Abstract
The EFSA Panel on Food Additives and Flavourings was requested to evaluate 43 flavouring substances assigned to the Flavouring Group Evaluation 63 (FGE.63), using the Procedure as outlined in the Commission Regulation (EC) No 1565/2000. Twenty‐nine substances have already been considered in FGE.63 and its revisions ([FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 02.252, 07.015, 07.069, 07.081, 07.099, 07.100, 07.101, 07.102, 07.114, 07.123, 07.151, 07.190, 07.240, 07.247, 07.249, 07.256, 09.281, 09.282, 09.657, 09.658, 09.923, 09.924, 09.925 and 09.936]). The remaining 14 flavouring substances have been cleared with respect to genotoxicity in FGE.204Rev1 ([FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244]) and they are considered in this revision 4 of FGE.63. The substances were evaluated through a stepwise approach that integrates information on the structure–activity relationships, intake from current uses, toxicological threshold of concern (TTC) and available data on metabolism and toxicity. The Panel concluded that none of these 43 substances gives rise to safety concerns at their levels of dietary intake, when estimated on the basis of the ‘Maximised Survey‐derived Daily Intake’ (MSDI) approach. Besides the safety assessment of the flavouring substances, the specifications for the materials of commerce have also been considered and found adequate for 43 flavouring substances. However, for 14 of these flavouring substances in the present revision and for 10 of the substances in the previous revision (FGE.63Rev3), the ‘modified Theoretical Added Maximum Daily Intakes’ (mTAMDIs) values are equal to or above the TTCs for their structural classes (I and II). For 15 substances previously evaluated in FGE.63Rev3, use levels are still needed to calculate the mTAMDI estimates. Therefore, in total for 39 flavouring substances, more data on uses and use levels should be provided to finalise their safety evaluations.
Keywords: Flavourings; α,β‐unsaturated carbonyls and precursors; FGE.07Rev6; JECFA
1. Introduction
The present revision of this Flavouring Group Evaluation (FGE) concerns the inclusion of 14 α,β‐unsaturated aliphatic secondary alcohols and ketones ([FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 1 07.139, 07.177, 07.188 and 07.244]) which have been evaluated with respect to genotoxicity in FGE.204Rev1. According to the Mandate and Terms of Reference of this FGE, when for a flavouring substance, the concern for genotoxicity is ruled out, the European Food Safety Authority (EFSA) proceeds to the full evaluation of these flavouring substances, taking into account the requirements of the Commission Regulation (EC) No 1565/2000 and of Regulation (EU) No 1334/2008.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background to Mandate from FGE.204Rev1 (M‐2015‐0114)
The use of flavourings is regulated under Regulation (EC) No 1334/2008 2 of the European Parliament and Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/2012 3 . The list contains flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/2000 4 .
On 21 November 2012, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids adopted an opinion on Flavouring Group Evaluation 204 (FGE.204): Consideration of genotoxicity data on 18 monounsaturated, aliphatic, α,β‐unsaturated ketones and precursors from chemical subgroup FGE.204 (FGE.19 s.g. 1.2.1).
The Panel concluded that for the representative substance 7‐Methyl‐3‐octen‐2‐one [FL‐no: 07.177] of subgroup 1.2.1 of FGE.19, the Panel’s concern with respect to genotoxicity could not be ruled out and consequently additional data are requested.
On 31 September 2014 (Ares (2014) 207551) the applicant submitted to the Commission and to EFSA data on the potential presence of the substance FL‐no 07.177 in plasma (analytical data).
On 9 January 2015 (Ares (2015) 202297) the applicant submitted additional studies on the representative substance [FL‐no: 07.177] in relation to this EFSA evaluation. This additional data examines the systemic exposure of rats following oral administration of this substance, using the same dosing regimen employed in the combined micronucleus and comet test previously submitted. The data on this representative substance is intended to cover the following 15 substances in this group, namely [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.187, 07.188, 07.244 and 07.258].
1.1.2. Terms of Reference of Mandate from FGE.204Rev1 (M‐2015‐0114)
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation of the flavouring substances mentioned above in accordance with Commission Regulation (EC) No 1565/2000.
1.2. Interpretation of the Terms of Reference
Flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] were evaluated by JECFA (JECFA, 2002a, 2008 (59th and 69th meetings)) and will be considered in the current revision of FGE.63.
Flavouring substances [FL‐no: 07.187 and 07.258] were not evaluated by JECFA and they have been evaluated in the revision 6 of FGE.07 (EFSA FAF Panel, 2022). For the flavouring substance 6‐methyl‐3‐hepten‐2‐one [FL‐no: 07.258], the industry informed that this substance is no longer supported (EFSA FAF Panel, 2022). On the basis that this flavouring substance is no longer supported by any interested party, the European Commission on 18/5/2020 (Ares(2020)2601393 – 18/5/2020) informed that it is going to proceed with the withdrawal of [FL‐no: 07.258] from the Union List of flavourings. Accordingly, the EFSA evaluation of this flavouring substance is closed and [FL‐no: 07.258] is no longer included in FGE.07Rev6.
The methodology for the evaluation of these substances is clarified in Appendix A.
2. Data and methodologies
2.1. Data
The present opinion is based on the data presented in Table 1.
Table 1.
Data considered in the current revision 4 of FGE.63 (FGE.63Rev4)
| FL‐no | Chemical name | Data provided for the current revision 4 of FGE.63 | Appendix (Table nr) and relevant section of the opinion | Documentation provided to EFSA nr: |
|---|---|---|---|---|
| 07.244 | trans‐6‐Methyl‐3‐hepten‐2‐one | Specifications, EU poundage data (MSDI), use levels, ADME data | Appendix B (Table B.1) Appendix C (Tables C.1 and C.4), Section 3.3.1 | Documentation provided to EFSA nr: 1 and 3 |
| 07.082 | Oct‐2‐en‐4‐one | Specifications, EU poundage data (MSDI), use levels, ADME data | Appendix B (Table B.1) Appendix C (Tables C.1 and C.4), Section 3.3.1 | Documentation provided to EFSA nr: 1, 3 and 4 |
| 07.188 | Non‐3‐en‐2‐one | Specifications, EU poundage data (MSDI), use levels | Appendix B (Table B.1) Appendix C (Tables C.1 and C.4) | Documentation provided to EFSA nr: 1 and 2 |
| 07.177 | 7‐Methyl‐3‐octenone‐2 | |||
| 07.139 | 5‐Methylhept‐2‐en‐4‐one | |||
| 07.121 | Dec‐3‐en‐2‐one | |||
| 07.106 | 5‐Methylhex‐3‐en‐2‐one | |||
| 07.105 | Hept‐3‐en‐2‐one | |||
| 07.104 | Hept‐2‐en‐4‐one | |||
| 07.048 | 4‐Hexen‐3‐one | |||
| 07.044 | Pent‐3‐en‐2‐one | |||
| 02.193 | Oct‐2‐en‐4‐ol | |||
| 02.102 | Oct‐3‐en‐2‐ol | |||
| 07.107 | Oct‐3‐en‐2‐one |
In addition, the following documentation has been consulted for the safety evaluation of FGE.63Rev4:
-
–
JECFA specifications for the 14 flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] (JECFA, 2002a);
-
–
Genotoxicity data evaluated in FGE.204Rev1 (EFSA FAF Panel, 2019);
-
–
59th JECFA report (JECFA, 2002b);
-
–
EFSA Scientific Opinion on FGE.63Rev3 (and the previous revisions) (EFSA CEF Panel, 2016a);
-
–
EFSA Scientific Opinion on FGE.07Rev5 (EFSA CEF Panel, 2017).
2.1.1. History of the evaluation of the substances in FGE.63
In the first version of Flavouring Group Evaluation 63 (FGE.63) (EFSA AFC Panel, 2008), EFSA considered a group of 13 aliphatic saturated and unsaturated secondary alcohols, ketones and related esters ([FL‐no: 07.015, 07.069, 07.100, 07.114, 07.123, 07.151, 07.240, 07.249, 09.657, 09.658, 09.923, 09.924 and 09.925]) which had been evaluated by the JECFA at its 59th meeting (JECFA, 2002b).
The revision 1 of FGE.63, FGE.63Rev1 (EFSA CEF Panel, 2012a) was prepared due to the inclusion of six additional substances [FL‐no: 02.252, 07.099, 07.190, 07.247, 07.256 and 09.936] evaluated by JECFA at their 59th and 69th meetings. Furthermore, information on the stereoisomeric composition for six substances ([FL‐no: 07.069, 07.114, 09.657, 09.658, 09.923 and 09.925]) and European poundage data for three substances ([FL‐no: 07.069, 07.100 and 09.658]) had been submitted since the first publication of FGE.63.
The second revision of FGE.63, FGE.63Rev2 (EFSA CEF Panel, 2013) included the consideration of one additional substance, 4‐methylpent‐3‐en‐2‐one [FL‐no: 07.101]. This substance is an α,β‐unsaturated ketone and was originally allocated in FGE.204 (EFSA CEF Panel, 2012a) where the concern for genotoxicity was assessed and ruled out.
The third revision of FGE.63, FGE.63Rev3 (EFSA CEF Panel, 2016a) dealt with the consideration of six additional substances ([FL‐no: 02.023, 02.099, 02.104, 02.136, 07.081 and 07.102]), evaluated at the 59th meeting of JECFA, and three substances ([FL‐no: 02.155, 09.281 and 09.282]), evaluated at the 69th meeting of JECFA. These substances are α,β‐unsaturated secondary alcohols and ketones and they were originally evaluated in FGE.205 (EFSA CEF Panel, 2012b) and FGE.205Rev1 (EFSA CEF Panel, 2016b) to assess their genotoxicity potential. Since the concern for genotoxicity was ruled out for all nine substances, they had been evaluated through the Procedure in FGE.63Rev3. In addition, specifications, use levels and poundage data have also become available for these substances and this information was considered by the Panel in FGE.63Rev3.
