Abstract
Background:
For patients with stages I-III colon cancer who have undergone surgical resection, guidelines recommend surveillance colonoscopy at 1 year. However, limited data exist on adherence and associated factors. We aimed to determine the rate of adherence to surveillance colonoscopy at 1 year among nonmetastatic colon cancer patients who underwent resection and factors associated with adherence.
Methods:
In this population-based retrospective cohort study, the Surveillance, Epidemiology, and End Results (SEER)–Medicare database was used. We identified patients with stages I-III colon cancer who underwent surgical resection and survived >3 years without recurrence (no chemotherapy after 8 months) from 2002–2011. Our primary outcome was a colonoscopy claim 10–15 months after resection. We used multivariable regression analysis to assess associations between sociodemographic and clinical factors and receipt of timely colonoscopy.
Results:
Among 28,732 patients who survived >3 years without recurrence, 7967 (28%) did not undergo colonoscopy; 12,033 (42%) had it at one year, with 3159 (11%) before 10 months and 5573 (19%) after 15 months. Decreased adherence was associated with older age; being male versus female; being black or Hispanic versus white; higher tumor stage; left-sided tumors versus right sided; and increased comorbidities. Chemotherapy receipt was associated with increased adherence (odds ratio 2.06; 95% confidence interval 1.88–2.24).
Conclusions:
In a large population-based sample of individuals aged ⩾ 65 years, only 42% of colon cancer survivors underwent 1-year surveillance colonoscopy. Demographic and clinical factors were associated with adherence.
Keywords: epidemiology, colonoscopy, colorectal cancer, quality of health care
Introduction
With the exception of colon cancer, most national guidelines discourage surveillance screening for patients with nearly all types of nonmetastatic cancer. For colon cancer, in contrast, the American Society of Clinical Oncology (ASCO), 1 the American Cancer Society, 2 and the National Comprehensive Cancer Network (NCCN) 3 suggest a physician encounter every 3–6 months for the first 3 years along with testing of the serum carcinoembryonic antigen (CEA) level, as well as CT scans of the chest, abdomen and pelvis annually. The rationales for these recommendations stem from observational studies and clinical trials that have shown improved progression-free and overall survival rates for those found to have liver or lung metastases who have undergone metastasectomies.4,5
The guidelines1–3,6 also recommend a surveillance colonoscopy at 1 year following surgery. This has two goals: to remove metachronous lesions which may arise rapidly after the initial resection or which may represent missed lesions;7–11 and to identify anastomotic recurrences which may develop in up to 4% of patients.10,12,13 One study, 14 using a VA database which followed over 3500 resected colon cancer patients who underwent resection, found a 43% reduction in 5-year mortality for the group who underwent follow-up colonoscopy compared with those who did not [hazard ratio (HR) 0.57; 95% confidence interval (CI) 0.51–0.64]. The reduction in mortality was similar in another study among patients followed a median of 3.6 years in a health maintenance organization (HR 0.58; 95% CI 0.44–0.75). 15 Notably, one case-control study that was conducted in the SEER–Medicare database did not find a benefit for follow-up colonoscopy. 16
We utilized a large population-based database to investigate factors associated with adherence to the surveillance colonoscopy recommendation.
Methods
Data source
Our study utilized data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare database. 17 SEER combines population-based tumor registries from around the country for approximately 28% of the population and provides information on tumor histology, site, stage of disease, treatment, and survival, along with demographic and census tract-level information. SEER is linked to the Medicare database, which includes Medicare A (inpatient) and Medicare B (outpatient) eligibility status, billed claims, and diagnoses. For colonoscopies specifically, we used CPT/HCPCS and ICD9 codes from Medpar and NCH files for the years 2002–2011.
Cohort selection
In our cohort, we included men and women aged 65 years and older who were diagnosed with and underwent surgical resection for nonmetastatic colon cancer from 1 January 2002 through 31 December 2011. To ensure complete information, we excluded any patients who were not enrolled in both Medicare Part A and Part B, as well as those who were members of health maintenance organizations (HMOs). In addition, we excluded those who had rectal cancer or a history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease) recorded in the year prior.
