Abstract
Background:
The relative role of coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) with stent implantation in patients with chronic kidney disease (CKD) and complex coronary artery disease (CAD) remains debatable due to the lack of randomized controlled trials (RCTs). We therefore performed this meta-analysis to compare the outcomes of the two strategies in CKD patients with multivessel and/or left main disease.
Methods:
Electronic databases including PubMed, EMBASE and Cochrane Library were comprehensively searched to identify the eligible subgroup analysis of RCTs and propensity-matched registries. The primary endpoint was all-cause mortality during the longest follow-up.
Results:
Five subgroup analyses of RCTs and six propensity-matched registries involving 26,441 patients were analyzed. Overall, the strategy of CABG was associated with lower risks of long-term mortality [odds ratio (OR) 0.83, 95% confidence interval (CI) 0.74–0.93], myocardial infarction (OR, 0.41; 95% CI, 0.27–0.62), and repeat revascularization (OR, 0.25; 95% CI, 0.16–0.39) compared with PCI in CKD patients with complex CAD. However, CABG was slightly associated with higher risk of stroke than PCI (OR, 1.33; 95% CI, 1.00–1.77). Nonetheless, the higher stroke risk in the CABG group no longer existed during long-term follow-up (OR, 0.92; 95% CI, 0.37–2.25) (>3 years).
Conclusion:
This meta-analysis supports the current guideline advising CABG for patients with CKD and complex CAD. At the expense of slightly increased risk of stroke, CABG reduces the incidences of long-term all-cause death, myocardial infarction and repeat revascularization compared with PCI.
Keywords: chronic kidney disease, complex coronary artery disease, coronary artery bypass grafting, outcome, percutaneous coronary intervention
Introduction
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD). 1 In clinical scenarios, CKD patients are prone to complex coronary artery disease (CAD) characterized by diffuse lesions, marked calcification, and small vessel diameters, rendering percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) challenging. 2 Unsurprisingly, CKD patients, especially those with multivessel or left main disease, often have an increased risk of unfavorable outcomes after revascularization compared with patients with preserved renal function.3,4
However, the relative role of PCI and CABG on survival in this population remains debatable. Although current the guideline supports CABG over PCI in patients with CKD and multivessel disease, the evidence mainly dates from retrospective registries since this high-risk group of patients is generally excluded or under included by randomized controlled trials (RCTs).5,6 Actually, patients still prefer PCI over surgery due to fewer complications and quicker recovery in real world practice. 7 The advent of the drug-eluting stent (DES) and more potent antiplatelet strategy has strikingly reduced the incidence of ischemic events, broadening the indications for PCI to include high-risk patients with complex lesions. 8 Although previous meta-analysis with non-randomized trials found that CABG had a survival advantage over PCI in patients with CKD and multivessel disease, the unadjusted confounding factors definitely had an effect on the results. 9 Therefore, we performed this meta-analysis including sub-analysis of RCTs and propensity-matched studies with high quality to compare the two revascularization strategies for patients with complex CAD and CKD.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, as well as Meta-analysis Of Observational Studies in Epidemiology checklist.10,11
Search strategy
A comprehensive search of the electronic databases including PubMed, EMBASE, and Cochrane Library from inception to 30 November 2019 was conducted by two independent investigators (JFT and DFZ) to identify pertinent articles comparing clinical outcomes of CABG and PCI with stent implantation in CKD patients with multivessel and/or left main disease. The following medical subject headings and search terms were used: “chronic kidney disease”, “end-stage renal disease”, “dialysis”, “percutaneous coronary intervention”, “coronary artery bypass”, “stent”, “revascular-ization”, “outcome”, “survival”, “mortality”, “randomized controlled trial”, “clinical trial”, “propensity-score matched”, and “propensity-score matching”. We also examined the references of the identified articles and relevant reviews to include other potentially eligible studies.
Study selection
Studies satisfying the following criteria were eligible: (1) patients with multivessel and/or left main CAD and concomitant CKD with an estimated glomerular filtration rate of <60 ml/min per 1.73 m2 or creatinine clearance <60 ml/min, or on dialysis; (2) sub-analysis of RCTs and propensity-matched observational studies comparing the two alternative approaches, that is, PCI and CABG; and (3) studies reporting endpoint data of interest. In addition, we did not include studies published as abstracts or conference proceedings. Only studies published in English were taken into account. When several reports overlapped with each other, we selected the largest and the latest one. The studies were reviewed by two investigators independently (WW and MDZ) to determine whether they met the inclusion criteria and any disagreement was resolved by consensus.
Data extraction and quality assessment
The following data was independently extracted by two authors (MZ and FX) through a standardized form for each study: study design, patient characteristics, quality indicators, and clinical outcomes. Differences in assessments were resolved by discussing with a third investigator (FY). The quality of RCTs was assessed by evaluating the following methodological criteria recommended by the Cochrane Collaboration: sequence generation, concealment of allocation, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias, 12 whereas the observational studies were evaluated by the Newcastle–Ottawa Scale criteria. 13 Studies with a Newcastle–Ottawa score of ⩾6 (maximum, 9) were considered high quality.
