Key Points
Question
What is the role of radiofrequency ablation in the treatment of low-risk papillary microcarcinoma of the thyroid?
Findings
In this systematic review and meta-analysis that included 15 studies comprising 1770 patients with 1822 tumors treated with radiofrequency ablation, the pooled proportion of complete tumor disappearance was 79%.
Meaning
This study suggests that radiofrequency ablation is a safe and efficient method to treat selected low-risk papillary microcarcinoma of the thyroid.
Abstract
Importance
Papillary microcarcinomas of the thyroid (mPTCs) account for an increasing proportion of thyroid cancers in past decades. The use of radiofrequency ablation (RFA) has been investigated as an alternative to surgery. The effectiveness and safety of RFA has yet to be determined.
Objective
To evaluate the effectiveness and safety of RFA for low-risk mPTC.
Data Sources
Embase, MEDLINE via Ovid, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and the top 100 references of Google Scholar were searched from inception to May 28, 2021.
Study Selection
Articles reporting on adult patients with mPTC treated with RFA were included. Studies that involved patients with pre-ablation lymph node or distant metastases, recurrence of disease, or extrathyroidal extension were excluded. Final article selection was conducted by multiple reviewers based on consensus. The proportion of eligible articles was 1%.
Data Extraction and Synthesis
This meta-analysis was conducted in accordance with the MOOSE guidelines. Random and fixed-effect models were applied to obtain pooled proportions and 95% CIs.
Main Outcomes and Measures
The primary outcome was the complete disappearance rate of mPTC. Secondary outcomes were tumor progression and complications.
Results
Fifteen studies were included in this meta-analysis. A total of 1770 patients (1379 women [77.9%]; mean [SD] age, 45.4 [11.4] years; age range, 42.5-66.0 years) with 1822 tumors were treated with RFA; 49 tumors underwent 1 additional RFA session and 1 tumor underwent 2 additional RFA sessions. Mean (SD) follow-up time was 33.0 (11.4) months (range, 6-131 months). The pooled complete disappearance rate at the end of follow-up was 79% (95% CI, 65%-94%). The overall tumor progression rate was 1.5% (n = 26 patients), local residual mPTC in the ablation area was found in 7 tumors (0.4%), new mPTC in the thyroid was found in 15 patients (0.9%), and 4 patients (0.2%) developed lymph node metastases during follow-up. No distant metastases were detected. Three major complications occurred (2 voice changes lasting >2 months and 1 cardiac arrhythmia). Minor complications were described in 45 patients.
Conclusions and Relevance
The findings of this systematic review and meta-analysis suggest that RFA is a safe and efficient method to treat selected low-risk mPTCs. Radiofrequency ablation could be envisioned as step-up treatment after local tumor growth under active surveillance for an mPTC or initial treatment in patients with mPTCs with anxiety about active surveillance.
This systematic review and meta-analysis evaluates the effectiveness and safety of radiofrequency ablation for low-risk papillary microcarcinoma of the thyroid.
Introduction
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid malignant neoplasm, accounting for approximately 85% of thyroid cancers.1 Papillary thyroid carcinomas measuring 10 mm or less were first defined by the World Health Organization as papillary thyroid microcarcinomas (mPTCs).2 Papillary thyroid microcarcinomas account for an increasing proportion of thyroid cancers; in some countries, almost half of all PTCs are classified as an mPTC.3,4,5 Patients with a diagnosis of mPTC have a disease-specific survival of more than 99% after 10 years of follow-up.6,7
Most guidelines concerning thyroid cancer care recommend a lobectomy as the first-line treatment strategy for low-risk unifocal mPTC.8,9 However, thyroid surgery comes with significant costs and morbidity rates caused by iatrogenic hypothyroidism and recurrent laryngeal nerve damage, resulting in poor quality of life.10,11,12,13,14,15 To de-escalate the treatment of mPTC and reduce surgery-related morbidity, less-aggressive treatment strategies such as active surveillance16 and thermal ablation17 for patients with low-risk mPTC have been proposed.
Thermal ablation primarily includes 3 techniques: microwave ablation, laser ablation, and radiofrequency ablation (RFA). Radiofrequency ablation is a nonsurgical, minimally invasive technique that relies on alternating electromagnetic current to cause molecular frictional heating to control tissue mass.18 Although RFA is currently used mostly in patients with benign nodules, recurrent PTC, and inoperable disease,19,20,21,22 recent evidence suggests that RFA could be an efficient treatment for patients with low-risk mPTC and has been shown to be more effective than microwave ablation or laser ablation.17 In this systematic review and meta-analysis of current literature, the primary goal is to analyze the effectiveness and safety of RFA for low-risk mPTC in a large number of patients.