For the analysis of the colonoscopies (see below), we included those patients who survived for at least 36 months without a recurrence during the 3 years. We defined a recurrence for the purposes of this study as undergoing any colon cancer-directed chemotherapy more than 8 months after surgery. (We chose 2011 as the cutoff year to allow for an additional 3 years of observation for all patients, thus ensuring this population was free of recurrence 3 years after cancer resection.)
Colonoscopies
We included in the analysis any colonoscopy claim following the surgical resection of the cancer. The HCPCS (Healthcare Common Procedure Coding System) and CPT (Current Procedural Terminology) codes for colonoscopy that were included are listed in Appendix Table 1.
We reviewed the indications for colonoscopies performed prior to 10 months following resection and found that the indications for the vast majority of these colonoscopies were considered part of the initial staging/diagnostic work up, conducted for the purpose of completing the initial staging work up, or for symptoms. We therefore excluded colonoscopies performed prior to 10 months following resection from the analysis.
Clinical and demographic characteristics
For each patient, we analyzed demographic data obtained from SEER at the time of the colon cancer diagnosis and resection, including age (65–69, 70–74, 75–79, 80–84, 85+ years), sex, race/ethnicity (white, black, Hispanic, other), marital status (married, single, other, unknown), region (eastern, midwest, western), and neighborhood of residence (large metropolitan, metropolitan, nonmetropolitan urban/rural), and socioeconomic status (SES) stratified into quintiles. Variables used to determine SES were education (percentage of adults aged > 25 years who had 12 years of education), poverty (the percentage of individuals living below the poverty line) and income (the median annual household income) and a composite variable using these three socioeconomic variables. 18 We also included the year of colon cancer diagnosis (2002–2004, 2005–2007, 2008–2011). We also collected clinical characteristics, including tumor location (right sided proximal to splenic flexure, left sided), receipt of chemotherapy, stage (I, II, III), and the presence of comorbid disease. To assess the prevalence of comorbid disease (0, 1, 2+), we used the Charlson comorbidity index, defined using ICD-9 coding, as reported by Deyo et al. 19 This was assessed at the time of surgery using the 12 months of claims data prior to that date.
Statistical analysis
Frequency distributions were compared between categorical variables. Then, we assessed the associations between these variables and the outcome of interest using both univariate logistic regression and multivariable logistic regression, controlling for the covariates described above. The results are reported with odds ratios (OR) and 95% confidence intervals, with an elevated OR indicating increased adherence. All analyses were performed with SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA).
Institutional Review Board approval
Since all data were de-identified, this study was considered ‘nonhuman subjects research’ and exemption from the Institutional Review Board of Columbia University Medical Center was obtained. Informed consent was not required for this retrospective study.
Results
We identified 50,173 people with a stage I-III colon cancer who underwent surgical resection between 2002 and 2011 and who survived at least 1 year. Of these, 28,732 (57%) had at least 3 years of follow up without recurrence and form the sample population for this study (see Appendix Table 2). We found that 7967 (28%) did not undergo surveillance colonoscopy at all during the 3-year time frame, while 12,033 (42%) had a surveillance colonoscopy between 10 and 15 months after surgery (see Figure 1). The remaining 8732 (30%) had a colonoscopy either before (3159; 11%) or after (5573; 19%) the recommended 1 year.
Figure 1.
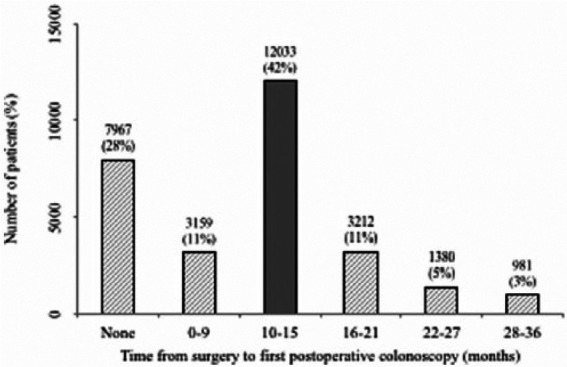
Distribution of colon cancer patients, stages I-III, who underwent surgery between 2002 and 2011 with 3+ years of follow up without recurrence by time from surgery to first postoperative colonoscopy.