For all 29 JECFA‐evaluated aliphatic secondary alcohols, ketones and related esters [FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 02.252, 07.015, 07.069, 07.081, 07.099, 07.100, 07.101, 07.102, 07.114, 07.123, 07.151, 07.190, 07.240, 07.247, 07.249, 07.256, 09.281, 09.282, 09.657, 09.658, 09.923, 09.924, 09.925 and 09.936] considered in FGE.63Rev3, the Panel agreed with the JECFA conclusion: ‘No safety concern at current levels of intake when used as flavouring agents, based on the ‘Maximized Survey‐derived Daily Intake’ (MSDI).
For 14 of the 29 substances in FGE.63Rev3 use levels have been provided ([FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 02.252, 07.081, 07.099, 07.101, 07.102, 07.190, 09.281, 09.282 and 09.936]). Four flavouring substances [FL‐no: 02.252, 07.099, 07.101 and 09.936] have mTAMDI intake estimates below the toxicological threshold of concern (TTC) for their structural class. For 10 substances [FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 07.081, 07.102, 07.190, 09.281 and 09.282], the mTAMDI values are above the TTC for their structural class II. Therefore, for these 10 substances, more reliable data on uses and use levels are required in order to finalise the evaluation.
For the remaining 15 [FL‐no: 09.657, 09.658, 09.923, 09.924, 09.925, 07.015, 07.081, 07.100, 07.114, 07.123, 07.151, 07.240, 07.247, 07.249, 07.256] substances, evaluated through the Procedure in FGE.63Rev3, use levels are still missing.
The present revision 4 of FGE.63 (FGE.63Rev4) deals with 14 flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] which were evaluated by JECFA in its 59th meeting (JECFA, 2002b). These substances were evaluated by EFSA in FGE.204Rev1 (EFSA FAF Panel, 2019), where it was concluded that the concern for genotoxicity for these substances could be ruled out. Therefore, they could be evaluated through the Procedure.
Together with the 29 substances that were already considered in FGE.63Rev3, the current revision comprises 43 substances. The 29 flavouring substances, for which the evaluation was finalised in FGE.63Rev3, will not be further discussed. Nevertheless, for the sake of completeness, the information for all the 43 substances is maintained in the various tables in this FGE.
| FGE | Adopted by EFSA | Link | No of substances |
|---|---|---|---|
| FGE.63 | 7 July 2007 | https://www.efsa.europa.eu/en/efsajournal/pub/706.htm | 13 |
| FGE.63Rev1 | 26 September 2012 | https://www.efsa.europa.eu/en/efsajournal/pub/2900.htm | 19 |
| FGE.63Rev2 | 09 April 2013 | https://www.efsa.europa.eu/en/efsajournal/pub/3188 | 20 |
| FGE.63Rev3 | 30 November 2016 | https://www.efsa.europa.eu/en/efsajournal/pub/4662 | 29 |
| FGE.63Rev4 | 15 December 2021 | https://www.efsa.europa.eu/en/efsajournal/pub/7102 | 43 |
2.2. Methodologies
This opinion was elaborated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee. The assessment strategy applied for the evaluation programme of flavouring substances, as laid down in Commission Regulation (EC) No 1565/2000, is based on the Opinion on a Programme for the Evaluation of Flavouring substances of the Scientific Committee on Food (SCF, 1999).
2.2.1. Procedure for the safety evaluation of flavouring substances
The approach for safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is described in Appendix A.
2.2.2. Approach used for the calculation of exposure
The approach used for calculation of the intake of the flavouring substances is described in Appendix A (point ‘a) Intake’) and in Appendix C (Section C.2 ‘mTAMDI calculation’).
3. Assessment
3.1. Specifications
JECFA status
JECFA specifications are available for all the flavouring substances in FGE.63Rev4, including the 14 newly included flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] (JECFA, 2002a).
EFSA considerations
Table 1 shows the chemical structures of the candidate substances which are considered in this revision of FGE.63 (FGE.63Rev4).
Table 2:Flavouring substances under evaluation in FGE.63Rev4
| FL‐no | Chemical name | Structural formula | Structural class* |
|---|---|---|---|
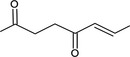
| 07.244 | trans‐6‐Methyl‐3‐hepten‐2‐one |
|
Class I |
| 07.188 | Non‐3‐en‐2‐one |

|
Class I |
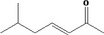
| 07.177 | 7‐Methyl‐3‐octenone‐2 |
|
Class I |
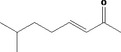
| 07.104 | Hept‐2‐en‐4‐one |
|
Class I |
| 07.121 | Dec‐3‐en‐2‐one |
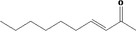
|
Class I |
| 07.106 | 5‐Methylhex‐3‐en‐2‐one |
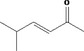
|
Class I |
| 07.105 | Hept‐3‐en‐2‐one |
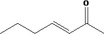
|
Class I |
| 07.107 | Oct‐3‐en‐2‐one |
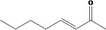
|
Class I |
| 07.048 | 4‐Hexen‐3‐one |
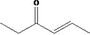
|
Class I |
| 07.044 | Pent‐3‐en‐2‐one |
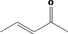
|
Class I |
| 02.193 | Oct‐2‐en‐4‐ol |
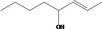
|
Class II |
| 02.102 | Oct‐3‐en‐2‐ol |
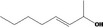
|
Class II |
| 07.139 | 5‐Methylhept‐2‐en‐4‐one |
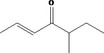
|
Class II |
| 07.082 | Oct‐2‐en‐4‐one |
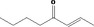
|
Class II |
Determined with OECD Toolbox (version 4.3.1 available at https://www.oecd.org/chemicalsafety/risk‐assessment/oecd‐qsar‐toolbox.htm).
The newly included flavouring substances in FGE.63Rev4 can exist as geometrical stereoisomers due to the presence of a double bond.
With regard to composition of the stereoisomeric mixtures, adequate information to describe the materials of commerce for these flavouring substances has been submitted by industry (Documentation provided to EFSA nr: 1). Based on this information on stereoisomerism, the chemical names and the CAS numbers for flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177 and 07.188] should be changed in the Union List (UL) to reflect their stereochemical configuration (see ‘EFSA comments’ column in Table B.1 – Appendix B).
Flavouring substances [FL‐no: 02.102 and 02.193] can exist also as optical stereoisomers due to the presence of a chiral centre in their structures. Industry informed that these flavouring substances occur as racemates (Documentation provided to EFSA nr: 2).
In addition, with regard to flavouring substance [FL‐no: 07.123], previously considered in FGE.63Rev3, the Panel noted that its chemical name should be changed to (E)‐geranylacetone.
The most recent specifications data for all 43 substances in FGE.63Rev4 are summarised in Table B.1 – Appendix B.
3.2. Estimation of intake
JECFA status
For 43 flavouring substances in FGE.63Rev4, including 11 newly allocated flavouring substances [FL‐no: 02.102, 07.044, 07.048, 07.082, 07.104, 07.105, 07.107, 07.121, 07.139, 07.188 and 07.244], intake data are available for the EU (JECFA, 2002b). For flavouring substances [FL‐no: 02.193 (JECFA‐no: 1141), 07.106 (JECFA‐no: 1132) and 07.177 (JECFA‐no: 1135)], no EU poundage data were available to JECFA and they concluded on the basis of USA poundage data (JECFA, 2002b).
EFSA considerations
For all 14 candidate substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] updated EU production figures have been submitted by industry (Documentation provided to EFSA nr. 1 and 3). The MSDI values range from 0.01 to 41.44 µg/capita per day (Table C.4 – Appendix C).
Table C.4.
