Abstract
Epileptic seizures are associated with excessive neuronal spiking. Perisomatic γ-aminobutyric acid (GABA)ergic interneurons specifically innervate the subcellular domains of postsynaptic excitatory cells that are critical for spike generation. With a revolution in transcriptomics-based cell taxonomy driving the development of novel transgenic mouse lines, selectively monitoring and modulating previously elusive interneuron types is becoming increasingly feasible. Emerging evidence suggests that the three types of hippocampal perisomatic interneurons, axo-axonic cells, along with parvalbumin- and cholecystokinin-expressing basket cells, each follow unique activity patterns in vivo, suggesting distinctive roles in regulating epileptic networks.
Keywords: GABA, inhibition, hippocampus, interneuron, perisomatic, basket cells, axo-axonic cells, epilepsy, CB1, cannabinoid, temporal lobe epilepsy, cholecystokinin, parvalbumin
Cellular Diversity of Perisomatic Inhibition
A prevalent strategy to combat epilepsy and seizures is to suppress neuronal hyperexcitability. Inhibition by GABAergic interneurons is an intrinsic mechanism in the brain to constrain glutamatergic neuron excitability, 1 and impaired inhibitory function is an important mechanism of epileptogenesis. 2 Some of our oldest anticonvulsants (benzodiazepines), as well as latest experimental therapeutic strategies (implanting interneuron progenitors or optogenetically driving interneuron activity) act by enhancing inhibition.3-5 However, multiple generations of antiseizure medications have failed to improve upon the unacceptably high proportion of patients with pharmacoresistant seizures, particularly in temporal lobe epilepsy (TLE). 6 Even with successful seizure control, patient quality of life is adversely affected by the frequent side effects of antiseizure medications and the various cognitive comorbidities of epilepsy.7,8 Thus, there is an immense unmet need for effective, disease-modifying, antiepileptic treatments, and the brain’s innate inhibitory system is a promising target for new strategies.
As an analogy to the epileptic brain with impaired inhibition, consider a truck with bad brakes. If a seizure is a runaway truck on a downhill slope, are interneurons brakes that we can engage to stop seizures? Unfortunately, the picture is more complicated. Interneurons can be recruited in seizures prior to excitatory neurons, and inhibition may even actively contribute to generating pathological, hypersynchronous oscillations.9-14 Interneurons have diverse developmental origins, connectivity, activity patterns, and synaptic properties, manifesting in at least 20 distinct types within individual brain regions.15-17 The limited success and frequent side effects of various previous treatment strategies that aimed to indiscriminately boost inhibition indicate that we need to target specific inhibitory microcircuit elements that are particularly effective in preventing seizures. However, our understanding of functional interneuron diversity remains limited in healthy brains and even more so in epilepsy.
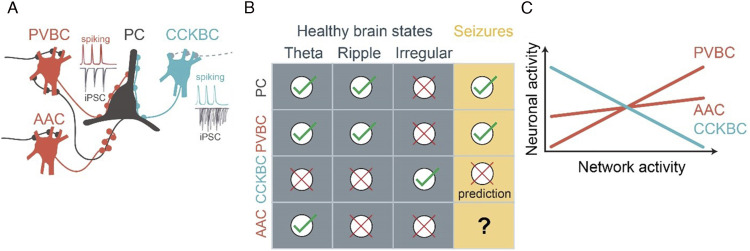
Perisomatic inhibition controls excitatory cell output, regulating both action potential rates and their precise timing in relation to rhythmic network activity. 18 Therefore, perisomatic inhibition is well positioned to regulate both network excitability and propensity to hyper-synchronization. Cortical pyramidal cells receive convergent input from distinct perisomatic GABAergic interneuron types (Figure 1a): basket cells (BCs) innervate the somata and proximal dendrites, while axo-axonic cells (AACs) innervate the axon initial segment, the site of action potential generation. There are two types of BCs, expressing either cholecystokinin (CCK) or parvalbumin (PV). PVBCs receive strong feed-forward and feed-back excitatory inputs and give rise to temporally precise, fast synaptic currents. In contrast, CCKBCs receive relatively weak excitatory inputs and evoke synaptic currents that are temporally imprecise and longer lasting.19,20 In the healthy brain, while PVBCs critically maintain theta- and gamma-range synchrony required for cognitive functions, 21 the circuit functions of CCKBCs and AACs remain largely unknown. In epilepsy, PVBC properties predict pronounced, yet complex, roles in synchrony during recurrent seizures that are supported by ample experimental data. However, the roles of CCKBCs and AACs in epileptic brain activity remain an open question.