Dark gray shading: Within recommended guidelines.
Light gray shading: Not within recommended guidelines.
As noted in the methods section, we assumed that colonoscopies prior to 10 months were either to complete the initial staging work up or for diagnostic purposes rather than for surveillance and so they were excluded from the analysis of predictors of surveillance colonoscopy, leaving a sample of 25,573 (89%) patients for analysis. Therefore, adherence was defined as receiving a colonoscopy between 10 and 15 months after resection. As described below, we also investigated a time frame of 7–18 months.
Table 1 shows the univariate and multivariable associations between sociodemographic, clinical, and other patient characteristics and the receipt of a surveillance colonoscopy between 10 and 15 months after surgery. The multivariable analysis shows that increasing age was associated with a decreased likelihood of adherence to colonoscopy at 1 year, with an adjusted odds ratio (OR) of 0.25 [95% confidence interval (CI) 0.23–0.28] for those 85+ years and 0.64 (95% CI 0.60–0.67) for those 65–69 years. Males were less likely than females to be adherent (OR 0.85; 95% CI 0.80–0.90) and married subjects were more likely than single (OR 1.44; 95% CI 1.36–1.53). Both Hispanics (OR 0.80; 95% CI 0.70–0.92) and Blacks (OR 0.75; 95% CI 0.68–0.84) were less likely to be adherent with the colonoscopy recommendation than Whites. People in metropolitan and rural areas were more likely to be compliant with colonoscopy than those in large metropolitan areas, and increasing SES was also associated with increased adherence (OR 1.34; 95% CI 1.22–1.48 for the highest SES quintile versus the lowest).
Table 1.
Univariate and multivariate analysis of receiving first postoperative colonoscopy during 10–15 months after surgery for colon cancer (stage I-III) patients who had 3+ years follow up without recurrence after the surgery.
| Risk factor | Total n | Colonoscopy (10–15 m) |
No Colonoscopy (10–15 m) |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Total n | 25573 | 12033 | 47% | 13540 | 53% | ||
| Age at diagnosis, (years) | p < 0.01 | p < 0.01 | |||||
| 65–74 (ref) | 9557 | 5667 | 59% | 3890 | 41% | – | – |
| 75–84 | 11715 | 5367 | 46% | 6348 | 54% | 0.58 (0.55–0.61) * | 0.64 (0.60–0.67) * |
| 85+ | 4301 | 999 | 23% | 3302 | 77% | 0.21 (0.19–0.23) * | 0.25 (0.23–0.28) * |
| Sex | p < 0.01 | p < 0.01 | |||||
| Female (ref) | 14997 | 6902 | 46% | 8095 | 54% | – | – |
| Male | 10576 | 5131 | 49% | 5445 | 51% | 1.11 (1.05–1.16) * | 0.85 (0.80–0.90) * |
| Year of diagnosis | p = 0.08 | p < 0.01 | |||||
| 2002–2004 (ref) | 9468 | 4396 | 46% | 5072 | 54% | – | – |
| 2005–2007 | 8719 | 4083 | 47% | 4636 | 53% | 1.02 (0.96–1.08) | 1.05 (0.98–1.11) |
| 2008–2011 | 7386 | 3554 | 48% | 3832 | 52% | 1.07 (1.01–1.14) * | 1.16 (1.08–1.24) * |
| Race | p < 0.01 | p < 0.01 | |||||