Endpoints
The primary endpoint was all-cause mortality during the longest follow-up. Secondary outcomes included short-term mortality (within 30 days), myocardial infarction (MI), stroke, and repeat revascularization. All the endpoints were defined as reported in each study (Supplemental material Table S1 online). Of note, we did not analyze cardiac mortality as an endpoint, since few studies reported the incidence of cardiac death.
Statistical analysis
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated with the Dersimonian and Lair random-effects model to account for heterogeneity. Potential heterogeneity among studies was quantified with the I2 statistic, which was classified as mild, moderate, and severe according to I2 values of <25%, 25% ⩽ I2 ⩽50%, and >50%, respectively. 14 To demonstrate the robustness of the results, we investigated the influence of each single study on the overall results by omitting each in turn. Moreover, we performed separate analyses according to the following variables: (1) RCTs or propensity-matched studies; (2) studies with long-term (>3 years) or midterm (⩽3 years) follow-up; (3) studies with multivessel disease; (4) studies using DES exclusively. Meta-regression analysis was also conducted to assess the correlation of patient characteristics, that is, age, gender, diabetes, hypertension, dyslipidemia, and previous MI with all the outcomes. The risk of potential publication bias was assessed by visual inspection of funnel plots, and the Begg and the Egger tests.15,16 All p values were two-sided, and results were considered statistically significant at p < 0.05. Computations were performed using Stata/SE12.0 (StataCorp, College Station, Texas, USA).
Results
Eligible studies
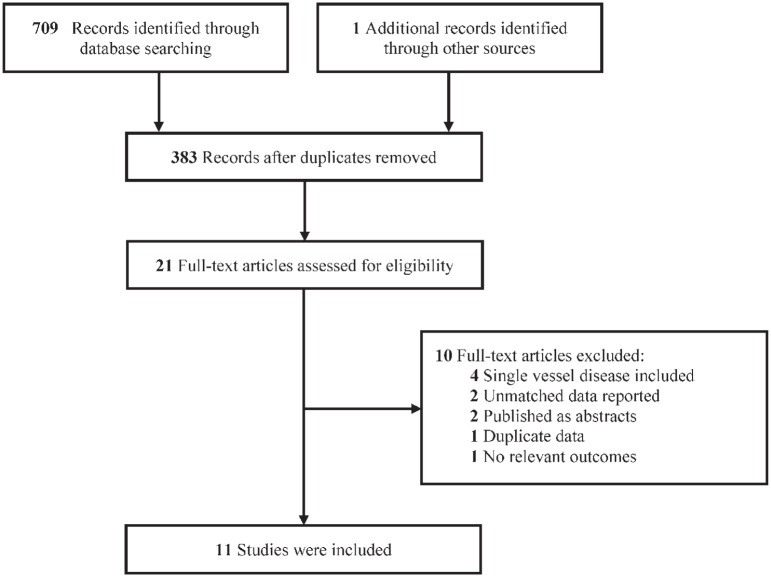
Eleven studies involving 26,441 patients (PCI group: 13,217; CABG group: 13,224) were included in the final analysis, including five sub-analyses of RCTs and six propensity-matched registries,17–27 published between 2005 and 2019 (Figure 1). The main characteristics of the eligible studies are presented in Table 1. Of the 11 studies, two compared PCI using bare-metal stent (BMS) versus CABG,17,25 five compared PCI using DES versus CABG,20,21,23,24,26 and the remaining four compared PCI using mixtures of BMS and DES versus CABG.18,19,22,27 Overall, nine studies included multivessel disease with no or very few left main disease,17–23,25,27 one study enrolled both multivessel and left main disease, 26 and the remaining one study enrolled only left main disease. 24 The clinical characteristics of the patients appear in Table 2. Quality assessment results are summarized in Supplemental Tables S2 and S3, and all the observational studies were considered to be of high quality.
Figure 1.
Flow diagram of included studies.
Table 1.
Main characteristics of the eligible studies in the meta-analysis.