Methods
Literature Search and Patient Selection
A systematic literature search was performed using the databases Embase, MEDLINE via Ovid, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and the top 100 references of Google Scholar. The search strategy is illustrated in the eTable in the Supplement. The search strategy was created by a qualified librarian of the University Medical Center Rotterdam (S.T.G.G.). Two reviewers (S.P.J.v.D. and H.I.C.) independently screened titles and abstracts of articles published until May 28, 2021. In cases of disagreement in the selection of articles, a third reviewer (T.M.v.G.) was consulted to make the final decision. Studies were included if they involved patients who had a primary mPTC and were treated with RFA for the first time. Exclusion criteria were: (1) case reports, case series of less than 5 patients, letters, conference abstracts, (systematic) reviews, meta-analyses, guidelines, study protocols, statements, or non-English articles; (2) patients with preablation lymph node or distant metastases, recurrence, or extrathyroidal extension; and (3) patients treated with other thermal ablation techniques such as laser, ethanol, or microwave ablation. This meta-analysis of scientific literature was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.23
Data Extraction and Outcomes
The following data were extracted: author names, year of publication, type of study, study period, total number of patients, total number of tumors, patient age, patient sex, total and mean number of RFA sessions, largest tumor diameter, volume of tumor, volume reduction rate, complications, and tumor progression. The primary outcome of this meta-analysis was the complete disappearance rate of mPTCs. Secondary outcomes were tumor progression and complications. Complete disappearance rate was defined by the percentage of patients with a completely absorbed tumor volume on results of ultrasonography after RFA. Incomplete ablation was defined as incompletely absorbed tumor volume on results of ultrasonography after RFA with benign fine-needle aspiration (FNA) results. Tumor progression was defined as cytologically or histologically confirmed residual mPTC in the ablation area, newly found mPTC elsewhere in the thyroid, or lymph node or distant metastases.
Complications were assessed using the reporting standards of the Society of Interventional Radiology.24 Cardiac events and/or arrhythmias, surgical intervention owing to bleeding after RFA, and voice changes (lasting >1 month) were considered major complications. Pain, hematoma, skin burn, temporary voice changes (lasting ≤1 month), hypoparathyroidism, fever, and neck swelling were considered minor complications. Volume reduction rate was calculated and defined in the included studies as following: volume reduction rate (%) = (initial volume – final volume)/initial volume ×100%.
Statistical Analysis
Descriptive statistics were presented as counts (numbers and percentages) and means with SDs. Medians were used as approximations of the mean in case of skewed variables. Meta-analysis of proportions and means was performed with 95% CIs. Between-study heterogeneity was calculated by the Higgins inconsistency index I2. If there was no statistical proof for heterogeneity (P ≥ .05), the assumption of homogeneity was deemed valid and a fixed-effect model was applied. Otherwise, a random-effect model was used. The risk of publication bias of the included studies was analyzed by using visual checking of symmetry in funnel plots and the Egger regression test. Meta-analysis was performed using metafor for R, version 4.0.3 (R Group for Statistical Computing) and Comprehensive Meta-Analysis, version 3.3.070 (Biostat Inc).
Results
Systematic Literature Search
The literature search was performed on May 28, 2021. A total of 1045 articles were found in the updated search through May 28, 2021. After removal of duplicates, 667 articles were screened and 63 full-text articles were assessed for eligibility. After careful selection of the articles, 15 studies were eligible for the final synthesis and were included in this review (eFigure 1 in the Supplement). Eleven articles potentially used overlapping patient cohorts based on author names, time period of patient inclusion, and affiliations.25,26,27,28,29,30,31,32,33,34,35
Study Characteristics and Quality Assessment
Twelve studies were case series (11 retrospective and 1 prospective),25,26,27,28,29,30,31,32,33,36,37,38 which are generally considered to have a high risk of bias and low certainty.39,40 Three studies were retrospective cohort studies.34,35,41 All included studies originated from China (n = 11) or Korea (n = 4) and were published after 2016.
Clinical Characteristics
A total of 1770 patients (1379 women [77.9%] and 391 men [22.1%]; mean [SD] age, 45.4 [11.4] years; age range, 42.5-66.0 years) with 1822 tumors were treated with 1872 RFA sessions. All patients had mPTC confirmed by ultrasonography and FNA or core needle biopsy without signs of lymph node metastases or extrathyroidal extension on ultrasonography before RFA. Seven articles including 1069 patients reported on the exclusion of aggressive histologic variants of mPTC. In total, 49 tumors received 1 additional RFA session and 1 tumor received 2 additional RFA sessions. The mean (SD) follow-up (reported in all 15 studies) was 33.0 (11.4) months (range, 6-131 months). Further baseline characteristics of the included studies can be seen in Table 125,26,27,28,29,30,31,32,33,34,35,36,37,38,41 and Table 2.25,26,27,28,29,30,31,32,33,34,35,36,37,38,41
Table 1. Characteristics of Included Studies.