| Non-Hispanic white (ref) | 21319 | 10201 | 48% | 11118 | 52% | – | – |
| Non-Hispanic black | 1929 | 770 | 40% | 1159 | 60% | 0.72 (0.66–0.80) * | 0.75 (0.68–0.84) * |
| Hispanic | 1064 | 437 | 41% | 627 | 59% | 0.76 (0.67–0.86) * | 0.80 (0.70–0.92) * |
| Others | 1261 | 625 | 50% | 636 | 50% | 1.07 (0.96–1.20) | 1.14 (1.01–1.29) * |
| Region | p < 0.01 | p < 0.01 | |||||
| Eastern (ref) | 5594 | 2464 | 44% | 3130 | 56% | – | – |
| Midwest | 10829 | 5480 | 51% | 5349 | 49% | 1.30 (1.22–1.39) * | 1.16 (1.08–1.25) * |
| Western | 9150 | 4089 | 44% | 5061 | 55% | 1.03 (0.96–1.10) | 0.95 (0.89–1.02) |
| Neighborhood | p < 0.01 | p < 0.01 | |||||
| Large metropolitan (ref) | 13140 | 5828 | 44% | 7312 | 56% | – | – |
| Metropolitan | 7714 | 3776 | 49% | 3938 | 51% | 1.20 (1.14–1.27) * | 1.14 (1.07–1.21) * |
| Nonmetro urban/rural | 4719 | 2429 | 51% | 2290 | 49% | 1.33 (1.25–1.42) * | 1.23 (1.13–1.33) * |
| Marital status | p < 0.01 | p < 0.01 | |||||
| Single/divorced/widow (ref) | 11489 | 4547 | 40% | 6942 | 60% | – | – |
| Married/domestic partner | 13172 | 7090 | 54% | 6082 | 46% | 1.78 (1.69–1.87) * | 1.44 (1.36–1.53) * |
| Unknown | 912 | 396 | 43% | 516 | 57% | 1.17 (1.02–1.34) * | 1.08 (0.93–1.24) |
| SES rank § | p < 0.01 | p < 0.01 | |||||
| 1 (ref) | 4542 | 1949 | 43% | 2593 | 57% | – | – |
| 2 | 5413 | 2576 | 48% | 2837 | 52% | 1.21 (1.12–1.31) * | 1.20 (1.10–1.31) * |
| 3 | 3967 | 1898 | 48% | 2069 | 52% | 1.22 (1.12–1.33) * | 1.23 (1.12–1.35) * |
| 4 | 6606 | 3168 | 48% | 3438 | 52% | 1.23 (1.14–1.32)* | 1.31 (1.20–1.43) * |
| 5 | 5045 | 2442 | 48% | 2603 | 52% | 1.25 (1.15–1.35) * | 1.34 (1.22–1.48) * |
| Tumor location | p = 0.23 | p < 0.01 | |||||
| Right-sided colon (ref) | 17551 | 8303 | 47% | 9248 | 53% | – | – |
| Left-sided colon | 8022 | 3730 | 47% | 4292 | 54% | 0.97 (0.91–1.02) | 0.85 (0.81–0.91) * |
| Chemotherapy used | P<0.01 | P<0.01 | |||||
| No (ref) | 21662 | 9589 | 44% | 12073 | 56% | – | – |
| Yes | 3911 | 2444 | 62% | 1467 | 38% | 2.10 (1.96-2.25) * | 2.06 (1.88-2.24) * |
| Stage | p < 0.01 | p < 0.01 | |||||
| I (ref) | 8904 | 4480 | 50% | 4424 | 50% | – | – |
| II | 10909 | 4904 | 45% | 6005 | 55% | 0.86 (0.76–0.85) * | 0.82 (0.77–0.87) * |
| III | 5760 | 2649 | 46% | 3111 | 54% | 0.84 (0.79–0.90) * | 0.61 (0.56–0.66) * |
| Comorbidity (modified CCI) | P<0.01 | P<0.01 | |||||
| 0 (ref) | 13868 | 6996 | 50% | 6872 | 50% | – | – |
| 1 | 7132 | 3264 | 46% | 3868 | 54% | 0.83 (0.78–0.88) * | 0.86 (0.81–0.91) * |
| 2+ | 4573 | 1773 | 39% | 2800 | 61% | 0.62 (0.58–0.67) * | 0.68 (0.63–0.73) * |
Higher odds ratio (OR) indicates more likely to receive colonoscopy during 10–15 months after surgery of colon cancer.
Indicates significant at α = 0.05.
Higher SES rank indicates higher family income and education level.
CI, confidence interval; CCI, Charlson comorbidity index; SES, socioeconomic status.
