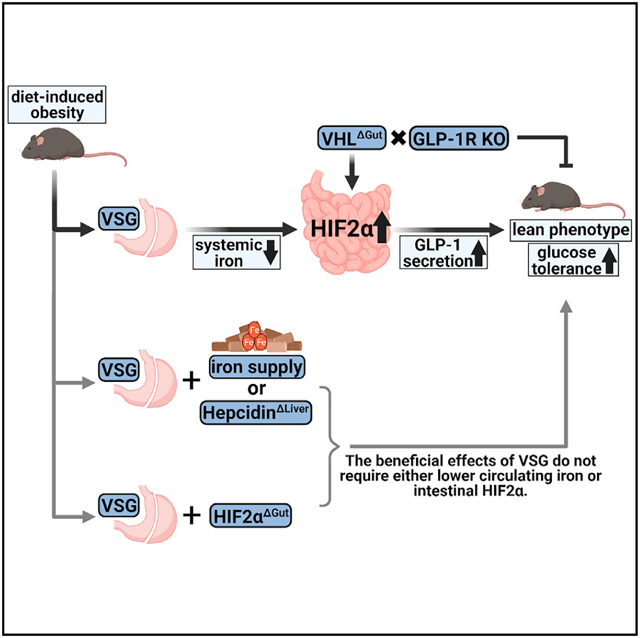
SUMMARY
Gastric bypass and vertical sleeve gastrectomy (VSG) remain the most potent and durable treatments for obesity and type 2 diabetes but are also associated with iron deficiency. The transcription factor HIF2α, which regulates iron absorption in the duodenum, increases following these surgeries. Increasing iron levels by means of dietary supplementation or hepatic hepcidin knockdown does not undermine the effects of VSG, indicating that metabolic improvements following VSG are not secondary to lower iron levels. Gut-specific deletion of Vhl results in increased constitutive duodenal HIF2α signaling and produces a profound lean, glucose-tolerant phenotype that mimics key effects of VSG. Interestingly, intestinal Vhl deletion also results in increased intestinal secretion of GLP-1, which is essential for these metabolic benefits. These data demonstrate a role for increased duodenal HIF2α signaling in regulating crosstalk between iron-regulatory systems and other aspects of systemic physiology important for metabolic regulation.
Graphical Abstract
In brief
Bariatric surgery remains the most potent treatment for obesity and type 2 diabetes but also reduces iron levels. Evers et al. find that the machinery for absorbing iron is activated after VSG. Activation of this machinery recapitulates multiple effects of VSG. These findings may lead to less invasive therapies.
INTRODUCTION
Bariatric surgery remains the most effective treatment for obesity and type 2 diabetes (Cummings et al., 2016; Schauer et al., 2017; Mingrone et al., 2021). This includes procedures such as vertical sleeve gastrectomy (VSG), which is now the most common bariatric procedure (Welbourn et al., 2019). However, VSG is invasive and requires permanent anatomical alteration of the gastrointestinal (GI) tract. Consequently, surgical interventions cannot be scaled to provide relief for the large populations of individuals with obesity and/or type 2 diabetes. This emphasizes the need to understand how VSG and other bariatric procedures exert their potent beneficial effects at a mechanistic level.
Although physical restriction and malabsorption remain common explanations (le Roux and Bueter, 2014; Arble et al., 2018; Evers et al., 2019), a wide range of data points toward these being inadequate to account for the many physiological changes that occur after VSG (Stefater et al., 2010; Ryan et al., 2014). One important example is that VSG results in a weight-independent effect to profoundly increase post-prandial secretion of gut hormones such as GLP-1 (Chambers et al., 2011). These changes emphasize the idea that critical for the effects of bariatric surgery are alterations in signals coming from the gut that affect a wide range of physiologies across many organ systems (Abu-Gazala et al., 2018; Ben-Zvi et al., 2018).
Use of bariatric surgery is not limited just by its invasiveness but by some long-term unwanted side effects, which include persistent hypoglycemia, acid reflux, and nutrient deficiencies. One of the most common nutrient deficiencies is a reduction in circulating iron and related increased rates of iron deficiency anemias (Ruz et al., 2009; Steenackers et al., 2018; Gowanlock et al., 2020; Mechanick et al., 2020). Iron regulation involves several organ systems, starting with the proximal portion of the small intestine. Iron absorption from the apical side of the intestine involves several enzymes and transporters whose expression is controlled by the transcription factor hypoxia-inducible factor (HIF) 2α (Anderson et al., 2013; Shah and Xie, 2014). Low iron conditions increase HIF2α signaling in the epithelium of the intestine. In turn, HIF2α stimulates transcription of the apical iron transporters divalent metal transporter 1 (Dmt1) and duodenal cytochrome b (Dcytb), which facilitate transport of iron from the chyme into intestinal cells.
HIF2α also stimulates expression of the basolateral iron transporter ferroportin to transport iron from intestinal cells into the circulation (Lane et al., 2015). Because iron is not only an essential micronutrient but also toxic at higher concentrations, circulating iron levels are tightly regulated. Iron entering the circulation from the basolateral side of the intestine can be inhibited by the hepatic hormone hepcidin (Ganz and Nemeth, 2012). Increased circulating iron levels stimulate hepcidin, which blocks the ferroportin transporter and inhibits iron entering the circulation. Increased iron levels, in turn, reduce expression of HIF2α, and, consequently, iron absorption from the chyme reduces (Mastrogiannaki et al., 2009; Taylor et al., 2011).
Intracellular HIFα expression (HIF1α and HIF2α) is regulated through prolyl hydroxylase (PHD) and the von Hippel-Lindau (VHL) proteins. Under sufficient oxygen conditions, HIFs are hydrolyzed by PHD and ubiquitinated by VHL, which sets them up for proteasomal degradation, preventing HIFs from going to the nucleus and influencing gene transcription (Ramakrishnan and Shah, 2017; Lee et al., 2020). Under insufficient oxygen conditions or hypoxia, HIFα binds to HIFβ and, in the nucleus, forms a complex with Creb binding protein (CBP)/P300 that induces target gene transcription (Lando et al., 2002; Lee et al., 2020). The key point is that iron levels are regulated at multiple levels, including absorption in the gut, so that this necessary but toxic nutrient remains at appropriate levels.
It is commonly believed that bariatric surgery results in reduced iron levels because of less exposure of the duodenum to ingested food (Kotkiewicz et al., 2015). However, lower iron levels are also observed in surgeries with widely varying effects on duodenal exposure to chyme. Moreover, lowered iron levels can also be observed in well-controlled rodent models of disparate procedures (Arble et al., 2018). Because iron deficiency and related anemia are commonly observed following bariatric surgery, iron supplementation is recommended as part of post-operative care (Aarts et al., 2011; Mechanick et al., 2020). Nonetheless, apparent beneficial effects of iron supplementation seem to be limited as a successful strategy to reduce iron deficiency and are related to the type of iron supplemented (Mischler et al., 2018). This suggests that bariatric surgery specifically affects iron homeostasis at a mechanistic level.
We report here that, in the mouse and rat, transcriptional changes point toward an increase in HIF2α signaling in the duodenum following bariatric surgery. This leads to the hypothesis, tested here, that VSG’s ability to alter the iron-regulatory system also contributes to beneficial effects on body weight, glucose regulation, and gut hormone secretion and identifies HIF2α-related signaling in the gut as an important regulator of systemic metabolism, body weight, and hormone secretion.
RESULTS
Bariatric surgery in rodents results in increased duodenal HIF2α target gene expression
We first performed an unbiased transcriptomics analysis, comparing the duodenum of rats receiving VSG or Roux-en-Y Gastric Bypass (RYGB) surgery (see in vivo data from this cohort in Figure S1). Despite the profound differences in the functional effect on the duodenum between these two surgeries, there is an unbiased common response to upregulate HIF2α and its target genes (Figures 1A–1C). To further determine whether HIF is upregulated following VSG, we performed VSG in a cohort of oxygen-dependent domain (ODD)-luciferase mice. These mice express luciferase at the ODD of HIFα and can therefore be used as a measure of HIFα activation. In this cohort, we found that luciferase activity, and therefore HIFα expression, was upregulated in the duodenum and ileum of mice with VSG (Figure 1D). However, luciferase activity at the ODD does not discriminate between HIF1α or HIF2α expression. We therefore compared the expression levels of both genes in the upper and lower small intestine (Figure 1E). This comparison showed that, in the proximal part of the small intestine, the duodenum, Hif2α and its protein target genes Dmt1, Dcytb, and Neu3, are more highly expressed, whereas, in the distal part of the small intestine, the ileum, expression levels of Vhl and Phd2 as well as the HIF1α target gene Glut1 are higher. In a separate cohort of mice (Figure 1F), we found that expression of HIF2α target genes following VSG surgery was also upregulated in the duodenum, specifically of genes transcribing the iron transporters Dmt1 (p < 0.05) and Dcytb (p < 0.0001). In contrast, the HIF1α target genes Pgk1 and Glut1 were not upregulated.
Dietary iron supplementation or genetic manipulation to increase circulating iron does not alter the effectiveness of VSG to reduce body weight or glycemia
Figure 1. HIF2α target genes are overexpressed specifically in the duodenum following bariatric surgery.
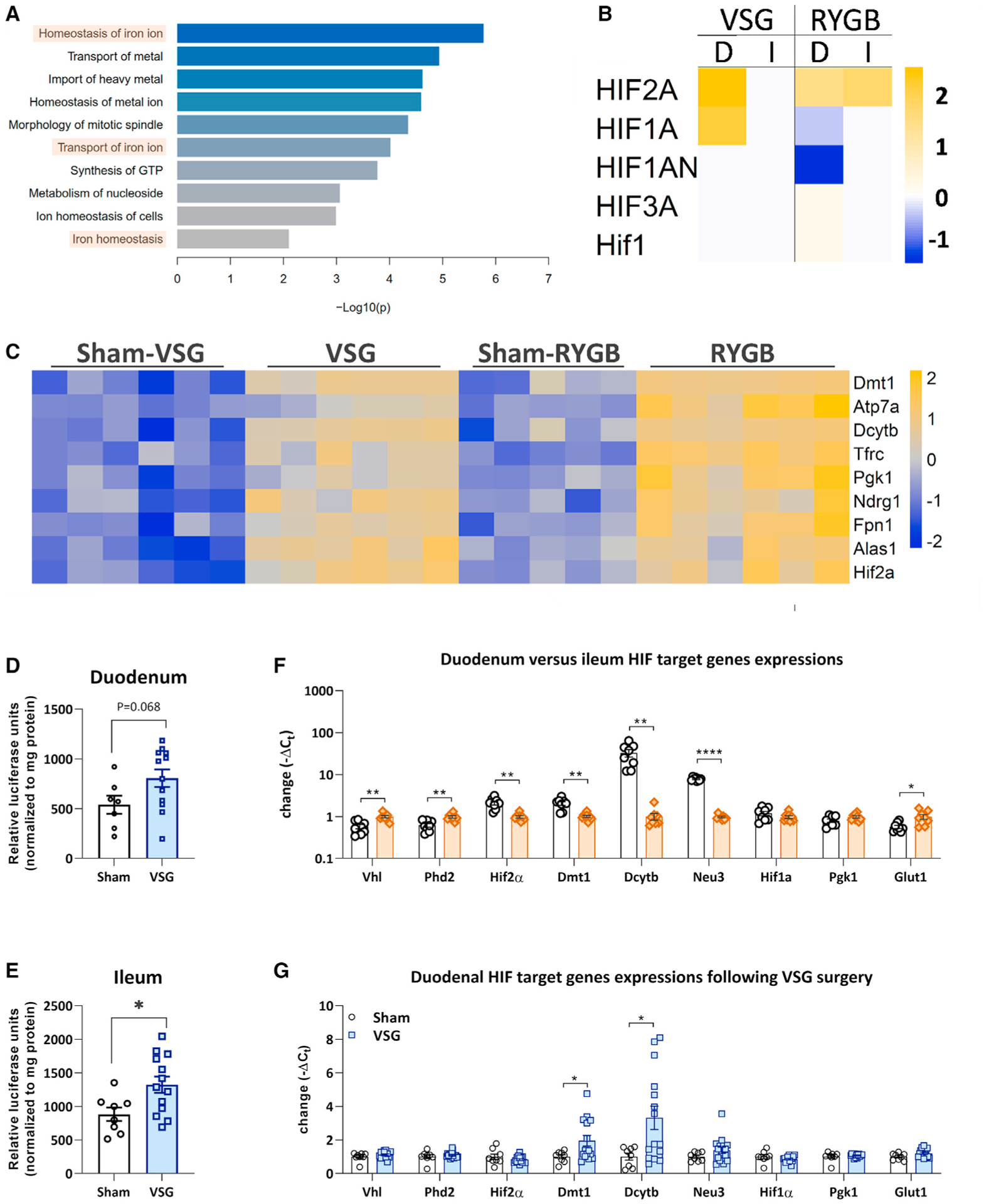
(A) Unbiased enrichment analysis based on the “Function and Disease” category in Ingenuity Pathway Analysis (IPA) shows strong commonality in iron transport homeostatic pathway effects following both RYGB and VSG. These results are based on the overlap of 100 regulated genes in the duodenum upon RYGB and VSG treatment. Shown are −log10 (p values).
