Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread worldwide and coronavirus disease 2019 remains a major health concern. Several SARS-CoV-2 vaccines have been produced and are playing important roles in the prevention of infection and worsening to a severe state, and in improving mortality [1]. However, some concerns about adverse effects related to SARS-CoV-2 vaccination have been raised. Recently, various adverse effects have been reported, but there is little data on adverse effects on the lungs [2].
Short abstract
Although the association between acute exacerbation of interstitial lung disease and vaccination is not yet clarified, this is the first case report describing the possibility that SARS-CoV-2 vaccination may trigger acute exacerbation https://bit.ly/3tpu6Cz
To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread worldwide and coronavirus disease 2019 remains a major health concern. Several SARS-CoV-2 vaccines have been produced and are playing important roles in the prevention of infection and worsening to a severe state, and in improving mortality [1]. However, some concerns about adverse effects related to SARS-CoV-2 vaccination have been raised. Recently, various adverse effects have been reported, but there is little data on adverse effects on the lungs [2].
We treated a patient with fibrotic interstitial lung disease (f-ILD) who needed hospitalisation for acute respiratory failure and newly arising bilateral ground-glass opacities (GGO) on chest high-resolution computed tomography (HRCT) within 9 days after his second mRNA SARS-CoV-2 vaccination. A full inpatient evaluation was performed and the patient was diagnosed with acute exacerbation of f-ILD, raising a question on the relationship between SARS-CoV-2 vaccination and acute exacerbation. Since recent studies have reported high mortality among f-ILD patients with SARS-CoV-2 infection [3, 4], this case has potential clinical significance and we present the clinical course below with some discussion.
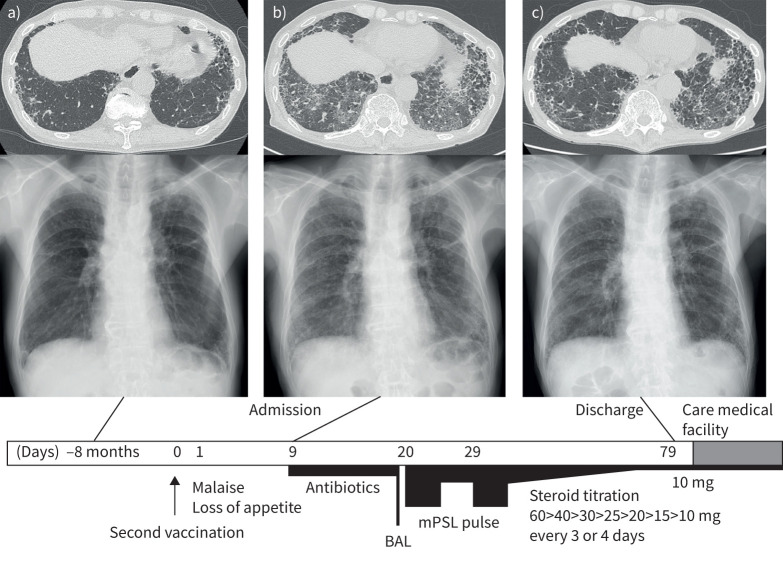
An 84-year-old male was previously diagnosed with f-ILD (figure 1a) and regularly followed at another hospital from October 2020. He was not receiving any medication. He had a former smoking history of 34 pack-years, and no significant past medical history. In mid-May 2021, he received his first dose of BNT162b2 vaccine (BioNTech/Pfizer), which produced no adverse effects. In June 2021, he received his second vaccine dose and gradually developed malaise and loss of appetite from the day following the vaccination. The symptoms persisted without improvement for 9 days after he received the second vaccine, and he visited a nearby hospital. His chest HRCT revealed diffuse GGO with basal and subpleural honeycomb and reticulation (figure 1b), and he was referred to our hospital.
FIGURE 1.
High-resolution computed tomography (HRCT) and chest radiograph findings with simple clinical course. a) 8 months prior to admission, subpleural reticulation and honeycomb structure are present, compatible with fibrotic interstitial lung disease. b) Chest images on admission. Newly developed bilateral and diffuse ground-glass opacities (GGO) and underlying subpleural honeycomb and reticulation are seen on HRCT. c) Chest images before discharge. GGO has improved. Below, the clinical course is shown. Numbers show clinical days from second vaccination. BAL: bronchoalveolar lavage; mPSL: methylprednisolone.
