and eTables 3 and 4 in Supplement 2). No differences were observed between the DCVAC/PCa and placebo groups for any of the secondary efficacy end points (eTable 5 and eFigure 2 in Supplement 2) or for quality-of-life data.
Safety
The safety analysis set comprised 749 and 379 patients in the DCVAC/PCa and placebo groups, respectively. The proportion of patients with at least 1 TEAE was slightly lower in the DCVAC/PCa group (677 [90.4%]) than in the placebo group (369 [97.4%]), as were the percentages of patients with TEAEs related to the study drug or chemotherapy, serious TEAEs, TEAEs leading to death, and grade 3 or higher TEAEs (Table 2). The other AE categories were comparable between treatment groups. The most common TEAEs in the DCVAC/PCa vs placebo groups were fatigue (271 [36.2%] vs 152 [40.1%]), alopecia (222 [29.6%] vs 130 [34.3%]), and diarrhea (206 [27.5%] vs 117 [30.9%]) (eTable 6 in Supplement 2). Serious TEAEs and fatal TEAEs are listed in eAppendix 2 in Supplement 2.
Table 2. Adverse Events (AEs), Safety Analysis Set.
| AEs | No. (%) | |
|---|---|---|
| DCVAC/PCa group (n = 749) | Placebo group (n = 379) | |
| Any TEAEsa | 677 (90.4) | 369 (97.4) |
| TEAE related to the DCVAC/PCa or placebo | 69 (9.2) | 48 (12.7) |
| Chemotherapy-related TEAEs | 599 (80.0) | 336 (88.7) |
| SREs | 41 (5.5) | 25 (6.6) |
| Serious TEAEs | 237 (31.6) | 150 (39.6) |
| TEAEs leading to death | 39 (5.2) | 30 (7.9) |
| Grade ≥3 TEAEs | 375 (50.1) | 216 (57.0) |
| TEAEs leading to DCVAC/PCa or placebo discontinuation | 31 (4.1) | 13 (3.4) |
| TEAEs leading to chemotherapy discontinuation | 101 (13.5) | 55 (14.5) |
| TEAEs leading to withdrawal from the trial | 13 (1.7) | 11 (2.9) |
Abbreviations: SRE, skeletal-related event; TEAE, treatment-emergent AE.
See eTable 6 in Supplement 2 for TEAEs in 2% or greater of patients in either group.
Discussion
The hypothesis behind the VIABLE phase 3 study in patients with mCRPC was that DCVAC/PCa pulsed with LNCaP would deliver prostate cancer antigens for effective in vivo T-cell activation to mount an immune response against the tumor, resulting in tumor regression and prolonging OS. VIABLE sought to confirm the results of the prior phase 1/2 trial, which combined DCVAC/PCa with docetaxel11 and revealed a promising OS in patients with mCRPC (19 months) relative to the predicted survival using the Halabi13 nomogram (12 months) and Memorial Sloan Kettering Cancer Center14 nomogram (13 months).
In the present phase 3 study, DCVAC/PCa combined with docetaxel did not lead to an OS improvement in mCRPC. Furthermore, no difference between study groups was observed in any of the secondary efficacy end points.
Because DCVAC/PCa could not be produced for 119 patients, their inclusion in the efficacy analysis set might adversely influence the efficacy outcomes. However, in a per-protocol analysis (comprising patients who [1] had at least 1 postbaseline efficacy assessment; [2] did not have any major protocol violation that would affect the end points being assessed; and [3] received at least 8 doses of DCVAC/PCa or placebo), OS was not significantly different between DCVAC/PCa and placebo (29.7 months [95% CI, 26.9-32.3] vs 26.7 months [95% CI, 24.7-28.8]; HR, 0.91 [95% CI, 0.75-1.11]; P = .34; 252 [57.5%] vs 174 [61.3%] deaths).
In the DCVAC/PCa group, we found that the median OS measured from randomization in patients pretreated with abiraterone or enzalutamide was about 10.7 months shorter than that of abiraterone- or enzalutamide-naive patients, a finding that may be attributed to emergence of drug resistance after androgen receptor therapy. The cause of drug resistance is speculative but could be due to the microenvironment of mCRPC, which has distinct features of tumor-infiltrating lymphocytes and immunosuppressive cell populations.15 However, it might also indicate that patients had worse prognosis at the time of trial entry. These findings provide a testable hypothesis for examining the interaction between prior abiraterone or enzalutamide exposure and the effects of DCVAC/PCa. Nevertheless, some abiraterone- or enzalutamide-naive patients received these treatments as second-line therapy following completion of DCVAC/PCa, with a potential benefit on OS.
Post hoc analyses showed a possible trend toward an effect of DCVAC/PCa on OS in patients who received 10 or more doses of DCVAC/PCa plus chemotherapy or placebo plus chemotherapy. Although no significant differences in baseline patient characteristics were identified, these patients must have had better prognosis at study entry to be able to remain in the study long enough to receive 10 or more doses of DCVAC/PCa or placebo.