(B) IPA upstream regulator analysis predicts activation of Hif2α in the duodenum, D, following RYGB and VSG, based on increased downstream target gene expression levels. Note that Hif2α activation is specific to the VSG duodenum and not the ileum, I.
(C) Heatmap of selected significantly regulated genes related to iron absorption (false discovery rate [FDR] < 10%) in the duodenum upon VSG and RYGB treatments compared with sham-operated rats. Shown are Z scores indicating significant activation (Z score > 2) or inhibition (Z score < −2).
(D and E) In ODD-luciferase mice, luciferase expression is increased in the (D) duodenum and (E) ileum, indicative of increased HIF1/2α protein activity. Average ± SEM; sham, n = 7/8; VSG, n = 12/13; *p < 0.05, unpaired t test.
(F) mRNA expression analyses comparing epithelium of the duodenum and ileum from sham-operated mice, showing relatively higher expression levels for Hif2α and its target genes Dmt1, Dcytb, and Neu3 in the duodenum than the ileum. Average ± SEM; duodenum, n = 17; ileum, n = 17; *p < 0.05, **p < 0.01, ****p < 0.0001, multiple t test with Holm-Sidak correction.
(G) mRNA expression analyses of duodenal epithelium from mice following VSG confirmed increased expression levels for the HIF2α target genes Dmt1 and Dcytb. Average ± SEM; sham, n = 8; VSG, n = 15/16; *p < 0.05; multiple t test with Holm-Sidak correction.
Average Ct data of gene expression is available in Tables S1 and S2.
Unlike in most rodent experiments, humans are told to take considerable iron supplements after bariatric surgery to reduce potential anemias. If HIF2α is a component of the surgical response, then higher iron levels could suppress HIF2α signaling and may therefore undermine the effectiveness of the procedure. Hence, to increase circulating iron levels, we tested B6C57/j mice supplemented with a high-fat/high-iron (350 ppm) diet and mice with deletion of hepcidin in the liver (HepcidinΔliver). In both cases, VSG showed clear benefits to glucose regulation and body weight (Figure S2). In the case of dietary iron supplementation, no effect of supplementation on hematocrit was observed (Figure 2A), whereas a trend toward restored iron levels was observed (Figure 2B). Surprisingly, circulating hepcidin levels were increased in VSG mice fed a high-iron diet (Figure 2C). Likewise, HepcidinΔliver did not alter the effect of VSG to increase total iron binding capacity (Figure 2D), and VSG did not lower circulating iron levels in the HepcidinΔliver (Figure 2E) or transferrin saturation (Figure 2F). These data indicate that the benefits of surgery are not secondary to lowering iron levels. Additionally, these data do not support use of VSG as a therapeutic option to treat high-iron conditions such as hemochromatosis. Moreover, these results suggest that intestinal iron transport into the circulation is actively suppressed even in the presence of lower hematocrit and circulating iron levels. Consequently, it appears that VSG surgery results in broad physiological changes that predispose the organism to maintaining lower circulating iron levels. The inability to reduce iron levels in hepcidin knockout (KO) mice points toward hepcidin being an important component of the effect of VSG to lower iron, but further work is warranted to fully identify the mechanisms by which VSG regulates hepcidin secretion and lowers iron levels.
Intestinal Hif2α is not essential for the metabolic effects of VSG
Figure 2. High dietary iron supplementation does not affect circulating iron following VSG.
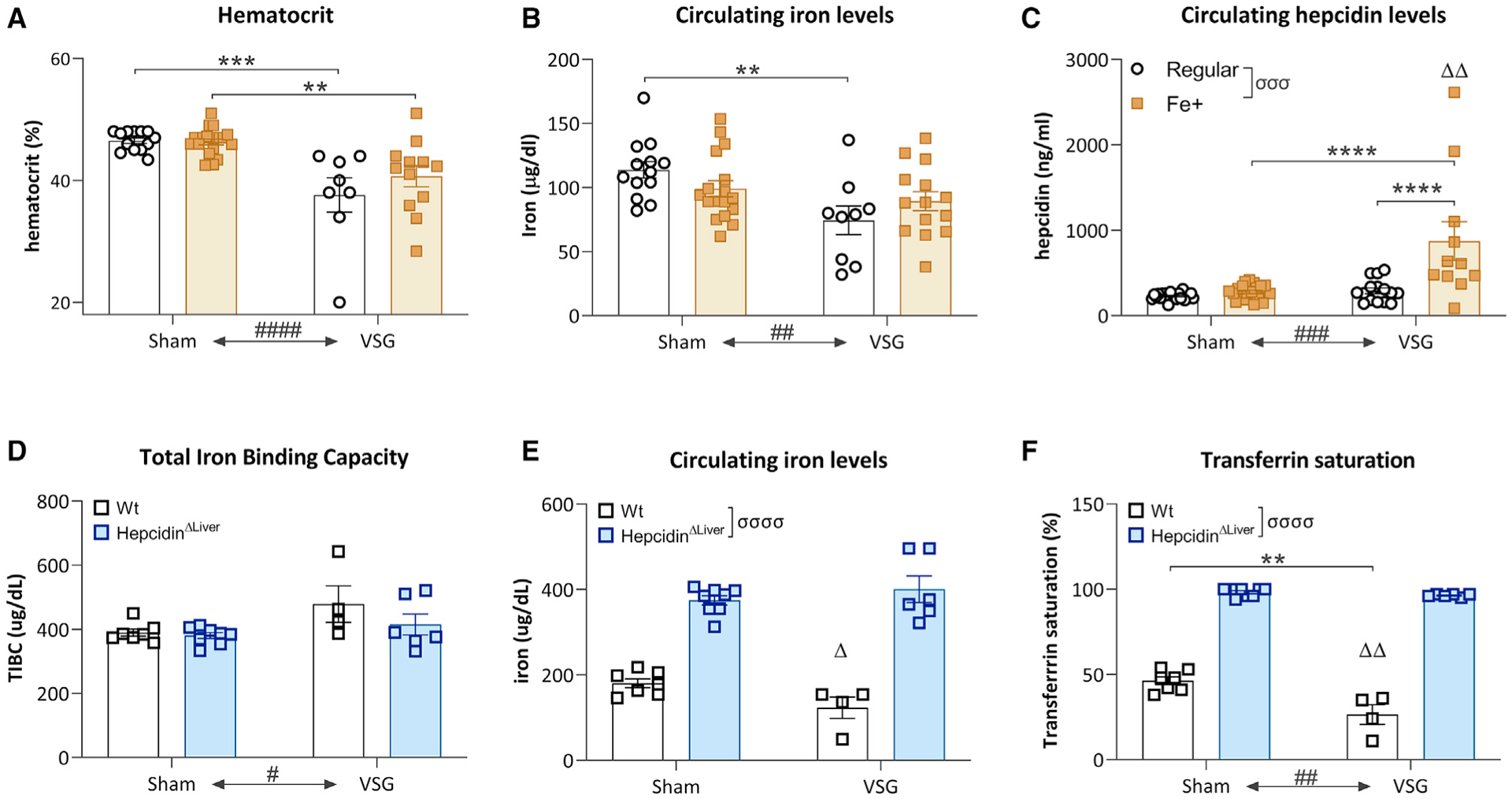
(A) VSG induced a reduction in hematocrit levels that could not be countered by high dietary iron levels. Average ± SEM; sham-regular, n = 13; sham-Fe+, n = 20; VSG-regular, n = 8; VSG-Fe+, n = 12; two-way ANOVA, main effect ####p < 0.0001 surgery effect, multiple comparisons post hoc Tukey test, **p < 0.01, ***p < 0.001.
(B) VSG induced a reduction in circulating iron levels that could not be countered by high dietary iron levels. Two-way ANOVA, main effect ##p < 0.01 surgery effect, multiple comparisons post hoc Tukey test, **p < 0.01.
(C) Circulating hepcidin levels were mostly increased in mice with VSG on a high-iron diet. Two-way ANOVA, main effects σσσP<0.001: dietary iron effect, ###p < 0.001: surgery effect, ΔΔp<0.01: interaction dietary iron*surgery; multiple comparisons post hoc Tukey test, ****p < 0.0001.
(D) VSG surgery, but not HepcidinΔliver, increased total iron binding capacity (TIBC). Average ± SEM; sham-WT, n = 7; VSG-WT, n = 4; sham-HepcidinΔliver, n = 8; VSG-HepcidinΔliver, n = 6; #p < 0.05, two-way ANOVA, main effect of surgery.
(E) HepcidinΔLiver increased circulating iron levels independent of surgery, although an interaction of lower iron levels in WT VSG was observed. Δp<0.05: two-way ANOVA, interaction of genotype*surgery.
(F) At the level of transferrin saturation, a main effect of HepcidinΔLiver, VSG surgery, and an interaction of genotype*surgery were observed. Two-way ANOVA, ****p < 0.0001: main effect of genotype, ###p < 0.001: main effect of surgery, ΔΔp < 0.01: interaction genotype*surgery.
