Abstract
Aim:
The correlation between soluble Klotho (sKlotho) levels and clinical outcomes remains inconclusive for patients undergoing maintenance haemodialysis (MHD). We aimed to evaluate the potential predictive significance of sKlotho in this population by conducting a meta-analysis.
Methods:
PubMed, Embase, Web of Science and Cochrane Library were comprehensively searched for studies concerning the association between sKlotho level and clinical outcomes including cardiovascular (CV) events and all-cause mortality. The pooled hazard ratios (HR) and 95% confidence intervals (CI) were generated using either random or fixed effects models. Sensitivity and subgroup analyses were used to explore heterogeneity sources.
Results:
Eight prospective studies with 992 MHD participants were included and reduced sKlotho levels predicted more adverse outcomes in this meta-analysis. The pooled HRs and 95% CIs related to CV events, mortality, or composite outcomes were 1.73 (95% CI 1.08–2.76, p = 0.02), 2.34 (95% CI 1.34–2.07, p = 0.003) or 1.75 (95% CI 1.19–2.57, p = 0.005). Moderate heterogeneity was observed in the composite adverse outcomes (I2 = 57%, p = 0.05). Age and sKlotho level were the main sources of heterogeneities in the subgroup analysis.
Conclusion:
Lower sKlotho levels were associated with more CV events and all-cause mortality, suggesting that sKlotho may have predictive value in CKD patients receiving haemodialysis.
Keywords: cardiovascular event, haemodialysis, mortality, outcome, sKlotho
Introduction
Klotho is an identified gene in 1997 and exerted anti-aging effect. 1 It encodes a single chain transmembrane protein (mKlotho), which functions as a co-receptor for fibroblast growth factor-23. 2 There is another form of Klotho, secreted Klotho or soluble (sKlotho), which is generated from alternative splicing of the Klotho gene or ectodomain cleavage of mKlotho. 2 sKlotho can be released into the circulation and functions as a circulating factor, with anti-inflammatory, anti-oxidative, anti-apoptotic and anti-fibrotic effects.3,4 sKlotho is suggested to be a novel kidney protective protein because it has various biological activities. 5 Although Klotho has also been found in multiple organs, the kidney has the highest level of Klotho and Klotho is significantly decreased when kidney is diseased, demonstrating that the kidney is an important organ that is associated with the production of Klotho.6–8 Because Klotho is predominantly expressed in the kidney, there is a close association between sKlotho levels and kidney disease. 9 The sKlotho level decreased significantly in patients in early and intermediate chronic kidney disease (CKD) stages, and it decreased further in advanced CKD stages.10–12 For pre-dialysis patients, decreased sKlotho levels are associated with more adverse kidney outcomes including creatinine doubling, renal replacement therapy and death.11,13,14 These observations suggested that sKlotho may be a potential biomarker for the timely diagnosis and prognosis of CKD patients without dialysis.
Patients undergoing maintenance haemodialysis (MHD) had lower sKlotho levels and a higher risk of cardiovascular (CV) events and mortality than pre-dialysis CKD patients.14–17 Furthermore, sKlotho deficiency is accompanied by abnormal calcium and phosphorus metabolism or prominent vascular calcification (VC) in this population.17–20 It is well established that disturbed mineral metabolism and VC are common complications in patients on MHD, and these independently predict poor prognosis in this patient population. 21 This suggests that reduced sKlotho levels may also be correlated with increased adverse outcomes in haemodialysis patients. An increasing number of studies have suggested that reduced sKlotho levels predict a poor prognosis such as death or CV morbidity in MHD patients,15,22,23 indicating that sKlotho is a potential predictor for MHD patients. However, other studies have shown conflicting results in which sKlotho level was not associated with adverse outcomes in MHD patients.24–26 Thus, the prognostic role of sKlotho in MHD patients remains uncertain and it has not been systematically reviewed. We conducted this meta-analysis and systematic review in MHD patients to investigate the relationship between the sKlotho level and adverse outcomes including CV events and all-cause mortality.
Methods and materials
The present meta-analysis was conducted in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria 27 and A Guide to Systematic Review and Meta-Analysis of Prognostic Factor studies. 28
Search strategy
Medical databases such as PubMed, Embase, Web of Science and the Cochrane Library (from their inception to 27 October 2019) were systematically searched to identify studies that assessed the prognostic value of sKlotho in patients with MHD. Two independent investigators (QFL and LXY) performed the search. Reference lists from each identified article were also examined. The following search terms were used: End stage renal disease or ESRD or end stage kidney disease or ESKD or uremic or dialysis or haemodialysis or haemodialysis or hemofiltration or HD or renal dialysis or renal dialysis; and Klotho or KL or alpha-Klotho or α-Klotho or α-KL or sKlotho or sKL; and death or mortality or all-cause mortality or total mortality or morbidity or cardiovascular event or cardiovascular disease or stroke or coronary artery disease or ischaemic heart disease or myocardial ischaemia or myocardial infarction or prediction or predictive or endpoint or outcome or survival or prognostic or prognosis.