On admission, his vital signs were a temperature of 38.4°C, respiratory rate of 20 breaths per min, and peripheral oxygen saturation of 91% in ambient air. Physical examination revealed bilateral fine crackles and no signs of heart murmur, leg oedema, or clubbed fingers. Arterial blood gas analysis on admission revealed hypoxaemia and respiratory alkalosis (pH 7.493, arterial carbon dioxide tension 33.5 mmHg, arterial oxygen tension 56.4 mmHg, HCO3− 25.7 mmol·L−1). Laboratory findings were as follows: white blood cells 9300 cells·mL−1 (neutrophils 82.3%, lymphocytes 6.9%, eosinophils 3.0%), C-reactive protein (CRP) 4.09 mg·dL−1, lactate dehydrogenase 591 U·L−1, KL-6 1837 U·mL−1, SP-D 693 ng·mL−1 and procalcitonin 0.111 ng·mL−1. The remaining serological tests were all unremarkable, including various factors related to connective tissue disease (CTD) (rheumatoid factor, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, anti-ARS antibody and others). SARS-CoV-2 antigen test and PCR were both negative. His sputum culture revealed no significant bacteria.
We began treatment with antibiotics to observe his initial response. However, despite antibiotic treatment for 7 days, the bilateral GGO spread and respiratory failure remained, requiring supplemental oxygen of 1 L per min with a nasal canula. His CRP level remained high and KL-6 was elevated to 2042 U·mL−1 on day 11 after admission, which led us to perform bronchoalveolar lavage (BAL) on day 12. The BAL fluid analysis was 14.0% neutrophils, 36.5% lymphocytes and 5.5% eosinophils. Cultures of BAL fluid showed no evidence of bacterial infection. Since his previous chest HRCT showed subpleural reticulation and honeycomb compatible with idiopathic pulmonary fibrosis (IPF), we diagnosed his condition as acute exacerbation of IPF.
Treatment with intravenous corticosteroids (two cycles of methylprednisolone 1000 mg per day for 3 days followed by 1 mg per kg per day) was initiated, after which methylprednisolone was gradually titrated from 60 mg to 10 mg, by 10 mg every 3 or 4 days. After steroid pulse treatment, the GGO on chest HRCT improved but subpleural reticulation remained and progressed (figure 1c). His respiratory condition stabilised without supplemental oxygen. After 2 weeks of rehabilitation, he was discharged to a care medical facility while receiving 10 mg of oral corticosteroids on the 71st day from admission.
We treated a patient with ILD who had acute respiratory failure and new GGO soon after receiving the SARS-CoV-2 vaccine. Even though he did not undergo a full evaluation for ILD when it was revealed at his former hospital, we retrospectively diagnosed him with IPF. This case met the criteria of acute exacerbation defined in an international working group report [5]. We diagnosed the condition as acute exacerbation of IPF, of which the exacerbation could have been triggered by SARS-CoV-2 vaccination. The aetiology of acute exacerbation of ILD remains unknown, but causes such as bacterial/viral infection, certain procedures or surgeries, and drug toxicity have been reported to trigger the pathogenesis of acute exacerbation [6]. Some vaccinations have also been reported to be a potential cause of acute exacerbation. previous case reports showed possible triggering of acute exacerbation of IPF by influenza A vaccination [7, 8]. A case of acute exacerbation of CTD-associated f-ILD resulting from an influenza vaccine has also been reported [9]. These three cases were diagnosed as f-ILD (two IPF and one CTD-ILD) before vaccination. Their HRCT showed bilateral GGO and they had respiratory failure several days after vaccination. All of them were treated with corticosteroid pulse therapy and their respiratory condition and computed tomography findings improved after initial treatment. Although the relationship between ILD and vaccination has not been fully elucidated, these findings suggest that vaccination could trigger acute exacerbation of ILD. No firm conclusion can be reached from this small number of case reports, but those earlier cases and our case share a similar clinical course and radiological features. Therefore, it is reasonable to conclude that our patient experienced acute exacerbation of IPF triggered by SARS-CoV-2 vaccination.
No previous study on acute exacerbation of f-ILD triggered by SARS-CoV-2 vaccine has been reported. Park et al. [10] reported a case of SARS-CoV-2 vaccine-related ILD, and the World Health Organization global pharmacovigilance database (VigiAccess) showed 464 cases of suspected ILD, 87 cases of suspected organising pneumonia, eight cases of acute lung injury and two cases of acute interstitial pneumonitis and idiopathic interstitial pneumonia from the BNT162b2 vaccine. However, no cases of acute exacerbation of f-ILD were seen [11], nor was acute exacerbation of f-ILD reported in a clinical trial [2]. Therefore, to our knowledge, this is the first report of acute exacerbation of f-ILD that could have been triggered by SARS-CoV-2 vaccination.