Circulating iron levels do not seem to affect the metabolic consequences of VSG. We tested whether intestinal HIF2α is essential for the effects of VSG. To do this, we developed a mouse with conditional KO of Hif2α (Hif2αΔGut) in VillinCreERT-expressing intestinal epithelial cells using tamoxifen administration 14 days prior to surgery. To confirm that Hif2α was conditionally knocked out over the duration of the study, we measured mucosal duodenal Hif2α (Figure 3A) and Cre (Figure 3B) mRNA expression and found that, after 84 days of induction, Cre was still highly expressed, whereas Hif2α mRNA was decreased significantly in Hif2αΔGut mice. We found that Hif2αΔGut animals respond to VSG surgery in a similar fashion as their Hif2αF/F wild-type (WT) littermates in terms of body weight (Figure 3C). Furthermore, following an intraperitoneal (i.p.; 2 g/kg glucose) or oral nutrient (2 g/kg glucose in Ensure Plus formula) challenge, we did not observe an effect of intestinal Hif2α deletion on glucose response (Figure 3D) or post-prandial total GLP-1 levels (Figure 3E). Even though Hif2αΔGut did not affect food intake compared with WT littermates (Figure 3F), it did result in lower hepatic triglyceride levels compared with the WT, which was independent of surgical treatment (Figure 3G). No effects of either surgery or genotype were observed on hepatic total cholesterol levels (Figure 3H). These data reveal that, although intestinal Hif2αΔGut lowers liver triglyceride levels, intestinal HIF2α is not essential for the beneficial metabolic effects observed following VSG. A possibility is that other HIFs may compensate for the lack of cellular HIF2α (Xie et al., 2017). For example, in this cohort under ad libitum feeding conditions, we found an increase in duodenal Hif1α expression between sham-operated mice (Figure S3). However, similar to previous observations by others (Das et al., 2019), knockdown of Hif2αΔGut did not result in a reduction of expression of the iron-related target genes Dmt1 and Dcytb. Nonetheless, the HIF2α target gene Neu3, which has been shown to be under strict regulation of HIF2α (Xie et al., 2017), was downregulated (Figure S3).
VhlΔGut results in increased HIF2α target gene expressions and a lean diet-induced obesity (DIO)-resistant and glucose-tolerant phenotype with increased circulating hepcidin and basal GLP-1 levels
Figure 3. Hif2αΔGut does not affect the response to VSG surgery.
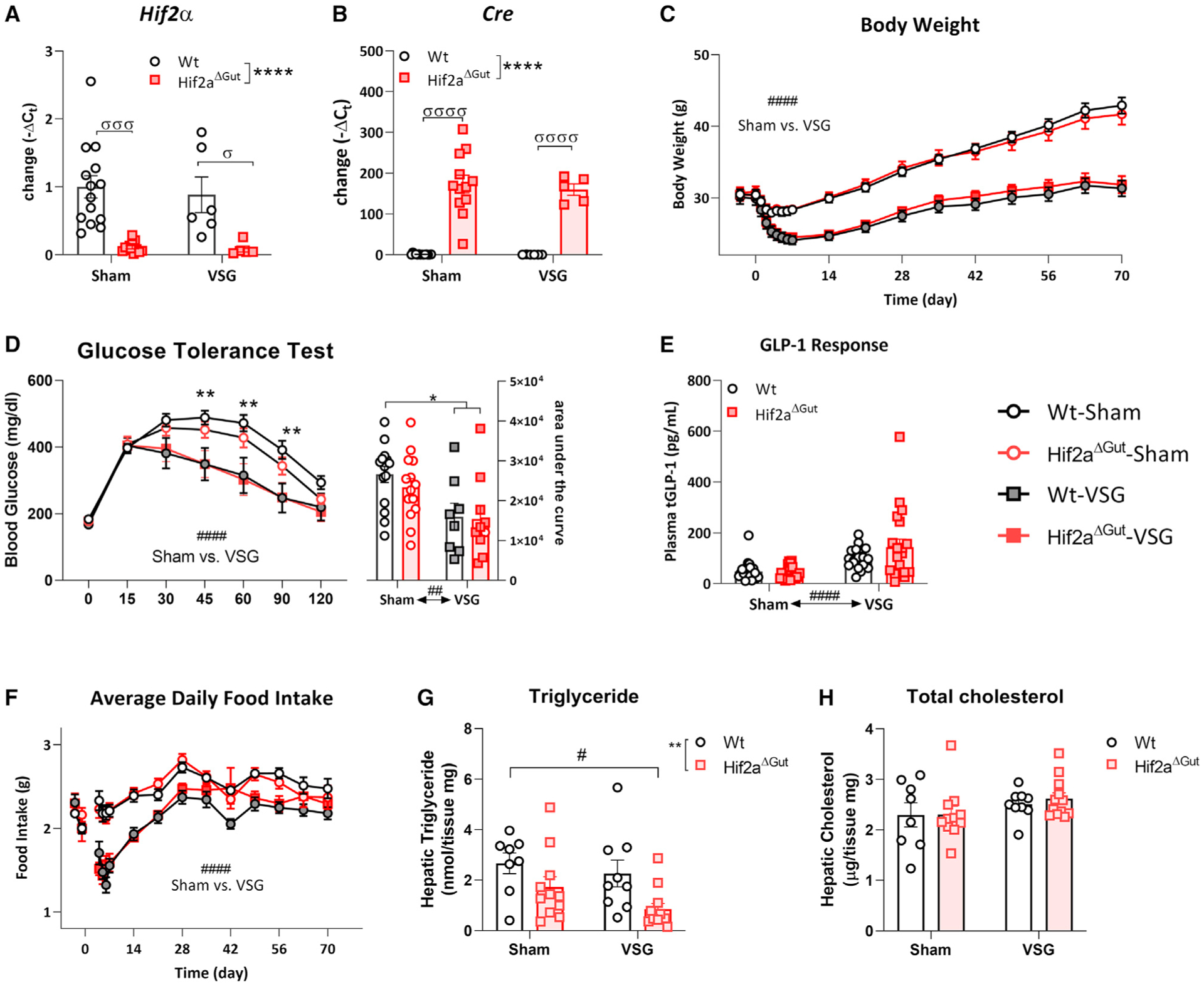
(A) At termination, Hif2α is effectively knocked down in duodenal mucosal samples from Cre-expressing mice. Average ± SEM; WT-sham, n = 25; WT-VSG, n = 18; HifαΔGut-sham, n = 25; HifαΔGut-VSG, n = 20. Two-way ANOVA: F1, 33 = 25.56, ****p < 0.001.
(B) At termination, Cre is expressed in the duodenum of Hif2αf/fVillinCreERT (HifαΔGut) mice 84 days after tamoxifen Cre induction independent of surgery. Two-way ANOVA: F1, 33 = 105.6, ****p < 0.001. Average Ct data are available in Table S3.
(C) Following VSG, mice follow a similar BW trajectory independent of genotype. rm-ANOVA: F51, 1638 = 4.851, ####p < 0.0001 post hoc Tukey test.
(D) Glucose levels following i.p. glucose administration (2 g/kg glucose) are lowered after VSG compared with sham surgery independent of genotype. rm-ANOVA: F18, 301 = 1.575, ####p < 0.0001 post hoc Tukey test, multiple comparisons **p < 0.05. The glucose area under the curve is lowered in VSG mice. Two-way ANOVA: F1, 43 = 0.2606, ##p < 0.01 surgery effect, *p < 0.05 post hoc Tukey test.
(E) Total GLP-1 response 15 min after oral nutrient exposure (2 g/kg glucose in Ensure Plus) was increased after VSG; two-way ANOVA: F1, 84 = 2.619, ####p < 0.0001), but not dependent on genotype.
(F) Food intake is reduced following VSG compared with sham surgery independent of genotype. rm-ANOVA: F42, 1250 = 2.826, ####p < 0.0001 post hoc Tukey test.
(G) Hepatic triglyceride levels were lower in Hif2αΔGut mice. Average ± SEM; WT-sham, n = 8; WT-VSG, n = 9; HifαΔGut-sham, n = 11; HifαΔGut-VSG, n = 12. Two-way ANOVA: F1, 36 = 8.891, **p < 0.01 main effect Hif2αΔGut, #p < 0.05 post hoc Tukey test.
(H) No effects of surgery or genotype on hepatic total cholesterol levels were observed.
Although we found that the lack of intestinal Hif2α did not affect the benefits of VSG surgery, we sought to identify the systemic effects of increased HIF and its target genes in the intestine. Therefore, we used another Cre-LoxP mouse model to delete Vhl in intestinal epithelial cells expressing VillinCRE (VhlΔGut). As expected, the mRNA levels of HIF1α target genes (Glut1, Pdk1, and Pgk1) and HIF2α target genes (Dmt1, Dcytb, and Neu3) were increased in the duodenum (Figure 4A) and ileum (Figure 4B) of VhlΔGut mice. Although the potent increase in target gene expression indicates an increase in HIF1α and HIF2α activity at the protein level, the increase in Phd2 (PHD domain 2) gene expression suggests a feedback mechanism to downregulate HIF protein levels in VhlΔGut mice. Interestingly, although Hif1α gene expression is reduced in both parts of the small intestine in VhlΔGut mice, expression of Hif2α is unaffected in the ileum and even increased in the duodenum, suggesting negative feedback on Hif1α expression that is not present for Hif2α expression.
Figure 4. Intestinal Vhl deletion (VhlΔGut).
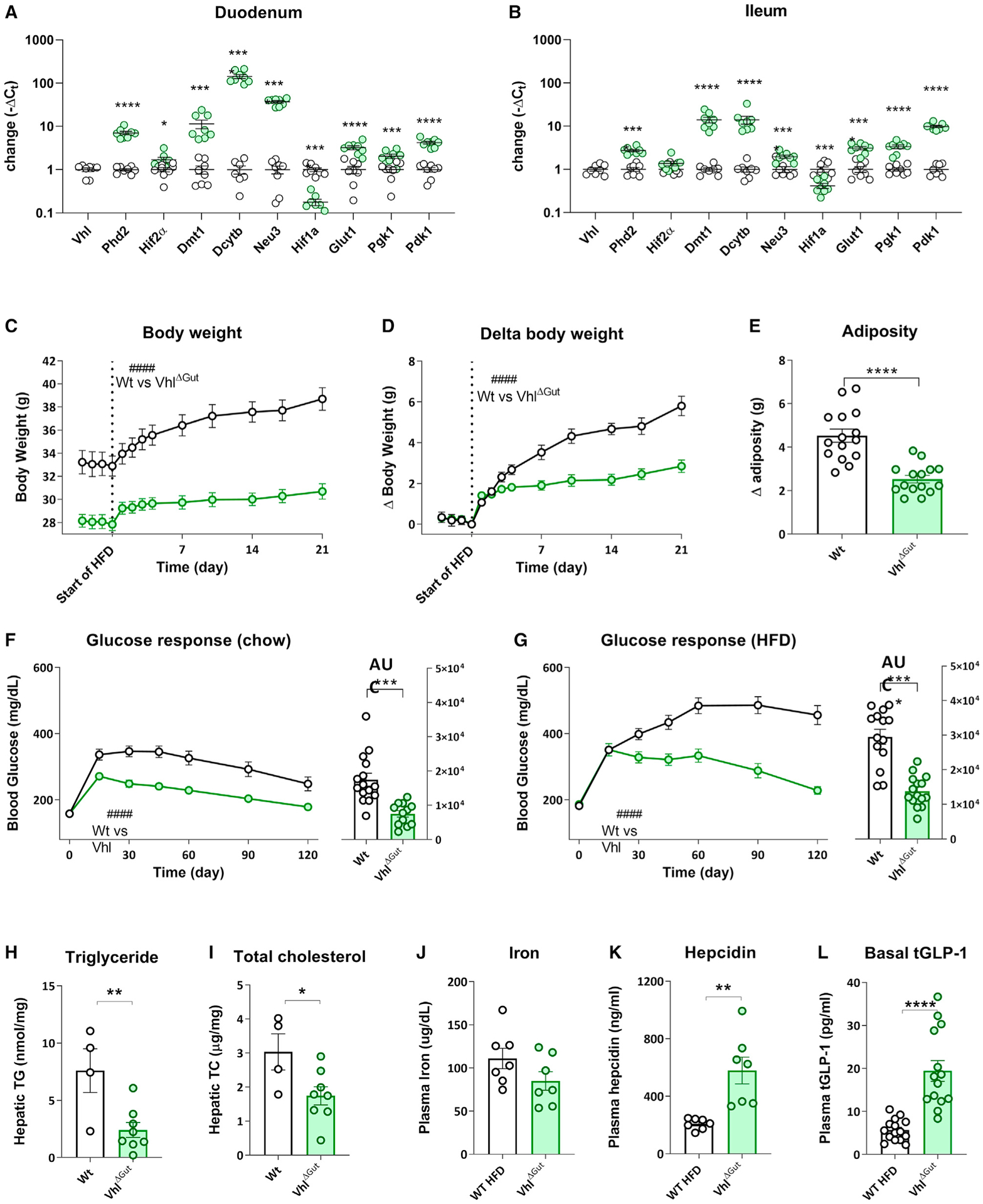
(A and B) In the duodenum (A) and ileum (B), VhlΔGut results in increased expression of the HIF2α target genes DMT1, Dcytb, and Neu3 and increased levels of the HIF1α target genes Glut1, Pgk1, and Pdk1. Additionally, expression of Phd2 is upregulated in the duodenum and ileum. Although Hif2α gene expression is upregulated in the duodenum, it is not affected in the ileum of VhlΔGut mice. In contrast, Hif1α gene expression is downregulated in the duodenum and ileum of VhlΔGut mice. Average ± SEM; WT, n = 8; VhlΔGut, n = 8; multiple comparisons Holm-Sidak: *p < 0.05, ***p < 0.001, ****p < 0.0001. Average Ct data are available in Tables S5 and S6.