Study selection and inclusion criteria
Titles and abstracts were screened by two reviewers (JHF and LLX) for potentially relevant studies and the full text of the relevant studies was evaluated for eligible studies based on the inclusion and exclusion criteria. The inclusion criteria were set as the following: (a) Patients with MHD history >3 months and age >18 years; (b) Cohort or longitudinal studies; and (c) Studies reporting the relationship between sKlotho levels and adverse clinical outcomes (such as mortality and CV events). The exclusion criteria were set as the following: (a) Patients with history of organ transplantation or immunosuppressants; (b) Retrospective cohort studies or observational studies, or publications other than English; (c) Non clinical studies including in vivo and in vitro studies; and (d) Lack of complete and essential data. The primary outcome was all-cause mortality, which was defined as subsequent death from CV or no-CV cause. Secondary outcomes were CV events, which were defined as new subsequent events such as coronary artery disease (CAD), heart dysfunction or heart failure, atrial fibrillation (AF) and cerebrovascular accidents.
Data extraction and quality assessment
Two reviewers (SS Li and QF Liu) extracted relevant data from the full text of the included studies using a standardised data extraction form. The obtained information was expressed as follows: first author, publication year, study characteristics (age, gender, location, study design and sample), assay utilisation of sKlotho, duration of follow-up and outcomes assessment including relative risk (RR), hazard ratio (HR) and odds ratio (OR). Any disagreements concerning date extraction were resolved by a third reviewer after re-examining the original data. The Newcastle–Ottawa Scale (NOS) was used to evaluate the risk of bias in the eligible publications. 29 The NOS assigned three key points including the selection of study participants, comparability of study groups and assessment of outcome. Each study was awarded several stars depending on the quality of the items. Studies that scored ⩾7 stars were considered to have a low bias risk and were rated as high-quality studies.
Data synthesis and statistical analysis
The HR and the 95% confidence interval (CI) that were reported in the study were directly extracted and converted to the pooled HR and 95% CI. If the HR was not available directly in the study, the indirect HR was calculated based on Kaplan–Meier curves, as previously described. 30 Otherwise, OR was estimated based on the provided data and combined with HRs to generate a pooled effect size. Heterogeneity among the studies was examined by the I2 statistic. Fixed-effect models were used if the I2 value <50%; otherwise, random-effect models were used in the pooled results. Publication bias was examined using a funnel plot and Egger’s test. Sensitivity and subgroup analyses were conducted to search the causes of heterogeneity. Data analysis was performed using Review Manager software 5.3 (Cochrane Collaboration, Copenhagen, Denmark) and Stata software 12.0 (Stata Corp, College Station, TX, USA). p < 0.05 using two-tailed tests was considered to represent statistical significance.
Results
Literature search
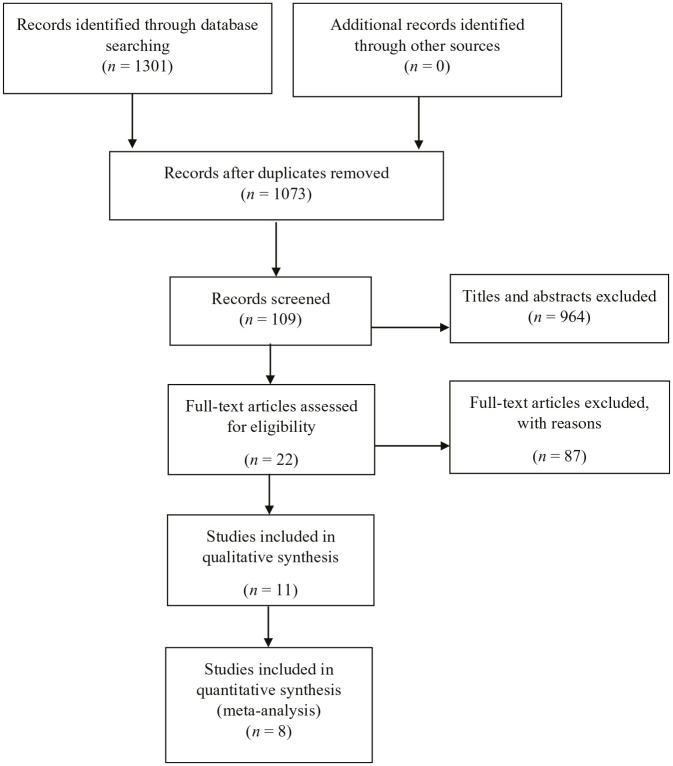
There were 1301 studies that were retrieved from medical databases and 228 studies were removed after duplication. Among the remaining 1073 studies, 964 were excluded after screening the titles and abstracts, and 87 studies were excluded after examining the full texts in the remaining 109 studies. Among the remaining 22 full articles, 11 articles were included for qualitative synthesis and 8 eligible articles were selected for quantitative synthesis.15,22–26,31,32 The literature screening flow chart is summarised in Figure 1.
Figure 1.
Study screening flow.
Study characteristics
The current meta-analysis included eight eligible prospective studies with 992 MHD participants. The sample size ranged from 30–239 and follow-up period ranged from 18–66 months. The effect indicators, including four HRs,15,22,23,25 one RR 32 and one OR, 24 and 95% CIs were retrieved directly from six of the eight studies.26,31 Based on the median sKlotho or sKlotho tertiles levels, two studies reported CV events,24,31 four studies reported all-cause mortality25,26,32,22 and two studies reported composite CV events and all-cause mortality.15,23 The study characteristics are summarized in Table 1. The NOS scores ranged from 6–8 stars and the average score was 7.3 stars. Four studies were of good quality. The detailed NOS information is displayed in Table 2.