| Source | Affiliation | Study period | Study design | Patients, No. | Tumors, No. | RFA sessions, No. | Age, mean (SD), y | Sex ratio (female:male) | Follow-up, mean (SD or range), mo | Initial tumor diameter, mean (SD), mm | Initial tumor volume, mean (SD), mm3 | Presence of other treatment group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al,26 2016, China | PLA Bejing | 9/2013-10/2014 | Prospectivea | 92 | 98 | 98 | 45 (11) | 69:23 | 8 (3) | 6 (2) | 119 (107) | No |
| Jeong et al,36 2018, Korea | University of Ulsan College of Medicine | 3/2011-4/2016 | Retrospectivea | 6 | 7 | 7 | 64 (17) | 3:3 | 19 (4) | 7 (1) | 160 (80) | No |
| Lim et al,30 2019, Korea | Haeundae Hospital | 5/2008-1/2017 | Retrospectivea | 133 | 152 | 167 | 46 (12) | 114:19 | 39 (25) | 4 (1) | 300 (400) | No |
| Ding et al,37 2019, China | Renji Hospital, Shanghai | 9/2014-12/2017 | Retrospectivea | 37 | 38 | 38 | 45 (13) | 29:8 | 6 | 7 (4) | 120 (100) | No |
| Zhang et al,33 2019, China | PLA Bejing | 2/2013-3/2017 | NSa | 60 | 60 | 60 | 43 (9) | 48:12 | NS | NS | 110 (170) | No |
| Zhang et al,35 2020, China | PLA Bejing | 1/2013-11/2013 | Retrospectiveb | 94 | 94 | 94 | 45 (11) | 70:24 | 64 (3) | 6 (3) | 176 (228) | Surgery (n = 80) |
| Cho et al,32 2020, Korea | Haeundae Hospital | 9/2008-1/2017 | Retrospectivea | 74 | 84 | 98 | 46 (12) | 66:8 | 72 (18) | 4 (2) | 20 | No |
| Wu et al,28 2020, China | PLA Bejing | 2/2014-8/2016 | Retrospectivea | 198 | 204 | 205 | 43 (10) | 141:57 | 26 (5) | 6 (2) | 99 (84) | No |
| Yan et al,25 2020, China | PLA Bejing | 6/2016-11/2018 | Retrospectivea | 202 | 211 | 214 | 43 (10) | 152:50 | 24 (9) | 5 (2) | 102 (94) | No |
| Yan et al,27 2021, China | PLA Bejing | 6/2014-12/2017 | Retrospectivea | 414 | 414 | 428 | 44 (10) | 323:91 | 42 (12) | 5 (2) | 93 (83) | No |
| Seo,38 2021, Korea | Kangwon National University | 11/2006-12/2009 | Retrospectivea | 5 | 5 | 7 | NS | 5:0 | 131 (121-159) | 5 | NS | No |
| Song et al,29 2021, China | PLA Bejing | 5/2014-4/2018 | Retrospective | 112 | 112 | 112 | 45 (11) | 94:18 | 30 (14) | 7 (2) | 182 | No |
| Song et al,34 2021, China | PLA Bejing | 5/2014-5/2018 | Retrospectiveb | 115 | NS | 115 | 45 (10) | 97:18 | 26 (11-60) | 7 (2) | 182 (157) | Surgery (n = 103) |
| He et al,31 2021, China | PLA Bejing | 6/2014-1/2019 | Retrospectivea | 95 | 95 | 96 | 66 (4) | 71:24 | 37 (17) | 6 (2) | 107 (99) | No |
| Zhang et al,41 2021, China | Zhejiang University Hangzhou | 11/2017-3/2020 | Retrospectiveb | 133 | 133 | 133 | 46 (10) | 97:36 | 6 (5) | 5 (2) | 58 (53) | Surgery (n = 133) |
Abbreviations: NS, not specified; PLA, People’s Liberation Army; RFA, radiofrequency ablation.
Case series.
Cohort studies.
Table 2. Effectiveness and Safety of RFA in Patients With mPTC.
| Source | Complete disappearance rate, No./No. (%) | VRR, % | Tumor volume reduction, mean (SD), mm3 | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| After 12 mo | At end of follow-up | After 12 mo | At end of follow-up | Overall tumor progression | mPTC residue in ablation area | Newly discovered mPTCa | Lymph node metastases | Major complications | Minor complications | ||
| Zhang et al,26 2016 | NS | NS | 96 | 100 | 110 (70) | 0 | 0 | 0 | 0 | 0 | 4/92 (4) |
| Jeong et al,36 2018 | NS | 3/6 (50) | NS | 48 | 90 (40) | 0 | 0 | 0 | 0 | 0 | 0 |
| Lim et al,30 2019 | 108/152 (71) | 139/152 (91) | −16 | 100 | 109 (87) | 0 | 0 | 0 | 0 | 1/133 (1) | 3/133 (2) |
| Ding et al,37 2019 | NS | 37/38 (97) | 99 | 99 | NS | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhang et al,33 2019 | 49/60 (82) | 60/60 (100) | NS | 100 | 104 (170) | 0 | 0 | 0 | 0 | 0 | 4/60 (7) |
| Zhang et al,35 2020 | NS | NS | NS | NS | NS | 1/94 (1) | 0 | 1/94 (1) | 0 | 0 | 0 |
| Cho et al,32 2020 | 62/84 (74) | 84/84 (100) | 54 | 100 | NS | 3/74 (4) | 0 | 3/74 (4) | 0 | 1/74 (1) | 3/74 (4) |
| Wu et al,28 2020 | NS | NS | 99 | 99 | 86 (24) | 1/198 (1) | 1/198 (1) | 0 | 0 | 0 | 9/198 (5) |
| Yan et al,25 2020 | 110/211 (52) | 139/211 (66) | 84 | 99 | 101 | 3/211 (2) | 3/211 (2) | 0 | 0 | 0 | 0 |
| Yan et al,27 2021 | NS | 366/414 (88) | 87 | 99 | 82 (57) | 15/414 (4) | 1/414 (0.2) | 10/414 (2) | 4/414 (1) | 0 | 16/414 (4) |
| Seo,38 2021 | 3/5 (60) | 3/5 (60) | NS | NS | NS | 0 | 0 | 0 | 0 | 0 | 0 |
| Song et al,29 2021 | 102/112 (91) | 112/112 (100) | NS | NS | NS | 1/112 (1) | 1/112 (1) | 0 | 0 | 0 | 0 |
| Song et al,34 2021 | 104/115 (90) | 115/115 (100) | 99 | 100 | NS | 1/115 (1) | 0 | 1/115 (1) | 0 | 0 | 2/115 (2) |
| He et al,31 2021 | 44/95 (46) | 44/95 (46) | 78 | 99 | 107 | 1/95 (1) | 1/95 (1) | 0 | 0 | 1/95 (1) | 1/95 (1) |
| Zhang et al,41 2021 | 37/133 (28) | 39/133 (29) | 99 | 99 | 58 | 0 | 0 | 0 | 0 | 0 | 3/133 (3) |
Abbreviations: mPTC, papillary microcarcinoma of the thyroid; NS, not specified; RFA, radiofrequency ablation; VRR, volume reduction rate.