| Study | No. patients |
Period | Region | Design, center | Inclusion criteria | Stent type | Follow-up, years | |
|---|---|---|---|---|---|---|---|---|
| PCI | CABG | |||||||
| Aoki et al. 17 | 69 | 73 | 1997–1998 | 19 countries | Post-hoc analysis of RCT, multi | MVD, Ccr <60 ml/min | BMS | 5 |
| Chang et al. 18 | 7049 | 7049 | 1997–2009 | USA | PSM registry, multi | MVD, on dialysis | BMS/DES | 1.7 (median) |
| Chang et al. 19 | 1458 | 1458 | 1996–2008 | USA | PSM registry, multi | MVD, eGFR <60 ml/min per 1.73 m2, not on dialysis | BMS/DES | 3.9 (median) |
| Bangalore et al. 20 | 2960 | 2960 | 2008–2011 | USA | PSM registry, multi | MVD, eGFR <60 ml/min per 1.73 m2 | EES | 2.9 (mean) |
| Chan et al. 21 | 893 | 893 | 2008–2011 | Canada | PSM registry, multi | MVD, Ccr <60 ml/min | DES | 1.8 (mean) |
| Komiya et al. 22 | 77 | 77 | 2005–2007 | Japan | PSM registry, multi | MVD, eGFR <30 ml/min per 1.73 m2, not on dialysis | BMS/DES | 2.5 (median) |
| Baber et al. 23 | 225 | 226 | 2005–2010 | 18 countries | Post-hoc analysis of RCT, multi | MVD, diabetes, eGFR <60 ml/min per 1.73 m2 | PES/SES | 3.8 (median) |
| Giustino et al. 24 | 177 | 184 | 2010–2014 | 17 countries | Post-hoc analysis of RCT, multi | LM, eGFR <60 ml/min per 1.73 m2 | EES | 3 (median) |
| Lima et al. 25 | 47 | 49 | 1995–2000 | Brazil | Post-hoc analysis of RCT, single | MVD, eGFR <60 ml/min per 1.73 m2 | BMS | 9.4 (median) |
| Milojevic et al. 26 | 158 | 151 | 2005–2007 | 17 countries | Post-hoc analysis of RCT, multi | LM/MVD, eGFR <60 ml/min per 1.73 m2 | PES | 5 |
| Gaipov et al. 27 | 104 | 104 | 2007–2014 | USA | PSM registry, multi | MVD, on dialysis | BMS/DES | 1.5 (median) |
BMS, bare-metal stent; CABG, coronary artery bypass grafting; Ccr, creatinine clearance; DES, drug-eluting stent; EES, everolimus-eluting stent; eGFR, estimated glomerular filtration rate; LM, left main; MVD, multivessel disease; PCI, percutaneous coronary intervention; PES, paclitaxel-eluting stent; PSM, propensity-score matching; RCT, randomized controlled trial.
Table 2.
Clinical characteristics of the patients.
| Study | Mean age, years | Male | Current smoker | Diabetes | Hypertension | Dyslipidemia | Prior MI | Mean LVEF, % | Mean SYNTAX score | Mean eGFR, ml/min per 1.73 m2 | Dialysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aoki et al. 17 | 70.5 | 68.3% | 13.4% | 18.3% | 49.3% | 54.9% | N/A | 59.5 | N/A | 52 † | N/A |
| Chang et al. 18 | 64.3 | 57.5% | 4.3% | 66.5% | 80.0% | 25.5% | 17.5% | N/A | N/A | N/A | 100% |
| Chang et al. 19 | 72.0 | 69.2% | 32.9%* | 35.6% | 73.2% | 88.4% | 37.7% | N/A | N/A | N/A | 0% |
| Bangalore et al. 20 | 69.8 | 61.7% | 26.6%* | 48.6% | 77.6% | 62.9% | 21.4% | N/A | N/A | N/A | 8.3% |
| Chan et al. 21 | 75.1 | 52.7% | 48.8%* | 44.3% | 84.0% | 75.2% | N/A | N/A | N/A | 43.5 † | 6.2% |
| Komiya et al. 22 | 72.7 | 59.7% | 21.4% | 60.4% | 92.9% | 48.1% | 26.6% | N/A | 29.4 | 21.9 | 0% |
| Baber et al. 23 | 67.9 | 63.2% | 10.6% | 100% | 93.8% | 84.3% | 25.7% | N/A | 26.9 | 47.4 | 0% |
| Giustino et al. 24 | 72.7 | 66.2% | 12.3% | 40.4% | 84.8% | 73.9% | 21.6% | 55.5 | 26.5 | 48.6 | 0.8% |
| Lima et al. 25 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0% |
| Milojevic et al. 26 | 71.8 | 67.6% | 10.7% | 30.4% | 85.1% | 78.0% | 34.0% | N/A | 29.5 | 47.6 | N/A |
| Gaipov et al. 27 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 100% |
Represents current or former smoker.
Represents creatinine clearance (ml/min).
eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Primary endpoint
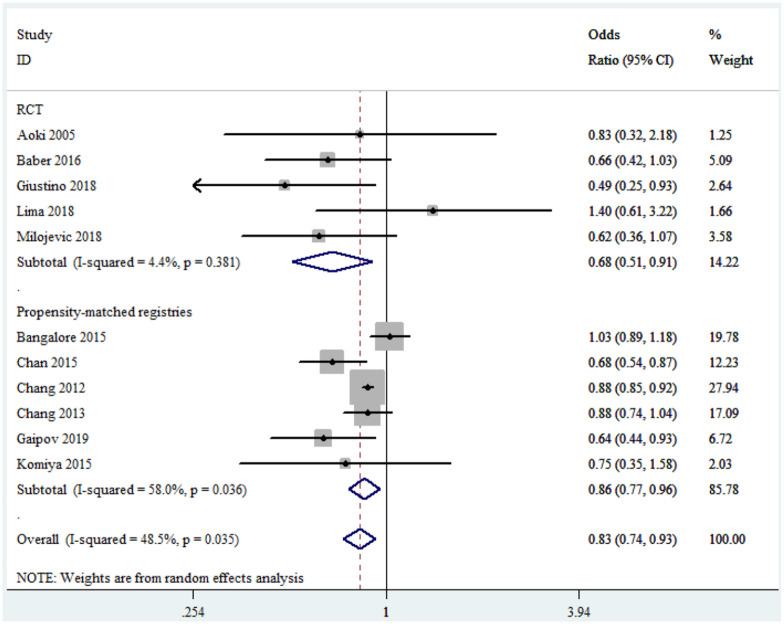
In summary, all the included studies reported all-cause mortality. CABG was significantly associated with lower risk of long-term mortality versus PCI with moderate heterogeneity (OR, 0.83; 95% CI, 0.74–0.93; I2 = 48.5%) (Figure 2). Subgroup analyses showed that there was mild heterogeneity in the sub-analysis of RCTs (OR, 0.68; 95% CI, 0.51–0.91; I2 = 4.4%) and no signs of heterogeneity in studies with long-term follow-up (OR, 0.84; 95% CI, 0.72–0.98; I2 = 0%) (Figure 2 and Table 3). In addition, in the propensity-matched registries (OR, 0.86; 95% CI, 0.77–0.96), in studies with midterm follow-up (OR, 0.81; 95% CI, 0.70–0.95), in studies with multivessel disease (OR, 0.86; 95% CI, 0.77–0.95), and in studies using DES exclusively (OR, 0.72; 95% CI, 0.54–0.97) the results were consistent with the overall analysis (Table 3). Moreover, sensitivity analysis conducted through the removal of any single trial showed that it did not essentially affect the overall pooled estimate (Supplemental Figure S1).
Figure 2.
Forest plot of long-term all-cause mortality for coronary artery bypass grafting versus percutaneous coronary intervention.
CI, confidence interval; RCT, randomized controlled trial.
Table 3.
Sensitivity analysis.
| Outcome | Subgroup | No. studies | OR (95% CI) | I 2 | p heterogeneity |
|---|---|---|---|---|---|
| All-cause death | RCTs | 5 | 0.68 (0.51–0.91) | 4.4% | 0.381 |
| PSM studies | 6 | 0.86 (0.77–0.96) | 58.0% | 0.036 | |
| Long-term | 5 | 0.84 (0.72–0.98) | 0% | 0.410 | |
| Midterm | 6 | 0.81 (0.70–0.95) | 66.9% | 0.010 | |
| MVD | 9 | 0.86 (0.77–0.95) | 45.6% | 0.065 | |
| DES exclusively | 5 | 0.72 (0.54–0.97) | 73.8% | 0.004 | |
| Short-term death | RCTs | 2 | 1.45 (0.27–7.80) | 29.7% | 0.233 |
| PSM studies | 2 | 0.77 (0.41–1.44) | 69.5% | 0.070 | |
| Long-term | 1 | 3.90 (0.43–35.26) | N/A | N/A | |
| Midterm | 3 | 0.76 (0.46–1.24) | 39.2% | 0.193 | |
| MVD | 2 | 0.77 (0.41–1.44) | 69.5% | 0.070 | |
| DES exclusively | 4 | 0.84 (0.48–1.47) | 44.5% | 0.144 | |
| Myocardial infarction | RCTs | 5 | 0.50 (0.26–0.97) | 58.1% | 0.049 |
| PSM studies | 4 | 0.35 (0.20–0.63) | 90.5% | <0.001 | |
| Long-term | 5 | 0.35 (0.23–0.55) | 40.6% | 0.151 | |
| Midterm | 4 | 0.46 (0.22–0.96) | 86.3% | <0.001 | |
| MVD | 7 | 0.37 (0.23–0.60) | 84.1% | <0.001 | |
| DES exclusively | 5 | 0.42 (0.23–0.74) | 84.2% | <0.001 | |
| Stroke | RCTs | 4 | 1.05 (0.55–2.00) | 26.7% | 0.251 |
| PSM studies | 3 | 1.49 (1.18–1.90) | 0% | 0.368 | |
| Long-term | 3 | 0.92 (0.37–2.25) | 49.2% | 0.140 | |
| Midterm | 4 | 1.49 (1.18–1.88) | 0% | 0.567 | |
| MVD | 5 | 1.33 (0.94–1.90) | 31.4% | 0.212 | |
| DES exclusively | 5 | 1.48 (1.18–1.86) | 0% | 0.459 | |
| Repeat revascularization | RCTs | 4 | 0.33 (0.24–0.47) | 0% | 0.484 |
| PSM studies | 4 | 0.19 (0.09–0.38) | 94.3% | <0.001 | |
| Long-term | 4 | 0.24 (0.19–0.29) | 0% | 0.500 | |
| Midterm | 4 | 0.23 (0.08–0.63) | 92.1% | <0.001 | |
| MVD | 6 | 0.21 (0.12–0.36) | 90.6% | <0.001 | |
| DES exclusively | 5 | 0.27 (0.14–0.53) | 89.2% | <0.001 |
CI, confidence interval; DES, drug-eluting stent; MVD, multivessel disease; OR, odds ratio; PSM, propensity-score matched; RCT, randomized controlled trial.