Table 1.
Characteristics of the included studies.
| First Author | Year | Country | Study design | Number | Follow-up period | Average age | Low versus high sKlotho group | Outcomes | Adjusted HR, RR or and 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Memmos 23 | 2019 | Greece | Prospective | 79 | 5.5 years | 59.7 ± 15.8 | Median sKlotho level | Combined CV events and | |
| all-cause mortality | Direct, HR, 2.759(1.223–6.224) | ||||||||
| Wei 31 | 2019 | China | Prospective | 88 | 24 months | 57 ± 12 | Overall sKlotho level | CV disease | Direct, HR, 0.975(0.960–0.990) |
| Cognitive function | Direct, HR, 1.002(0.986–1.018) | ||||||||
| Valenzuela 32 | 2019 | Spain | Prospective | 30 | 18 months | 71 ± 9 | Median sKlotho level | All-cause mortality | Direct, RR, 1.6(0.65–1.35) |
| Zheng 26 | 2018 | China | Prospective | 128 | 36 months | 61.91 ± 15.39 | Median sKlotho level | CAC score | Direct, OR, 1.033(1.020–1.044) |
| Observational | All-cause mortality | None | |||||||
| Marçais 15 | 2017 | France | Prospective | 238 | 24 months | 69.9 (57.0–78.4) | sKlotho quartile level | Combined CV morbidity and | |
| mortality | Direct, HR,1.163 (1.011–1.316) | ||||||||
| Otani-Takei 22 | 2015 | Japan | Prospective | 63 | 65 months | 64.2 ± 13.0 | sKlotho tertile level | Mortality | Direct, HR, 4.14(1.29–13.48) |
| CV events | None | ||||||||
| Buiten 24 | 2014 | UK | Sectional Prospective | 127 | 48 months | 67 ± 7 | Median sKlotho level | AAC + CAC score | None |
| LV-dysfunction | Direct, OR,1.515(0.901–2.564) | ||||||||
| CAD | Direct, OR,1.099(0.813–1.515) | ||||||||
| Nowak 25 | 2014 | Germany | Sectional | 239 | 924 days | 68 ± 14 | sKlotho tertile level | AF | Direct, OR, 3.02(1.03–8.82) |
| Prospective | All-cause mortality | Direct, HR, 2.42(1.00–5.87) |
AAC, abdominal aorta calcification; AF, atrial fibrillation; CAC, coronary artery calcification; CAD, coronary artery disease; CI, confidence interval; CV, cardiovascular; HRs, hazard ratios; LV, left ventricular; OR, odds ration; RR, relative risk.
Table 2.
NOS scores of the cohort studies included.
| Cohort study | Selection representativeness the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Comparability Control for important factor or additional factor* | Outcome assessment | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total quality scores |
|---|---|---|---|---|---|---|---|---|---|
| Memmos 23 | / | ★ | ★ | ★ | ★★ | ★ | ★ | / | 7 |
| Wei 31 | / | ★ | ★ | ★ | ★★ | ★ | ★ | / | 7 |
| Valenzuela 32 | / | ★ | ★ | ★ | ★★ | ★ | / | / | 6 |
| Zheng 26 | / | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Marçais 2017 | / | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Otani-Takei 22 | / | ★ | ★ | ★ | ★ | ★ | ★ | / | 6 |
| Buiten 24 | / | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Nowak 25 | / | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 |
Two stars could be awarded for this item. Studies that controlled for age or eGFR were awarded one star, respectively.
eGFR, estimated glomerular filtration rate; NOS, Newcastle–Ottawa Scale.
Correlation between sKlotho and CV events
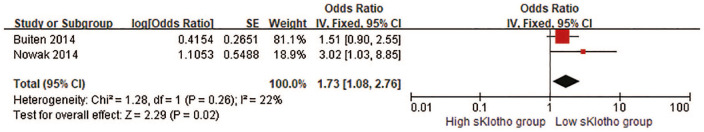
One study reported that overall sKlotho level was correlated with cerebrovascular disease [HR, 0.975 (0.960–0.990)] 31 and one study demonstrated that median sKlotho was not associated with CV events such as heart dysfunction (OR, 1.515, 95% CI 0.901–2.564) or CAD (OR, 1.099, 95% CI 0.813–1.515). 24 Another study demonstrated that the sKlotho level was associated with the development of AF (OR, 1.515, 95% CI 0.901–2.564). 25 The pooled result of the association between the sKlotho level and CV events was a pooled HR of 1.73 (95% CI, 1.08–2.76, p = 0.02; Figure 2). No obvious heterogeneity was found (I2 = 22%, p = 0.26; Figure 2).
Figure 2.
Forest plots of low sKlotho levels and cardiovascular events.
CI, confidence interval.