Found in other place than ablated area.
Effectiveness
Complete Disappearance Rates
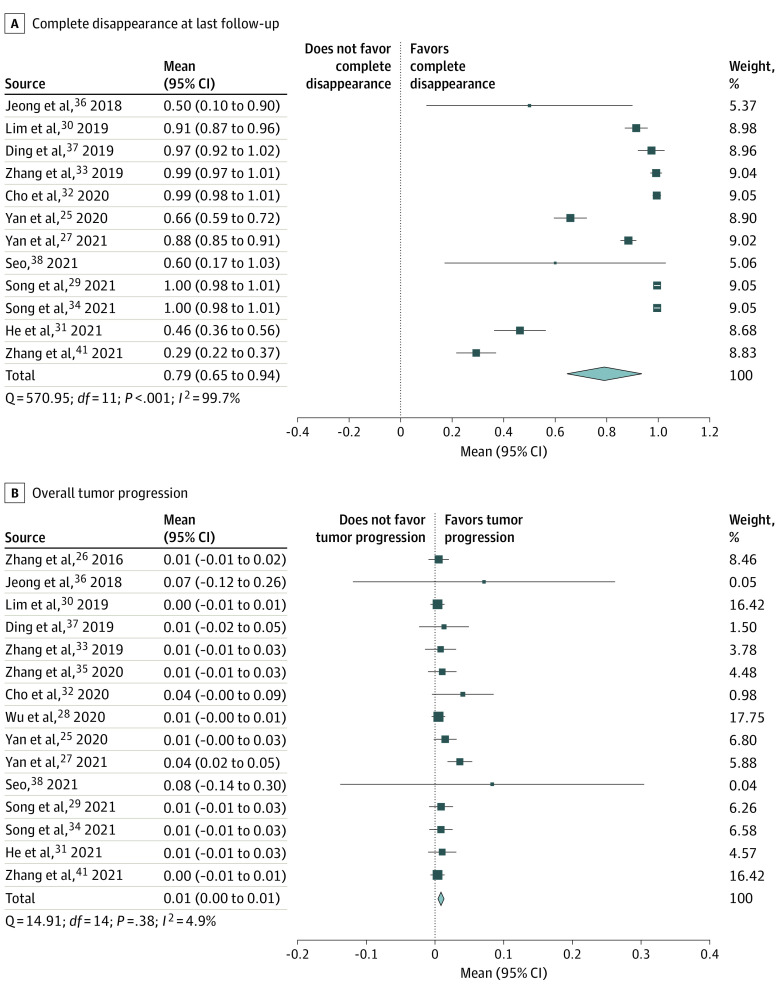
Twelve studies reported on complete disappearance rates of tumor tissue on results of ultrasonography after RFA, with a mean (SD) follow-up of 34.0 (20.8) months.25,27,29,30,31,32,33,34,36,37,38,41 The frequency of complete disappearance after 12 months ranged between 27.8% and 91.0%, whereas the complete disappearance rate at the end of follow-up ranged between 29.3% and 100%. The pooled proportion of the complete disappearance rate at 12 months was 66% (95% CI, 52%-81%) and at the end of follow-up was 79% (95% CI, 65%-94%) (Table 3, Figure, A). There was high heterogeneity between the studies (I2 = 96.8% at 12 months; P < .001 and I2 = 99.7% at the end of follow-up; P < .001). In 3 studies30,32,38 a small number of patients whose mPTC was incompletely ablated without pathologically confirmed mPTC after initial ablation received a second ablation instead of follow-up (n = 24).
Table 3. Meta-analysis of RFA in Patients With mPTC.
| Characteristic | No. | Follow-up, mean (SD), mo | Pooled proportion (95% CI) | Heterogeneity test | Publication bias (Egger test) | |||
|---|---|---|---|---|---|---|---|---|
| Studies | Patients | I2, % | P value | z Score | P value | |||
| Complete disappearance | ||||||||
| After 12 mo | 9 | 929 | 12 | 0.66 (0.52-0.81) | 96.8 | <.001 | −0.39 | .69 |
| At end of follow-up | 12 | 1386 | 34 (21) | 0.79 (0.65-0.94) | 99.7 | <.001 | −1.87 | .06 |
| Volume reduction rate after 12 mo | 7 | 1025 | 12 | 0.92 (0.85-0.99) | 99.8 | <.001 | −5.17 | <.001 |
| Mean volume reduction, mm3 | 6 | 937 | 30 (18) | 95 (83-107) | 87.7 | <.001 | 0.26 | .79 |
| Tumor progression rate | 15 | 1770 | 33 (11) | 0.01 (0.00-0.01) | 4.9 | .38 | 2.22 | .03 |
| Total complications | 15 | 1770 | 33 (11) | 0.02 (0.01-0.03) | 60.9 | <.001 | 4.21 | <.001 |
| Major complications | 15 | 1770 | 33 (11) | 0.00 (0.00-0.01) | 0.00 | .99 | 4.21 | <.001 |
Abbreviations: mPTC, papillary microcarcinoma of the thyroid; RFA, radiofrequency ablation.