Figure 1.
Activity patterns of perisomatic interneurons. (a) Pyramidal cells (PC) receive convergent perisomatic input from PV- and CCK-expressing basket cells, and axo-axonic cells. The inserts symbolize the timing and precision of postsynaptic currents during rhythmic network activity. (b) Brain state–specific recruitment of pyramidal cells and perisomatic interneurons. (c) Scaling modes of perisomatic interneurons with average network activity. Note: PV: parvalbumin; CCK: cholecystokinin.
New Tools Make Perisomatic Interneurons Accessible
Interneurons display strikingly specific expression of certain neuropeptides and calcium-binding proteins, such as PV, CCK, somatostatin (SST), and vasoactive intestinal polypeptide (VIP). These classical markers are often used as proxies to define interneuron classes and for genetically targeting indicators (to record) or actuators (to modulate) the activity of each class. Despite proving extremely useful for earlier investigations, classical marker expression cannot differentiate interneuron types along all dimensions of diversity. For example, in the hippocampus, the brain area most impacted by TLE, PV expresses in multiple anatomical cell types: not only in perisomatic BCs and AACs, but also in dendrite-innervating bistratified cells. Classical CCKBC markers, CCK and the cannabinoid receptor type 1 (CB1), are also expressed by pyramidal cells. Consequently, there has previously been a lack of genetic tools to selectively label each perisomatic interneuron type, which has significantly hindered our understanding of their precise roles in epilepsy and relative utility as targets of future antiepileptic therapies.
Fortunately, the set of available tools is expanding at a staggering pace. Interneuron taxonomy based on single-cell whole-transcriptome RNA sequencing has identified interneuron types that are conserved across brain areas and species.22-25 Integrated analysis of morphologic, electrophysiologic, and transcriptomic features revealed 28 distinct interneuron types. 26 As opposed to loosely related groups of cells expressing an arbitrary marker gene, cell types defined based on the whole transcriptome (i.e., by clustering cells considering the quantitative expression levels of all genes) are more likely to correspond to distinct morpho-functional cell types representing circuit elements with unique roles in brain function. 26 Importantly, such advances in transcriptomics allow researchers to computationally identify differentially expressed genes (typically transcription factors or cell adhesion molecules) in each cell type. As a result, interneuron type-specific transgenic mouse lines or synthetic viral enhancers are rapidly becoming available,27-31 including transgenic lines for selectively labeling CCKBCs (Sncg-Flp) 32 or AACs (Nkx2.1-CreER, Unc5b-CreER, or Vipr2-Cre).27,33,34
Distinct Activity Patterns of Perisomatic Interneurons in vivo
Seizures do not emerge from a vacuum: the striking association of seizures with diurnal and ultradian patterns and sleep stages highlight the critical role of brain states in seizure generation both in patients and in animal models.35-37 Mechanistic understanding of unprovoked, spontaneously occurring seizures requires model systems with the excitatory, inhibitory, and modulatory connections, and energy and metabolic homeostasis intact, preferably in vivo. However, methods that allow recording identified interneurons in vivo, such as the sharp electrode or juxtacellular techniques, have historically been extremely laborious. Interneuron type-specific transgenic driver lines now open the door to using high-throughput in vivo recording methods, such as multielectrode arrays and two-photon calcium imaging.
In awake non-epileptic mice, the dominant brain states include periods of theta-frequency (5–10 Hz) rhythmic activity (typically associated with exploratory behavior, such as locomotion), alternating with periods of irregular activity (associated with quiet wakefulness). Intermittently, during these quiet periods, hippocampal ensembles are briefly reactivated during high-frequency (120–200 Hz) sharp wave-ripple episodes. 38 Perisomatic interneuron activity is remarkably brain state–specific (Figure 1b). 15 Interestingly, PVBC activity is high in the same brain states in which pyramidal cell activity is also relatively high, including theta- and ripple oscillations. On the contrary, CCKBCs are suppressed in these brain states, yet they are active when the rest of the network is suppressed, potentially owing to a mutual inhibitory connectivity between the PVBC and CCKBC populations. 32 AACs are recruited during theta-, but not during ripple oscillations.33,39,40 Similar trends were found on the time scale of seconds, during smaller activity fluctuations within behavioral states: while PVBC activity is strongly positively correlated to pyramidal cell activity, AAC activity is only weakly correlated and CCKBC activity is clearly negatively correlated (Figure 1c).32,33
This latter observation is especially surprising because previous reports (lacking specific CCKBC labeling) suggested that interneuron activity typically scales positively with network activity, both in control and epileptic brains. Positively scaled, also known as balanced inhibition, contributes to stabilizing network dynamics across a wide range of activity levels. 41 In sensory cortices, presentations of simple tactile, olfactory, auditory, or visual stimuli lead to coordinated activation of pyramidal cells and fast-spiking interneurons alike. 42 However, the significance of negatively scaled inhibition is less obvious.