Correlation between sKlotho and all-cause mortality
Four studies reported the relationship of sKlotho level and all-cause mortality.22,25,26,32 Among the four studies, one study demonstrated that sKlotho level was strongly correlated with all-cause mortality, 22 but the remaining three studies did not show this association.25,26,32 Zheng et al. observed that sKlotho level was related with all-cause mortality in the model using the raw data, but the prominent association disappeared in the adjusted models. 26 Because the adjusted HR was not obtained directly or indirectly in this study, the other three HRs were pooled. The combined results demonstrated that patients with reduced sKlotho levels had a high risk of elevated all-cause mortality in the fixed-effect model (pooled HR, 2.34, 95% CI 1.34–4.07, p = 0.003, Figure 3) without heterogeneity (I2 = 0, p = 0.45; Figure 3). When we calculated the unadjusted OR from Zheng et al.’s study 26 and pooled it for analysis, the result did not change (pooled HR, 2.34, 95% CI 1.47–3.72, p < 0.001) and there was no heterogeneity (I2 = 0, p = 0.66).
Figure 3.
Forest plots of low sKlotho levels and all-cause mortality.
CI, confidence interval.
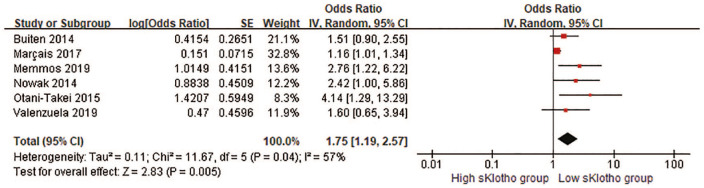
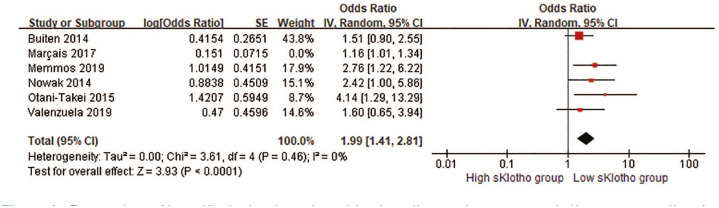
Correlation between sKlotho and composite adverse clinical outcomes
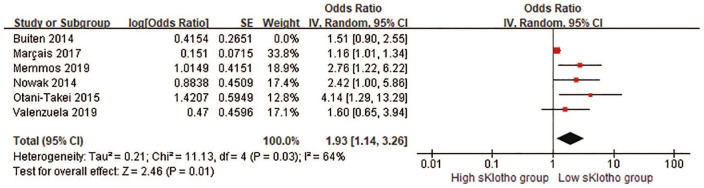
CV events and all-cause mortality were combined into the composite adverse clinical outcomes for further analysis. Five HRs and one OR were pooled in our meta-analysis. Overall, there was a remarkable difference in the rate of composite adverse clinical outcomes (pooled HR, 1.75, 95% CI 1.19–2.57, p = 0.005, Figure 4) with moderate heterogeneity (I2 = 57%, p = 0.05; Figure 4) in a random-effect model. This suggested that reduced sKlotho levels were related with an increased risk of combined CV events and all-cause mortality. When HR related to AF from Nowak et al.’s study 25 instead of HR related to all-cause mortality was pooled for the overall effect, the significant association persisted (pooled HR 1.79, 95% CI 1.19–2.68, p = 0.005). Notably, HR and OR represented different outcome measurements in the cohort studies, and it was not reasonable to combine them directly although they were still pooled in published studies due to lack of effect measures, such as HR or RR.30,33,34 Because the HR from Buiten’s study was not obtained or extracted, instead, the OR was combined with other five HRs for the analysis in this study. Interestingly, if we removed Buiten’s study in the sensitivity analysis, the overall effect still reached the statistical significance (pooled HR 1.93, 95% CI 1.14–3.26, p = 0.01, Figure 5), indicating our result was still reliable despite the inclusion of Buiten’s study.
Figure 4.
Forest plots of low sKlotho levels and combined cardiovascular events and all-cause mortality.
CI, confidence interval.
Figure 5.
Forest plots of low sKlotho levels and combined cardiovascular events and all-cause mortality after excluding Buiten’s study.
CI, confidence interval.
Sensitivity and subgroup analysis of included studies
Although the positive association remained significant after omitting any a single study in the sensitivity analysis, moderate heterogeneity was no longer observed after removing Marçais et al.’s study 15 (I2 = 0%, p = 0.46; Figure 6). Because this study has the second largest sample size (238 participants), the study was still included in our meta-analysis. The funnel plot was asymmetrical based on visual inspection and indicated that there was publication bias, which was confirmed by Egger’s test (Egger’s test, p = 0.006).
Figure 6.
Forest plots of low sKlotho levels and combined cardiovascular events and all-cause mortality after excluding Marçais 2017’s study.
CI, confidence interval.