Estimated intakes based on the MSDI approach and the mTAMDI approach for substances in FGE.63Rev4
| Estimated intakes based on the MSDI approach and the mTAMDI approach | ||||||
|---|---|---|---|---|---|---|
| FL‐no | EU Union List name | MSDI – EU (µg/capita per day) | MSDI – USA (µg/capita per day) | mTAMDI (µg/person per day) | Structural class | TTC (µg/person per day) |
| 02.102 | Oct‐3‐en‐2‐ol | 0.01 | ND | 1,700 | Class II | 540 |
| 02.193 | Oct‐2‐en‐4‐ol | 1.84 | ND | 1,700 | Class II | 540 |
| 02.252 | 4,8‐Dimethyl‐3,7‐nonadien‐2‐ol | 3 | 0.1 | 19 | Class I | 1,800 |
| 07.044 | Pent‐3‐en‐2‐one | 0.25 | ND | 1,800 | Class I | 1,800 |
| 07.048 | 4‐Hexen‐3‐one | 41.44 | 1 | 1,800 | Class I | 1,800 |
| 07.104 | Hept‐2‐en‐4‐one | 0.01 | ND | 2,400 | Class I | 1,800 |
| 07.139 | 5‐Methylhept‐2‐en‐4‐one | 32.60 | 1 | 1,700 | Class II | 540 |
| 09.657 | 1‐Methylbutyl acetate | 2.9 | 3 | NA | Class I | 1,800 |
| 09.658 | 1‐Methylbutyl butyrate | 0.47 | 1 | NA | Class I | 1,800 |
| 09.923 | Hept‐2‐yl butyrate | 3 | 3 | NA | Class I | 1,800 |
| 09.924 | 3‐Heptyl acetate (mixture of R and S) | 3 | 3 | NA | Class I | 1,800 |
| 09.925 | Nonan‐3‐yl acetate | 3 | 3 | NA | Class I | 1,800 |
| 02.023 | Oct‐1‐en‐3‐ol | 390 | 23 | 1,800 | Class II | 540 |
| 02.099 | Pent‐1‐en‐3‐ol | 4.3 | 1 | 2,300 | Class II | 540 |
| 02.104 | Hex‐1‐en‐3‐ol | 0.012 | 2 | 2,300 | Class II | 540 |
| 02.136 | Dec‐1‐en‐3‐ol | 0.012 | 0.1 | 2,300 | Class II | 540 |
| 02.155 | 1‐Hepten‐3‐ol | 0.13 | – | 3,900 | Class II | 540 |
| 07.015 | 6‐Methylhept‐5‐en‐2‐one | 100 | 44 | NA | Class II | 540 |
| 07.069 | Tetrahydro‐pseudo‐ionone | 0.012 | 0.01 | NA | Class II | 540 |
| 07.081 | Oct‐1‐en‐3‐one | 1.5 | 0.1 | 1,600 | Class II | 540 |
| 07.082 | Oct‐2‐en‐4‐one | 13.78 | 3 | 2,400 | Class II | 540 |
| 07.099 | 6‐Methylhepta‐3,5‐dien‐2‐one | 13 | 5 | 190 | Class II | 540 |
| 07.100 | 5‐Methylhex‐5‐en‐2‐one | 0.24 | 0.3 | NA | Class II | 540 |
| 07.101 | 4‐Methylpent‐3‐en‐2‐one | 0.34 | ND | 340 | Class II | 540 |
| 07.102 | Pent‐1‐en‐3‐one | 1.6 | 0.1 | 1,600 | Class II | 540 |
| 07.105 | Hept‐3‐en‐2‐one | 0.01 | 0.07 | 2,400 | Class I | 1,800 |
| 07.106 | 5‐Methylhex‐3‐en‐2‐one | 0.01 | 0.1 | 2,400 | Class I | 1,800 |
| 07.107 | Oct‐3‐en‐2‐one | 0.63 | 1 | 2,500 | Class I | 1,800 |
| 07.114 | 6,10,14‐Trimethylpentadeca‐5,9,13‐trien‐2‐one | 0.085 | ND | NA | Class II | 540 |
| 07.121 | Dec‐3‐en‐2‐one | 0.17 | ND | 2,400 | Class I | 1,800 |
| 07.123 | Geranylacetone | 41 | 2 | NA | Class II | 540 |
| 07.151 | Decan‐3‐one | 3 | 3 | NA | Class II | 540 |
| 07.177 | 7‐Methyl‐3‐octenone‐2 | 0.04 | 2 | 2,400 | Class I | 1,800 |
| 07.188 | Non‐3‐en‐2‐one | 0.05 | 13 | 2,400 | Class I | 1,800 |
| 07.190 | Octa‐1,5‐dien‐3‐one | 0.061 | ND | 1,600 | Class II | 540 |
| 07.240 | 2‐Methylheptan‐3‐one | 3 | 3 | NA | Class II | 540 |
| 07.244 | (6E)‐Methyl‐3‐hepten‐2‐one | 0.01 | 3 | 2,400 | Class I | 1,800 |
| 07.247 | (E,E)‐3,5‐Octadien‐2‐one | 3 | 4 | NA | Class II | 540 |
| 07.249 | Undecan‐6‐one | 3 | 3 | NA | Class II | 540 |
| 07.256 | (E) & (Z)‐4,8‐Dimethyl‐3,7‐nonadiene‐2‐ one | 6.1 | 6.6 | NA | Class II | 540 |
| 09.281 | Oct‐1‐en‐3‐yl acetate | 2.1 | – | 3,900 | Class II | 540 |
| 09.282 | Oct‐1‐en‐3‐yl butyrate | 0.0012 | – | 3,900 | Class II | 540 |
| 09.936 | 4,8‐Dimethyl‐3,7‐nonadien‐2‐yl acetate | 3 | 0.2 | 19 | Class II | 540 |
For these 14 newly included flavouring substances, normal and maximum use levels have been submitted (Documentation provided to EFSA nr. 1) and mTAMDI intake values can be calculated. The mTAMDI intake estimates calculated from these data for two substances [FL‐no: 07.044 and 07.048] are equal to the toxicological threshold of concern (TTC) of their structural class I. For the remaining 12 substances ([FL‐no: 02.102, 02.193, 07.104, 07.139, 07.082, 07.105, 07.106, 07.107, 07.121, 07.177, 07.188, 07.244]), the mTAMDI intake estimates are above the TTC for their structural classes (I and II). Therefore, for all 14 flavouring substances, more detailed data on uses and use levels should be provided in order to refine the exposure assessment and to finalise their safety evaluation.
No normal and maximum use levels have been provided for 15 flavouring substances [FL‐no: 09.657, 09.658, 09.923, 09.924, 09.925, 07.015, 07.081, 07.100, 07.114, 07.123, 07.151, 07.240, 07.247, 07.249, 07.256], previously considered in FGE.63Rev3.
The MSDI values and the mTAMDI intake estimates for the flavouring substances in FGE.63Rev4 are shown in Table C.4 – Appendix C.
3.3. Biological and toxicological data
3.3.1. ADME data
At its 59th meeting, JECFA evaluated a group of aliphatic acyclic secondary alcohols and ketones and esters derived from aliphatic secondary alcohols (JECFA, 2002b). JECFA describes that alcohols and their corresponding ketones are interconvertible under physiological conditions and the principal elimination pathway for ketones is their reduction to their corresponding secondary alcohols and subsequent conjugation with glucuronic acid and excretion. If the substance is α,β unsaturated, such as the candidate substances in the present revision of this FGE, conjugation with glutathione can also occur. The glutathione conjugates are transformed to their corresponding mercapturic acid derivatives and excreted.
JECFA concluded that none of the flavouring substances within this flavouring group would be expected to be metabolised to noxious metabolites and agreed to evaluate these substances along the A‐side of the Procedure.
EFSA consideration
Based on the data described in FGE.07Rev5 (EFSA CEF Panel, 2017; see Appendix D), and in line with JECFA, the main elimination pathway expected for aliphatic ketones with chain lengths equal to or above five carbon atoms, such as the candidate substances in the present revision, would be the (enzymatic) reduction of the carbonyl function to the corresponding secondary alcohol followed by subsequent conjugation to glucuronic acid and excretion. However, ω‐ and ω‐1 oxidation (i.e. oxidation of the terminal or one‐but‐last carbon atoms of a chain) are alternative metabolic pathways that can be competing with the ketone reduction pathway at high tissue concentrations (Topping et al., 1994). While ω oxidation would lead to the formation of primary alcohols, which are converted into carboxylic acids; ω‐1 oxidation of a ketone results in a hydroxy‐ketone which subsequently can be oxidised to a diketone. If the two ketone functions are in γ position relative to each other, such diketones are known to be neurotoxic (e.g. axonal swelling, axonal atrophy) (Topping et al.; 1994). For substances that are subject to ω‐oxidation, no formation of such a diketone is possible. One of the candidate substances, 6‐Methyl‐(3E)‐hepten‐2‐one [FL‐no: 07.244], is structurally related to 5‐methylheptan‐3‐one [FL‐no: 07.182] which can be oxidised to a γ‐diketone as outlined in FGE.07Rev5 (EFSA CEF Panel, 2017). The Panel investigated the metabolism of [FL‐no: 07.244] using the OECD QSAR Toolbox (V. 4.3.1; in vivo rat metabolism simulator). The toolbox did not predict the formation of a γ‐diketone, although it indicated formation of hydroxy‐ketones. The Panel noted that such hydroxy‐ketones might be converted into γ‐diketones.
If such a γ‐diketone were formed, its conversion into stable pyrrole‐protein adducts would be required to express neuropathy. Such stable protein‐pyrrole adducts result from a first nucleophilic attack by a free protein amine to the γ‐diketones leading to imines in equilibrium with a secondary amine which, upon ring closure, yields a dihydro pyrrolidino moiety. Abstraction of two water molecules from this intermediate results in the formation of the stable neurotoxic protein‐pyrrole adduct (Documentation provided to EFSA nr: 3). This mechanism underlying neurotoxicity is analogous to that reported for 2,5 hexanedione (Couri and Milks, 1982).
The formation of the putative stable protein‐pyrrole adduct from the respective γ‐diketone outlined above is depicted in Figure 1.
Figure 1.
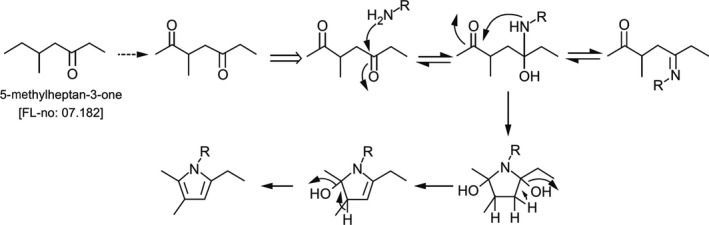
Proposed mechanism of conversion of 5‐methylheptan‐3‐one [FL‐no: 07.182] to a stable pyrrole‐protein adduct (R = protein portion) (Documentation provided to EFSA nr: 3)
For this reaction sequence, the capacity of proton abstraction from C3 and C4 of the intermediate dihydro pyrrolidino moiety is of critical importance for the formation of the neurotoxic protein‐pyrrole adduct. This is supported by the fact that the γ‐diketone 3,3‐dimethyl‐2,5‐hexanedione does not cause neurotoxic effects because the lack of hydrogen at C3 does not allow the formation of a pyrrole (Sayre et al., 1986). Conversely, the γ‐diketone 3,4‐dimethyl‐2,5‐hexanedione can undergo pyrrolisation (i.e. ring closure followed by loss of water), as this structure includes protons which can be abstracted at both C3 and C4; consequently, this compound displays neurotoxic effects (Sayre et al., 1986).
Taking this knowledge of the mechanism of the formation of neurotoxic protein‐pyrrole adducts from γ‐diketones into account, the Panel considered whether this might also be applicable to the flavouring substance 6‐Methyl‐(3E)‐hepten‐2‐one [FL‐no: 07.244] and requested industry provide evidence documenting that this flavouring substance could, or could not, be oxidised to a neurotoxic γ‐diketone. In this respect, the industry stated that upon oxidation at position C5 of [FL‐no: 07.244], an unsaturated γ‐diketone, i.e. 6‐methyl‐3‐heptene‐2,5‐dione, would be generated. This γ‐diketone would be prone to undergo nucleophilic attack by a free protein amine, leading to an imine. A ring closure and the formation of a dihydro pyrrolidino moiety may energetically not be favoured due to the conformational restrictions around the 3,4 olefinic bond. Even if this dihydro pyrrolidino moiety were formed, the subsequent conversion to a stable protein‐pyrrole adduct is not expected since this would require abstraction of the protons in positions 3 and 4 across the double bond with concomitant loss of two water molecules (Figure 2) (Documentation provided to EFSA nr: 3).
Figure 2.