Figure. Pooled Proportion of Complete Disappearance and Tumor Progression.
Tumor Progression
All 15 studies reported on the possible occurrence of tumor progression.25,26,27,28,29,30,31,32,33,34,35,36,37,38,41 The overall tumor progression rate was 1.5% (n = 26 patients), residual mPTC in the ablation area was found in 7 patients (0.4%), new mPTC in the thyroid was found in 15 patients (0.9%), and 4 patients (0.2%) developed lymph node metastases during follow-up. No distant metastases were detected. Yan et al27 reported the highest rate of tumor progression (3.6% [15 of 414]), whereas 7 studies reported no tumor progression in patients with mPTC treated with RFA.26,30,33,36,37,38,41 The pooled proportion of tumor progression was 1% (95% CI, 0%-1%) (Figure, B). There was no evidence of between-study heterogeneity (I2 = 4.9%; P = .38). Patients with tumor progression were treated with additional RFA sessions (n = 25) or active surveillance was initiated (n = 1).
Safety
Complications were evaluated in all 15 studies and 9 studies26,27,28,30,31,32,33,34,41 reported complications. Three major complications occurred: 2 patients experienced voice changes, which spontaneously resolved after 2 months, and 1 patient experienced temporary cardiac arrhythmias during the RFA procedure. In addition, 45 minor complications occurred (2.5%), which included 20 patients with postoperative pain, 14 patients with transient voice changes that resolved within 1 month, 5 patients with skin burns, 4 patients with hematomas, 1 patient with transient hypoparathyroidism, and 1 patient with fever. The pooled proportion of total complication rate was 2% (95% CI, 1%-3%), with evidence of moderate heterogeneity between studies (I2 = 60.9%; P < .001). The pooled proportion of major complication rate was 0% (95% CI, 0%-1%), without evidence of heterogeneity between studies (I2 = 0.00%; P = .99). Forest plots of the studies can be seen in eFigure 2 in the Supplement.
Other Effect Measures
Volume Reduction Ratio
The volume reduction rate after 12 months was reported by 10 studies.25,26,27,28,30,31,32,34,37,41 The pooled proportion of the mean tumor volume reduction ratio after 12 months was 92.1% (95% CI, 85.0%-99.2%). There was high heterogeneity between the studies (I2 = 99.8%; P < .001). Twelve studies reported on the volume reduction rate at the end of follow-up.25,26,27,28,30,31,32,33,34,36,37,41 In 11 of those 12 studies, the volume reduction rate was higher than 98.0%.25,26,27,28,30,31,32,33,34,37,41
Mean Volume Tumor Reduction
Nine studies reported on the absolute volume reduction of the mPTC after RFA.25,26,27,28,30,31,33,36,41 In most studies, tumor volume increased immediately after ablation and decreased gradually between 1 and 6 months of follow-up. Tumor volume reduction over time can be seen in eFigure 3 in the Supplement. The weighted pooled proportion of the mean tumor volume reduction was 95.0 mm3 (95% CI, 83.2-106.8 mm3), with evidence of high heterogeneity between the studies (I2 = 87.7%; P < .001). The mean (SD) follow-up was 29.8 (17.7) months.
Publication Bias
Asymmetrical distribution in the funnel plots, suggestive of publication bias, was found for overall tumor progression, major complications, and total complications. Evidence of publication bias was found by the Egger test (Table 3) for volume reduction ratio (z score, –5.17; P < .001), overall tumor progression rates (z score, 2.22; P = .03), total complications (z score, 42.1; P < .001), and major complications (z score, 42.1; P < .001). Funnel plots can be found in eFigure 4 in the Supplement.
Discussion
In this study, the effectiveness and safety of RFA as a treatment for mPTC in 1770 patients was analyzed. This study demonstrates that 79% of all patients with mPTC who underwent RFA had complete disappearance of tumor tissue on results of ultrasonography after RFA. Although complete disappearance of the tumor was not achieved in 21% of the patients undergoing RFA, only 7 patients (0.4%) received a diagnosis of FNA-confirmed residual mPTC cells. This finding emphasizes that the assessment of tumor response in patients with mPTC after RFA is complicated. Most studies applied ultrasonography and FNA of the residual tumor volume and, if no cancer cells were seen on cytologic examination, patients generally received follow-up. The diagnostic accuracy of FNA after RFA has shown to be reduced because of insufficient cellularity in the ablation area.42,43 Core needle biopsy is thought to have a higher diagnostic accuracy for detecting residual cancer cells and could be valuable in increasing the certainty of “complete mPTC disappearance” in case of residual tumor volume on ultrasonography after RFA.25 To assess the oncologic acceptability of RFA as a treatment option, complete tumor response after RFA has to be clearly defined, as no criterion standard exists yet.