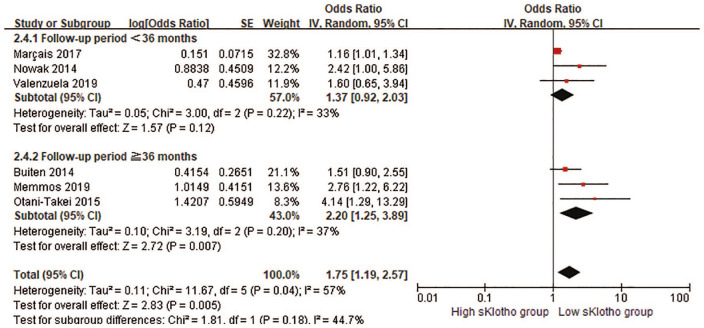
A subgroup meta-analysis was conducted to investigate the possible origins of heterogeneity in this study (Table 3). The eligible studies were divided into subgroups by age (⩾65 years or <65 years), sample size (⩾200 or <200), sKlotho level (median sKlotho and not median sKlotho), follow-up time (⩾36 months or <36 months) and study quality (⩾7 stars or <7 stars). The positive association was still observed in the subgroups by age and sKlotho level, but it was attenuated by a shorter follow-up time (<36 months, Figure 7), lower quality of the study (<7 stars) and a larger sample size (⩾100). No significant heterogeneity was detected in all subgroups except for age and sKlotho level, indicating that age and sKlotho level may be the main origins of heterogeneity.
Table 3.
Results of subgroup analysis by age, sample size, follow-up time and study quality.
| Subgroup | Studies | Effect estimate pooled HR (95% CI) |
Heterogeneity within each group |
Heterogeneity between subgroup |
|---|---|---|---|---|
| Age | 6 | 1.75 (1.19, 2.57) | p = 0.02; I² = 83% | |
| Age ⩾65 years | 4 | 1.31 (1.03, 1.67) | p = 0.29; I² = 20% | |
| Age <65 years | 2 | 3.15 (1.62, 6.14) | p = 0.58; I² = 0% | |
| Sample size | 6 | 1.75 (1.19, 2.57) | p = 0.46; I² = 0% | |
| Sample size ⩾200 | 2 | 1.47 (0.75, 2.86) | p = 0.11; I² = 61% | |
| Sample size <200 | 4 | 1.97 (1.31, 2.96) | p = 0.34; I² = 11% | |
| sKlotho level | 6 | 1.75 (1.19, 2.57) | p = 0.07; I² = 69.2% | |
| Median sKlotho | 4 | 1.44 (1.03, 2.01) | p = 0.15; I² = 44% | |
| Not median sKlotho | 2 | 2.94 (1.46, 5.95) | p = 0.47; I² = 0% | |
| Follow-up time | 6 | 1.75 (1.19, 2.57) | p = 0.18; I² = 44.7% | |
| Follow-up time ⩾36 months | 3 | 2.20 (1.25, 3.89) | p = 0.20; I² = 37% | |
| Follow-up time <36 months | 3 | 1.37 (0.92, 2.03) | p = 0.22; I² = 33% | |
| Study quality | 6 | 1.75 (1.19, 2.57) | p = 0.43; I² = 0% | |
| High-quality study(⩾7 stars) | 4 | 1.59 (1.06, 2.38) | p = 0.06; I² = 59% | |
| Low-quality study(<7 stars) | 2 | 2.39 (0.95, 5.99) | p = 0.21; I² = 31% |
CI, confidence interval; HR, hazard ratio.
Figure 7.
Forest plots of low sKlotho levels and combined cardiovascular events and all-cause mortality in the subgroup by follow-up time.
CI, confidence interval.
Discussion
In this study, we systematically investigated the association of the sKlotho level with adverse clinical outcomes in MHD patients. We showed that a reduction in the sKlotho level was strongly associated with an increase in CV events and all-cause mortality in this population. To the best of our knowledge, this is the first meta-analysis and systematic review providing insights into the association of sKlotho level with adverse outcomes in MHD patients. The results suggested that sKlotho may have prognostic role for MHD patients.
sKlotho is generated from mKlotho ectodomain cleavage or alternative splicing, so sKlotho can be released into the extracellular space and it functions as a circulating hormone. The kidney is the major organ that is responsible for the production and metabolism of Klotho (both mKlotho and sKlotho). 6 Thus, if the kidney is diseased, sKlotho loss immediately occurs and the sKlotho level reflects the state of kidney function. 35 A strong positive association between sKlotho and kidney function has been well demonstrated in a recent meta-analysis that involved nine publications with 1457 patients. 9 sKlotho deficiency also caused deterioration of kidney function and accelerated CKD progression. Consistent with this notion, we recently reviewed the predictive value of sKlotho in CKD patients who were not receiving dialysis. We found that patients with a low sKlotho level had a high risk of kidney function deterioration or death (pooled HR, 1.64, 95% CI 1.19–2.26). 30 The findings suggested that sKlotho is a feasible biomarker for CKD diagnosis and progression.
MHD patients had more CV complications and a higher mortality rate despite past improvements in prognosis. 36 Screening potential biomarkers is of importance to reduce CV events and mortality in MHD patients. Unfortunately, an ideal biomarker has not been available until now in clinical practice. 37 Available evidence for patients with MHD is characterised by progressive sKlotho deficiency that is caused by almost or complete loss of kidney function.11,15,17,22 As a potential cardio-renal protective factor, theoretically, progressive reduction in sKlotho in patients with MHD may affect clinical outcomes. Previous observational studies demonstrated that the reduced sKlotho level was correlated with cardiac parameters such as left ventricular hypertrophy, heart dysfunction, coronary artery calcification and AF, independent of traditional CV risk factors.19,20,38,39 This means that sKlotho may have a beneficial effect against CV complications and mortality. A previous prospective study by Otani-Takei et al. reported that patients with lower sKlotho levels had higher all-mortality rate (HR, 4.14, 95% CI 1.29–13.48) in fully adjusted models. 22 This study enrolled 63 MHD patients with a 65-month follow-up. 22 Memmos et al. recently observed that low sKlotho was independently associated with more CV events and all-mortality after adjustment for traditional risk factors (HR, 2.759, 95% CI 1.223–6.224). 23 Their study enrolled 79 MHD patients, and it had the longest follow-up time (66 months). 23 A similar result has also been shown in another recent study with a larger sample size. 15 These publications supported the correlation between the reduced sKlotho level and more detrimental outcomes in MHD patients.