(C) VhlΔGut results in mice that are lower in body weight on chow as well as a 60% HFD; day 0–21; average ± SEM; WT, n = 15; VhlΔGut, n = 15; rm-ANOVA: F12, 336 = 18.54, ####p < 0.0001.
(D) VhlΔGut mice gain less weight and are DIO resistant to a 60% HFD; rm-ANOVA: F12, 336 = 18.30, ####p < 0.0001.
(E) VhlΔGut mice gain less adipose mass when fed a 60% HFD; t test: t28 = 5.763, ****p < 0.01.
(F) During an ipGTT (2 g/kg glucose), chow-fed VhlΔGut mice have reduced circulating glucose levels compared with the WT (mixed-effects analysis: F6,150 = 6.616, ####p < 0.0001), resulting in a lower area under the curve of the glucose response (t test: t25 = 4.444, ***p < 0.001).
(G–I) During an ipGTT (2 g/kg glucose) following 14 days of HFD feeding, VhlΔGut mice have (G) lower circulating glucose levels compared with the WT (mixed-effects analysis: F6,162 = 18.27, ####p < 0.0001), resulting in a lower area under the curve of the glucose response; t test: t27 = 6.487, ****p < 0.0001. Under HFD conditions, VhlΔGut mice have (H) lower hepatic triglyceride levels (average ± SEM; WT, n = 4; VhlΔGut, n = 8; t test: t10 = 3.253, **p < 0.01) and (I) lower hepatic total cholesterol levels (t test: t10 = 2.445, **p < 0.05).
(J) VhlΔGut mice do not have altered circulating iron levels. average ± SEM; WT, n = 7l VhlΔGut, n = 6.
(K) VhlΔGut mice have increased circulating hepcidin levels. t test: t12 = 3.943, **p < 0.01.
(L) Following 4 h of fasting, VhlΔGut mice have increased basal circulating total GLP-1 levels. Average ± SEM; WT, n = 15; VhlΔGut, n = 15; t test: t27 = 5.689, ****p < 0.001.
Strikingly, VhlΔGut mice are lower in body weight compared with littermate controls when fed a standard chow diet (Figure 4C). Furthermore, when fed a 60% high-fat diet (HFD), VhlΔGut mice showed profound resistance to DIO by gaining considerably less weight (Figures 4C and 4D) and adipose tissue mass (Figure 4E). Under chow-fed (Figure 4F) and HFD-fed (Figure 4G) conditions, VhlΔGut mice had an improved glucose response during an ipGTT (2 g/kg glucose), suggesting improved glucose tolerance compared with WT mice. Furthermore, VhlΔGut mice on an HFD had lower hepatic triglyceride (Figure 4H) and total cholesterol (Figure 4I) levels. Even though expression of iron transporter genes is increased in the intestine of VhlΔGut mice, circulating iron levels were not different compared with littermate controls (Figure 4J). Similar to VSG, normal iron levels are likely the result of an increase in circulating hepcidin levels preventing excessive iron from entering the circulation (Figure 4K). Interestingly, we found that, following 4 h of fasting, VhlΔGut mice have higher circulating levels of total GLP-1 at baseline compared with WT littermates (Figure 4L).
Increased GLP-1 in VhlΔgut mice originates from the intestine and is essential for its glucose-tolerant phenotype
We showed that intestinal Hif2α is not essential to induce an increased GLP-1 response following VSG and that VhlΔGut mice have increased basal circulating GLP-1 levels. To examine the importance of increased basal total GLP-1 levels on the profound metabolic phenotype of the VhlΔGut, we first determined the origin of the increased GLP-1; i.e., whether it comes from the intestine or pancreas. To do so, we bred VhlF/FVillinCre (VhlΔGut) with preproglucagon (Gcg)stopflox mice. This resulted in mice that do not express VHL in the intestine and are total Gcg body KO mice except for the intestine, in which Gcg is reactivated (RA). Because these mice only express Gcg in the intestine, any circulating GLP-1 is limited to what is secreted from villin-expressing cells in the intestine. We found that circulating GLP-1 levels are indeed increased similarly in VhlΔGut mice and VhlΔGut_GcgRAΔGut mice (Figure 5A), confirming that the intestine is the source of increased circulating GLP-1. Similarly, GcGRA mice have similar circulating levels of GLP-1 as littermate controls, suggesting that any circulating GLP-1 is secreted primarily from the intestine. To further confirm that circulating GLP-1 levels in these mice originate from the intestine, we measured pancreatic GLP-1 levels and indeed found that intestinal GcgRA resulted in limited to non-measurable pancreatic GLP-1 levels (Figure 5B).
Figure 5. Diet-induced obesity resistance of the VhlΔGut depends on Glp1R signaling.
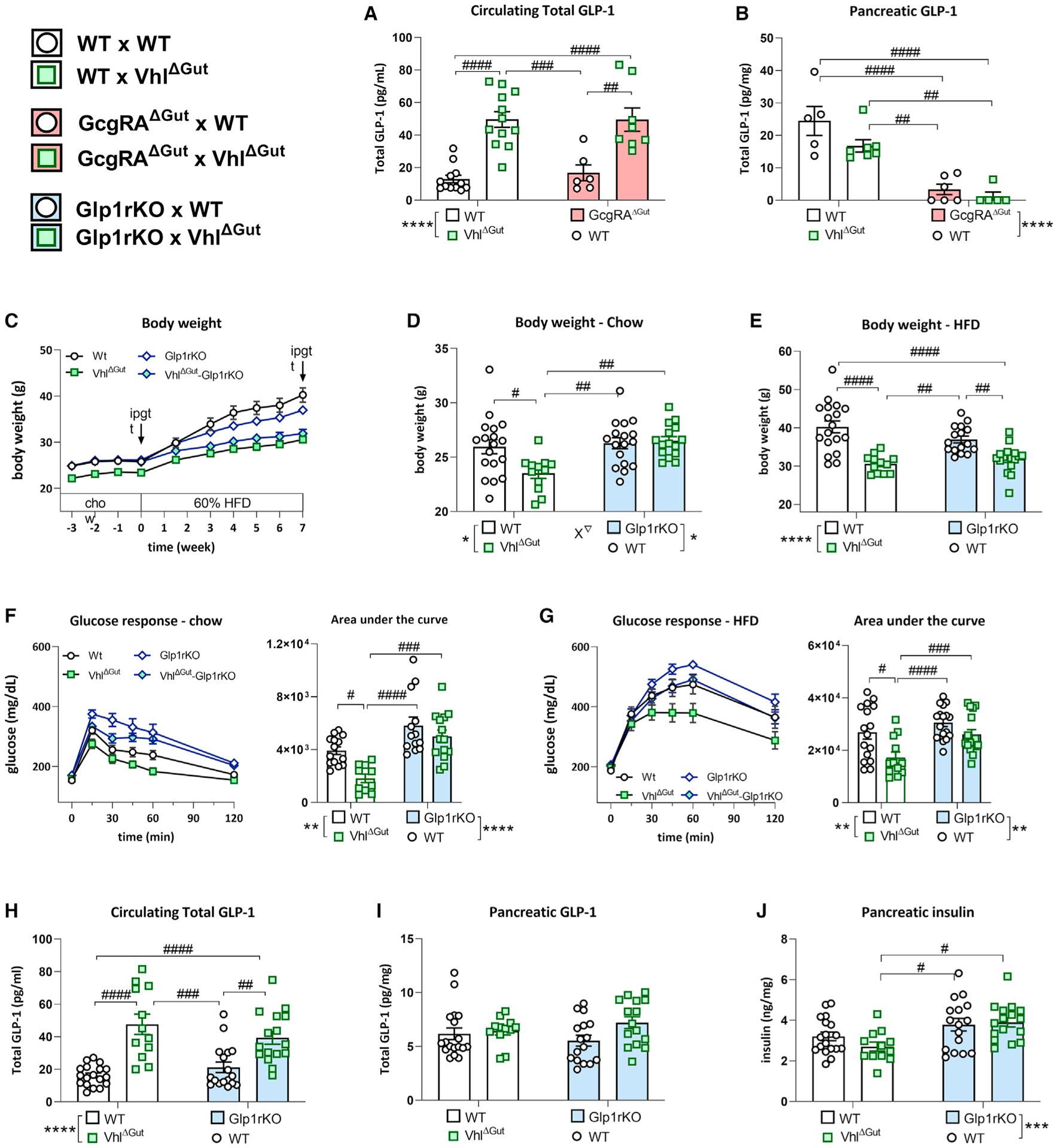
(A and B)The combination of VhlΔGut and intestinal Gcg reactivation (GcgRAΔGut) show that (A) increased circulating total GLP-1 levels in VhlΔGut mice (average ± SEM; WT × WT, n = 12; WT × VhlΔGut, n = 12; GcgRAΔGut × WT, n = 6; GcgRAΔGut ×VhlΔGut, n = 8; two-way ANOVA: F1,34 = 48.18, ****p < 0.0001 effect of VhlΔgut; ####p < 0.0001 post hoc Tukey test) originates from the intestine and not (B) from the pancreas (two-way ANOVA: F1,19 = 53.78, ****p < 0.0001 effect of GcgRAΔGut).
(C) Body weight over the duration of the study reveals that VhlΔgut mice are lower in body weight while fed a chow diet. During 7 weeks of 60% HFD diet feeding, VhlΔgut-Glp1rKO mice attenuate weight gain to the level of VhlΔgut mice, revealing that DIO resistance is not dependent on GLP1R action.
(D) Under standard chow diet conditions, VhlΔGut mice are lower in body weight compared with WT, Glp1rKO, or VhlΔGutGlp1rKO mice. Average ± SEM; WT × WT, n = 18; WT × VhlΔGut, n = 12; Glp1rKO × WT, n = 16; Glp1rKO × VhlΔGut, n = 15; two-way ANOVA: F1, 58 = 5.986, ∇p < 0.05 interaction, #p < 0.05, ##p < 0.01 post hoc Tukey test).