The stark contrast of positively and negatively scaled activity of the two basket cell populations raises a point critically important for epilepsy research. Returning to our metaphor: can we stop the runaway truck by pressing harder on a brake pedal that is already maximally engaged? One may argue that further boosting positively scaled inhibition, which is already highly active during seizures, is unlikely to be the most efficient intervention. However, engaging negatively scaled inhibition that remains underutilized in the first place may have a greater effect during seizures, much like activating emergency brakes on a speeding vehicle.
Unfortunately, data on interneuron type-specific activity patterns during spontaneous seizures in chronic epilepsy models are scant. Due to the relative sparsity of seizures in most chronic epilepsy models (such as mouse models of TLE), only certain types of seizures have been amenable for interneuron type-specific in vivo recording. One such example is the Scn1a +/− model of Dravet syndrome, in which seizures are provoked by increasing the body temperature of head-restrained mice, while recording neocortical interneuron activity using two-photon calcium imaging. 43 PV interneuron activity was found to be already elevated interictally compared to controls at baseline temperatures, and activity rose further with increasing temperature, before reaching maximal activation at seizure onset. In this paradigm, PV, SST, and VIP INs and pyramidal cells all increase their activity leading up to, and during, epileptic seizures. 44 Such coordination indicated that just like in control brains, most interneurons are positively scaled in epilepsy. Of note, VIP cells that innervate other interneurons and thus have a net disinhibitory effect45,46 are also recruited by seizures and thus may change inhibitory dynamics in complex ways by differentially affecting other interneuron subtypes in epilepsy.47,48 However, given the prior inability to identify CCKBCs during in vivo recordings, we do not know yet if CCKBCs are negatively scaled in epilepsy. Speculatively, the demonstrated high PVBC (and perhaps VIP) interneuron activity may contribute to CCKBC suppression during seizure generation.
Brake or Accelerator: Antiepileptic Effects of PV Interneurons
The ultimate test of cell type-specific roles of interneurons in epilepsy is to determine whether interventions targeting each type are effective at preventing or curtailing seizures, and at improving comorbidities. In the case of PVBCs, the results are mixed. Optogenetically driving PV cells within PV–Cre transgenic mice reliably terminated spontaneous seizures when photostimulation was triggered by an on-line seizure detection system. 49 Such precise intervention may also have disease-modifying effects, as similarly suppressing seizures over weeks leads to increased cognitive performance in mice with chronic TLE. 50 Permanent PV cell silencing reduced seizure threshold, 51 corroborating their antiepileptic effects. However, chronically driving PV cells may eventually elicit seizures. 52 The loss of efficacy with prolonged PVBC activation may be due to postsynaptic GABAergic responses becoming depolarizing 53 or PV cells entering the depolarization block. 54 As mentioned, an important caveat is that PV–Cre driver lines label multiple cell types, including PVBCs and AACs. Axons of both PVBCs and AACs are reorganized in epilepsy, but their numbers may change in opposite directions, a conundrum reviewed extensively elsewhere.55-62
The strategic positioning of axo-axonic GABAergic inputs near the site of action potential generation, where voltage-gated sodium channel density is maximal, predicts a decisive role of AAC activity in gating pyramidal cell output. However, the axon initial segment also has low levels of potassium chloride cotransporters. 63 Because GABAA receptors are ligand-gated chloride channels, their postsynaptic effect is strongly influenced by the function of chloride pumps. 64 For example, extreme chloride accumulation inside glutamatergic neurons may produce excitatory GABAergic responses and contribute to epileptic seizures.65-68 While AAC activation suppresses CA1 pyramidal cell spiking in non-epileptic mice in vivo, 33 whether AACs have excitatory or inhibitory effects in epilepsy remains to be determined.