However, several studies contribute to these inconsistent results. In Nowak et al.’s study, sKlotho levels, either as a categorical variable or a continuous variable, failed to predict death in MHD patients in the univariate or multivariate Cox regression analysis (HR, 2.42, 95% CI 1.00–5.87). 25 This study had the largest sample size. Another study showed patients with low sKlotho level had more CV events, such as CAD and heart dysfunction, but the correlation was not statistically significant. 24 Several other studies also showed that the sKlotho level was not associated with adverse clinical outcomes.25,32 These controversial results suggested that the predictive role of sKlotho in MHD patients has not been determined. Thus, it is important to address this inconsistency using a meta-analysis. Our findings in this meta-analysis including 992 MHD patients from eight studies revealed that reduced sKlotho levels were significantly associated with an increased risk of CV outcomes and all-cause mortality (HR, 1.75, 95% CI 1.19–2.57), providing evidence for the use of sKlotho as a possible prognostic biomarker. Although moderate heterogeneity was observed (I2 = 57%, p = 0.05), this relationship persisted in the sensitivity analysis, indicating the stability of our results. In the subgroup analysis, the association still persists with regard to age and sKlotho level, however it became weak with a larger sample size and shorter duration of follow-up. Interestingly, studies with a larger sample size had a shorter duration of follow-up, which affects the development of adverse outcomes.
There are several reasonable explanations for this association. In vivo and in vitro studies provided direct and indirect evidence that the sKlotho level was associated with cardio-protective effects. sKlotho directly suppressed isoproterenol-induced cardiomyocyte apoptosis via endoplasmic reticulum stress signalling. 40 Oxidative stress and inflammation were predictors of CV events and mortality, 41 and sKlotho exerted cardio-beneficial effects by modulating oxidative stress and inflammation. 42 sKlotho has also been reported to ameliorate cardiac hypertrophy or fibrosis and improve heart function by regulating abnormal calcium signalling (TRPC6 channels) 43 and fibrotic signalling such as transforming growth factor-β1 and Wnt signalling.44,45 In addition, the presence of the abnormalities in mineral metabolism, secondary hyperparathyroidism (SHPT) and VC further enhanced the risk of CV events and the mortality rate in MHD patients.21,46 sKlotho deficiency plays pathogenic roles in the development of these pathophysiological disorders.20,47–49 sKlotho, with or without FGF23, was shown to decrease blood phosphorus level, maintain blood calcium level and inhibit VC via multiple mechanisms. 49 First, sKlotho served as a co-receptor for FGF23 and decreased hyperphosphatemia by enhancing renal phosphate excretion via mediating renal phosphate transporters activity.49,50 Second, Klotho is also located in parathyroid tissue and aggressive loss of the Klotho–FGF receptor complex in end-stage renal disease contributed to overproduction of intact parathyroid hormone and SHPT.51–53 Third, sKlotho independently promoted calcium reabsorption and reduced renal calcium loss by regulating calcium-selective channels that are expressed in the kidney. 54 Finally, sKlotho was demonstrated to suppress VC through inhibiting vascular osteoblastic differentiation and the Wnt/β-catenin signalling pathway.55–57 Overall, sKlotho exerted pleiotropic direct or indirect CV protective effects. These may at least partly explain the relationship of decreased sKlotho level with increased VC events and mortality in MHD patients, which further confirmed our results.
This study has several limitations. First, the meta-analysis enrolled a small number of studies with a relatively small sample size and thus the strength of our results may be reduced. Second, there is moderate heterogeneity in this study. To find the sources of heterogeneity, we conducted a subgroup analysis. We found that there was no heterogeneity among subgroups except for the subgroups by age and sKlotho level. Thus, heterogeneity may result from age and sKlotho level. Because sKlotho was originally identified as a life-span factor, it is not surprising that sKlotho level is decreased with aging. 58 This means that sKlotho expression is influenced by aging. Aging is also associated significantly with increased adverse outcomes in MHD patients in previous studies.26,59,60 In this context, the effect of sKlotho on clinical outcomes may be weakened by aging. There may be an interaction between sKlotho and aging. Thus, aging is an important confounder and the source of heterogeneity. In addition, sKlotho level (median sKlotho level or not median sKlotho level) was another source of heterogeneity among studies, although no heterogeneity exits within this subgroup. We observed that studies with median sKlotho levels had a shorter follow-up and smaller sample size, thus, differences in methodologies among studies may explain the heterogeneities. Third, we only included eight English articles and excluded non-English articles. Publication bias could not be avoided. Finally, although six adjusted effect measures (five HRs and one OR) were obtained directly from the full text of the manuscript, the other two adjusted HRs were not obtained directly. Therefore, our conclusion may be less convincing due to the limitations after omitting these two studies.