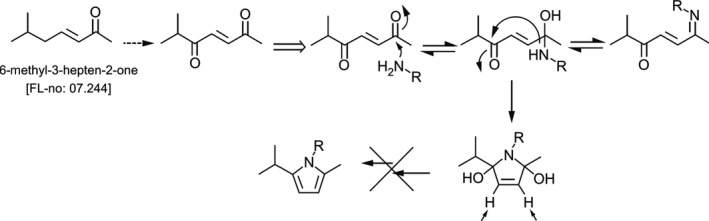
Proposed mechanism of potential formation of stable protein‐pyrrole adducts from γ‐diketone derived from flavouring substance [FL‐no: 07.244] (Documentation provided to EFSA nr: 3)
The Panel concurred with the proposed mechanism described by industry, that ω‐1 or ω‐2 oxidation and formation of γ‐diketones along the unsaturated part of the aliphatic chain would not be expected to form stable neurotoxic protein‐pyrrole adducts.
Thus, the Panel considered that the formation of a neurotoxic stable pyrrole‐protein is not expected for flavouring substance [FL‐no: 07.244] since the concerned γ‐diketone would be formed along the unsaturated part of the carbon chain and this would not allow the formation of the neurotoxic protein‐pyrrole adduct. Similarly, this would also be the case for candidate flavouring substances [FL‐no: 02.102, 07.105, 07.107, 07.121, 07.177and 07.188] (see Table 3). In addition, in flavouring substances [FL‐no: 02.102, 07.107, 07.121, 07.177, 07.188 and 07.244], the oxidation leading to the formation of the γ‐diketone would be at mid‐chain (ω‐3, ω‐4 or ω‐5). This oxidation would not be sufficiently energetically favourable to get sufficient γ‐diketone formed to result in neurotoxicity (levels of γ‐diketone not toxicologically relevant) (EFSA CEF Panel, 2015). In flavouring substances [FL‐no: 07.044, 07.048, 07.104, 07.106 and 07.139], there is no carbon atom at a γ position that can be oxidised to yield a γ‐diketone (at the γ‐position only primary or tertiary alcohols can be formed) (Topping et al., 1994) (see Table 3). Therefore, the Panel concluded that flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] can be evaluated along the A‐side of the Procedure.
Table 3.
Flavouring substances in FGE.63Rev4 and the potentially resulting γ‐diketones
| FL‐no | Chemical name | Chemical structure | γ‐diketone | Comments |
|---|---|---|---|---|
| 02.102 | oct‐3‐en‐2‐ol |
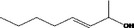
|
|
via ω‐3 oxidation: no neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
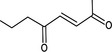
| 07.044 | pent‐3‐en‐2‐one |
|
Not possible | oxidation at the γ position results in a primary alcohol and not in a ketone |
| 07.048 | 4‐hexen‐3‐one |
|
Not possible | oxidation at the γ position results in a primary alcohol and not in a ketone |
| 07.104 | hept‐2‐en‐4‐one |
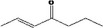
|
Not possible | oxidation at the γ positions results in primary alcohols and not in ketones |
| 07.105 | hept‐3‐en‐2‐one |
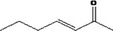
|
|
Via ω‐2 oxidation: no neurotoxicity is expected because of prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
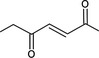
| 07.106 | 5‐methylhex‐3‐en‐2‐one |
|
Not possible | ω‐1 oxidation results in a tertiary alcohol and not in a ketone at the γ position; ω oxidation results in the formation of a primary alcohol at the δ position (relative to the keto‐function) |
| 07.107 | oct‐3‐en‐2‐one |
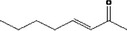
|
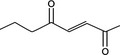
|
Via ω‐3 oxidation: no neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
| 07.121 | dec‐3‐en‐2‐one |
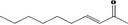
|
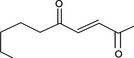
|
Via ω‐5 oxidation: no neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
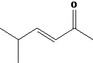
| 07.139 | 5‐methylhept‐2‐en‐4‐one |
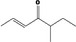
|
Not possible | Oxidation at the γ positions results in primary alcohols and not in ketones |
| 07.177 | 7‐Methyl‐3‐octenone‐2 |
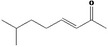
|
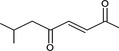
|
Via ω‐4 oxidation: No neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
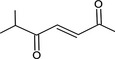
| 07.188 | Non‐3‐en‐2‐one |
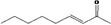
|
|
Via ω‐4 oxidation: no neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
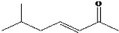
| 07.244 | trans‐6‐methyl‐3‐hepten‐2‐one |
|
|
Via ω‐3 oxidation: no neurotoxicity is expected because of limited formation of the γ‐diketone and prevention of formation of the stable pyrrole‐protein adduct by the unsaturation |
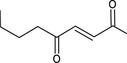
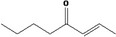
| 07.082 | oct‐2‐en‐4‐one |
|
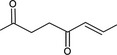
|
Via ω‐1 oxidation: neurotoxicity may be expected because of probable formation of stable pyrrole‐protein adduct and no prevention by the unsaturation |
| 02.193 | oct‐2‐en‐4‐ol |
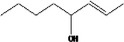
|
Corresponding γ‐diketone is the same as for [FL‐no: 07.082] but formation is very limited. |
Via ω‐1 oxidation: no neurotoxicity is expected because of ready conjugation of the secondary alcohol with e.g. glucuronic acid and long aliphatic chain (C > 7). |
The Panel observed that, for the candidate substance oct‐2‐en‐4‐one [FL‐no: 07.082] and the corresponding alcohol oct‐2‐en‐4‐ol [FL‐no: 02.193], if ω‐1 oxidation occurs, γ‐diketones would be generated along the saturated part of the aliphatic chain (see Table 3). Therefore, the double bond in [FL‐no: 07.082 and 02.193] cannot prevent the formation of the neurotoxic protein‐pyrrole adduct for these flavouring substances.
For oct‐2‐en‐4‐ol [FL‐no: 02.193], the Panel noted that, according to FGE.25Rev3 (EFSA CEF Panel, 2015) for secondary alcohols, the γ‐diketone formation is not expected to be relevant, because these alcohols are readily conjugated with e.g. glucuronic acid. This would reduce the amount of secondary alcohol available for conversion into a γ‐diketone. Further supporting this view is a paper by Sanz et al. (1995) in which different derivatives of n‐hexane and n‐heptane have been tested in vitro for their neurotoxic potential (the pyrrole‐protein adduct formation capability in rat liver microsomal fraction was tested, but also the potential to induce neurotoxicity in vivo was studied). The paper indicated that the neurotoxic potencies of 2‐hexanol and 2‐heptanol are much less than those of the ketone derivatives. Additionally, as stated in FGE.07Rev5 (EFSA CEF Panel, 2017), the length of the aliphatic chain would affect the neurotoxic potency of the formed γ‐diketones. Therefore, the Panel considered that the secondary alcohol oct‐2‐en‐4‐ol [FL‐no: 02.193] does not raise a concern with respect to neurotoxicity and it can be evaluated along the A‐side of the Procedure.
Conversely, for the candidate substance oct‐2‐en‐4‐one [FL‐no: 07.082], the Panel requested industry to investigate the possible generation of the γ‐diketone oct‐2‐en‐4,7‐dione and its neurotoxic potency. To this aim, following a clarification teleconference 5 in November 2020, industry provided an in vitro biotransformation study of oct‐2‐en‐4‐one [FL‐no: 07.082] using cryopreserved rat and human hepatocytes (Documentation provided to EFSA nr: 4). The in vitro biotransformation of 2‐hexanone was studied in parallel, as confirmation of the potential for formation of a diketone metabolite (2,5‐hexanedione). Incubations were carried out in crimp‐sealed vials with suspensions of one commercial batch of pooled human hepatocytes from 10 male donors and with one commercial batch of pooled rat hepatocytes from 24 male donors. For both species, cell viability was confirmed to be approximately 90% using the trypan blue exclusion test. The specifications for the human hepatocytes stated metabolic proficiency for several cytochrome P450 substrates. For the rat hepatocytes, only a clarification that the cells were proficient in 7‐ethoxycoumarin deethylation was noted. For both human and rat hepatocytes, proficiency for 7‐ethoxycoumarin deethylation was experimentally confirmed by the test laboratory, showing adequate formation of 7‐hydroxycoumarin and its glucuronide and sulfate conjugates. For oct‐2‐en‐4‐one biotransformation, incubations were done in triplicate; for biotransformation of 2‐hexanone, incubations were done in duplicate. Chromatographic methods were developed for the quantitative measurement of oct‐2‐en‐4,7‐dione and 2,5‐hexanedione using LC‐MS/MS. Limits of quantification and detection were 0.05 μg/mL and 0.01 μg/mL, respectively, for both metabolites.
Results with rat hepatocytes
Following the incubation of 10 μmol/L (1.260 μg/mL) oct‐2‐en‐4‐one with rat hepatocytes for 4 h, in one of the replicates, oct‐2‐en‐4,7‐dione was generated at a concentration above the limit of detection but it remained below the limit of quantification. Following the incubation of 10 μmol/L (1.002 μg/mL) 2‐hexanone (positive control) with rat hepatocytes for 4 h, the specific metabolite product 2,5‐hexanedione was not generated in detectable amounts, but it was observed above the limit of detection in one incubate at incubation time 0.
Results with human hepatocytes
Following the incubation of 10 μmol/L of oct‐2‐en‐4‐one with human hepatocytes for 4 h, in two of the replicates, oct‐2‐en‐4,7‐dione was generated at a concentration above the limit of detection, but it remained below the limit of quantification. Following the incubation of 10 μmol/L of 2‐hexanone (positive control) with human hepatocytes for 4 h, the specific metabolite 2,5‐hexanedione was not generated in quantities greater than the limit of detection.
For both starting substances, peaks co‐eluting with the diketones were observed in incubates where biotransformation was not anticipated: a peak at the retention time of the octenedione standard was seen in an incubate without hepatocytes at incubation time T = 0 and a peak at the retention time of the hexadione standard was observed in a rat hepatocyte incubate also at T = 0 (see above). Both peaks were below the limit of quantification.
In relation to this in vitro biotransformation study, the Panel noted the following:
The limits of detection and quantification determined for the target metabolites demonstrated that the chosen analytical approach was suitable for investigation of the potential formation of the respective γ‐diketones.