Secondary endpoints
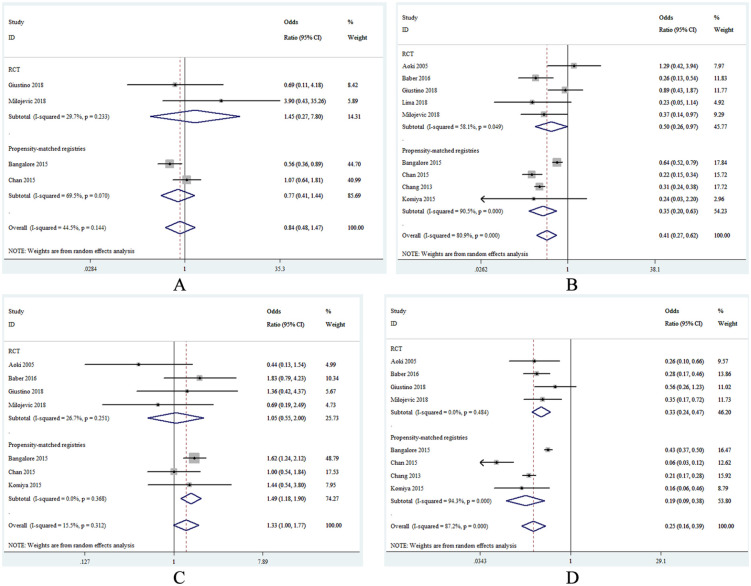
Four studies provided information regarding short-term death. Overall, short-term mortality did not differ between CABG and PCI with moderate heterogeneity (OR, 0.84; 95% CI, 0.48–1.47; I2 = 44.5%; Figure 3A). Also, the lack of statistically significant difference was consistent across all the subset analyses (Table 3).
Figure 3.
Forest plots of the secondary endpoints for coronary artery bypass grafting versus percutaneous coronary intervention. (A) short-term mortality, (B) myocardial infarction, (C) stroke, (D) repeat revascularization. CI, confidence interval; RCT, randomized controlled trial.
The incidence of MI was reported in nine studies. Compared with the PCI group, patients who received CABG were associated with lower risk of MI (OR, 0.41; 95% CI, 0.27–0.62; I2 = 80.9%) (Figure 3B). When analyzing the studies with long-term follow-up exclusively, the heterogeneity was significantly reduced (OR, 0.35; 95% CI, 0.23–0.55; I2 = 40.6%). Additionally, in subset analysis of RCTs (OR, 0.50; 95% CI, 0.26–0.97), in propensity-matched registries (OR, 0.35; 95% CI, 0.20–0.63), in studies with midterm follow-up (OR, 0.46; 95% CI, 0.22–0.96), in studies with multivessel disease (OR, 0.37; 95% CI, 0.23–0.60), and in studies using DES exclusively (OR, 0.42; 95% CI, 0.23–0.74) the results were in line with the overall analysis (Table 3).
Seven studies reported the endpoint of stroke. There was a slightly significant increased risk of stroke in the CABG group compared with the PCI group (OR, 1.33; 95% CI, 1.00–1.77; I2 = 15.5%), which was largely driven by the results of the propensity-matched registries (OR, 1.49; 95% CI, 1.18–1.90), whereas no difference was found between the two strategies in subset analysis of RCTs (OR, 1.05; 95% CI, 0.55–2.00) (Figure 3C). The higher risk of stroke in the CABG group was consistent in studies with midterm follow-up (OR, 1.49; 95% CI, 1.18–1.88), whereas it was similar between the two therapies in studies with long-term follow-up (OR, 0.92; 95% CI, 0.37–2.25). Notably, the higher risk of stroke in the CABG group was more evident when compared with patients who used DES exclusively in the PCI group (OR, 1.48; 95% CI, 1.18–1.86). Besides, the strategy of CABG tended to be associated with higher risk of stroke versus PCI in studies with multivessel disease (OR, 1.33; 95% CI, 0.94–1.90).
As shown in Figure 3D, the use of CABG versus BMS produced a 75% significant reduction in the risk of repeat revascularization (OR, 0.25; 95% CI, 0.16–0.39), with severe heterogeneity (I2 = 87.2%). When analyzing the sub-analysis of RCTs (OR, 0.33; 95% CI, 0.24–0.47; I2 = 0%) or studies with long-term follow-up (OR, 0.24; 95% CI, 0.19–0.29; I2 = 0%), no signs of heterogeneity were found and CABG remained to be associated with lower risk of repeat revascularization in comparison with PCI. In the propensity-matched registries (OR, 0.19; 95% CI, 0.09–0.38), in studies with midterm follow-up (OR, 0.23; 95% CI, 0.08–0.63), in studies with multivessel disease (OR, 0.21; 95% CI, 0.12–0.36), and in studies using DES exclusively (OR, 0.27; 95% CI, 0.14–0.53) the results were in concordance with the overall analysis (Table 3).