Do We Have Emergency Brakes? Loss of CCKBC Function in Epilepsy
While epilepsy is frequently accompanied by loss of interneurons and their inhibitory function, the precise extent and type of interneuron loss remained difficult to determine. Loss of multiple interneuron types, including PV, SST, and CCK interneurons, has been reported in various models.69,70 CCKBC loss may differ depending on the model system and brain area, as well as between anatomical or functional measures, and therefore remained imprecisely understood. Anatomical evidence shows that the CA1 density of CCK-positive axons was reduced in the mouse pilocarpine model of TLE. 70 However, perisomatic synapses, including from PV and CCKBCs, were preserved in human TLE patients.59,71 In a genetic lissencephaly model featuring seizures, the sublayer-specificity of CCKBC synapses in the CA1 was impaired, but the synapses themselves were not eliminated. 54 CCKBC function, including the cholinergic signaling mechanisms that preferentially depolarize CCK-, rather than PVBCs, 72 may be impaired even without apparent anatomical synapse loss. Carbachol-induced (presumably CCKBC) postsynaptic currents were reduced in the hippocampus of TLE mice, supporting a reduced CCKBC perisomatic control of pyramidal cell activity in epilepsy.70,73 In addition, CCKBCs express presynaptic CB1 cannabinoid receptors at high levels, 74 which can silence GABA release (note that CB1 receptor expression may be reduced on glutamatergic and perhaps even on GABAergic terminals in certain chronic epilepsy models).70,75,76 Cannabinoid-mediated, depolarization-induced suppression of inhibition becomes permanently potentiated in a febrile seizure model, 77 which may reduce CCKBC GABA release. Furthermore, endocannabinoid levels elevated by seizures may also mute CCKBC synapses.78,79 Of note, reduced release of the CCK peptide by CCKBCs may also reduce PVBC synapse efficacy. 80
Altogether, these data indicate that CCKBC synapses are anatomically preserved in epilepsy, although their function may diminish due to some combination of reduced cholinergic drive, reduced cellular excitability, and altered cannabinoid signaling. Notably, the “dormant basket cell hypothesis” has proposed the anatomical presence, but functional deficit, of perisomatic inhibition in the epileptic dentate gyrus, resulting from the loss of excitatory drive to PVBCs, weakening recurrent inhibitory mechanisms.81-84 Somewhat similarly, silenced CCKBC synapses may also contribute to epileptic pathology in various brain areas.
Summary and Outlook
Because temporally precise inhibition is necessary for hippocampal function, impaired interictal interneuronal activity in epilepsy may contribute to cognitive performance deficits.85-87 Therefore, future therapeutic strategies aimed at enhancing inhibition to combat seizures should ideally avoid compromising the critical role of interneurons in synchronizing networks outside seizures. Altogether, CCKBC properties suggest they are ideally suited to reduce postsynaptic pyramidal cell excitability, without affecting network rhythmicity underlying cognitive function. Future studies will be necessary to test this hypothesis in chronic epilepsy models. Overall, through enabling a better understanding of the diverse roles of inhibitory interneurons, the emergence of novel tools for interneuron type-specific recordings and interventions may lead to identifying better targets for anti-epileptic treatments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) under Award Numbers NINDSR01NS99457 (to IS), K99NS117795 (to BD), and 5T32NS007280 (to PMK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iDs
Barna Dudok https://orcid.org/0000-0001-5078-0090
Peter M. Klein https://orcid.org/0000-0002-3232-3387
Ivan Soltesz https://orcid.org/0000-0003-1493-3744
References
- 1.Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 2000;41(suppl 6):S90-S95. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 2.Dudek FE. Loss of GABAergic interneurons in seizure-induced epileptogenesis-two decades later and in a more complex world. Epilepsy Current. 2020;20(suppl 6):70S-72S. doi: 10.1177/1535759720960464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialer M, Johannessen SI, Koepp MJ, et al. Progress report on new antiepileptic drugs: A summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). I. Drugs in preclinical and early clinical development. Epilepsia. 2020;61(11):2340-2364. doi: 10.1111/epi.16725. [DOI] [PubMed] [Google Scholar]