The demonstration of metabolic proficiency using 7‐ethoxycoumarin as an indicator substrate is limited. The specifications for the human cells are helpful in this respect because they indicated biotransformation activity for several types of reactions. Nevertheless, for the formation of the putative diketones, ω‐1 oxidation is necessary. Use of a substrate that would show the metabolic proficiency for this particular biotransformation would have been informative.
It is of concern that the positive control (2‐hexanone) was not metabolised into detectable amounts of the corresponding γ‐diketone 2,5‐hexanedione. This puts the sensitivity of the experimental design into question.
The concentration of the substrate is quite low (1 µg/mL), but no explanation is given for this choice. Although the sensitivity of the analytical method is sufficient, with such a low substrate concentration, it is not clear that detectable amount of metabolites could be formed.
Additional studies with hepatic microsomes, supersomes and/or other in vitro models for metabolism would have been informative. With respect to risk assessment, results using microsomes/supersomes are of limited relevance since they have limited capacity for phase II metabolism. Therefore, they may not reflect the real formation and liberation of metabolites.
No other metabolites were monitored. When the intermediate for the diketones (i.e. the corresponding keto‐alcohol) is formed, then this may be conjugated so quickly in hepatocytes that subsequent detectable oxidation of hydroxylated ketones is not seen. In that respect, a higher substrate concentration might have revealed the formation of the diketone. In addition, if the keto‐alcohols reach the blood stream, they may also be oxidised to the diketones in extra‐hepatic tissues.
The observations of peaks assigned by the study authors to the putative metabolites under conditions where such metabolism would not have been possible, casts doubt on the relevance of the peaks in the incubates where biotransformation could have occurred.
Overall, the Panel concluded that the information provided is not sufficient to judge whether oct‐2‐en‐4‐one [FL‐no: 07.082] can be converted to the corresponding γ‐diketone oct‐2‐en‐4,7‐dione. In particular, the fact that the positive control (2‐hexanone) was not metabolised into detectable amounts of 2,5‐hexandione demonstrates the non‐suitability of the experimental design. Consequently, the flavouring substance [FL‐no: 07.082] should be evaluated along the B‐side of the Procedure.
3.3.2. Genotoxicity data
This revision involves the inclusion of 14 flavouring substances ([FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244]), which have a structural alert for genotoxicity (i.e. α,β‐unsaturated carbonyl substance or precursor), preventing their evaluation through the Procedure (see also Appendix A). Therefore, these substances were evaluated in FGE.204Rev1 where their genotoxic potential has been assessed and ruled out (EFSA FAF Panel, 2019). Accordingly, the safety evaluation through the Procedure can be performed for these 14 flavouring substances.
3.3.3. Toxicological data
No subacute, subchronic/chronic toxicity and carcinogenicity studies are available on any of the candidate substances.
For structurally related substances in FGE.07Rev5, acute, subacute and subchronic toxicity studies and developmental and reproductive toxicity studies are listed in FGE.07Rev5 (see Appendix E of EFSA CEF Panel, 2017). Particularly, for flavouring substance 5‐methylheptan‐3‐one [FL‐no: 07.182], evaluated in FGE.07, a subchronic 13‐week study is available in which a no observed adverse effect level (NOAEL) of 82 mg/kg body weight (bw) per day was identified based on neurotoxicity endpoints (behavioural effects) and microscopic changes. The details of the study are available in FGE.07Rev5 (EFSA CEF Panel, 2017).
EFSA considerations
The Panel noted that flavouring substance 5‐methylheptan‐3‐one [FL‐no: 07.182], according to its chemical structure, can be considered structurally related to the flavouring substance oct‐2‐en‐4‐one [FL‐no: 07.082]. Based on the available information on absorption, distribution, metabolism and excretion (ADME) from the CEF Panel opinion on FGE.07Rev5 (see Appendix D of EFSA CEF Panel, 2017), 5 methylheptan‐3‐one [FL‐no: 07.182], similar to the candidate substance [FL‐no: 07.082], may potentially undergo ω‐1 oxidation leading first to a hydroxy‐ketone and then to a γ‐diketone (3‐methyl‐2,5‐heptanedione). The Panel also noted that in the γ‐diketone, potentially generated by 5‐methylheptan‐3‐one [FL‐no: 07.182], there is a single methyl group on one of the carbons located between the two carbonyl groups of the γ‐diketone (see Figure 1); this would increase the potential neurotoxicity of the compound (Topping et al., 1994). Thus, the use of 5‐methylheptan‐3‐one [FL‐no: 07.182] as reference substance represents a conservative scenario with regard to the formation of neurotoxic metabolites. Therefore, the Panel used the NOAEL of 82 mg/kg bw per day identified for 5‐methylheptan‐3‐one [FL‐no: 07.182] from a 13‐week study to derive a margin of safety for the flavouring substance oct‐2‐en‐4‐one [FL‐no: 07.082].
3.4. Application of the Procedure
Application of the Procedure to 14 aliphatic α,β unsaturated secondary alcohols and aldehydes by JECFA (2002a)
JECFA allocated six flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.104 and 07.139], currently under evaluation in FGE.63Rev4, to structural class I according to the decision tree approach presented by Cramer et al. (1978). The remaining eight flavouring substances [FL‐no: 07.082, 07.105, 07.106, 07.107, 07.121, 07.177, 07.188 and 07.244] were allocated to structural class II.
JECFA considered that these flavouring substances can be anticipated to be metabolised to innocuous products (step 2). The intakes, based on MSDI approach, for all 14 substances are below the thresholds of concern for structural class I and II (1,800 and 540 µg/person per day, respectively) (step A3). Therefore, JECFA concluded that these 14 flavouring substances would pose no safety concern at their estimated level of use, based on the MSDI approach.
The JECFA safety evaluations of the flavouring substances in FGE.63Rev4 are summarised in Table D.1 – Appendix D.
EFSA considerations
The FAF Panel partially agrees with JECFA with respect to the allocation to structural class of the 14 flavouring substances. According to the predictions run in OECD (Q)SAR Toolbox (version 4.3), four out of the 14 candidate substances ([FL‐no: 02.102, 02.193, 07.082 and 07.139]) are assigned to structural class II. The remaining 10 substances ([FL‐no: 07.244, 07.188, 07.177, 07.104, 07.121, 07.106, 07.105, 07.107, 07.048 and 07.044]) are structural class I.
The FAF Panel agrees with the way of the application of the Procedure that has been performed by JECFA for all flavouring substances with the exception of oct‐2‐en‐4‐one [FL‐no: 07.082]. The MSDI exposure estimates for the all flavouring substances are below the thresholds of concern for their structural classes (I and II) (see Table C.4 – Appendix C). Therefore, the FAF Panel concludes, at step A3 of the Procedure scheme, that flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] do not raise a safety concern when used as flavouring substances at the current levels of use, based on the MSDI approach.
For flavouring substance oct‐2‐en‐4‐one [FL‐no: 07.082], the Panel considers that the available NOAEL on the structurally related substance 5 methylheptan‐3‐one [FL‐no: 07.182] is suitable for a further evaluation of this substance (see EFSA considerations in Section 3.3.3). Comparison of the MSDI of [FL‐no: 07.082] (13.78 µg/capita per day) with the NOAEL of the 90‐day study with 5 methylheptan‐3‐one [FL‐no: 07.182] (82 mg/kg bw per day) provides an adequate margin of safety of 360,000 for the candidate substance [FL‐no: 07.082].
Therefore, the Panel concluded, at step B4 of the Procedure scheme, that the flavouring substance oct‐2‐en‐4‐one [FL‐no: 07.082] does not pose a safety concern when used as a flavouring substance at the estimated levels of intake, based on MSDI approach.
For all the 14 flavouring substances, normal and maximum use levels are available and mTAMDI values have been calculated (see Table C.4 – Appendix C). The mTAMDI intake estimates for two substances [FL‐no: 07.044 and 07.048] are equal to the toxicological threshold of concern (TTC) of their structural class I. For the remaining 12 substances ([FL‐no: 02.102, 02.193, 07.104, 07.139, 07.082, 07.105, 07.106, 07.107, 07.121, 07.177, 07.188, 07.244]), the mTAMDI intake estimates are above the TTC for their structural classes (I and II). Therefore, for all 14 flavouring substances, more detailed data on uses and use levels should be provided in order to refine the exposure assessment and to finalise their safety evaluation.
4. Discussion
This revision 4 of FGE.63 comprises in total 43 JECFA‐evaluated flavouring substances, 29 of which have already been considered in FGE.63 and its three revisions. The remaining 14 substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244] have been included in this revision, following evaluation in FGE.204Rev1 of their genotoxic potential due to the presence of a structural alert for genotoxicity (i.e. α,β‐unsaturated carbonyl or precursors for that) in which the concern for the genotoxicity was ruled out.
The FAF Panel concludes in this revision of FGE.63 as follows:
at step A3 of the Procedure: the flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244];
at step B4 of the Procedure: the flavouring substance oct‐2‐en‐4‐one [FL‐no: 07.082]
do not raise a safety concern at the estimated levels of exposure, when based on MSDI approach.
For all 14 flavouring substances, normal and maximum use levels have been provided. The mTAMDI intake estimates for two substances [FL‐no: 07.044 and 07.048] are equal to the toxicological threshold of concern (TTC) of their structural class I. For the remaining 12 substances ([FL‐no: 02.102, 02.193, 07.104, 07.139, 07.082, 07.105, 07.106, 07.107, 07.121, 07.177, 07.188, 07.244]), the mTAMDI intake estimates are above the TTC for their structural classes (I and II). Therefore, for all 14 flavouring substances, more detailed data on uses and use levels should be provided in order to refine the exposure assessment and to finalise their safety evaluation.
No normal and maximum use levels have been provided for 15 flavouring substances [FL‐no: 09.657, 09.658, 09.923, 09.924, 09.925, 07.015, 07.081, 07.100, 07.114, 07.123, 07.151, 07.240, 07.247, 07.249, 07.256], previously considered in FGE.63Rev3. Therefore, for these 15 flavouring substances, normal and maximum use levels are needed to calculate the mTAMDI estimates in order to identify those flavouring substances that need more refined exposure assessment and to finalise the evaluation accordingly. The Panel also noted that in the previous revision (FGE.63Rev3), 10 substances [FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 07.081, 07.102, 07.190, 09.281 and 09.282] had mTAMDI values above their respective TTCs. For these 10 substances, more reliable data on uses and use levels are required in order to finalise the evaluation.