Meta-regression analysis and publication bias
Funnel plot assessment was performed, and no publication bias was found for all the outcomes (Supplemental Figure S2 and Supplemental Table S4). In addition, meta-regression analyses revealed significant association between previous MI and the endpoints of MI (regression coefficient, –0.045; 95% CI –0.089 to –0.001; p = 0.047) as well as repeat revascularization (regression coefficient, –0.042; 95% CI –0.068 to –0.016; p = 0.011). No interaction was found between the aforementioned age, gender, diabetes mellitus, hypertension, dyslipidemia, and all the clinical outcomes (Supplemental Table S5).
Discussion
The present meta-analysis involving 26,441 patients showed that the strategy of CABG reduced the risk of long-term mortality, MI, and repeat revascularization compared with PCI in CKD patients with multivessel and/or left main disease. However, CABG was slightly associated with higher risk of stroke than PCI and the short-term mortality was similar between the two treatment strategies. Subgroup analysis of RCTs, propensity-matched registries, studies with long-term or midterm follow-up, studies with multivessel disease, and studies using DES exclusively obtained mostly similar results compared with the overall analysis.
It is well-recognized that complex CAD characterized by diffuse lesions, extensive calcification, and small vessel diameters is more common in CKD patients, making coronary revascularization difficult. 2 During PCI, stents cannot expand adequately in heavily calcified lesions. The increasingly used rotational atherectomy is sufficient to modify physical attributes of calcified plaque to facilitate balloon dilatation and stent implantation. 28 Although it is associated with greater acute diameter gain, less final residual stenosis after stent implantation, and higher procedural success, routine use of rotational atherectomy did not reduce the long-term ischemic events in randomized trials.29,30 Likewise, calcified vessels also pose technical challenges during the performance of distal anastomoses. Not surprisingly, patients with CKD often have an increased risk of unfavorable outcomes after revascularization compared with those without CKD.3,4
However, the relative role of the two revascularization approaches has not been fully demonstrated in this population. Until now, no randomized trial has been performed to address this issue. Current observational studies have shown conflicting results: some reported better outcomes in patients who underwent CABG, whereas others reported similar survival rates between the two groups. Previous meta-analysis conducted by Wang et al. showed that CABG could reduce the all-cause mortality compared with PCI in CKD patients with multivessel disease. 9 Nonetheless, our study has several strengths. First of all, all the available sub-analysis of RCTs and propensity-matched registries were included in our analysis to improve the power and reliability of the results. Contrarily, that review did not include the recently published data from large-scale RCTs and high quality registries, and most of the included studies reported unmatched data severely influenced by confounding factors. Second, we included only complex disease including multivessel disease and/or left main disease in our study, whereas four of the 11 studies enrolled patients with single-vessel disease (17.9–59%) in that review. Moreover, the study by Shroff et al., which accounted for over 60% of the sample size in that meta-analysis, did not report the data of diseased vessel number. Third, more diverse subgroup analyses were performed in our study, and the results of all the outcomes were largely consistent with the overall analysis, confirming the robustness of our findings.
Similar to the recently published meta-analysis of five randomized trials conducted by Gallo et al. in patients with left main disease not stratified for CKD, 31 our study found that CABG reduced the risk of long-term MI and repeat revascularization compared with PCI in CKD patients with multivessel and/or left main disease. Moreover, CABG was also associated with lower long-term all-cause mortality than PCI in the current meta-analysis. Furthermore, this conclusion was reinforced by the fact that the results of subgroup analyses based on different study design, follow-up time, type of stent, and type of disease were totally consistent with the overall population. Theoretically, CABG offers prophylactic protection by virtue of bypassing a larger extent of obstructive lesions or vulnerable plaques, minimizing the effect of progressive disease in the entire upstream proximal vessel. Meanwhile, the use of internal mammary artery ensures the long-term patency of the conduits. 32 In contrast, PCI addresses short segments of severe stenosis where progressive atherosclerosis forms new severe significant stenosis and plaque ruptures. Moreover, the advantage of CABG over PCI may be partially due to the completeness of revascularization, since complete revascularization is more often achieved in patients who underwent CABG rather than PCI, especially in complex disease. 33
Of note, the predominantly applied first-generation DES or BMS and the suboptimal antiplatelet therapies may contribute to the poor outcomes in patients who underwent PCI than those who received CABG. New-generation DESs with novel stent platforms and more biocompatible polymers are associated with enhanced endothelialization, fewer stent fractures and less endothelial dysfunction.34–36 In fact, robust evidence has confirmed the superiority of new-generation DESs regarding lower risks of stent thrombosis, MI, and repeat revascularization compared with first-generation DES and BMS. 37 Also, new antiplatelet drugs have a particular advantage over clopidogrel regarding MI and stent thrombosis. 38 Previous studies have illustrated the more beneficial effect of intravascular ultrasound or fractional flow reserve on prognosis than routine PCI in complex disease. Thus, the use of new-generation DESs, more potent antiplatelet drugs, and new interventional techniques will likely reduce the incidence of ischemic events for patients who received PCI.