We also examined clinical characteristics and their association with adherence to surveillance colonoscopy. Higher tumor stage was associated with decreased compliance (OR 0.61; 95% CI 0.56–0.66 for stage III and OR 0.82; 95% CI 0.77–0.87 for stage II versus 1.00 for stage I) and increased comorbidity score was also associated with worse compliance (OR 0.68; 95% CI 0.63–0.73 for two or more comorbidities versus 1.00 for those with no comorbidities). Those with left-sided colon cancers were also less likely to be adherent to the surveillance colonoscopy recommendation (OR 0.85; 95% CI 0.81–0.91). Finally, receipt of adjuvant chemotherapy (after controlling for tumor stage) was associated with increased adherence to the colonoscopy recommendation (OR 2.06; 95% CI 1.88–2.24).
We conducted a sensitivity analysis using 7–18 months as the range for adherence to the surveillance colonoscopy recommendation rather than 10–15 months and found that 16,147 (56%) of patients had colonoscopies during that time frame. Of the 3159 subjects (11%) who underwent colonoscopy in the 0–9-month time frame following surgery, 1217 (4%) received the colonoscopy during the first 6 months, while 1942 (7%) underwent colonoscopy in the 7–9-month time interval.
Discussion
While surveillance colonoscopy is almost universally recommended at 1 year following colon resection in individuals with nonmetastatic colon cancer, our study found that only 42% received it within the recommended 1-year time frame. Nonreceipt of timely colonoscopy was associated with increasing age, male sex, black race or Hispanic ethnicity, living in a metropolitan area, being single, having lower SES, higher stage at diagnosis, left-sided tumor, nonreceipt of adjuvant chemotherapy, and increased comorbidities.
The appropriate measures to utilize for surveillance for cancer survivors have become an increasingly debated topic. Randomized trials and cohort studies have often failed to find a survival benefit for the use of tumor markers or radiologic procedures that have typically been used in the first few years following the primary treatment of cancers, such as breast or ovarian cancer, and so they are not routinely recommended for most cancers.20–22 Colon cancer has been an exception to that trend, largely because resection of limited metastases has often proven to be efficacious in prolonging life and curing patients.23–25 Thus, studies have demonstrated that intensive follow-up strategies that encompass office visits, tumor markers, radiologic procedures, and endoscopy are both life prolonging and cost effective.4,26–29 Usually included in these algorithms is the 1-year surveillance colonoscopy.
Roughly 1.5–4% of colon cancer survivors will develop a metachronous colon cancer within the first 2 years after resection.10–13 However, these studies were done prior to the routine use of adjuvant chemotherapy. At least two studies have demonstrated in large administrative databases that surveillance colonoscopy at 1 year is associated with reduced odds of metachronous colon cancer,14,15 while one study did not find such an effect. 16 Two meta-analyses have also supported the use of 1-year follow-up colonoscopies.4,29
Despite the fact that most guidelines recommend 1-year follow-up surveillance colonoscopy, we found that nonadherence was substantial. Four other studies conducted in large population-based administrative databases found comparable results. Cooper and colleagues 30 investigated the use of guideline-recommended surveillance studies in the follow up of 9426 colorectal cancer patients who underwent resection in 2000–2001. They found that 74% of patients had colonoscopy within 3 years of diagnosis; if we include those who had a colonoscopy by 36 months, the percentage in our cohort is 61%. Decreased adherence to the recommendation was found for those with advanced age, who were black and with increased comorbidities. A second large population-based study 31 utilized 1423 colorectal cancer survivors within the Cancer Care Outcomes Research and Surveillance Study (CANCORS) who underwent surgery between 2003 and 2005 and were alive without recurrence for at least 14 months. They found that 49% received follow-up colonoscopy within 14 months of surgery. Surveillance colonoscopy was more probable in those with colon (versus rectal) cancer; those who had visited a primary care physician; and those who had received adjuvant chemotherapy (OR 1.75; 95% CI 1.27–2.41). The presence of comorbidities reduced the probability of undergoing surveillance colonoscopy. Stage III patients in this study were less likely to undergo surveillance colonoscopy (OR 0.68; p = 0.04). Race, age and marital status had no effect. Vargas and colleagues 32 found that among 12,381 patients from the Texas Cancer Registry who were recurrence free for 3 years, 75.3% received at least one colonoscopy in a 3-year surveillance period. Salloum and colleagues 33 found that among 2297 patients, only 55.0% received one complete exam of the colon during the 18 months after treatment with curative intent.