(E) Following 7 weeks of 60% HFD feeding, VhlΔGut are similar in weight to VhlΔgut-Glp1rKO mice. Two-way ANOVA: F1,58 = 40.66, ****p < 0.0001 main effect of VhlΔGut, #p < 0.05, ###p < 0.001, ####p < 0.0001 post hoc Tukey test.
(F) Under chow conditions, the lower glucose response during an ipGTT (2 g/kg glucose) in VhlΔGut mice depends on Glp1R action. Area under the curve: two-way ANOVA: F1, 47 = 29.49, ****p < 0.0001 main effect of Glp1rKO, **p < 0.01 main effect of VhlΔGut, #p < 0.05, ###p < 0.001, ####p < 0.0001 post hoc Tukey test).
(G) Under 60% HFD conditions, the lower glucose response during an ipGTT in VhlΔGut mice does depend on Glp1r action. Area under the curve: two-way ANOVA: F1,58 = 46.75, ****p < 0.0001 main effect of VhlΔGut; F1,58 = 6.951, ∇p < 0.05 interaction, ##p < 0.01, ####p < 0.0001 post hoc Tukey test.
(H and I) VhlΔGut and VhlΔGutGlp1rKO mice have increased circulating total GLP-1 levels (two-way ANOVA: F1,34 = 48.18, ****p < 0.0001 main effect of VhlΔGut, ##p < 0.01, ###p < 0.001, ####p < 0.0001 post hoc Tukey test) but (I) comparable levels of pancreatic GLP-1.
(J) Pancreatic insulin was higher in the Glp1rKO groups compared with VhlΔGut. Two-way ANOVA: F1,58 = 12.43, ***p < 0.001 main effect of Glp1rKO, #p < 0.05 post hoc Tukey test).
Because the Gcgstopflox lacks glucagon as well, assessing improvements in their glucose regulation is not informative. Hence, to study whether the increased basal total GLP-1 levels observed in the VhlΔGut mice is necessary for the lean and glucose-tolerant phenotype, we bred VhlΔGut with GLP1 receptor stop-flox mice (Glp1rstopflox). This resulted in mice that are total body GLP1R KO mice except for VillinCre-expressing cells. However, because others have shown that GLP1R is not expressed in intestinal cells (Richards et al., 2014), we consider these mice to functionally be complete body Glp1r KO mice as well as intestine-specific VHL KO mice. VhlΔGut and VhlΔGutGlp1rKO mice have increased circulating total GLP-1 levels (Figure 5H) without increased pancreatic GLP-1 (Figure 5I). Remarkably, under chow conditions, only VhlΔGut mice are lower in body weight (Figures 5C and 5D) compared with all other groups, revealing that GLP-1 receptor action is essential for the lower-body-weight phenotype of VhlΔGut mice. However, when switched to a 60% HFD, VhlΔGut and VhlΔGutGlp1rKO mice show resistance to DIO (Figures 5C and 5E). Because Glp1rKO mice are known to be resistant to DIO (Ayala et al., 2010), the decreased weight gain in VhlΔGutGlp1rKO mice cannot be fully attributed to the inhibited action of GLP-1 and may be explained partly by increased pancreatic insulin levels (Figure 5J). Nonetheless, under chow conditions (Figures 5F) and 60% HFD conditions (Figure 5G), VhlΔGut mice have a lower glucose response compared with VhlΔGutGlp1rKO mice even though body weights were similar between groups following 7 weeks of 60% HFD feeding. These data demonstrate that increased GLP-1R signaling in VhlΔGut mice is essential for the lean phenotype under chow conditions and improved glucose tolerance under chow as well as HFD conditions.
DISCUSSION
Bariatric surgeries, including VSG, have profound effects on a number of physiological systems. These include obvious ones, such as regulation of food intake and body weight, and less obvious ones, such as the drive to breathe in response to escalating levels of CO2 (Arble et al., 2019). Here we find that this includes an effect on regulation of iron and HIF in the proximal small intestine. Clinically, bariatric surgeries result in lower circulating iron levels, and this has largely been deemed a result of malabsorption from an altered GI tract that is physically less capable of absorbing iron (Ruz et al., 2009; Steenackers et al., 2018; Gowanlock et al., 2020). In the clinic, individuals undergoing bariatric surgery are given iron supplements to reduce this deleterious effect. In two different models where the ability of VSG to reduce iron levels was abrogated, the benefits of VSG on glucose and body weight were normal. This indicates that the beneficial metabolic effects of VSG are not secondary to lowering iron.
If the effects of VSG are not secondary to reduced circulating iron levels, then the next question is whether intestinal HIF2α is necessary for the effects of VSG. To test this hypothesis, we created a mouse that had specifically reduced HIF2α signaling in the intestine. Despite profound reductions in HIF2α in the duodenum, the ability of VSG to reduce food intake, body weight, and body fat and improve glucose tolerance was normal in intestinal Hif2αΔGut mice. These data make a strong case that HIF2α is not required for the metabolic effects of VSG. However, there are a number of HIFs expressed in the intestine that share similar signaling pathways; therefore, the potential crosstalk between these pathways may partly compensate for HIF2α deficiency (Ramakrishnan and Shah, 2017; Xie et al., 2017).
Although HIF2α is not necessary for the metabolic effects of VSG, the question remains as to whether activation of the HIF2α pathway will mimic the beneficial effects of VSG on weight, glucose, and gut hormone secretion. To examine this, we took advantage of a mouse where Vhl was deleted specifically from the intestine (Shah et al., 2008, 2009). VHL is critical for the effects of HIF2α (and other HIFs) because it ubiquitinates HIFs and targets them for peroxisomal degradation and, therefore, keeps HIF from entering the nucleus to stimulate target transcription. Through KO of Vhl, HIFs are not degraded, and this results in increased target gene transcription. Indeed, intestinal VhlΔGut mice have profound increases in the expression of several HIF2α target genes. Moreover, the lean phenotype of these mice is quite profound when they are placed on chow or an HFD. On chow, VhlΔGut mice are considerably leaner than their littermate controls and have better glucose tolerance. This effect becomes substantially bigger when the mice are placed on an HFD, with ~50% less body fat after 21 days on an HFD (Figure 4E). On an HFD, VhlΔGut mice have a much lower glucose excursion during a glucose tolerance test compared with their littermate controls (Figure 4G). Even though all intestinal iron transporters are highly upregulated in VhlΔGut mice, these mice tend to have lower circulating iron levels. This reduction in circulating iron levels is accompanied by significantly higher circulating hepcidin levels, such as what occurs after VSG on a high-iron diet. Finally, we found that VhlΔGut mice have increased basal GLP-1 levels.
We will admit being surprised by the profound and consistent phenotype of these mice. The selective upregulation of HIF-related signaling in the intestine had a profound effect on several varied parameters that mimic disparate physiologic effects of what occurs after VSG. Among the most curious of these is the robust increase in circulating GLP-1 levels. GLP-1 receptor activation can produce weight loss and glucose improvements in rodents and humans and is the basis for currently approved therapies for obesity and type 2 diabetes (Astrup et al., 2009; Barrera et al., 2011; Burmeister et al., 2012; Heppner and Perez-Tilve, 2015). GLP-1 is just one peptide product of the large pro-hormone Gcg (Müller et al., 2019). Gcg is expressed in a number of tissues, including the gut, pancreas, and central nervous system. Consequently, we sought to determine whether the increased circulating levels of GLP-1 were derived primarily from the intestine. We crossed VhlΔGut mice with a mouse where we could selectively reactivate the endogenous Gcg allele in the gut (Chambers et al., 2017). In mice where the endogenous allele for Gcg was RA only in the intestine, the effect of VHL deficiency to increase basal GLP-1 circulating levels was similar to intestinal VHL deficiency alone.
These data make a compelling case that the increased circulating levels of GLP-1 in VhlΔGut mice are a product of increased secretion of GLP-1 from the gut. Interpreting other metabolic parameters from these mice is complicated because these mice not only lack GLP-1 but also other Gcg-derived peptides. These mice have exceptionally good glucose tolerance, presumably because of their lack of glucagon (Chambers et al., 2017). Consequently, they are a poor model on which to test whether the effect of intestinal VHL deficiency depends on the increased secretion of GLP-1. To test the role of GLP-1 signaling in the phenotype of VhlΔGut mice, we crossed these mice with GLP-1 receptor KO mice. Again, we observed the effect of intestinal VHL deficiency to increase basal GLP-1 secretion, and this was not altered by KO of the GLP-1 receptor (Figure 5J). Interestingly, loss of GLP-1R signaling completely abrogated the lean phenotype of VhlΔGut mice on chow but not when switched to an HFD. In the case of glucose levels, loss of GLP-1R signaling clearly abrogated the beneficial effects observed on glucose tolerance in VhlΔGut mice under both diet conditions.
These data point toward a profound effect of bariatric surgery to increase HIF2α signaling in the gut. The effect of VSG to lower iron likely depends on the ability of VSG to increase hepcidin secretion from the liver. What remains unclear is how the signal from the surgically altered GI tract affects hepcidin secretion from the liver. However, a wide range of data points to the important effects of VSG to alter liver function, which includes hepatic glucose production (Chambers et al., 2011), bile acid secretion (Myronovych et al., 2014; Ryan et al., 2014), and triglyceride accumulation (Myronovych et al., 2014).
Although the effect of VSG does not depend on HIF2α activation in the intestine, increasing HIF2α signaling in the gut by selective disruption of VHL in the intestine results in reduced weight gain and improved glucose tolerance on an HFD. VSG and selective disruption of VHL in the intestine result in increased basal secretion of GLP-1 from the intestine. The improved metabolic phenotype of VhlΔGut mice does not easily fit with previous observations that expression of HIF2α in the ileum is positively correlated with increasing BMI and that genetic or pharmacological inhibition of HIF2α improved metabolism by reducing ceramide levels (Xie et al., 2017). Our work focused on regulation of HIF2α in the duodenum, whereas the measurements from Xie et al. (2017) were from the ileum. We focused on the duodenum because expression of HIF2α and many of its key target genes is considerably higher in the duodenum than in the ileum, and this is consistent with its role in iron absorption, which occurs mostly in the duodenum (Figure 1). Moreover, conditional versus constitutive intestine-specific HIF2α KO used by Xie et al. (2017) could contribute to the discrepancy in metabolic endpoints. Nonetheless, comparable with Xie et al., we also found a reduction of liver triglyceride levels in conditional Hif2αΔGut mice, confirming a role of intestinal Hif2α in liver lipid metabolism.
These data point to a clear effect of multiple bariatric surgeries to increase HIF2α signaling in the small intestine. The current data indicate that manipulations of HIF2α signaling, whether they are the result of altered GI anatomy or genetic deletion of VHL, result in similar effects on weight, metabolism, and gut hormone secretion. There are a number of potential ways to manipulate HIF2α signaling, particularly because the key cells are found facing the lumen in the upper small intestine. Such manipulations could replicate the effects of VSG without the need for permanent alterations of GI anatomy.