The overall complication rate due to RFA was low (48 [2.7%]) and 3 (0.2%) major complications occurred. All complications, minor and major, resolved spontaneously within 3 months. Twenty-two patients (1.2%) experienced FNA-confirmed residual mPTC or new mPTC, which all were permanently removed by additional ablations. Surgical complications such as permanent hypothyroidism and recurrent laryngeal nerve damage occur in 30% and 1% to 2% of patients, respectively, after unilateral thyroid lobectomy.12,44,45 In the current study, less-severe complications in patients treated with RFA, such as pain, hematoma, skin burn, and temporary voice hoarseness, occurred in 2.5% of patients.
All included studies were conducted in China and Korea, where thyroid cancer guidelines differ significantly from guidelines in Europe. Although North American and European guidelines focus mainly on reducing overdiagnosis of mPTC by applying restrictive diagnostic workup strategies,8,46,47 Asian guidelines often aim to reduce overtreatment using active surveillance and thermal ablation techniques.48,49,50 Active surveillance instead of immediate surgery has proven to be a safe and viable treatment option for patients with low-risk mPTC.51,52,53 However, this treatment strategy has also been shown to have low potential in countries in which restrictive diagnostic workup strategies are applied.54 In these countries, the number of patients with mPTC are limited and, when encountered, the mPTC is often further progressed (ie, lymph node metastases or extrathyroidal extension), resulting in a high level of reluctance among thyroid specialists to use active surveillance.54 The effectiveness and safety of RFA in a population with a restrictive diagnostic workup strategy is unknown. With a 79% complete disappearance rate of tumor tissue, RFA could also be a valuable treatment option for patients with low-risk mPTC in these countries. The question whether RFA can aid in preventing lymph node metastases remains to be investigated.
Although this study suggests that RFA is a safe and efficient method to treat low-risk mPTCs, there is no evidence that treatment of low-risk mPTC is associated with any clinical benefit. Especially in populations with less-restrictive diagnostic workup protocols, patients with low-risk mPTC should generally receive active surveillance. However, in case of local tumor growth under active surveillance or in case of patient anxiety about active surveillance, RFA could be a valuable minimally invasive strategy in the management of low-risk mPTC. Different studied treatment options for mPTC and its advantages and disadvantages are described in Table 4.52,55,56,57,58
Table 4. Different Studied mPTC Treatment Options With Advantages and Disadvantages.
| Characteristic | Surgerya | RFA | Active surveillance |
|---|---|---|---|
| Complete disappearance of mPTC, % | 100 | 80 | 0 |
| Progression of disease, %b | 355 | Unknown | 752 |
| Overall complications (eg, infection, bleeding, transient voice problems, or hypoparathyroidism), % | 3-856,57 | 2 | 0 |
| Advantages |
|
|
|
| Disadvantages |
|
|
|
Abbreviations: mPTC, papillary thyroid microcarcinoma; RFA, radiofrequency ablation.
Lobectomy.
Surgery: recurrence of disease more than 5 years after initial treatment (in other thyroid lobe or nodal metastasis); RFA: recurrence of disease more than 5 years after initial treatment (in ablation area, other thyroid lobe, or nodal metastasis); active surveillance: progression of disease more than 5 years after start of active surveillance (tumor growth, new mPTC in other lobe, or nodal metastasis).
Limitations
This study has some limitations, the most important of which is the inclusion of mostly retrospective case series with small sample sizes that use several RFA techniques (eg, ablation energy, time of ablation, electrode tip diameter) and follow-up schedules. These studies have a higher risk of bias and low certainty.39,40 In addition, the likelihood of patient selection bias is increased in the included studies (eg, smaller tumors, healthier patients). There were 11 studies with potential overlapping patient cohorts based on author names, inclusion periods, and affiliations. Finally, owing to the only recent developments in the field of thermal ablation techniques for patients with mPTC, follow-up periods of the patients included in the analyzed studies were relatively short (mean [SD], 33.0 [11.4] months). Despite these limitations, this review managed to illustrate the available evidence on the effectiveness and safety of RFA in patients with mPTC.
The results in the current study suggest that RFA could function as a useful alternative treatment strategy in which patients are treated minimally invasively with curative intentions. Future studies may focus on improving complete disappearance rates of the tumor volume, possibly with more advanced or longer RFA procedures. To properly assess and compare oncologic outcomes with surgery and/or active surveillance in populations with restrictive diagnostic workup strategies, prospective trials or registration studies with long-term follow-up should be conducted. Although 3 Chinese studies showed that RFA was less expensive than surgery in patients with mPTC,34,35,41 future research may also focus on evaluating the long-term cost-effectiveness of RFA in other national health care environments.
Conclusions
The findings of this systematic review and meta-analysis suggest that RFA is a safe and efficient method to treat low-risk mPTC, with 79% complete disappearance rates of tumor tissue. Future research may focus on determining what role RFA could play in the treatment of mPTC, especially in countries with restrictive diagnostic workup protocols. Radiofrequency ablation could be envisioned as step-up treatment after local tumor growth under active surveillance or initial treatment in patients with anxiety about active surveillance and wishing to avoid surgery.