We found multiple demographic and clinical factors to be associated with nonadherence to this recommendation. Many of these factors have also been reported in other studies to be associated with nonadherence to other interventions and recommendations. Some of these factors are quite prevalent and thus their effects can have a substantial impact on the nonreceipt of colonoscopy, as we observed. For example, 20% of the US population lives in the rural/small metropolitan area and thus the lower colonoscopy rate in the larger metropolitan area 34 could have a much more substantial effect.
We found a 20–25% decreased receipt of colonoscopy among Blacks and Hispanics as compared with Whites (Table 1). We have found previously that racial/ethnic disparities that favor Whites exist for a substantial number of treatments and interventions.35–38 This was found in addition to the fact that lower SES patients were also less likely to undergo colonoscopy at 1 year; thus, both factors were associated with receipt of this procedure independently. Our studies have also found that being married versus being single is usually associated with increased compliance with the appropriate guidelines and receipt of the appropriate treatment or intervention, as in this study. 39
More advanced stage at diagnosis also seemed to be associated with lower odds of undergoing colonoscopy at 1 year, also seen in the study by Salz and colleagues. 31 Several reasons may account for this. Increased stage would lead to receipt of adjuvant chemotherapy, and thus one could be more fatigued and have a lower performance status and reduced desire to undergo further testing. Higher stage also identifies a higher risk group for whom colonoscopy might be considered more necessary, and therefore more emphasis would have been placed on colonoscopy by the treating doctors. It is possible that more of the later-stage patients underwent the follow-up colonoscopy in the time intervals following the 10–15-month time frame in our study. It is also important to recognize that the estimate of the association with stage may have been reduced because chemotherapy receipt was also associated with receipt of colonoscopy and could have confounded the association with stage.
We also found that the receipt of adjuvant chemotherapy was associated with increased adherence to the surveillance colonoscopy guideline, as found by others. 31 This may result from the fact that these patients are under closer medical monitoring and thus more likely to be advised to undergo screening and more likely to be returning regularly for interval check ups with their oncologists.
Finally, it is noteworthy that those with left-sided tumors were less likely to undergo surveillance colonoscopy. It has been found that patients with rectal cancer are less likely to undergo surveillance colonoscopy than patients with colon cancer, 30 but we chose not to include rectal cancer in these analyses, as the presence of an ostomy further may impact the timing of surveillance. Why this difference occurred remains speculative.
While our study has several major strengths, such as a large population-based high-quality database, we recognize that there are some important potential limitations as well. Some misclassification may have occurred with regard to the identification of diagnostic versus surveillance colonoscopy. With any study that utilizes administrative and billing data, we can define and report associations, but we are unable to determine causality that would require randomized controlled trials. Further, we do not have data on some potentially important variables; most notably, performance status, which would be an important covariate. While the comorbidity index accounts for this to some degree, it is not a perfect replacement. Furthermore, this database is limited to those over 65 years of age and so its results would not necessarily be generalizable to a younger age range of colorectal cancer patients. We did not have any information as to reasons for why surveillance colonoscopy was not specifically done, that is, whether the patient or the physician was responsible for the nonperformance. We did not have physician-related factors available for analysis.
We used a 10- to 15-month time interval to define adherence. This is, of course, to some degree arbitrary. When we did the same analyses with a wider time interval (7–18 months) we found essentially the same predictors of adherence. Another potential issue is that we included patients without recurrence for 36 months, and defined recurrence as receipt of a new chemotherapy regimen. Despite the fact that we used methods similar to those used in other studies to define recurrence,40–43 we acknowledge that this approach may not capture patients who chose not to receive chemotherapy, as suggested by a recent paper. 44 We do not feel this would have had a large impact on our sample population, as we required that patients survive 3 years, which would have been less likely without chemotherapy. Further, our study sample was from 2002 and forward (rather than in the 1990s, as in the Warren paper) and we suspect that the improvements in chemotherapy in that 10-year time interval will have led to greater utilization of chemotherapy by the elderly.