We found that DCVAC/PCa was well tolerated without any observed systemic adverse effects. Indeed, the vast majority of TEAEs observed in this trial were related to chemotherapy rather than DCVAC/PCa.
Limitations
Some limitations of this study include the definition of the efficacy analysis set, which included 119 patients for whom DCVAC/PCa could not be produced. The median OS was 18.7 months in these patients, which was shorter than that in the DCVAC/PCa group overall (23.9 months). Thus, the inclusion of these patients may attenuate the OS in the DCVAC/PCa group. Another limitation is that we did not evaluate the tumor microenvironment, tumor-infiltrating lymphocytes, or immunological status to consider their prognostic relevance to the effects of DCVAC/PCa. Despite these limitations, the VIABLE study confirmed the feasibility of conducting a large-scale trial, which involved inadvertent logistic complexities and challenges of generating and providing autologous DCVAC/PCa for 169 sites located in 21 countries across Europe and the US.
Conclusions
In this phase 3 randomized clinical trial, results showed that DCVAC/PCa combined with docetaxel plus prednisone and continued as maintenance treatment did not extend OS in these patients with mCRPC. Nevertheless, DCVAC/PCa was well tolerated, with the majority of TEAEs being related to chemotherapy rather than DCVAC/PCa, thus providing evidence for its safety.
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Further patient characteristics at baseline, efficacy analysis set
eTable 2. Exposure to DCVAC/PCa or placebo, efficacy analysis set
eTable 3. Post-hoc analysis of overall survival in subgroups of patients who received ≥10, ≥12, and 15 doses of DCVAC/PCa or placebo
eTable 4. Post hoc analysis of patient characteristics at baseline according by number of DCVAC/PCa doses
eTable 5. Secondary endpoints, efficacy analysis set
eTable 6. TEAEs in ≥2% of patients in either treatment group, safety analysis set
eFigure 1. Kaplan–Meier estimates of overall survival in subgroups of patients who received ≥10 (A), ≥12 (B), and 15 doses (C) of DCVAC/PCa plus chemotherapy or placebo plus chemotherapy (post hoc analysis)
eFigure 2. Kaplan–Meier Estimates of Progression-Free Survival, Efficacy Analysis Set
eAppendix 1. List of investigators
eAppendix 2. Serious adverse events
Nonauthor Collaborators: VIABLE Investigators
Data Sharing Statement
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29. doi: 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Maluf FC, Smaletz O, Herchenhorn D. Castration-resistant prostate cancer: systemic therapy in 2012. Clinics (Sao Paulo). 2012;67(4):389-394. doi: 10.6061/clinics/2012(04)13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520. doi: 10.1056/NEJMoa041318 [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242-245. doi: 10.1200/JCO.2007.12.4008 [DOI] [PubMed] [Google Scholar]
- 5.Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rijnders M, de Wit R, Boormans JL, Lolkema MPJ, van der Veldt AAM. Systematic review of immune checkpoint inhibition in urological cancers. Eur Urol. 2017;72(3):411-423. doi: 10.1016/j.eururo.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, et al. ; IMPACT Study Investigators . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Xiang Y, Xin VW, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13(1):107. doi: 10.1186/s13045-020-00939-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099-1105. doi: 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulley JL, Borre M, Vogelzang NJ, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051-1061. doi: 10.1200/JCO.18.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podrazil M, Horvath R, Becht E, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6(20):18192-18205. doi: 10.18632/oncotarget.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute . Common Terminology Criteria for Adverse Events (version 4.03). Accessed January 3, 2022. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/
- 13.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232-1237. doi: 10.1200/JCO.2003.06.100 [DOI] [PubMed] [Google Scholar]
- 14.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20(19):3972-3982. doi: 10.1200/JCO.2002.11.021 [DOI] [PubMed] [Google Scholar]
- 15.Dallos MC, Drake CG. Blocking PD-1/PD-L1 in genitourinary malignancies: to immunity and beyond. Cancer J. 2018;24(1):20-30. doi: 10.1097/PPO.0000000000000302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Further patient characteristics at baseline, efficacy analysis set
eTable 2. Exposure to DCVAC/PCa or placebo, efficacy analysis set
eTable 3. Post-hoc analysis of overall survival in subgroups of patients who received ≥10, ≥12, and 15 doses of DCVAC/PCa or placebo
eTable 4. Post hoc analysis of patient characteristics at baseline according by number of DCVAC/PCa doses
eTable 5. Secondary endpoints, efficacy analysis set
eTable 6. TEAEs in ≥2% of patients in either treatment group, safety analysis set
eFigure 1. Kaplan–Meier estimates of overall survival in subgroups of patients who received ≥10 (A), ≥12 (B), and 15 doses (C) of DCVAC/PCa plus chemotherapy or placebo plus chemotherapy (post hoc analysis)
eFigure 2. Kaplan–Meier Estimates of Progression-Free Survival, Efficacy Analysis Set
eAppendix 1. List of investigators
eAppendix 2. Serious adverse events
Nonauthor Collaborators: VIABLE Investigators
Data Sharing Statement