The benefit of CABG over PCI with DES comes at the expense of slightly increased stroke risk. Given the serious consequences of stroke, this finding may have important clinical implications. It is generally believed that aortic manipulation and consequent atherosclerotic debris embolization are the most common mechanisms of stroke in on-pump CABG. 39 Hopefully, off-pump CABG with the “aortic no-touch” technique appears to be of special value for patients with CKD. 40 Also, the lower incidence of dual antiplatelet therapy after revascularization might also contribute to the bad result of CABG. Similar to prior observations from the NOBLE and the SYNTAX trials,41,42 our study demonstrated that PCI was associated with an increase in late stroke (>3 years), which might completely counteract the early benefit of PCI.
Limitations
Our study presents several limitations that cannot be ignored. First, considering the lack of RCTs, we included both sub-analysis of RCTs and propensity-matched registries in our study, thus the results should be considered exploratory and hypothesis generating. Second, trials included in this meta-analysis were varied in study design, inclusion criteria, stent type, and follow-up time. Not surprisingly, heterogeneity was noted in the analyses of certain endpoints. Thus, a random-effect model was applied all across the study, and subgroup analyses as well as meta-regression analyses were conducted to explore the heterogeneity. Third, subgroup analysis according to CKD stages was not performed due to lack of data. In fact, our meta-analysis mainly included patients with stage 3–4 CKD, with only two study enrolled patients on dialysis. Thus, the results should be extrapolated carefully to patients on dialysis. Finally, first-generation DES, applied mostly in the original studies, could not fully reflect the clinical practice in the new-generation DES era.
Conclusion
This meta-analysis supports current guideline advising CABG for patients with complex CAD and CKD. At the expense of slightly increased risk of stroke, CABG reduces the incidences of long-term all-cause death, MI, and repeat revascularization compared with PCI. More importantly, the results of all the subgroup analyses were mostly consistent with the overall population. Randomized trials are warranted to investigate the relative benefit of CABG and PCI with new-generation DESs, more potent antiplatelet therapy and new interventional techniques in the future.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_2040622321990273 for Coronary artery bypass graft surgery versus stenting for patients with chronic kidney disease and complex coronary artery disease: a systematic review and meta-analysis by Kongyong Cui, Hong Liu, Fei Yuan, Feng Xu, Min Zhang, Mingduo Zhang, Wei Wang, Dongfeng Zhang, Jinfan Tian, Shuzheng Lyu and Kefei Dou in Therapeutic Advances in Chronic Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Kongyong Cui  https://orcid.org/0000-0002-2313-5368
https://orcid.org/0000-0002-2313-5368
Shuzheng Lyu  https://orcid.org/0000-0002-4243-2341
https://orcid.org/0000-0002-4243-2341
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kongyong Cui, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Hong Liu, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Fei Yuan, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Feng Xu, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Min Zhang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Mingduo Zhang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Wei Wang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Dongfeng Zhang, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Jinfan Tian, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing, China.
Shuzheng Lyu, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University and Beijing Institute of Heart, Lung and Blood Vessel Diseases, 2 Anzhen Road, Chaoyang District, Beijing 100029, China.
Kefei Dou, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
References
- 1. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007; 116: 85–97. [DOI] [PubMed] [Google Scholar]
- 3. Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the society of thoracic surgeons national adult cardiac database. Circulation 2006; 113: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 4. Baber U, Giustino G, Sartori S, et al. Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug-eluting stents: a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv 2016; 9: 28–38. [DOI] [PubMed] [Google Scholar]
- 5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 6. Baber U, Mehran R. Coronary artery revascularization in chronic kidney disease: time for a randomized trial. Circ Cardiovasc Interv 2015; 8: e002140. [DOI] [PubMed] [Google Scholar]
- 7. Kipp R, Lehman J, Israel J, et al. Patient preferences for coronary artery bypass graft surgery or percutaneous intervention in multivessel coronary artery disease. Catheter Cardiovasc Interv 2013; 82: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bangalore S, Kumar S, Fusaro M, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ 2012; 345: e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Zhu S, Gao P, et al. Comparison of coronary artery bypass grafting and drug-eluting stents in patients with chronic kidney disease and multivessel disease: a meta-analysis. Eur J Intern Med 2017; 43: 28–35. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 12. Lundh A, Gotzsche PC. Recommendations by Cochrane review groups for assessment of the risk of bias in studies. BMC Med Res Methodol 2008; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aoki J, Ong AT, Hoye A, et al. Five year clinical effect of coronary stenting and coronary artery bypass grafting in renal insufficient patients with multivessel coronary artery disease: insights from ARTS trial. Eur Heart J 2005; 26: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 18. Chang TI, Shilane D, Kazi DS, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 2012; 23: 2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang TI, Leong TK, Kazi DS, et al. Comparative effectiveness of coronary artery bypass grafting and percutaneous coronary intervention for multivessel coronary disease in a community-based population with chronic kidney disease. Am Heart J 2013; 165: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bangalore S, Guo Y, Samadashvili Z, et al. Revascularization in patients with multivessel coronary artery disease and chronic kidney disease: everolimus-eluting stents versus coronary artery bypass graft surgery. J Am Coll Cardiol 2015; 66: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan W, Ivanov J, Ko D, et al. Clinical outcomes of treatment by percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with chronic kidney disease undergoing index revascularization in Ontario. Circ Cardiovasc Interv 2015; 8: e001973. [DOI] [PubMed] [Google Scholar]
- 22. Komiya T, Ueno G, Kadota K, et al. An optimal strategy for coronary revascularization in patients with severe renal dysfunction. Eur J Cardiothorac Surg 2015; 48: 293–300. [DOI] [PubMed] [Google Scholar]
- 23. Baber U, Farkouh ME, Arbel Y, et al. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J 2016; 37: 3440–3447. [DOI] [PubMed] [Google Scholar]
- 24. Giustino G, Mehran R, Serruys PW, et al. Left main revascularization with PCI or CABG in patients with chronic kidney disease: EXCEL trial. J Am Coll Cardiol 2018; 72: 754–765. [DOI] [PubMed] [Google Scholar]
- 25. Lima EG, Charytan DM, Hueb W, et al. Long-term outcomes of patients with stable coronary disease and chronic kidney dysfunction: 10-year follow-up of the medicine, angioplasty, or surgery study II trial. Nephrol Dial Transplant. Epub ahead of print 24 December 2018. DOI: 10.1093/ndt/gfy379. [DOI] [PubMed] [Google Scholar]
- 26. Milojevic M, Head SJ, Mack MJ, et al. The impact of chronic kidney disease on outcomes following percutaneous coronary intervention versus coronary artery bypass grafting in patients with complex coronary artery disease: five-year follow-up of the SYNTAX trial. EuroIntervention 2018; 14: 102–111. [DOI] [PubMed] [Google Scholar]
- 27. Gaipov A, Molnar MZ, Potukuchi PK, et al. Predialysis coronary revascularization and postdialysis mortality. J Thorac Cardiovasc Surg 2019; 157: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma SK, Tomey MI, Teirstein PS, et al. North American expert review of rotational atherectomy. Circ Cardiovasc Interv 2019; 12: e007448. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann R, Mintz GS, Popma JJ, et al. Treatment of calcified coronary lesions with Palmaz-Schatz stents. An intravascular ultrasound study. Eur Heart J 1998; 19: 1224–1231. [DOI] [PubMed] [Google Scholar]
- 30. Abdel-Wahab M, Richardt G, Joachim Buttner H, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv 2013; 6: 10–19. [DOI] [PubMed] [Google Scholar]
- 31. Gallo M, Blitzer D, Laforgia PL, et al. Percutaneous coronary intervention versus coronary artery bypass graft for left main coronary artery disease: a meta-analysis. J Thorac Cardiovasc Surg. Epub ahead of print 15 April 2020. DOI: 10.1016/j.jtcvs.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 32. Kuntz RE. Importance of considering atherosclerosis progression when choosing a coronary revascularization strategy: the diabetes-percutaneous transluminal coronary angioplasty dilemma. Circulation 1999; 99: 847–851. [DOI] [PubMed] [Google Scholar]
- 33. Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol 2013; 61: 282–294. [DOI] [PubMed] [Google Scholar]
- 34. Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 2008; 52: 333–342. [DOI] [PubMed] [Google Scholar]
- 35. Pendyala LK, Yin X, Li J, et al. The first-generation drug-eluting stents and coronary endothelial dysfunction. JACC Cardiovasc Interv 2009; 2: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 36. Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014; 129: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015; 65: 2496–2507. [DOI] [PubMed] [Google Scholar]
- 38. Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation 2008; 118: 1626–1636. [DOI] [PubMed] [Google Scholar]
- 39. Newman MF, Mathew JP, Grocott HP, et al. Central nervous system injury associated with cardiac surgery. Lancet 2006; 368: 694–703. [DOI] [PubMed] [Google Scholar]
- 40. Palmerini T, Biondi-Zoccai G, Riva DD, et al. Risk of stroke with percutaneous coronary intervention compared with on-pump and off-pump coronary artery bypass graft surgery: evidence from a comprehensive network meta-analysis. Am Heart J 2013; 165: 910–917. [DOI] [PubMed] [Google Scholar]
- 41. Makikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016; 388: 2743–2752. [DOI] [PubMed] [Google Scholar]
- 42. Mack MJ, Head SJ, Holmes DR, Jr, et al. Analysis of stroke occurring in the SYNTAX trial comparing coronary artery bypass surgery and percutaneous coronary intervention in the treatment of complex coronary artery disease. JACC Cardiovasc Interv 2013; 6: 344–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_2040622321990273 for Coronary artery bypass graft surgery versus stenting for patients with chronic kidney disease and complex coronary artery disease: a systematic review and meta-analysis by Kongyong Cui, Hong Liu, Fei Yuan, Feng Xu, Min Zhang, Mingduo Zhang, Wei Wang, Dongfeng Zhang, Jinfan Tian, Shuzheng Lyu and Kefei Dou in Therapeutic Advances in Chronic Disease