Limitations of the study
The experiments described here are technically demanding and occurred over more than a 5-year period and not in the order in which they are now presented. Therefore, not all experimental measurements and timelines are parallel among all experiments. In some cases, the same measurement was made with different assays. Further, the complex breeding involved in the various tissue-specific knockdowns prevented us from having all mice on exactly the same genetic background. All of these issues limit direct comparisons of actual values among the various experiments. Nevertheless, each experiment includes critical control groups to assess the effect of the surgery and genotype and their potential interactions in that experiment. Throughout these experiments, we used mixtures of oral glucose tolerance tests and i.p. glucose tolerance tests. I.p. glucose tolerance tests have the limitation that they do not engage the multiple components of the GI tract that contribute to regulation and potential dysregulation of glucose levels. In all cases, however, we documented the effect of our surgical or genetic manipulation on multiple measures of overall glucose regulation. Finally, it is important to note that VHL’s ability to target proteins for degradation are not specific to HIF2α. VHL is an important component of degradation of various forms of HIF (Shah and Xie, 2014; Yang et al., 2015; Ramakrishnan and Shah, 2016; Xie et al., 2017). Consequently, the substantial phenotype of intestine-specific deletion of VHL cannot be unambiguously attributed entirely to activation of HIF2α. Although HIF2α is the logical target, given that its signaling is upregulated exclusively after VSG and RYGB, these experiments cannot exclude the possibility that other targets of VHL may contribute to the observed effects.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Randy J. Seeley (seeleyrj@med.umich.edu).
Material availability
Mouse lines generated in this study have not been deposited. Sources for breeding pairs are available in the key resources table. Requests for available resources (mouse lines, tissue samples, etc.) and reagents should be directed to and will be fulfilled by the lead contact.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| T-PER Tissue Protein Extraction Reagent | Thermo Fisher Scientific | Cat# 78510 |
| protease inhibitor (1 pill/100ml T-PER) | Sigma-Aldrich | Cat# S8820 |
| DPP-4 inhibitor (20μl/ml T-PER) | Millipore-Sigma | Cat# SKUDPP4 |
| BCA protein analysis | Pierce-Fisher | Cat#23225 |
| FailSafe PCR PreMix | Lucigen Corp, Middleton,WI | Cat# FS99250 |
| SYBR Safe | Invitrogen | Cat# S33102 |
| TRIzol reagent | Thermo Fisher Scientific | Cat# 15596026 |
| PureLink RNA mini kit | Thermo Fisher Scientific | Cat# K157001 |
| RNeasy Mini kit | Qiagen | Cat#74106 |
| iScript cDNA synthesis kit | BioRad | Cat# 1708891 |
| SsoAdvanced Universal Probes Supermix | BioRad | Cat# 1725281 |
| Critical commercial assays | ||
| Dual Dual-Luciferase Assay System | Promega | Cat# E1910 |
| Ultra-Sensitive Mouse Insulin ELISA | Crystal Chem | Cat# 90080 |
| QuantiChrom Iron Assay | BioAssay Systems | Cat# DIFE-250 |
| Hepcidin-Murine CompeteTM ELISA | Intrinsic Life Sciences | Cat# HMC-001 |
| V-PLEX GLP-1 Total Kit | Meso Scale Discovery | Cat# K1503PD |
| TaqMan gene expression Assay - Hif2a (Epas1) | Thermofisher Scientific | Mm01236112_m1 |
| TaqMan gene expression Assay - Hif1a | Thermofisher Scientific | Mm00468869_m1 |
| TaqMan gene expression Assay - Vhl | Thermofisher Scientific | Mm00494137_m1 |
| TaqMan gene expression Assay - Dmt1 (Slc11a2) | Thermofisher Scientific | Mm00435363_m1 |
| TaqMan gene expression Assay - Dcytb (Cybrd1) | Thermofisher Scientific | Mm01335930_m1 |
| TaqMan gene expression Assay - Phd2 (Egln1) | Thermofisher Scientific | Mm00459770_m1 |
| TaqMan gene expression Assay - Neu3 | Thermofisher Scientific | Mm00479379_m1 |
| TaqMan gene expression Assay - Pdk1 | Thermofisher Scientific | Mm01276567_m1 |
| TaqMan gene expression Assay - Pgk1 | Thermofisher Scientific | Mm00435617_m1 |
| TaqMan gene expression Assay - Glut1 (Slc2a1) | Thermofisher Scientific | Mm05908127_s1 |
| TaqMan gene expression Assay - Rpl32 | Thermofisher Scientific | Mm07306626_gH |
| TaqMan gene expression Assay - Actb | Thermofisher Scientific | Mm02619580_g1 |
| Deposited data | ||
| Array data submitted to GEO database | This paper | GSE169403 |
| Experimental models: Organisms/strains | ||
| VhlF/F;Vilcre | Prof.dr. Y. Shah, Dept. Mol & Int Phys, University of Michigan | N/A |
| Hif2αF/F;VilWt/Wt, Hif2αF/F-VilCreERT | Prof.dr. Y. Shah, Dept. Mol & Int Phys, University of Michigan | N/A |
| HampF/F;AlbWt/Wt HampF/F-AlbCre | Prof.dr. Y. Shah, Dept. Mol & Int Phys, University of Michigan | N/A |
| Gcgstopflox | Prof.dr. R.J. Seeley, Dept. Surgery, University of Michigan | N/A |
| Glp1rstopflox | Prof.dr. R.J. Seeley, Dept. Surgery, University of Michigan | N/A |
| FVB.129S6Gt(ROSA) 26Sortm2(HIF1A/luc)Kael)/J – ODD-Luc | Jackson laboratory | Stock no.006206 |
| C57BL/6J | Jackson Laboratory | Stock no.000664 |
| Long-Evans rats | Harlan Laboratories | HsdBlu: LE |
| Oligonucleotides | ||
| Oligonucleotides are listed in Table S7 | N/A | N/A |
| Software and algorithms | ||
| Graphpad Prism | Graphpad Software | Version 8.1.2 |
| R A Language and Environment for Statistical Computing | R | R Core Team (2017) |
| Expression console v.1.3.0.187 | Thermo Fisher Scientific | N/A |
| CARMAweb | Medical University Innsbruck | Rainer et al. (2006) |
| QIAGEN Ingenuity Pathway Analysis, IPA | QIAGEN | www.qiagen.com/ingenuity |
| Other | ||
| Regular chow diet | Envigo Teklad | Cat# 7012 |
| 60% HFD | Research Diets, Inc | Cat# D12492 |
| Custom high fat (45% FDC) AIN-93G with 35ppm Fe | Dyets Inc, Pa | Cat# 115244 |
| Custom high fat (45% FDC) AIN-93G with 350ppm Fe | Dyets Inc, Pa | Cat# 115245 |
| Osmolite OneCal liquid diet | Abbott, Columbus, OH | Cat# 64633 |
| Ensure Plus | Abbott | Cat # 57263 |
| Accu-Chek Aviva Meter | Accu-Chek, Roche Diabetes Care | https://www.accu-chek.com |
| Endopath ETS-FLEX 35mm Stapler | Ethicon endo-surgery | VASECR35 |
| Nuclear Magnetic Resonance | EchoMRI LLC, USA | EchoMRI-900 |
| Ovation PicoSL WTA System V2 | Nugen | Part no. 3312 |
| GeneChip Rat Gene 2.1 ST Array Plate | Thermofisher Scientific | Cat# 902143 |
Data and code availability
Standardized RNA sequencing data have been deposited at GEO data base (GSE169403) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal study design
All animal studies were approved by and conducted according to the guidelines of the Institutional Animal Care and Use Committee at the University of Michigan.
Intestinal ODD-luciferase activity following VSG
Male ODD-luciferase reporter mice (FVB.129S6Gt(ROSA)26Sortm2(HIF1A/luc)Kael/J) at age 4–5 weeks were purchased from Jackson Laboratory. All mice had continuous access to 60% HFD (Research Diets) and water. Mice received either Sham (n=8) or VSG (n=13) following 8 weeks of diet induction and were terminated at 28 days post-surgery. Tissue luciferase activity was measured by homogenizing the tissues with the Dual Dual-Luciferase Assay System (Promega) according to the manufacturer’s instructions and measured with SpectraMax M5 (Molecular Devices, San Jose, CA).
Characterization of VhlF/F;VilCre mice
Breeder mice were kindly provided by Dr. Y.M Shah from the department of Molecular & Integrative Physiology at the University of Michigan. Prior to study, male VhlF/F;Vilcre;(intestinal Vhl knockout) and wildtype VhlF/F male littermate mice, had ad lib access to water and regular chow diet (Envigo Teklad). At study day 0, age 10–12 weeks, the diet was switched to a 60% HFD (Research Diets). At study day -1 and day 14 an ip-gtt (2 g/kg glucose) was performed. At day 21 following an overnight fast, animals were euthanized using CO2 and cardiac puncture. Whole blood was divided of EDTA coated tubes for Hepcidin and Total GLP-1 analysis and a heparin coated tube for plasma iron analysis. Blood samples were centrifuged at 3000g for 10min at 4°C. Plasma was stored at −80°C until further analysis. Duodenum and ileum were dissected and processed for mRNA expression determination (detailed description below).
Response to VSG in mice fed a high iron containing diet
Male C57B6/J mice (Jackson Laboratory), age 5 weeks at arrival had ad lib access for 6 weeks to 45% high-fat regular iron diet (35ppm Fe, Dyets Inc, #115244) and water before being randomized to 4 groups based on body weight. The groups consisted of two groups receiving Sham surgery of which one group was kept on the 45% high-fat regular iron diet and the other was switched to a 45% high-fat high iron diet (350ppm Fe, Dyets Inc, #115245), the other two groups received VSG surgery (details are described below) of which one group was kept on the 45% high-fat regular iron diet and the other was switched to 45% high-fat regular iron diet. Designated high iron diet groups were switched to the diet 2 weeks prior to surgery. At 56 days post-surgery an ip-gtt (2g/kg glucose) was performed. Animals were terminated at day 70 post surgery using CO2 and cardiac puncture. Animals were not fasted before termination. At termination, blood was collected to determine hematocrit, total iron levels, and hepcidin levels. Duodenum and ileum were dissected and processed for mRNA expression determination (detailed description below).
Response to VSG in a mouse model of hereditary hemochromatosis
Breeder (HampF/F;AlbWt/Wt and HampF/F-AlbCre) mice were kindly provided by Dr. Y.M Shah from the department of Molecular & Integrative Physiology at the University of Michigan. Male offspring were single housed (age 4–5 weeks) and had ad lib access to 60% high fat diet (Research Diets) and water. Following 8 weeks of diet induction mice were randomized to 4 groups of which 2 received Sham surgery, namely Wt-Sham (HampF/F;AlbWt/Wt) and HepcidinΔLiver-Sham (HampF/F;AlbCre) and 2 groups received VSG surgery, namely Wt-VSG (HampF/F;AlbWt/Wt) and HepcidinΔLiver-VSG (HampF/F;AlbCre). An ip-gtt (2 g/kg glucose) was performed at 5 weeks post-surgery. Following an overnight fast, animals were terminated 8 weeks post-surgery using CO2 and cardiac puncture. A whole blood sample (150μl) was collected in a heparin coated tube and send to Unit for Laboratory Animal Medicine (ULAM) at the University of Michigan for the analysis of circulating iron levels, total iron binding capacity (TIBC) and transferrin saturation (%).