The pathogenesis of acute exacerbation triggered by vaccination remains unclear. SARS-CoV-2 vaccination has been revealed to induce T cell responses reflected in production of some cytokines, such as interferon-γ and interleukin-2 [12]. Since a high percentage of the population that receives SARS-CoV-2 vaccination experiences fatigue and fever as side-effects, some sort of immune response likely occurs in the body, possibly provoking acute exacerbation in f-ILD patients. However, acute exacerbation of f-ILD occurs with a certain probability in patients with f-ILD, with an incidence rate reported to be around 5–10% per year [5, 6]. In this case, we cannot prove whether this event just happened to overlap with the timing of vaccination, or was triggered by the vaccination itself. Further study of the possible association between SARS-CoV-2 vaccination and acute exacerbation is needed, by evaluating numbers of vaccinated patients with ILD and the incidence of acute exacerbation among those patients.
In conclusion, this is the first case report describing acute exacerbation of f-ILD after SARS-CoV-2 vaccination. Unquestionably, vaccination is a very effective method of preventing SARS-CoV-2 infection and has more benefits than risk. However, little is known about whether vaccination itself could cause acute exacerbation of f-ILD, and further investigation of the relationship between vaccination and acute exacerbation is needed.
Shareable PDF
Footnotes
Author contributions: All authors fulfill the criteria for authorship. T. Bando, R. Takei, Y. Mutoh and Y. Kondoh wrote the original manuscript. All authors contributed to revisions of the manuscript, provided final approval of the version to be published and agreed to be accountable for all aspects of the work.
Conflict of interest: T. Bando, R. Takei, Y. Mutoh, H. Sasano, Y. Yamano, T. Yokoyama, T. Matsuda, K. Kataoka and T. Kimura have nothing to disclose. Y. Kondoh reports funding from Nippon Boehringer Ingelheim Co. Ltd, consulting fees from Asahi Kasei Pharma Corp., Shionogi & Co. Ltd, Boehringer Ingelheim Co. Ltd, Janssen Pharmaceutical K.K., Healios K.K. and Taiho Pharmaceutical Co. Ltd, and payment for lectures from Asahi Kasei Pharma Corp., Shionogi & Co. Ltd, Boehringer Ingelheim Co. Ltd, Actelion Pharmaceuticals Ltd, DAIICHI SANKYO Co. Ltd, Bristol Myers Squibb, AstraZeneca K.K., Eisai inc., KYORIN Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma and Novartis Pharma K.K., outside the submitted work.
Support statement: This study was partially supported by the Study Group on Diffuse Lung Disease, Scientific Research/Research on Intractable Diseases in the Ministry of Health, Labour and Welfare, Japan.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med 2021; 14: 1389–1401. doi: 10.2147/IJGM.S310497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med 2020; 202: 1656–1665. doi: 10.1164/rccm.202007-2794OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondoh Y, Kataoka K, Ando M, et al. COVID-19 and acute exacerbation of interstitial lung disease. Respir Investig 2021; 59: 675–678. doi: 10.1016/j.resinv.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Kondoh Y, Brown KK, et al. Acute exacerbations of fibrotic interstitial lung diseases. Respirology 2020; 25: 525–534. doi: 10.1111/resp.13682 [DOI] [PubMed] [Google Scholar]
- 7.Umeda Y, Morikawa M, Anzai M, et al. Acute exacerbation of idiopathic pulmonary fibrosis after pandemic influenza A (H1N1) vaccination. Intern Med 2010; 49: 2333–2336. doi: 10.2169/internalmedicine.49.3890 [DOI] [PubMed] [Google Scholar]
- 8.Hirasawa Y, Kono C, Yamada Y, et al. Case report: influenza vaccination-associated acute lung injury: two case report. Nihon Naika Gakkai Zasshi 2015; 104: 1457–1459. doi: 10.2169/naika.104.1457 [DOI] [PubMed] [Google Scholar]
- 9.Okusaki T, Fukuhara K. Exacerbation of connective tissue disease-associated interstitial lung disease due to influenza vaccination. Respir Med Case Rep 2021; 33: 101463. doi: 10.1016/j.rmcr.2021.101463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JY, Kim JH, Lee IJ, et al. COVID-19 vaccine-related interstitial lung disease: a case study. Thorax 2022; 77: 102–104. doi: 10.1136/thoraxjnl-2021-217609 [DOI] [PubMed] [Google Scholar]
- 11.VigiAccess . WHO Collaborating Centre for International Drug Monitoring. www.vigiaccess.org/ Date last accessed: 25 November 2021.
- 12.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020; 383: 2320–2332. doi: 10.1056/NEJMoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02806-2021.Shareable (275.7KB, pdf)