In conclusion, surveillance colonoscopy is recommended at 1 year of follow up for patients with stages I, II, and III colon cancer who have undergone resection of the tumor. In a large population-based sample of individuals age ⩾ 65 years, we found that 28% of survivors did not undergo this follow up at all and that only 42% underwent colonoscopy at the recommended 1-year time point. Knowledge regarding factors associated with nonadherence can be used to identify those at risk for nonadherence and to plan future interventions to improve adherence.
Supplemental Material
Supplemental material, ColonoscopySupplement for Adherence to colonoscopy at 1 year following resection of localized colon cancer: a retrospective cohort study by Alfred I. Neugut, Xiaobo Zhong, Benjamin Lebwohl, Grace C. Hillyer, Melissa K. Accordino, Jason D. Wright, Ravi P. Kiran and Dawn L. Hershman in Therapeutic Advances in Gastroenterology
Footnotes
Funding: Dr Hershman (NCI R01 CA166084) and Dr Wright (NCI R01CA169121) are recipients of grants from the National Cancer Institute. Dr Accordino is the recipient of a postdoctoral fellowship (R25 CA094061) from the National Cancer Institute and a Young Investigator Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Alfred I. Neugut, Department of Medicine, Columbia University, New York, USA Department of Epidemiology, Columbia University, New York, USA; Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA.
Xiaobo Zhong, Department of Biostatistics, Columbia University, New York, USA.
Benjamin Lebwohl, Department of Medicine, Columbia University Medical Center, 180 Fort Washington Avenue, Suite 936, New York, NY 10032, USA.
Grace C. Hillyer, Deparment of Epidemiology, Columbia University, New York, USA Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA.
Melissa K. Accordino, Department of Medicine, Columbia University, New York, USA Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA.
Jason D. Wright, Department of Epidemiology, Columbia University, New York, USA Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA; Department of Obstetrics and Gynecology, Columbia University, New York, USA.
Ravi P. Kiran, Department of Epidemiology, Columbia University, New York, USA Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA; Department of Surgery, Columbia University, New York, USA.
Dawn L. Hershman, Department of Medicine, Columbia University, New York, USA Department of Epidemiology, Columbia University, New York, USA; Herbert Irving Comprehensive Cancer Center, Columbia University, New York, USA.
References
- 1. Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013; 31: 4465–4470. [DOI] [PubMed] [Google Scholar]
- 2. El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin 2015; 65: 428–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benson AB, 3rd, Bekaii-Saab T, Chan E, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013; 11: 519–528. [DOI] [PubMed] [Google Scholar]
- 4. Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, et al. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015; 26: 644–656. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med 1998; 129: 27–35. [DOI] [PubMed] [Google Scholar]
- 6. Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology 2016; 150: 758–768.e711. [DOI] [PubMed] [Google Scholar]
- 7. Green RJ, Metlay JP, Propert K, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med 2002; 136: 261–269. [DOI] [PubMed] [Google Scholar]
- 8. Schoemaker D, Black R, Giles L, et al. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology 1998; 114: 7–14. [DOI] [PubMed] [Google Scholar]
- 9. Ringland CL, Arkenau HT, O’Connell DL, et al. Second primary colorectal cancers (SPCRCs): experiences from a large Australian Cancer Registry. Ann Oncol 2010; 21: 92–97. [DOI] [PubMed] [Google Scholar]
- 10. Barillari P, Ramacciato G, Manetti G, et al. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Dis Colon Rectum 1996; 39: 388–393. [DOI] [PubMed] [Google Scholar]
- 11. Mulder SA, Kranse R, Damhuis RA, et al. The incidence and risk factors of metachronous colorectal cancer: an indication for follow-up. Dis Colon Rectum 2012; 55: 522–531. [DOI] [PubMed] [Google Scholar]
- 12. Juhl G, Larson GM, Mullins R, et al. Six-year results of annual colonoscopy after resection of colorectal cancer. World J Surg 1990; 14: 255–260; discussion 260–251. [DOI] [PubMed] [Google Scholar]
- 13. Castells A, Bessa X, Daniels M, et al. Value of postoperative surveillance after radical surgery for colorectal cancer: results of a cohort study. Dis Colon Rectum 1998; 41: 714–723; discussion 723–714. [DOI] [PubMed] [Google Scholar]
- 14. Fisher DA, Jeffreys A, Grambow SC, et al. Mortality and follow-up colonoscopy after colorectal cancer. Am J Gastroenterol 2003; 98: 901–906. [DOI] [PubMed] [Google Scholar]