Response to VSG in intestinal Hif2α knockout mice
Breeder (Hif2α F/F;VilWt/Wt and Hif2α F/F-VilCreERT) mice were kindly provided by Dr. Y.M Shah from the department of Molecular & Integrative Physiology at the University of Michigan. Male offspring were single housed (age 4–5 weeks) and had ad lib access to 60% high fat diet (Research Diets) and water throughout the study. At 6 weeks of diet induction, two weeks prior to surgery, all mice received three doses of tamoxifen (s.c. 10mg/kg) with one day in between each dose to knock down Hif2α expression in villin expressing cells. The study consisted of 4 groups of which 2 received Sham surgery, namely Wt-Sham (Hif2αF/F;VilWt/Wt) and IntHif2αKO-Sham (Hif2αF/F;VilCreERT) and 2 groups received VSG surgery, namely Wt-VSG (Hif2αF/F;VilWt/Wt) and IntHif2αKO-VSG (Hif2αF/F;VilCreERT). At 28 days post-surgery, animals received an oral administration of EnsurePlus+2g/kg glucose to measure the Total GLP-1 response at 15min post administration. At 56 days post-surgery an ip-gtt (2 g/kg glucose) was performed. Following an overnight fast, animals were terminated at day 70 post-surgery using CO2 and cardiac puncture. Duodenum and ileum were dissected and processed for mRNA expression determination (detailed description below).
Origin of increased circulating total GLP-1 in VhlF/F;VilCRE mice
To study the origin of increased total GLP-1 levels in VhlF/F;VilCre mice, we crossbred VhlF/F;VilCre with Gcgstopflox mice. Gcgstopflox mice are functionally total body Gcg knockout mice and crossbreeding with VilCre mice would result in mice in which Gcg expression is reactivated (GcgRA) only in the Villin expressing intestinal cells. This resulted in 4 genetically different groups, namely Wt (VhlF/F; GcgWt/Wt-VilWt/Wt), Vhl-KO (VhlF/F;GcgWt/Wt;VilCre), GcgRA VhlWt/Wt;Gcgstopflox;VilCre), and VhlKO-GcgRA VhlF/F;Gcgstopflox-VilCre). At 6 weeks of age male offspring were terminated following an overnight fast using CO2 and cardiac puncture. Blood samples were collected for the determination of Total GLP-1. The pancreas was dissected and flash frozen in liquid nitrogen. Frozen pancreas was first grinded using a mortar and pestle and was lysed in 1mL T-PER Tissue Protein Extraction Reagent (Thermon Fisher Scientific) containing a protease inhibitor cocktail (Sigma-Aldrich; 1 pill/100ml T-PER) and DPP-4 inhibitor (Millipore-Sigma, 20μl/ml T-PER). Samples were centrifuged at 10000g for 5min at 4°C. The supernatant was collected to determine total protein concentration using BCA protein analysis (#23225, Pierce-Fisher) and Total GLP-1 analysis (specified description below).
VhlF/F-VilCRE mice phenotype dependency on GLP-1 action
To study the functionality of increased total GLP-1 levels in VhlF/F;VilCre mice, we crossbred VhlF/F-VilCre with Glp1rstopflox mice. Glp1rstopflox mice are functionally total body Glp1 receptor knockout mice and crossbreeding with VilCre mice would result in mice in which Glp1r expression is reactivated only in the Villin expressing intestinal cells. Because Glp1r is to the best of our knowledge not or barely expressed in intestinal cells (Richards et al., 2014), crossbreeding with VhlCre mice would result in a functional total body GLp1r knockout mouse (Glp1rKO). This resulted in 4 genetically different groups, namely Wt (VhlF/F-Glp1rWt/Wt-VilWt/Wt), Vhl-KO (VhlF/F-Glp1rWt/Wt-VilCre), Glp1rKO (VhlWt/Wt-Glp1rstopflox-VilCre), and VhlKO-Glp1rKO (VhlF/F-Glp1rstopflox-VilCre). At age 4–5 weeks, male mice were single housed and had ad lib access to chow and water. An ip-gtt (2 g/kg glucose) was performed 1 week prior and 4 weeks following the switch to a 60% HFD (Research Diets). Following an overnight fast, mice were terminated following 7 weeks of 60% HFD feeding using CO2 and cardiac puncture. Blood samples were collected for the determination of Total GLP-1 levels.
Rat cohort for RNA sequencing study
Male Long-Evans rats (250 – 300 g, 8 –10 weeks of age; Harlan Laboratories, Indianapolis, IN) were single housed at the Metabolic Diseases Institute of the University of Cincinnati under standard controlled conditions (12:12-h light-dark cycle, 50–60% humidity, 25°C). Animals had free access to water and a high-fat diet (HFD) (40% fat; 4.54 kcal/g, D03082706; Research Diets, New Brunswick, New Jersey) for eight weeks. Three days pre-operatively, rats were matched for body weight and fat mass and randomized to RYGB, VSG, RYGB-sham, and VSG-sham surgical groups (n=6) and the high-fat diet was replaced with Osmolite OneCal liquid diet (Abbott, Columbus, OH). VSG and RYGB surgery were performed in anesthetized rats as described previously (Chambers et al., 2011). All surgical groups received postoperative care consisting of subcutaneous injections of Metacam (0.25 mg/100 g body weight once daily for 4 days), Buprenex (0.3 mL twice a day for 5 days), and warm saline (10 mL and 5 mL twice daily for days 0–3 and 4–5, respectively). During the 5 days of postoperative care, rats had free access to Osmolite OneCal Liquid Diet (Abbott, Columbus, OH) until they were switched back to solid HFD diet. All rats were sacrificed 30 days after surgery.
METHOD DETAILS
Genotyping
Mice were genotyped based on DNA extraction of the tail tip using HotSHOT genomic DNA preparations. In short, tail tips were submerged in 75μl alkaline lysis buffer (125μl 10N NaOH+20μl 0.5M EDTA+50mlH2O) and heated to 95°C for 30min and cooled to 4°C until 75μl neutralization buffer (325mg Tris-HCl+50ml H2O) was added to each sample. For PCR, 1–5μl DNA template was used and mixed with FailSafe PCR PreMix (Lucigen Corp, Middleton,WI) and run in BioRAD thermal cycler. PCR products were run in a 1.5% agarose gel in 1X TBE with SYBRTM Safe (10μl/100ml 1.5%agarose; Invitrogen). Gels were imaged using a Chemiluminescent imager (BioRAD). Genotypes were identified with the following primer pair:
VHL F1 (5’-CTG GTA CCC ACG AAA CTG TC-3’), F2 (5’-CTA GGC ACC GAG CTT AGA GGT TTG CG-3’), and R1 (5’-CTG ACT TCA CTG ATG CTT GTC ACA G-3’); Cre F (5’-AGT GCG TTC GAA CGC TAG AGC CTG T-3’) and R (5’-GAA CCT GAT GGA CAT GTT CAG G-3’); GCGStopflox F1 (5’-CCT TCA GAA AAG CTG TCA GA-3’), F2 (5’-GCA TTC TAG TTG TGG TTT GTC C-3’), and RA (5’-TCC TAT GTA ACT GTT TGG CAT G-3’); GLP1RStopflox-WT F1 (5’- TGA GAG CTG ATG GAA GGT GTT G-3’), Mutant F2 (5’-CTG CAT TCT AGT TGT GGT TTG TCC-3’), and Common R1(5’-CCT TCA GAT GGG GAA ACA AAG C-3’); Hamp F (5-TAG GCT GCT TAC CTC TCT TTC TT-3’) and R (5’-AAT TCC AAG ACT TAG AAG GCA AA-3’).
Vertical sleeve gastrectomy surgery (VSG)
The day prior to surgery solid food was replaced with liquid diet (Osmolite). Under isoflurane/O2 mixture anesthesia, all mice received a midline incision in the ventral abdominal wall and the stomach was exposed. For VSG, approximately 80% of the stomach was transected along the greater curvature using an Endopath ETS-FLEX 35mm Stapler (Ethicon endo-surgery) creating a sleeve-like gastric remnant. For sham surgery, pressure was applied on the stomach imitating the VSG-transection line with nontoothed blunt forceps. The abdominal wall was closed using continuous absorbable 5–0 Vicryl Rapide sutures. Postoperatively, mice had ad lib access to Osmolite liquid diet for 4 consecutive days. All mice received 1 ml warm saline subcutaneously for fluid replacement on the first postoperative day and analgesic treatment with meloxicam (0.5 mg/kg) for 3 consecutive days following surgery. Solid diet access was resumed on post-surgery day 4. Body weight and food intake were measured daily for the first week post-surgery. General health status was checked for 10 consecutive days post-surgery.
Glucose tolerance test
During glucose tolerance tests, all mice were fasted for 4 hours prior to intraperitoneal injections of dextrose (2 g/kg body weight). Tail vein blood glucose levels were measured using Accu-Chek glucometers (Accu-Chek) at 0, 15, 30, 45, 60, 90, and 120 minutes post glucose administration. Area under the curve over 120 minutes was calculated.
ELISA
Plasma insulin levels were determined using ELISA colorimetric insulin assay kit (Crystal Chem). For iron measurement, plasma samples were collected from heparinized blood and used for measurement of total circulating iron (Fe2+ and Fe3+) using the QuantiChrom Iron Assay Kit (BioAssay Systems). Plasma hepcidin levels were determined using Hepcidin-Murine CompeteTM ELISA (Intrinsic Life Sciences). For GLP-1, whole blood samples were collected in microtubes treated with 1:10μl anti-proteolytic cocktail (EDTA/Aprotinin/Heparin) to prevent enzymatic GLP-1 degradation. Plasma total GLP-1 levels were determined using the Meso Scale Discovery Platform according to the manufacturer’s instructions (Meso Scale Discovery). All assays were performed according to the manufacturer’s instructions.
Luciferase assay
Tissue luciferase activity was measured by homogenizing the tissues of ODD-luciferase reporter mice with the Dual Dual-Luciferase Assay System (Promega) according to the manufacturer’s instructions and measured with SpectraMax M5 (Molecular Devices).
Body composition
In vivo lean and adipose tissue body composition in mice and rat was measured using nuclear magnetic resonance (EchoMRI™-900, EchMRI LLC, Houston, USA).
RT-PCR
To determine mRNA expression from duodenum and ileum, the small intestine was dissected. The duodenum or proximal small intestine was cut from the stomach to app. 3cm in length. The ileum or distal small intestine was cut at app. 5cm proximal to- and at the cecum. Intestinal sections were cut longitudinal and gently rinsed in saline to remove intestinal contents. Tissues were placed on a microscope slide with the epithelial layer facing upwards. Using a clean microscope slide the epithelial layer was scraped from the submucosal layer. The epithelial sample was frozen in liquid nitrogen and stored at −80°C. Epithelial tissue were homogenized using Trizol reagent (Thermo Fisher Scientific). RNA was then extracted using PureLink RNA mini kit (Thermo Fisher Scientific) and cDNA was isolated using iScript cDNA synthesis kit (BioRad). The real-time quantitative PCR was conducted using a CFX-96 Real-Time System (BioRad) with SsoAdvanced Universal Probes Supermix (BioRad) and TaqMan Gene Expression Assays (Thermo Fisher Scientific). Expression levels of target genes were normalized to the average of Rpl32 and Actb gene expressions. Probes used are shown at key resources table.