Thus, our findings demonstrated that a decreased the sKlotho level was associated with more detrimental clinical outcomes. The study provided evidence that sKlotho may have predictive performance in CKD patients who are receiving haemodialysis despite several limitations. Further larger scale prospective studies with high quality are needed to confirm our conclusions.
Acknowledgments
We thank Jodi Smith, PhD for editing the English text of a draft of this manuscript.
Footnotes
Author contributions: GYL conceived the study and revised the manuscript. QFL and LXY performed the literature search and analysed the original data. JHF and LLX screened the literature and identified the eligible studies. SSL and QFL extracted the data and wrote the manuscript. All authors had read, revised and approved this manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was granted partly from the Social Development Foundation of Kunshan (KS18058) and partly from the Technological Development Foundation of Clinical Medicine of Jiangsu University (JLY20180108), Jiangsu Province, China.
ORCID iD: Qi-feng Liu  https://orcid.org/0000-0002-1224-3750
https://orcid.org/0000-0002-1224-3750
Contributor Information
Qi-Feng Liu, Department of Nephrology, The First Affiliated Hospital of Soochow University, Jiangsu, China; Department of Nephrology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China.
Sha-Sha Li, Clinical Research & Lab Centre, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China; Immunology Laboratory, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China.
Li-Xia Yu, Department of Nephrology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China.
Jian-Hua Feng, Department of Nephrology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China.
Li-Li Xue, Department of Nephrology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, Jiangsu, China.
Guo-Yuan Lu, Department of Nephrology, The First Affiliated Hospital of Soochow University, 188 Shizi Road Suzhou, Jiangsu, 215006, China.
References
- 1. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 2. Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 1998; 424: 6–10. [DOI] [PubMed] [Google Scholar]
- 3. Zou D, Wu W, He Y, et al. The role of klotho in chronic kidney disease. BMC Nephrol 2018; 19: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalton GD, Xie J, An SW, et al. New insights into the mechanism of action of soluble klotho. Front Endocrinol (Lausanne) 2017; 8: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone 2017; 100: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 2016; 27: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim K, Groen A, Molostvov G, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab 2015; 100: E1308–E1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picciotto D, Murugavel A, Ansaldo F, et al. The organ handling of soluble klotho in humans. Kidney Blood Press Res 2019; 44: 715–726. [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, Su W, Shen Z, et al. Correlation between soluble α-Klotho and renal function in patients with chronic kidney disease: a review and meta-analysis. Biomed Res Int 2018; 2018: 9481475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimamura Y, Hamada K, Inoue K, et al. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 2012; 16: 722–729. [DOI] [PubMed] [Google Scholar]
- 11. Liu QF, Ye JM, Yu LX, et al. Plasma s-Klotho is related to kidney function and predicts adverse renal outcomes in patients with advanced chronic kidney disease. J Investig Med 2018; 66: 669–675. [DOI] [PubMed] [Google Scholar]
- 12. Sakan H, Nakatani K, Asai O, et al. Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 2014; 9: e86301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drew DA, Katz R, Kritchevsky S, et al. Association between soluble Klotho and change in kidney function: the health aging and body composition study. J Am Soc Nephrol 2017; 28: 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian J, Zhong J, Yan M, et al. Circulating α-Klotho is related to plasma aldosterone and its follow-up change predicts CKD progression. Kidney Blood Press Res 2018; 43: 836–846. [DOI] [PubMed] [Google Scholar]
- 15. Marçais C, Maucort-Boulch D, Drai J, et al. Circulating Klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab 2017; 102: 3154–3161. [DOI] [PubMed] [Google Scholar]
- 16. Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014; 383: 1831–1843. [DOI] [PubMed] [Google Scholar]
- 17. Yokoyama K, Imura A, Ohkido I, et al. Serum soluble α-klotho in hemodialysis patients. Clin Nephrol 2012; 77: 347–351. [DOI] [PubMed] [Google Scholar]
- 18. Zheng S, Chen Y, Zheng Y, et al. Correlation of serum levels of fibroblast growth factor 23 and Klotho protein levels with bone mineral density in maintenance hemodialysis patients. Eur J Med Res 2018; 23: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu L, Kang L, Ren XZ, et al. Circulating α-Klotho levels in hemodialysis patients and their relationship to atherosclerosis. Kidney Blood Press Res 2018; 43: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 20. Koga S, Ikeda S, Akashi R, et al. Circulating soluble Klotho is inversely associated with coronary artery calcification evaluated by three-dimensional intravascular ultrasound. Eur Heart J 2018; 39: 1170. [Google Scholar]
- 21. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530. [DOI] [PubMed] [Google Scholar]
- 22. Otani-Takei N, Masuda T, Akimoto T, et al. Association between serum soluble Klotho levels and mortality in chronic hemodialysis patients. Int J Endocrinol 2015; 2015: 406269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Memmos E, Sarafidis P, Pateinakis P, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol 2019; 20: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buiten MS, de Bie MK, Bouma-de Krijger A, et al. Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol 2014; 15: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowak A, Friedrich B, Artunc F, et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One 2014; 9: e100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng S, Zheng Y, Jin L, et al. Relationship between serum soluble Klotho protein and coronary artery calcification and prognosis in patients on maintenance hemodialysis. Iran J Public Health 2018; 47: 510–518. [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019; 364: k4597. [DOI] [PubMed] [Google Scholar]