- 4.Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: Selective circuit control for the epilepsies. Nat Neurosci. 2015;18(3):331-338. doi: 10.1038/nn.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt RF, Baraban SC. Interneuron Transplantation as a Treatment for Epilepsy. Cold Spring Harb Perspect Med. 2015;5(12):a022376. doi: 10.1101/cshperspect.a022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia. 2011;52(4):657-678. doi: 10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich T, Reyes A, Paul BM, Uttarwar V, Hartman S, Mathur K, et al. Beyond depression: The impact of executive functioning on quality of life in patients with temporal lobe epilepsy. Epilepsy Res. 2019;149:30-36. doi: 10.1016/j.eplepsyres.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsharkawy AE, May T, Thorbecke R, et al. Long-term outcome and determinants of quality of life after temporal lobe epilepsy surgery in adults. Epilepsy Res. 2009;86(2-3):191-199. doi: 10.1016/j.eplepsyres.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Magloire V, Mercier MS, Kullmann DM, Pavlov I. GABAergic interneurons in seizures: investigating causality with optogenetics. Neuroscientist. 2019;25(4):344-358. doi: 10.1177/1073858418805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miri ML, Vinck M, Pant R, Cardin JA. Altered hippocampal interneuron activity precedes ictal onset. Elife. 2018;7:1-20. doi: 10.7554/eLife.40750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elahian B, Lado NE, Mankin E, et al. Low-voltage fast seizures in humans begin with increased interneuron firing. Ann Neurol. 2018;84(4):588-600. doi: 10.1002/ana.25325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyoda I, Fujita S, Thamattoor AK, Buckmaster PS. Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. J Neurosci. 2015;35(16):6600-6618. doi: 10.1523/jneurosci.4786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann AR, Raedt R, Steenland HW, et al. Involvement of fast-spiking cells in ictal sequences during spontaneous seizures in rats with chronic temporal lobe epilepsy. Brain. 2017;140(9):2355-2369. doi: 10.1093/brain/awx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Righes Marafiga J, Vendramin Pasquetti M, Calcagnotto ME. GABAergic interneurons in epilepsy: More than a simple change in inhibition. Epilepsy Behav. 2021;121(Pt B):106935. doi: 10.1016/j.yebeh.2020.106935. [DOI] [PubMed] [Google Scholar]
- 15.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53-57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic inhibitory interneurons. Physiol Rev. 2017;97:1619-1747. 10.1152/physrev.00007.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci. 2017;18(5):299-309. doi: 10.1038/nrn.2017.30. [DOI] [PubMed] [Google Scholar]
- 18.Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26(9):489-495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. J Physiol. 2012;590(4):683-694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33-42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science. 80-2014;345(6196):1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 22.Bakken TE, Jorstad NL, Hu Q, et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 2020;589:111-119. doi: 10.1101/2020.03.31.016972. 10.1101/2020.03.31.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasic B, Menon V, Nguyen TN, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19(2):335-346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisel A, Manchado ABM, Codeluppi S, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138-1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 25.Fuzik J, Zeisel A, Máté Z, et al. Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat Biotechnol. 2016;34(2):175-183. doi: 10.1038/nbt.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouwens NW, Sorensen SA, Baftizadeh F, et al. Integrated morphoelectric and transcriptomic classification of cortical GABAergic cells. Cell. 2020;183(4):935-953. e19. doi: 10.1016/j.cell.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daigle TL, Madisen L, Hage TA, et al. A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell. 2018;174(2):465-480. e22. doi: 10.1016/j.cell.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta P, Kreeger L, Wylie DC, et al. Functional access to neuron subclasses in rodent and primate forebrain. Cell Rep. 2019;26(10):2818-2832. e8. doi: 10.1016/j.celrep.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vormstein-Schneider D, Lin JD, Pelkey KA, et al. Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans. Nat Neurosci. 2020;23(12):1629-1636. doi: 10.1038/s41593-020-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin AN, Malik R, Cho KKA, et al. Regulatory Elements Inserted into AAVs Confer Preferential Activity in Cortical Interneurons. Eneuro. 2020;7(6):0211-0220. doi: 10.1523/ENEURO.0211-20.2020.ENEURO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Hegarty S, Winter C, Wang F, He Z. Viral vectors for neuronal cell type-specific visualization and manipulations. Curr Opin Neurobiol. 2020;63:67-76. doi: 10.1016/j.conb.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudok B, Klein PM, Hwaun E, et al. Alternating sources of perisomatic inhibition during behavior. Neuron. 2021;109(6):997-1012. e9. doi: 10.1016/j.neuron.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudok B, Szoboszlay M, Paul A, et al. Recruitment and inhibitory action of hippocampal axo-axonic cells during behavior. Neuron 2021. doi: 10.1016/j.neuron.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339(6115):70-74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewell LA, Liang L, Armstrong C, Soltész I, Leutgeb S, Leutgeb JK. Brain State Is a Major Factor in Preseizure Hippocampal Network Activity and Influences Success of Seizure Intervention. J Neurosci. 2015;35(47):15635-15648. doi: 10.1523/jneurosci.5112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baud MO, Kleen JK, Mirro EA, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9(1):1-10. doi: 10.1038/s41467-017-02577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer DC, Sun FT, Brown SN, et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502. doi: 10.1111/epi.13455. [DOI] [PubMed] [Google Scholar]