- 15. Rulyak SJ, Lieberman DA, Wagner EH, et al. Outcome of follow-up colon examination among a population-based cohort of colorectal cancer patients. Clin Gastroenterol Hepatol 2007; 5: 470–476; quiz 407. [DOI] [PubMed] [Google Scholar]
- 16. Ramsey SD, Howlader N, Etzioni R, et al. Surveillance endoscopy does not improve survival for patients with local and regional stage colorectal cancer. Cancer 2007; 109: 2222–2228. [DOI] [PubMed] [Google Scholar]
- 17. Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 1993; 31: 732–748. [PubMed] [Google Scholar]
- 18. Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 2007; 110: 660–669. [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis Manag 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 20. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016; 34: 611–635. [DOI] [PubMed] [Google Scholar]
- 21. Rustin G, Van der Burg M, Griffin C, et al. Early versus delayed treatment of relapsed ovarian cancer. Lancet 2011; 377: 380–381. [DOI] [PubMed] [Google Scholar]
- 22. Rustin GJ. Follow-up with CA125 after primary therapy of advanced ovarian cancer has major implications for treatment outcome and trial performances and should not be routinely performed. Ann Oncol 2011; 22(Suppl. 8): viii45–viii48. [DOI] [PubMed] [Google Scholar]
- 23. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007; 25: 4575–4580. [DOI] [PubMed] [Google Scholar]
- 24. Robertson DJ, Stukel TA, Gottlieb DJ, et al. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer 2009; 115: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010; 97: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 26. Renehan AG, O’Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 2004; 328: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002; 324: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 2003; 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007; 50: 1783–1799. [DOI] [PubMed] [Google Scholar]
- 30. Cooper GS, Kou TD, Reynolds HL., Jr. Receipt of guideline-recommended follow-up in older colorectal cancer survivors: a population-based analysis. Cancer 2008; 113: 2029–2037. [DOI] [PubMed] [Google Scholar]
- 31. Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res 2010; 10: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vargas GM, Sheffield KM, Parmar AD, et al. Physician follow-up and observation of guidelines in the post treatment surveillance of colorectal cancer. Surgery 2013; 154: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salloum RG, Hornbrook MC, Fishman PA, et al. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer 2012; 118: 5644–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. US Census Bureau. GCT-P1. Urban/rural and metropolitan/nonmetropolitan population. 2000. Census 2000 summary file 1. Washington DUCB. Available at: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_GCTP1.US26&prodType=table (accessed 23 July 2014).
- 35. Hershman DL, Wilde ET, Wright JD, et al. Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer. J Clin Oncol 2012; 30: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundararajan V, Grann VR, Jacobson JS, et al. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: a population-based study. Cancer J 2001; 7: 213–218. [PubMed] [Google Scholar]
- 37. Neugut AI, Fleischauer AT, Sundararajan V, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. J Clin Oncol 2002; 20: 2643–2650. [DOI] [PubMed] [Google Scholar]
- 38. Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat 2012; 136: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cui Z, Wright JD, Accordino MK, et al. Safety, utilization, and cost of image-guided percutaneous liver biopsy among cancer patients. Cancer Invest 2016; 34: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hershman DL, Wright JD, Lim E, et al. Contraindicated use of bevacizumab and toxicity in elderly patients with cancer. J Clin Oncol 2013; 31: 3592–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 2013; 119: 3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Y, Mauldin PD, Ebeling M, et al. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer 2013; 119: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 43. Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment–a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev 2012; 21: 800–809. [DOI] [PubMed] [Google Scholar]
- 44. Warren JL, Mariotto A, Melbert D, et al. Sensitivity of medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care 2016; 54: e47–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ColonoscopySupplement for Adherence to colonoscopy at 1 year following resection of localized colon cancer: a retrospective cohort study by Alfred I. Neugut, Xiaobo Zhong, Benjamin Lebwohl, Grace C. Hillyer, Melissa K. Accordino, Jason D. Wright, Ravi P. Kiran and Dawn L. Hershman in Therapeutic Advances in Gastroenterology