Transcriptome analysis
Total RNA from the dissected duodenum, jejunum and ileum was isolated using the RNeasy Mini kit including on-column digestion of DNA during RNA purification (Qiagen, Valencia, CA, USA) and was amplified using the Ovation PicoSL WTA System V2 in combination with the Encore Biotin Module (Nugen). Amplified cDNA was hybridized on Rat Gene 2.1 ST arrays (Thermo Fisher Scientific Inc., Waltham, USA). Staining and scanning was done according to the Thermo Fisher Scientific expression protocol including minor modifications as suggested in the Encore Biotion protocol. Expression console (v.1.3.0.187, Thermo Fisher Scientific) was used for quality control and to obtain annotated normalized RNA gene-level data. Statistical analyses were performed by utilizing the statistical programming environment R (R Core Team (2017) R A Language and Environment for Statistical Computing.) implemented in CARMAweb (Rainer et al., 2006). Genewise testing for differential expression was done employing the limma t-test and Benjamini-Hochberg multiple testing correction (FDR <10%). Heatmaps were generated with R. Enrichment and upstream regulator analyses were generated through the use of QIAGEN’s Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, www.qiagen.com/ingenuity) using Fisher’s Exact Test p-values. Input for the enrichment analyses were regulated genes with FDR<10% in both surgeries and same direction of regulation. For the upstream regulator analysis VSG and RYGB were analyzed separately (FDR<10% and fold-change > 1.3x). Array data have been submitted to the GEO database at NCBI (GSE169403).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
Details of statistical analyses including group sizes (n) are presented in the figure legends. All data are presented as average ± SEM. All statistical analyses were performed using Graphpad Prism 8.1.2 software (La Jolla, CA). Two groups direct comparisons were performed using t-test. Two groups multiple comparisons were performed using Multiple t-test with Bonferroni correction. Multiple groups with one variable comparison were performed using one-way ANOVA post hoc Tukey. Multiple groups with two variables comparison were performed using two-way ANOVA post hoc Tukey. Multiple group comparisons over time were performed using repeated measures ANOVA post hoc Tukey. Data were considered statistically significant when P<0.05.
Supplementary Material
Highlights.
Bariatric surgeries result in reduced iron despite upregulation of absorption pathways
Dietary or genetic manipulation to increase iron does not affect VSG effectiveness
Genetic activation of intestinal HIF2α results in improved body fat glucose tolerance
Intestinal HIF2α activation increases GLP-1 secretion, which mediates effects
ACKNOWLEDGMENTS
This work was supported by University of Michigan DK020572 (MDRC), DK089503 (MNORC), NIH 5T32DK108740 (to N.B.-K.), 5T32DK071212-12 (to N.B.-K.), UL1TR002240 (to N.B.-K.), a China Scholarship Council grant (CSC#201606100218 to Y.S.), DK110537 (to S.K.R.), R01CA148828, R01DK095201, R01CA245546, GI Center P30DK034933 (to Y.M.S.), German Research Foundation grant SFB 1321/1 and German Center for Diabetes Research (to K.S.), and the Helmholtz Alliance “Aging and Metabolic Programming, AMPro” (to M.I. from support of Johannes Beckers). We would also like to thank Drs. Alfor Lewis, Andriy Myronovich, and Mouhamadoule Toure for the extensive surgical work on this project and Kelli Rule and Jack Magrisso for work on the mouse models. We would also like to thank Johannes Beckers for critical work on the arrays to identify gene similarities between VSG and RYGB.
DECLARATION OF INTERESTS
R.J.S. has received research support from Novo Nordisk, Astra Zeneca, Pfizer, Energesis, Kintai, and Ionis. R.J.S. has served as a paid consultant for Novo Nordisk, Scohia, Kintai, Eli Lilly, and Ionis. R.J.S. has equity positions in Calibrate and Rewind.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.110270.
REFERENCES
- Aarts EO, Janssen IMC, and Berends FJ (2011). The gastric sleeve: losing weight as fast as micronutrients? Obes. Surg 21, 207–211. 10.1007/s11695-010-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Gazala S, Horwitz E, Ben-Haroush Schyr R, Bardugo A, Israeli H, Hija A, Schug J, Shin S, Dor Y, and Kaestner KH (2018). Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes 67, 1079–1085. 10.2337/db17-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, Bredell BX, Gardenghi S, Rivella S, and Shah YM (2013). Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci U S A 110, 4922–30. 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Evers SS, Bozadjieva N, Frikke-Schmidt H, Myronovych A, Lewis A, Toure MH, and Seeley RJ (2018). Metabolic comparison of one-anastomosis gastric bypass, single-anastomosis duodenal-switch, Roux-en-Y gastric bypass, and vertical sleeve gastrectomy in rat. Surg. Obes. Relat. Dis 14, 1857–1867. 10.1016/j.soard.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Schwartz AR, Polotsky VY, Sandoval DA, and Seeley RJ (2019). Vertical sleeve gastrectomy improves ventilatory drive through a leptin-dependent mechanism. JCI Insight 4. 10.1172/jci.insight.124469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, and Lean ME (2009). Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616. 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, and Drucker DJ (2010). Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology 151, 4678–4687. 10.1210/en.2010-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, and Seeley RJ (2011). GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7, 507–516. 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi D, Meoli L, Abidi WM, Nestoridi E, Panciotti C, Castillo E, Pizarro P, Shirley E, Gourash WF, Thompson CC, et al. (2018). Time-dependent molecular responses differ between gastric bypass and dieting but are conserved across species. Cell Metab. 28, 310–323.e6. 10.1016/j.cmet.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, and Ayala JE (2012). Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am. J. Physiol. Endocrinol. Metab 302, E334–E343. 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson–Pérez HE, Stefater MA, Gaitonde S, Sorrell JE, Toure M, Berger J, et al. (2011). Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141, 950–958. 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim KS, Gutierrez-Aguilar R, Li B, Drucker DJ, D’Alessio DA, et al. (2017). The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 25, 927–934.e3. 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, Bock SN, Landers JT, Kratz M, Foster-Schubert KE, et al. (2016). Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 59, 945–953. 10.1007/s00125-016-3903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das NK, Shwartz AJ, Barthel G, Inohara N, Liu Q, Sankar A, Hill DR, Ma X, Lamberg O, Schnizlein MK, et al. (2019). Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers SS, Kim K-S, Bozadjieva N, Lewis AG, Farris D, Sorensen MJ, Kim Y, Whitesall SE, Kennedy RT, Michele DE, et al. (2019). Continuous glucose monitoring reveals glycemic variability and hypoglycemia after vertical sleeve gastrectomy in rats. Mol. Metabol 32, 148–159. 10.1016/j.molmet.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, and Nemeth E (2012). Hepcidin and iron homeostasis. Biochim. Biophys. Acta (BBA) Mol. Cell Res 1823, 1434–1443. 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowanlock Z, Lezhanska A, Conroy M, Crowther M, Tiboni M, Mbuagbaw L, and Siegal DM (2020). Iron deficiency following bariatric surgery: a retrospective cohort study. Blood Adv. 4, 3639–3647. 10.1182/bloodadvances.2020001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner KM, and Perez-Tilve D (2015). GLP-1 based therapeutics: simultaneously combating T2DM and obesity. Front. Neurosci 9. 10.3389/fnins.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotkiewicz A, Donaldson K, Dye C, Rogers AM, Mauger D, Kong L, and Eyster ME (2015). Anemia and the need for intravenous iron infusion after roux-en-Y gastric bypass. Clin Med. Insights Blood Disord 8. 10.4137/CMBD.S21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, and Whitelaw ML (2002). Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295, 858–861. 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Lane DJ, Bae DH, Merlot AM, Sahni S, and Richardson DR (2015). Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients 7, 2274–2296. 10.3390/NU7042274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Chandel NS, and Simon MC (2020). Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol 21, 268–283. 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, and Peyssonnaux C (2009). HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Invest 119, 1159–1166. 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, Kushner RF, Lindquist R, Pessah-Pollack R, Seger J, et al. (2020). Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: co-sponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis 16, 175–247. 10.1016/j.soard.2019.10.025. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, Chamseddine G, Bornstein SR, and Rubino F (2021). Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 397, 293–304. 10.1016/S0140-6736(20)32649-0. [DOI] [PubMed] [Google Scholar]
- Mischler RA, Armah SM, Craig BA, Rosen AD, Banerjee A, Selzer DJ, Choi JN, and Gletsu-Miller N (2018). Comparison of oral iron supplement formulations for normalization of iron status following roux-EN-y gastric bypass surgery: a randomized trial. Obes. Surg 28, 369–377. 10.1007/s11695-017-2858-4. [DOI] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt RR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. (2019). Glucagon-like peptide 1 (GLP-1). Mol. Metab, 72–130. 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, and Kohli R (2014). Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity 22, 390–400. 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2017). R A Language and Environment for Statistical Computing. - References - Scientific Research Publishing (No Date), . https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=2144573 [Google Scholar]
- Rainer J, Sanchez-Cabo F, Stocker G, Sturn A, and Trajanoski Z (2006). CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Res. 34, W498–W503, (WEB. SERV. ISS.). 10.1093/nar/gkl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan SK, and Shah YM (2016). Role of intestinal HIF-2α in health and Disease. Annu. Rev. Physiol 78, 301–325. 10.1146/annurev-physiol-021115-105202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan SK, and Shah YM (2017). A central role for hypoxia-inducible factor (HIF)-2α in hepatic glucose homeostasis. Nutr.Health. Aging, 207–216. 10.3233/NHA-170022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, and Reimann F (2014). Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233. 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, and Bueter M (2014). The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp. Physiol 99, 1128–1132. 10.1113/expphysiol.2014.078378. [DOI] [PubMed] [Google Scholar]
- Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, Basfi-fer K, Csendes A, Papapietro K, Pizarro F, et al. (2009). Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am. J. Clin. Nutr 90, 527–532. 10.3945/ajcn.2009.27699. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, et al. (2014). FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188. 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, et al. (2017). Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N. Engl. J. Med 376, 641–651. 10.1056/nejmoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, and Gonzalez FJ (2008). Hypoxia-inducible factor Augments experimental colitis through an MIF-dependent Inflammatory signaling Cascade. Gastroenterology 134, 2036–2048. 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Matsubara T, Ito S, Yim SH, and Gonzalez FJ (2009). Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9, 152–164. 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, and Xie L (2014). Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 146, 630–642. 10.1053/j.gastro.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenackers N, Van der Schueren B, Mertens A, Lannoo M, Grauwet T, Augustijns P, and Matthys C (2018). Iron deficiency after bariatric surgery: what is the real problem? Proc. Nutr. Soc 77, 1445–1455. 10.1017/S0029665118000149. [DOI] [PubMed] [Google Scholar]
- Stefater MA, Pérez–Tilve D, Chambers AP, Wilson–Pérez HE, Sandoval DA, Berger J, Toure M, Tschöp M, Woods SC, and Seeley RJ (2010). Sleeve Gastrectomy Induces Loss of Weight and Fat Mass in Obese Rats, but Does Not Affect Leptin Sensitivity. Gastroenterology 138, 2426–2436. 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, and Shah YM (2011). Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140, 2044–2055. 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, Ramos A, Våge V, Al-Sabah S, Brown W, et al. (2019). Bariatric surgery Worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry report 2018. Obes. Surg 29, 782–795. 10.1007/s11695-018-3593-1. [DOI] [PubMed] [Google Scholar]
- Xie C, Yagai T, Luo Y, Liang X, Chen T, Wang Q, Sun D, Zhao J, Ramakrishnan SK, Sun L, et al. (2017). Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med 23, 1298–1308. 10.1038/nm.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Wu C, Xiong ZF, and Fang X (2015). Progress on hypoxia-inducible factor-3: its structure, gene regulation and biological function (Review). Mol. Med. Rep 12, 2411–2416. 10.3892/MMR.2015.3689/HTML. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Standardized RNA sequencing data have been deposited at GEO data base (GSE169403) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