- 38.Buzsáki G. Hippocampal sharp wave‐ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073-1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci Unit States Am. 2012;109(40):E2726-E2734. doi: 10.1073/pnas.1210929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viney TJ, Lasztoczi B, Katona L, et al. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci. 2013;16(12):1802-1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vreeswijk Cv., Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274(5293):1724-1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- 42.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72(2):231-243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran CH, Vaiana M, Nakuci J, et al. Interneuron desynchronization precedes seizures in a mouse model of dravet syndrome. J Neurosci. 2020;40(13):2764-2775. doi: 10.1523/JNEUROSCI.2370-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somarowthu A, Goff KM, Goldberg EM. Two-photon calcium imaging of seizures in awake, head-fixed mice. Cell Calcium. 2021;96:102380. doi: 10.1016/j.ceca.2021.102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acsády L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73(2):317-334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 46.Khoshkhoo S, Vogt D, Sohal VS. Dynamic, cell-type-specific roles for GABAergic interneurons in a mouse model of optogenetically inducible seizures. Neuron. 2017;93(2):291-298. doi: 10.1016/j.neuron.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David LS, Topolnik L. Target-specific alterations in the VIP inhibitory drive to hippocampal GABAergic cells after status epilepticus. Exp Neurol. 2017;292:102-112. doi: 10.1016/j.expneurol.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Cunha-Reis D, Caulino-Rocha A, Correia-de-Sá P. VIPergic neuroprotection in epileptogenesis: Challenges and opportunities. Pharmacol Res. 2021;164:105356. doi: 10.1016/j.phrs.2020.105356. [DOI] [PubMed] [Google Scholar]
- 49.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HK, Gschwind T, Nguyen TM, et al. Optogenetic intervention of seizures improves spatial memory in a mouse model of chronic temporal lobe epilepsy. Epilepsy. 2020;61:1-11. doi: 10.1111/epi.16445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drexel M, Romanov RA, Wood J, et al. Selective silencing of hippocampal parvalbumin interneurons induces development of recurrent spontaneous limbic seizures in mice. J Neurosci. 2017;37(34):8166-8179. doi: 10.1523/jneurosci.3456-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lévesque M, Chen L-Y, Etter G, et al. Paradoxical effects of optogenetic stimulation in mesial temporal lobe epilepsy. Ann Neurol. 2019;86(5):714-728. doi: 10.1002/ana.25572. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Xu C, Xu Z, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95(1):92-105. e5. doi: 10.1016/j.neuron.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Ekins TG, Mahadevan V, Zhang Y, et al. Emergence of non-canonical parvalbumin-containing interneurons in hippocampus of a murine model of type I lissencephaly. Elife. 2020;9:1-29. doi: 10.7554/eLife.62373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard A, Tamas G, Soltesz I. Lighting the chandelier: New vistas for axo-axonic cells. Trends Neurosci. 2005;28(6):310-316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Arellano JI, Muñoz A, Ballesteros-Yáñez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127(1):45-64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Stornetta RL, Zhu JJ. Chandelier cells control excessive cortical excitation: Characteristics of whisker-evoked synaptic responses of layer 2/3 nonpyramidal and pyramidal neurons. J Neurosci. 2004;24(22):5101-5108. doi: 10.1523/JNEUROSCI.0544-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alhourani A, Fish KN, Wozny TA, Sudhakar V, Hamilton RL, Richardson RM. GABA bouton subpopulations in the human dentate gyrus are differentially altered in mesial temporal lobe epilepsy. J Neurophysiol. 2020;123(1):392-406. doi: 10.1152/JN.00523.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cossart R, Dinocourt C, Hirsch JC, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4(1):52-62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 60.Lillis KP. A tisket a tasket: Chandelier ≠ Basket (Cell Bouton Density in Sclerotic DG). Epilepsy Current. 2020;20(4):218-220. doi: 10.1177/1535759720930682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kisvárday ZF, Adams CBT, Smith AD. Synaptic connections of axo-axonic (chandelier) cells in human epileptic temporal cortex. Neuroscience. 1986;19(4):1179-1186. doi: 10.1016/0306-4522(86)90131-4. [DOI] [PubMed] [Google Scholar]