eTable. Terms Used to Search the Databases Until May 28, 2021
eFigure 1. PRISMA Flowchart of the Inclusion Process
eFigure 2. Forest Plots of the Efficacy and Safety of RFA in Patients With mPTC
eFigure 3. Change in Mean mPTC Volume Postablation
eFigure 4. Funnel Plots of the Included Studies
References
- 1.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(suppl 2):S1-S9. doi: 10.1038/modpathol.2010.129 [DOI] [PubMed] [Google Scholar]
- 2.Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989;63(5):908-911. doi: [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322. doi: 10.1001/jamaoto.2014.1 [DOI] [PubMed] [Google Scholar]
- 4.Leenhardt L, Grosclaude P, Chérié-Challine L; Thyroid Cancer Committee . Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? report from the French Thyroid Cancer Committee. Thyroid. 2004;14(12):1056-1060. doi: 10.1089/thy.2004.14.1056 [DOI] [PubMed] [Google Scholar]
- 5.Du L, Wang Y, Sun X, et al. Thyroid cancer: trends in incidence, mortality and clinical-pathological patterns in Zhejiang Province, Southeast China. BMC Cancer. 2018;18(1):291. doi: 10.1186/s12885-018-4081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo EJ, Roman SA, Sosa JA. Patients with follicular and Hurthle cell microcarcinomas have compromised survival: a population level study of 22,738 patients. Surgery. 2013;154(6):1246-1253. doi: 10.1016/j.surg.2013.04.033 [DOI] [PubMed] [Google Scholar]
- 7.vanVelsen EFS, Stegenga MT, vanKemenade FJ, et al. Comparing the prognostic value of the Eighth Edition of the American Joint Committee on Cancer/Tumor Node Metastasis Staging System Between Papillary and Follicular Thyroid Cancer. Thyroid. 2018;28(8):976-981. doi: 10.1089/thy.2018.0066 [DOI] [PubMed] [Google Scholar]
- 8.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perros P, Colley S, Boelaert K, et al. Guidelines for the management of thyroid cancer: third edition. Accessed January 6, 2022. https://onlinelibrary.wiley.com/doi/pdf/10.1111/cen.12515
- 10.McIntyre C, Jacques T, Palazzo F, Farnell K, Tolley N. Quality of life in differentiated thyroid cancer. Int J Surg. 2018;50:133-136. doi: 10.1016/j.ijsu.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 11.Joliat G-R, Guarnero V, Demartines N, Schweizer V, Matter M. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Incidence and postoperative evolution assessment. Medicine (Baltimore). 2017;96(17):e6674. doi: 10.1097/MD.0000000000006674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo C-Y, Kwok K-F, Yuen P-W. A prospective evaluation of recurrent laryngeal nerve paralysis during thyroidectomy. Arch Surg. 2000;135(2):204-207. doi: 10.1001/archsurg.135.2.204 [DOI] [PubMed] [Google Scholar]
- 13.Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2017;25(2):142-146. doi: 10.1097/MOO.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 14.Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243-2255. doi: 10.1210/jc.2012-1063 [DOI] [PubMed] [Google Scholar]
- 15.Büttner M, Musholt TJ, Singer S. Quality of life in patients with hypoparathyroidism receiving standard treatment: a systematic review. Endocrine. 2017;58(1):14-20. doi: 10.1007/s12020-017-1377-3 [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44(3):307-315. doi: 10.1016/j.ejso.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 17.Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720-731. doi: 10.1089/thy.2019.0707 [DOI] [PubMed] [Google Scholar]
- 18.Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16(4):192-200. doi: 10.1053/j.tvir.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JH, Baek JH, Ha EJ, Lee JH. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012;2012:919650. doi: 10.1155/2012/919650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172-185. doi: 10.1159/000508484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163-170. doi: 10.1007/s00330-014-3405-5 [DOI] [PubMed] [Google Scholar]
- 22.Chung SR, Baek JH, Choi YJ, Lee JH. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897-4903. doi: 10.1007/s00330-019-06063-5 [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 24.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9, pt 2):S199-S202. doi: 10.1097/01.RVI.0000094584.83406.3e [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Luo Y, Zhang Y, et al. The clinical application of core-needle biopsy after radiofrequency ablation for low-risk papillary thyroid microcarcinoma: a large cohort of 202 patients study. J Cancer. 2020;11(18):5257-5263. doi: 10.7150/jca.42673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581-1587. doi: 10.1089/thy.2015.0471 [DOI] [PubMed] [Google Scholar]
- 27.Yan L, Lan Y, Xiao J, Lin L, Jiang B, Luo Y. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol. 2021;31(2):685-694. doi: 10.1007/s00330-020-07128-6 [DOI] [PubMed] [Google Scholar]
- 28.Wu R, Luo Y, Tang J, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: a retrospective analysis of 198 patients. Int J Hyperthermia. 2020;37(1):168-174. doi: 10.1080/02656736.2019.1708480 [DOI] [PubMed] [Google Scholar]
- 29.Song Q, Gao H, Tian X, et al. Evaluation of ultrasound-guided radiofrequency ablation as a treatment option for papillary thyroid microcarcinoma in the isthmus: a retrospective study. Front Endocrinol (Lausanne). 2021;11:599471. doi: 10.3389/fendo.2020.599471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim HK, Cho SJ, Baek JH, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20(12):1653-1661. doi: 10.3348/kjr.2019.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He H, Song Q, Lan Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma in patients aged 55 years or older: a retrospective study. Int J Hyperthermia. 2021;38(1):604-610. doi: 10.1080/02656736.2021.1912416 [DOI] [PubMed] [Google Scholar]