- 29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 30. Liu QF, Yu LX, Feng JH, et al. The prognostic role of Klotho in patients with chronic kidney disease: a systematic review and meta-analysis. Dis Markers 2019; 2019: 6468729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei H, Li H, Song X, et al. Serum klotho: a potential predictor of cerebrovascular disease in hemodialysis patients. BMC Nephrology 2019; 20: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valenzuela PL, Cobo F, Diez-Vega I, et al. Physical performance, plasma S-klotho, and all-cause mortality in elderly dialysis patients: a prospective cohort study. Exp Gerontol 2019; 122: 123–128. [DOI] [PubMed] [Google Scholar]
- 33. Siristatidis C, Sergentanis TN, Kanavidis P, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer–a systematic review and meta-analysis. Hum Reprod Update 2013; 19: 105–123. [DOI] [PubMed] [Google Scholar]
- 34. Yang WS, Va P, Wong MY, et al. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr 2011; 94: 1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rotondi S, Pasquali M, Tartaglione L, et al. Soluble α-Klotho serum levels in chronic kidney disease. Int J Endocrinol 2015; 2015: 872193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang IK, Lu CY, Lin CL, et al. Comparison of the risk of de novo cardiovascular disease between hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Int J Cardiol 2016; 218: 219–224. [DOI] [PubMed] [Google Scholar]
- 37. Pichler G, Haller MC, Kainz A, et al. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2017; 32: 1566–1578. [DOI] [PubMed] [Google Scholar]
- 38. Kim HJ, Kang E, Oh YK, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study. BMC Nephrology 2018; 19: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizia-Stec K, Wieczorek J, Polak M, et al. Lower soluble Klotho and higher fibroblast growth factor 23 serum levels are associated with episodes of atrial fibrillation. Cytokine 2018; 111: 106–111. [DOI] [PubMed] [Google Scholar]
- 40. Song S, Gao P, Xiao H, et al. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One 2013; 8: e82968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cozzolino M, Mangano M, Stucchi A, et al. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018; 33(Suppl. 3): iii28–iii34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Y, Zhuang X, Huang Z, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-kappaB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 238–251. [DOI] [PubMed] [Google Scholar]
- 43. Xie J, Cha SK, An SW, et al. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 2012; 3: 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ding J, Tang Q, Luo B, et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-beta1 signaling pathway. Eur J Pharmacol 2019; 859: 172549. [DOI] [PubMed] [Google Scholar]
- 45. Liu Q, Zhu LJ, Waaga-Gasser AM, et al. The axis of local cardiac endogenous Klotho-TGF-β1-Wnt signaling mediates cardiac fibrosis in human. J Mol Cell Cardiol 2019; 136: 113–124. [DOI] [PubMed] [Google Scholar]
- 46. Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. J Am Soc Nephrol 2015; 26: 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch 2011; 462: 185–193. [DOI] [PubMed] [Google Scholar]
- 48. Hu MC, Shiizaki K, Kuro-o M, et al. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 2013; 75: 503–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuro OM. The FGF23 and Klotho system beyond mineral metabolism. Clin Exp Nephrol 2017; 21: 64–69. [DOI] [PubMed] [Google Scholar]
- 50. Andrukhova O, Bayer J, Schuler C, et al. Klotho lacks an FGF23-independent role in mineral homeostasis. J Bone Miner Res 2017; 32: 2049–2061. [DOI] [PubMed] [Google Scholar]
- 51. Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 2010; 77: 232–238. [DOI] [PubMed] [Google Scholar]
- 52. Yan J, Jingbo C, Wang D, et al. A correlation between decreased parathyroid α-Klotho and fibroblast growth factor receptor 1 expression with pathological category and parathyroid gland volume in dialysis patients. Int Urol Nephrol 2015; 47: 701–706. [DOI] [PubMed] [Google Scholar]
- 53. Fan Y, Liu W, Bi R, et al. Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc Natl Acad Sci USA 2018; 115: E3749–E3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolf MT, An SW, Nie M, et al. Klotho up-regulates renal calcium channel transient receptor potential vanilloid 5 (TRPV5) by intra- and extracellular N-glycosylation-dependent mechanisms. J Biol Chem 2014; 289: 35849–35857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fang Y, Ginsberg C, Sugatani T, et al. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int 2014; 85: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen T, Mao H, Chen C, et al. The role and mechanism of α-Klotho in the calcification of rat aortic vascular smooth muscle cells. Biomed Res Int 2015; 2015: 194362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seiler S, Wen M, Roth HJ, et al. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 2013; 83: 121–128. [DOI] [PubMed] [Google Scholar]
- 59. Msaad R, Essadik R, Mohtadi K, et al. Predictors of mortality in hemodialysis patients. Pan Afr Med J 2019; 33: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hatakeyama S, Murasawa H, Hamano I, et al. Prognosis of elderly Japanese patients aged ⩾80 years undergoing hemodialysis. ScientificWorldJournal 2013; 2013: 693514. [DOI] [PMC free article] [PubMed] [Google Scholar]