To determine whether the conclusions for the 43 JECFA‐evaluated substances can be applied to the materials of commerce, it is necessary to consider the available specifications. Adequate specifications, including complete purity criteria and identity, are available for all 43 flavouring substances in FGE.63Rev4.
5. Conclusions
In conclusion, for all 43 flavouring substances in FGE.63Rev4, the FAF Panel agrees with JECFA conclusions ‘No safety concern at estimated levels of intake as flavouring substances’ when based on the MSDI approach.
However, for 14 of these candidate substances in the present revision ([FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188 and 07.244]) and for 10 of the substances in the previous revision (FGE.63Rev3) ([FL‐no: 02.023, 02.099, 02.104, 02.136, 02.155, 07.081, 07.102, 07.190, 09.281 and 09.282]), the mTAMDI values are equal to or above the TTCs for their structural classes (I and II). For 15 substances [FL‐no: 09.657, 09.658, 09.923, 09.924, 09.925, 07.015, 07.081, 07.100, 07.114, 07.123, 07.151, 07.240, 07.247, 07.249 and 07.256], previously evaluated in FGE.63Rev3, use levels are still needed to calculate the mTAMDI estimates. Therefore, in total for 39 flavouring substances, more data on uses and use levels should be provided in order to finalise their safety evaluations.
6. Recommendations
The Panel recommends the European Commission to consider:
requesting normal and maximum uses and use levels for [FL‐no: 09.657, 09.658, 09.923, 09.924, 09.925, 07.015, 07.081, 07.100, 07.114, 07.123, 07.151, 07.240, 07.247, 07.249, 07.256];
requesting more detailed data on uses and use levels for substances ([FL‐no: 02.023, 02.099, 02.102, 02.104, 02.193, 02.136, 02.155, 07.044, 07.048, 07.081, 07.082, 07.102, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177, 07.188, 07.190, 07.244, 09.281 and 09.282]. When these data are received, the assessment for these flavouring substances should be updated accordingly and expanded if necessary (i.e. request of additional toxicity data);
in accordance with the latest specifications for the materials of commerce provided by industry, changing the chemical names and the CAS numbers in the Union List for flavouring substances [FL‐no: 02.102, 02.193, 07.044, 07.048, 07.082, 07.104, 07.105, 07.106, 07.107, 07.121, 07.139, 07.177 and 07.188] to reflect their stereochemical configuration (see Table B.1 of Appendix B);
changing the chemical name in the Union List for flavouring substance [FL‐no: 07.123], previously considered in FGE.63Rev3, as indicated in Table B.1 of Appendix B.
7. Documentation provided to EFSA
EFFA (European Flavour Association), 2019. Submission of additional information on isomeric composition, poundage and refined use levels data of substances of FGE.204 Rev1 (FGE.19 Subgroup 1.2.1).
EFFA (European Flavour Association), 2020. Submission of additional information on stereoisomers for two substances of FGE.204 ([FL‐no: 02.102 and 02.193]) for evaluation in FGE.63 Rev4.
EFFA (European Flavour Association), 2020a. Submission of additional information on EU poundage data, info on use and potential oxidation of substances of FGE.204 Rev1 (FGE.19 Subgroup 1.2.1).
EFFA (European Flavour Association), 2021. Submission of an in vitro comparative metabolism study of oct‐2‐en‐4‐one ([FL‐no: 07.082]).
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- bw
body weight
- EFFA
European Flavours Association
- FGE
Flavouring Group Evaluation
- JEFCA
Joint FAO/WHO Expert Committee on Food Additives
- MSDI
Maximised Survey‐derived Daily Intake
- mTAMDI
modified Theoretical Added Maximum Daily Intakes
- NOAEL
Mo observed adverse effect level
- SCF
Scientific Committee on Food
- TTC
toxicological threshold of concern
Appendix A – Procedure of the safety evaluation
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is shown in schematic form in Figure A.1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999), hereafter named the ‘JECFA Procedure’. 6
Figure A.1.
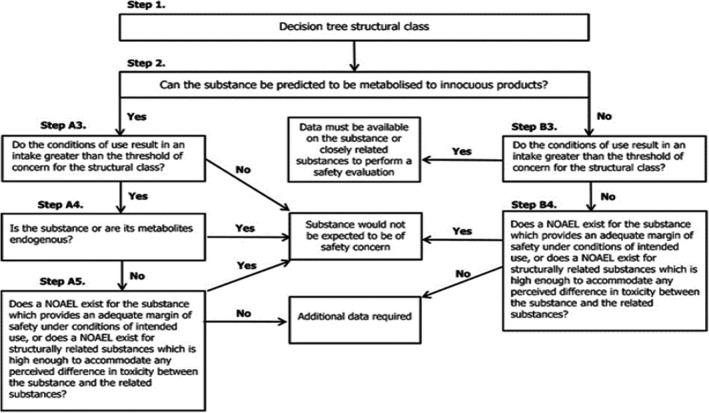
Procedure for the safety evaluation of chemically defined flavouring substances
The Procedure is a stepwise approach that integrates information on intake from current uses, structure–activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II and III) for which toxicological thresholds of concern (TTCs) (human exposure thresholds) have been specified. Exposures below these TTCs are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The TTCs for these structural classes of 1,800, 540 or 90 µg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous products 7 (step 2)?
Do their exposures exceed the TTC for the structural class (steps A3 and B3)?
Are the flavourings or their metabolites endogenous 8 (step A4)?
Does an NOAEL exist on the flavourings or on structurally related substances (steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure. The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions.
For the flavouring substances considered in this Flavouring Group Evaluation (FGE), the EFSA Panel on Food Additives and Flavourings (FAF) compares the JECFA evaluation of structurally related substances with the result of a corresponding EFSA evaluation, focussing on specifications, intake estimations and toxicity data, especially genotoxicity data. The considerations by EFSA will conclude whether the flavouring substances are of no safety concern at their estimated levels of intake, whether additional data are required or whether certain substances should not be evaluated through the EFSA Procedure.
The following issues are of special importance:
a) Intake
In its evaluation, the Panel as a default uses the ‘maximised survey‐derived daily intake’ (MSDI) 9 approach to estimate the per capita intakes of the flavouring substances in Europe.
In its evaluation, JECFA includes intake estimates based on the MSDI approach derived from both European and USA production figures. The highest of the two MSDI figures is used in the evaluation by JECFA. It is noted that in several cases, only the MSDI figures from the USA were available, meaning that certain flavouring substances have been evaluated by JECFA only on the basis of these figures. For substances in the Union List 10 of flavouring substances for which this is the case, the Panel will need European Union (EU) production figures in order to finalise the evaluation.
When the Panel examined the information provided by the European Flavour Industry on the use levels in various foods, it appeared obvious that the MSDI approach in a number of cases would grossly underestimate the intake by regular consumers of products flavoured at the use levels reported by the Industry, especially in those cases where the annual production values were reported to be small. In consequence, the Panel had reservations about the data on use and use levels provided and the intake estimates obtained by the MSDI approach. It is noted that JECFA, at its 65th meeting, considered ‘how to improve the identification and assessment of flavouring agents, for which the MSDI estimates may be substantially lower than the dietary exposures that would be estimated from the anticipated average use levels in foods’ (JECFA, 2005).
In the absence of more accurate information that would enable the Panel to make a more realistic estimate of the intakes of the flavouring substances, the Panel has decided also to perform an estimate of the daily intakes per person using a modified Theoretical Added Maximum Daily Intake (mTAMDI) approach based on the normal use levels reported by Industry (see Appendix C.2).
As information on use levels for the flavouring substances has not been requested by JECFA or has not otherwise been provided to the Panel, it is not possible to estimate the daily intakes using the mTAMDI approach for many of the substances evaluated by JECFA. The Panel will need information on use levels in order to finalise the evaluation.
b) Threshold of 1.5 microgram/person per day (step B5) used by JECFA
JECFA uses the threshold of concern of 1.5 j.tg/person per day as part of the evaluation procedure:
‘The Committee noted that this value was based on a risk analysis of known carcinogens which involved several conservative assumptions. The use of this value was supported by additional information on developmental toxicity, neurotoxicity and immunotoxicity. In the judgement of the Committee, flavouring substances for which insufficient data are available for them to be evaluated using earlier steps in the Procedure, but for which the intake would not exceed 1.5 j.tg/person per day would not be expected to present a safety concern. The Committee recommended that the Procedure for the Safety Evaluation of Flavouring Agents, used at the forty‐sixth meeting, should be amended to include the last step on the right‐hand side of the original procedure (‘Do the conditions of use result in an intake greater than 1.5 j.tg per day?’)’ (JECFA, 1999).
In line with the opinion expressed by the Scientific Committee on Food (SCF, 1999), the Panel does not make use of this threshold of 1.5 j.tg per person per day.
c) Genotoxicity
As reflected in the opinion of SCF (1999), the Panel has in its evaluation focussed on a possible genotoxic potential of the flavouring substances or of structurally related substances. Generally, substances for which the Panel has concluded that there is an indication of genotoxic potential in vitro, will not be evaluated using the EFSA Procedure until further genotoxicity data are provided. Substances for which a genotoxic potential in vivo has been concluded, will not be evaluated through the Procedure.
d) Specifications
Regarding specifications, the evaluation by the Panel could lead to a different opinion than that of JECFA, since the Panel requests information on e.g. isomerism.
e) Structural Relationship
In the consideration of the JECFA‐evaluated substances, the Panel will examine the structural relationship and metabolism features of the substances within the flavouring group and compare this with the corresponding FGE.
Appendix B – Specifications
Table B.1.