- 62.Wittner L, Maglóczky Z, Borhegyi Z, et al. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience. 2001;108(4):587-600. doi: 10.1016/s0306-4522(01)00446-8. [DOI] [PubMed] [Google Scholar]
- 63.Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311(5758):233-235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 64.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15(10):637-654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaila K, Ruusuvuori E, Seja P, Voipio J, Puskarjov M. GABA actions and ionic plasticity in epilepsy. Curr Opin Neurobiol. 2014;26:34-41. doi: 10.1016/j.conb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Glykys J, Dzhala V, Egawa K, Kahle KT, Delpire E, Staley K. Chloride dysregulation, seizures, and cerebral edema: A relationship with therapeutic potential. Trends Neurosci. 2017;40(5):276-294. doi: 10.1016/j.tins.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore YE, Kelley MR, Brandon NJ, Deeb TZ, Moss SJ. Seizing control of KCC2: A new therapeutic target for epilepsy. Trends Neurosci. 2017;40(9):555-571. doi: 10.1016/j.tins.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Kahle KT, Staley K. Altered neuronal chloride homeostasis and excitatory GABAergic signaling in human temporal lobe epilepsy. Epilepsy Current. 2008;8(2):51-53. doi: 10.1111/j.1535-7511.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol. 2003;459(4):407-425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- 70.Wyeth MS, Zhang N, Mody I, Houser CR. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010;30(26):8993-9006. doi: 10.1523/jneurosci.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wittner L, Maglóczky Z. Synaptic reorganization of the perisomatic inhibitory network in hippocampi of temporal lobe epileptic patients. BioMed Res Int. 2017;2017:1-13. doi: 10.1155/2017/7154295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78(3):1743-1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 73.Kang Y-J, Clement EM, Park I-H, Greenfield LJ, Jr, Smith BN, Lee S-H. Vulnerability of cholecystokinin-expressing GABAergic interneurons in the unilateral intrahippocampal kainate mouse model of temporal lobe epilepsy. Exp Neurol. 2021;342:113724. doi: 10.1016/j.expneurol.2021.113724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katona I, Sperlágh B, Sík A, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19(11):4544-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ludányi A, Eross L, Czirják S, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28(12):2976-2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maglóczky Z, Tóth K, Karlócai R, et al. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia. 2010;51(suppl 3):115-120. doi: 10.1111/j.1528-1167.2010.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen K, Ratzliff A, Hilgenberg L, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39(4):599-611. doi: 10.1016/S0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 78.Farrell JS, Colangeli R, Dong A, et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron. 2021;109(15):2398-2403. e4. doi: 10.1016/j.neuron.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Therapeut. 2003;307(1):129-137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 80.Földy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10(9):1128-1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 81.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: The ?dormant basket cell? hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1(1):41-66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 82.Bekenstein J, Lothman E. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993;259(5091):97-100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- 83.Bui AD, Nguyen TM, Limouse C, et al. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science. 2018;359(6377):787-790. doi: 10.1126/science.aan4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scharfman HE. Controlling learning and epilepsy together. Science. 80-2018;359(6377):740-741. doi: 10.1126/science.aas8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shuman T, Aharoni D, Cai DJ, et al. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nat Neurosci. 2020;23(2):229-238. doi: 10.1038/s41593-019-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muldoon SF, Villette V, Tressard T, et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain. 2015;138(10):2875-2890. doi: 10.1093/brain/awv227. [DOI] [PubMed] [Google Scholar]
- 87.Moxon KA, Shahlaie K, Girgis F, Saez I, Kennedy J, Gurkoff GG. From adagio to allegretto: The changing tempo of theta frequencies in epilepsy and its relation to interneuron function. Neurobiol Dis. 2019;129:169-181. doi: 10.1016/j.nbd.2019.02.009. [DOI] [PubMed] [Google Scholar]