- 32.Cho SJ, Baek SM, Lim HK, Lee KD, Son JM, Baek JH. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. 2020;30(12):1745-1751. doi: 10.1089/thy.2020.0106 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang MB, Luo YK, Li J, Zhang Y, Tang J. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8(12):5450-5458. doi: 10.1002/cam4.2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Q, Gao H, Ren L, et al. Radiofrequency ablation versus total thyroidectomy in patients with papillary thyroid microcarcinoma located in the isthmus: a retrospective cohort study. Int J Hyperthermia. 2021;38(1):708-714. doi: 10.1080/02656736.2021.1916625 [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years’ follow-up. Thyroid. 2020;30(3):408-417. doi: 10.1089/thy.2019.0147 [DOI] [PubMed] [Google Scholar]
- 36.Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34(5):611-616. doi: 10.1080/02656736.2018.1427288 [DOI] [PubMed] [Google Scholar]
- 37.Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712-717. doi: 10.1016/j.crad.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 38.Seo YK. Radiofrequency ablation of papillary thyroid microcarcinoma: a 10-year follow-up study. J Korean Soc Radiol. 2021;82(4):914-922. doi: 10.3348/jksr.2020.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines, 4: rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407-415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 40.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63. doi: 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Yin J, Hu C, Ye Q, Wang P, Huang P. Comparison of ultrasound guided percutaneous radiofrequency ablation and open thyroidectomy in the treatment of low-risk papillary thyroid microcarcinoma: a propensity score matching study. Clin Hemorheol Microcirc. Published online February 12, 2021. doi: 10.3233/CH-201087 [DOI] [PubMed] [Google Scholar]
- 42.Chung SR, Suh CH, Baek JH, Choi YJ, Lee JH. The role of core needle biopsy in the diagnosis of initially detected thyroid nodules: a systematic review and meta-analysis. Eur Radiol. 2018;28(11):4909-4918. doi: 10.1007/s00330-018-5494-z [DOI] [PubMed] [Google Scholar]
- 43.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199-208. doi: 10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 44.Said M, Chiu V, Haigh PI. Hypothyroidism after hemithyroidectomy. World J Surg. 2013;37(12):2839-2844. doi: 10.1007/s00268-013-2201-8 [DOI] [PubMed] [Google Scholar]
- 45.Zakaria HM, Al Awad NA, Al Kreedes AS, et al. Recurrent laryngeal nerve injury in thyroid surgery. Oman Med J. 2011;26(1):34-38. doi: 10.5001/omj.2011.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nederlands Huisartsen Genootschap. Links T. Richtlijn voor de diagnostiek, behandeling en follow-up van patiënten met gedifferentieerd (niet-medullair) schildkliercarcinoom. Accessed January 6, 2022. https://richtlijnen.nhg.org/multidisciplinaire-richtlijnen/schildkliercarcinoom
- 47.Perros P, Boelaert K, Colley S, et al. ; British Thyroid Association . Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(s1)(suppl 1):1-122. doi: 10.1111/cen.12515 [DOI] [PubMed] [Google Scholar]
- 48.Park S, Oh C-M, Cho H, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016;355:i5745. doi: 10.1136/bmj.i5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul). 2016;31(3):373-378. doi: 10.3803/EnM.2016.31.3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao M, Ge M, Ji Q, et al. ; Chinese Association of Thyroid Oncology Cato Chinese Anti-Cancer Association . 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med. 2017;14(3):203-211. doi: 10.20892/j.issn.2095-3941.2017.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walgama E, Sacks WL, Ho AS. Papillary thyroid microcarcinoma: optimal management versus overtreatment. Curr Opin Oncol. 2020;32(1):1-6. doi: 10.1097/CCO.0000000000000595 [DOI] [PubMed] [Google Scholar]
- 52.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27-34. doi: 10.1089/thy.2013.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222-1231. doi: 10.1007/s00268-009-0359-x [DOI] [PubMed] [Google Scholar]
- 54.Lončar I, van Dijk SPJ, Metman MJH, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic workup strategies. Thyroid. 2021;31(8):1219-1225. doi: 10.1089/thy.2020.0845 [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez Schaap PM, Botti M, Otten RHJ, et al. Hemithyroidectomy versus total thyroidectomy for well differentiated T1-2 N0 thyroid cancer: systematic review and meta-analysis. BJS Open. 2020;4(6):987-994. doi: 10.1002/bjs5.50359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeon YW, Gwak HG, Lim ST, Schneider J, Suh YJ. Long-term prognosis of unilateral and multifocal papillary thyroid microcarcinoma after unilateral lobectomy versus total thyroidectomy. Ann Surg Oncol. 2019;26(9):2952-2958. doi: 10.1245/s10434-019-07482-w [DOI] [PubMed] [Google Scholar]
- 57.Ji YB, Song CM, Kim D, et al. Efficacy of hemithyroidectomy in papillary thyroid carcinoma with minimal extrathyroidal extension. Eur Arch Otorhinolaryngol. 2019;276(12):3435-3442. doi: 10.1007/s00405-019-05598-z [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Qiu Y, Fei Y, Xing Z, Zhu J, Su A. Prevalence of and risk factors for hypothyroidism after hemithyroidectomy: a systematic review and meta-analysis. Endocrine. 2020;70(2):243-255. doi: 10.1007/s12020-020-02410-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Terms Used to Search the Databases Until May 28, 2021
eFigure 1. PRISMA Flowchart of the Inclusion Process
eFigure 2. Forest Plots of the Efficacy and Safety of RFA in Patients With mPTC
eFigure 3. Change in Mean mPTC Volume Postablation
eFigure 4. Funnel Plots of the Included Studies