Summary table on specifications data for flavouring substances in FGE.63Rev4, for chemical structures, see Appendix D
|
Information included in the EU Union list Regulation No (EU) 1334/2008 as amended |
Most recent available specifications data a |
EFSA Comments |
|||||
|---|---|---|---|---|---|---|---|
|
FL‐no JECFA‐no FEMA no CoE no CAS no |
Chemical name | Purity of the named compound |
Phys. form Mol. formula Mol. weight |
Solubility c Solubility in ethanol d |
Boiling point, °C e Melting point, °C ID test Assay minimum (isomers distribution/SC h ) |
Refrac. Index f Spec. gravity g |
|
|
02.023 1152 2805 72 3391‐86‐4 |
Oct‐1‐en‐3‐ol | (b) |
Liquid C8H16O 128.22 |
Insoluble Miscible |
175–175.2 NMR 96% |
1.431–1.442 0.835–0.845 |
|
|
02.099 1150 3584 11717 616‐25‐1 |
Pent‐1‐en‐3‐ol | (b) |
Liquid C5H10O 86.13 |
Sparsely soluble Miscible |
114 NMR 98% (racemate) |
1.419–1.427 0.831–0.837 |
|
|
02.102 1140 3602 76649‐14‐4 |
Oct‐3‐en‐2‐ol | (b) |
Liquid C8H16O 128.22 |
Insoluble Miscible |
73–76 (13 hPa) IR NMR MS 95% (E)‐isomer (racemate) |
1.422–1.428 0.826–0.836 |
The chemical name should be changed to Oct‐(3E)‐en‐2‐ol and the CAS number to 57648‐55‐2 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1 and 2) |
|
02.104 1151 3608 10220 4798‐44‐1 |
Hex‐1‐en‐3‐ol | (b) |
Liquid C6H12O 100.16 |
Insoluble Miscible |
133.5–134 NMR 98% (racemate) |
1.425–1.431 0.830–0.836 |
|
|
02.136 1153 3824 51100‐54‐0 |
Dec‐1‐en‐3‐ol | (b) |
Liquid C10H20O 156.27 |
Slightly soluble Miscible |
215 NMR MS 97% (racemate) |
1.439–1.446 0.836–0.842 |
|
|
02.155 1842 4129 10218 4938‐52‐7 |
1‐Hepten‐3‐ol | (b) |
Liquid C7H14O 114.19 |
Practically insoluble or insoluble Freely soluble |
155 MS 97%(racemate) |
1.431–1.437 0.834–0.837 |
|
|
02.193 1141 3888 4798‐61‐2 |
Oct‐2‐en‐4‐ol | (b) |
Liquid C8H16O 128.22 |
Insoluble 50% Soluble in ethanol |
174–176 IR NMR MS % (E)‐isomer 1–2% (Z) isomer (racemate) |
1.438–1.442 0.830–0.838 |
The chemical name should be changed to Oct‐(2E)‐en‐4‐ol and the CAS number to 20125‐81‐9 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1 and 2) |
|
02.252 1841 4102 67845‐50‐5 |
4,8‐Dimethyl‐3,7‐nonadien‐2‐ol | (b) |
Liquid C11H20O 168 |
Insoluble Soluble |
70 (2.6 hPa) IR NMR 95% (racemate and mixture of E/Z stereoisomers: 50–80% (E)) |
1.465–1.473 0.860–0.870 |
|
|
07.015 1120 2707 149 110‐93‐0 |
6‐Methylhept‐5‐en‐2‐one | (b) |
Liquid C8H14O 126.19 |
Insoluble Miscible |
173.1 NMR 97% |
1.435–1.445 0.846–0.854 |
|
|
07.044 1124 3417 666 625‐33‐2 |
Pent‐3‐en‐2‐one | (b) |
Liquid C5H8O 84.12 |
Slightly soluble Miscible at room temp. |
122 NMR At least 75% (E)‐isomer 25% (Z)‐isomer |
1.433–1.437 0.860–0.865 |
The chemical name should be changed to pent‐(3E)‐en‐2‐one and the CAS number to 3102‐33‐8 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.048 1125 3352 718 2497‐21‐4 |
4‐Hexen‐3‐one | (b) |
Liquid C6H10O 98.15 |
Slightly soluble Miscible |
93 (195 hPa) NMR 90–95% (E)‐isomer 4–5% (Z)‐isomer |
1.437–1.443 0.855–0.861 |
The chemical name should be changed to hex‐(4E)‐en‐3‐one and the CAS number to 50396‐87‐7 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.069 1121 3059 2053 4433‐36‐7 |
Tetrahydro‐pseudo‐ionone | (b) |
Liquid C13H24O 196.33 |
Insoluble Miscible |
234 NMR 95% (racemate) |
1.449–1.455 0.865–0.875 |
|
|
07.081 1148 3515 2312 4312‐99‐6 |
Oct‐1‐en‐3‐one | (b) |
Liquid C8H14O 126.20 |
Insoluble Miscible |
37–38 (3 hPa) NMR 96% |
1.428–1.439 0.813–0.819 |
|
|
07.082 1129 3603 2313 4643‐27‐0 |
Oct‐2‐en‐4‐one | (b) |
Liquid C8H14O 126.20 |
Insoluble Miscible at room temp. |
81 (26–27 hPa) IR NMR 90–91% (E)‐isomer 5–6% (Z)‐isomer |
1.440–1.446 0.835–0.842 |
The chemical name should be changed to oct‐(2E)‐en‐4‐one and the CAS number to 22286‐99‐3 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.099 1134 3363 11143 1604‐28‐0 |
6‐Methylhepta‐3,5‐dien‐2‐one | (b) |
Liquid C8H12O 124.18 |
Almost insoluble Miscible |
190 NMR 96% (mixture of E/Z stereoisomers: 60–90% (E)) |
1.528–1.537 0.895–0.899 |
|
|
07.100 1119 3365 11150 3240‐09‐3 |
5‐Methylhex‐5‐en‐2‐one | (b) |
Liquid C7H12O 112.17 |
Insoluble Miscible |
148–149 NMR 97% |
1.428–1.433 0.862–0.868 |
|
|
07.101 1131 3368 11853 141‐79‐7 |
4‐Methylpent‐3‐en‐2‐one | (b) |
Liquid C6H10O 98.14 |
Slightly soluble Miscible |
126.76 NMR 95% |
1.442–1.447 0.862–0.868 |
|
|
07.102 1147 3382 11179 1629‐58‐9 |
Pent‐1‐en‐3‐one | (b) |
Liquid C5H8O 84.12 |
Insoluble Miscible |
68–70 (260 hPa) NMR 97% |
1.417–1.422 0.842–0.848 |
|
|
07.104 1126 3399 11093 4643‐25‐8 |
Hept‐2‐en‐4‐one | (b) |
Liquid C7H12O 112.17 |
Slightly soluble Miscible |
156–157 IR NMR 95% (E)‐isomer |
1.440–1.445 0.845–0.852 |
The chemical name should be changed to hept‐(2E)‐en‐4‐one and the CAS number to 22286‐99‐3 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.105 1127 3400 11094 1119‐44‐4 |
Hept‐3‐en‐2‐one | (b) |
Liquid C7H12O 112.17 |
Slightly soluble Miscible |
162 NMR 95% (E)‐isomer |
1.439–1.448 0.841–0.847 |
The chemical name should be changed to hept‐(3E)‐en‐2‐one and the CAS number to 5609‐09‐6 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.106 1132 3409 11149 5166‐53‐0 |
5‐Methylhex‐3‐en‐2‐one | (b) |
Liquid C7H12O 112.17 |
Insoluble Miscible |
77.5 (65 hPa) NMR 95% (E)‐isomer |
1.437–1.441 0.838–0.843 |
The chemical name should be changed to 5‐Methylhex‐(3E)‐en‐2‐one and the CAS number to 1821‐29‐0 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.107 1128 3416 11170 1669‐44‐9 |
Oct‐3‐en‐2‐one |
At least 94%; secondary component 4‐6% 4‐octen‐2‐one |
Liquid C8H14O 126.19 |
Insoluble Miscible at room temp. |
75–79 (26 hPa) NMR 90–91% (E)‐isomer 3–4% (Z)‐isomer SC: 4‐6% 4‐octen‐2‐one |
1.445–1.449 0.834–0.839 |
The chemical name should be changed to Oct‐(3E)‐en‐2‐one and the CAS number to 18402‐82‐9 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.114 1123 3442 11206 762‐29‐8 |
6,10,14‐Trimethylpentadeca‐5,9,13‐trien‐2‐one | (b) |
Liquid C18H30O 262.44 |
Soluble Miscible |
147–148 NMR 96% (mixture of (5E,9E)‐, (5Z,9Z)‐, (5E,9Z)‐ and (5Z,9E)‐isomers) |
1.478–1.483 0.885–0.895 |
|
|
07.121 1130 3532 11751 10519‐33‐2 |
Dec‐3‐en‐2‐one | (b) |
Liquid C10H18O 154.25 |
Almost insoluble Miscible at room temp. |
125–126 NMR 95% (E)‐isomer |
1.446–1.452 0.809–0.813 |
The chemical name should be changed to Dec‐(3E)‐en‐2‐one and the CAS number to 18402‐84‐1 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1) |
|
07.123 1122 3542 11088 3796‐70‐1 |
Geranyl acetone | (b) |
Liquid C13H22O 194.32 |
Slightly soluble Miscible |
247 NMR 95% (E)‐isomer |
1.463–1.471 0.861–0.867 |
Chemical name in the Union List should be changed to (E)‐geranyl acetone |
|
07.139 1133 3761 81925‐81‐7 |
5‐Methylhept‐2‐en‐4‐one | (b) |
Liquid C8H14O 126.19 |
Slightly soluble Miscible |
86–87 (78 hPa) NMR 91–95% (E)‐isomer 1–5% (Z)‐isomer (racemate) |
1.440–1.445 0.845–0.852 |
The chemical name should be changed to 5‐Methylhept‐(2E)‐en‐4‐one and the CAS number to 102322‐83‐8 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1). |
|
07.151 1118 3966 11056 928‐80‐3 |
Decan‐3‐one | (b) |
Liquid C10H20O 156.27 |
Insoluble Miscible |
204‐205 NMR 97% |
1.421–1.427 0.820–0.830 |
|
|
07.177 1135 3868 33046‐81‐0 |
7‐Methyl‐3‐octenone‐2 |
At least 94%; secondary components 2‐4% 7‐methyl‐4‐octen‐2‐one, 5,6‐ dimethyl‐3‐hepten‐2one and 3‐nonen‐2‐one |
Liquid C9H16O 140.2 |
Slightly soluble Miscible |
198 n.a. IR NMR MS % (E)‐isomer SC: 2‐4% 7‐methyl‐4‐octen‐2‐one, 5,6‐dimethyl‐3‐hepten‐2‐one and 3‐nonen‐2‐one |
1.446–1.451 0.838–0.847 |
The chemical name should be changed to 7‐Methyl‐oct‐(3E)‐en‐2‐one and the CAS number to 1004754‐77‐1 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1). |
|
07.188 1136 3955 11163 14309‐57‐0 |
Non‐3‐en‐2‐one | (b) |
Liquid C9H16O 140.22 |
Insoluble Miscible at room temp. |
198 IR MS 95% (E)‐isomer |
1.443–1.452 0.843–0.846 |
The chemical name should be changed to Non‐(3E)‐en‐2‐one and the CAS number to 18402‐83‐0 to reflect the stereochemical configuration. (Documentation provided to EFSA nr: 1). |
|
07.190 1848 4405 65213‐86‐7 |
Octa‐1,5‐dien‐3‐one | (b) |
Liquid C8H12O 124.18 |
Practically insoluble or insoluble Freely soluble |
169 MS 95% (mixture of E/Z stereoisomers: 60–90% (E)) |
1.438–1.444 0.823–0.829 |
|
|
07.240 1156 4000 13019‐20‐0 |
2‐Methylheptan‐3‐one | (b) |
Liquid C8H16O 128.2 |
Insoluble Miscible |
158–160 NMR 98% |
1.408–1.413 0.811–0.821 |
|
|
07.244 1138 4001 20859‐10‐3 |
(6E)‐Methyl‐3‐hepten‐2‐one | (b) |
Liquid C8H14O 126.2 |
Insoluble Miscible at room temp. |
170–180 NMR 96% (E)‐isomer < 1% (Z)‐isomer |
1.438–1.447 0.840–0.850 |
|
|
07.247 1139 4008 30086‐02‐3 |
(E,E)‐3,5‐Octadien‐2‐one | (b) |
Liquid C8H12O 124.2 |
Insoluble Miscible |
220 NMR 95% |
1.508–1.516 0.880–0.890 |
|
|
07.249 1155 4022 927‐49‐1 |
Undecan‐6‐one | (b) |
Liquid C11H22O 170.3 |
Insoluble Miscible |
228 NMR 97% |
1.424–1.430 0.826–0.836 |
|
|
07.256 1137 3969 817‐88‐9 |
(E) & (Z)‐4,8‐Dimethyl‐3,7‐nonadiene‐2‐one | 94% Secondary component: 3–4% 4,8–dimethyl‐3,7‐nonadien‐2‐ol |
Liquid C11H18O 166.26 |
Insoluble Freely soluble |
200–201 n.a. IR NMR % (Mixture of E/Z stereoisomers: 60–90% (E)) Secondary component: 3–4% 4,8‐dimethyl‐3,7‐nonadien‐2‐ol |
1.473–1.477 0.869–0.875 |
|
|
09.281 1836 3582 11716 2442‐10‐6 |
Oct‐1‐en‐3‐yl acetate | (b) |
Liquid C10H18O2 170.25 |
Practically insoluble or insoluble Freely soluble |
80 (2 hPa) NMR 97% (racemate) |
1.418–1.428 0.865–0.886 |
|
|
09.282 1837 3612 16491‐54‐6 |
Oct‐1‐en‐3‐yl butyrate | (b) |
Liquid C12H22O2 198.32 |
Practically insoluble or insoluble Freely soluble |
81 (0.46 hPa) IR NMR MS 95% (racemate) |
1.418–1.428 0.865–0.875 |
|
|
09.657 1146 4012 10761 626‐38‐0 |
1‐Methylbutyl acetate | (b) |
Liquid C7H14O2 130.2 |
Insoluble Partially Soluble |
135 NMR 98% (racemate) |
1.369‐1.400 0.862‐0.866 |
|
|
09.658 1142 3893 10763 60415‐61‐4 |
1‐Methylbutyl butyrate | (b) |
Liquid C9H18O2 158.24 |
Insoluble 50% Soluble |
185–186 IR NMR MS 99% (racemate) |
1.409–1.415 0.862–0.868 |
|
|
09.923 1144 3981 39026‐94‐3 |
Hept‐2‐yl butyrate | (b) |
Liquid C11H22O2 186.3 |
Insoluble Miscible |
210 NMR 98% (racemate) |
1.413–1.417 0.855–0.860 |
|
|
09.924 1143 3980 5921‐83‐5 |
3‐Heptyl acetate (mixture of R and S) | (b) |
Liquid C9H18O2 158.2 |
Insoluble Miscible |
185 NMR 98% (racemate) |
1.406–1.414 0.858–0.867 |
|
|
09.925 1145 4007 60826‐15‐5 |
Nonan‐3‐yl acetate | (b) |
Liquid C11H22O2 186.3 |
Insoluble Miscible |
225 NMR 98% (racemate) |
1.416–1.423 0.854–0.864 |
|
|
09.936 1847 4103 91418‐25‐6 |
4,8‐Dimethyl‐3,7‐nonadien‐2‐yl acetate | (b) |
Liquid C13H22O2 210 |
Insoluble Soluble |
75–83 (3 hPa) IR NMR 95% (racemate and mixture of E/Z stereoisomers: 50–80% (E)) |
1.451–1.459 0.890–0.900 |
|
UL: Union List.
At least 95% unless otherwise specified.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
Secondary components.
Appendix C – Exposure estimates
C.1 Normal and Maximum Use Levels
Table C.1.
Normal and maximum use levels (mg/kg) of JECFA evaluated flavouring substances in FGE.63Rev4 in food categories listed in Annex III of Reg. (EC) 1565/2000 (EFSA CEF Panel, 2016a and Documentation provided to EFSA n. 1 and 3)
| FL‐no |
Normal use levels (mg/kg) a Maximum use levels (mg/kg) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 05.3b) | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 02.023 |
0.63 1.8 |
0.5 1 |
1 2 |
– – |
11.85 18.4 |
1.14 1.8 |
– – |
0.56 1.8 |
3.81 10.5 |
3.74 5.7 |
1 5 |
1 5 |
1 5 |
2 5 |
1 2 |
0.56 1.2 |
0.3 1 |
0.35 0.7 |
2 10 |
| 02.099 |
5 35 |
2 25 |
3 50 |
– – |
7 35 |
4 50 |
– – |
5 25 |
5 50 |
2 10 |
1 10 |
1 10 |
1 10 |
5 25 |
3 50 |
3 25 |
4 50 |
5 100 |
2 25 |
| 02.102 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5 10.4 |
5 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
2.5 4.63 |
0.67 10 |
1 2 |
– – |
| 02.104 |
5 35 |
2 25 |
3 50 |
– – |
7 35 |
4 50 |
– – |
5 25 |
5 50 |
2 10 |
1 10 |
1 10 |
1 10 |
5 25 |
3 50 |
3 25 |
4 50 |
5 100 |
2 25 |
| 02.136 |
5 35 |
2 25 |
3 50 |
– – |
7 35 |
4 50 |
– – |
5 25 |
5 50 |
2 10 |
1 10 |
1 10 |
1 10 |
5 25 |
3 50 |
3 25 |
4 50 |
5 100 |
2 25 |
| 02.155 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
– – |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 02.193 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5 10.4 |
5 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
2.5 4.63 |
0.67 10 |
1 2 |
– – |
| 02.252 |
0.0005 0.025 |
0.0005 0.025 |
0.005 0.25 |
0.0005 0.025 |
0.0005 0.025 |
0.05 2.5 |
– – |
– – |
0.005 0.25 |
0.0005 0.025 |
0.0005 0.025 |
– – |
– – |
0.0005 0.025 |
– – |
0.05 2.5 |
0.05 2.5 |
0.0005 0.025 |
0.0005 0.025 |
| 07.044 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5 10.4 |
5 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
2.5 4.63 |
0.67 10 |
1 2 |
– – |
| 07.048 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5 10.4 |
5 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
2.5 4.63 |
0.67 10 |
1 2 |
– – |
| 07.081 |
3 15 |
2 10 |
3 15 |
– – |
2 10 |
4 20 |
– – |
2 10 |
5 25 |
1 5 |
1 5 |
1 5 |
1 5 |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.082 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.099 |
0.05 0.05 |
– – |
0.5 2 |
– – |
– – |
1.1 9 |
– – |
1 4.5 |
1 4.5 |
– – |
– – |
– – |
– – |
0.5 2 |
1 4.5 |
0.05 0.05 |
0 0 |
– – |
– – |
| 07.101 |
0.4 0.4 |
– – |
0.75 0.75 |
– – |
– – |
1.12 1.12 |
– – |
– – |
2.25 2.25 |
– – |
– – |
– – |
– – |
0.5 0.5 |
0.5 0.5 |
– – |
0 0 |
– – |
– – |
| 07.102 |
3 5 |
2 10 |
3 15 |
– – |
2 10 |
4 20 |
– – |
2 10 |
5 25 |
1 5 |
1 5 |
1 5 |
1 5 |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.104 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.105 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.106 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.107 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.121 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.139 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5 10.4 |
5 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
2.5 4.63 |
0.67 10 |
1 2 |
– – |
| 07.177 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.188 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 07.190 |
3 15 |
2 10 |
3 15 |
2 10 |
– – |
4 20 |
– – |
2 10 |
5 25 |
1 5 |
1 5 |
– – |
– – |
2 10 |
3 15 |
2 10 |
4 20 |
5 25 |
2 10 |
| 07.244 |
5 11.5 |
0.5 1.25 |
1.55 2.28 |
– – |
4.6 8.36 |
6.92 11.63 |
2 6.63 |
5.71 10.4 |
8.75 15 |
2.13 2.6 |
1 1 |
– – |
– – |
2 5.6 |
– – |
3 4.63 |
0.67 10 |
1 2 |
– – |
| 09.281 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
– – |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.282 |
7 35 |
5 25 |
10 50 |
7 35 |
– – |
10 50 |
– – |
5 25 |
10 50 |
2 10 |
2 10 |
– – |
– – |
5 25 |
10 50 |
5 25 |
10 50 |
20 100 |
5 25 |
| 09.936 |
0.0005 0.025 |
0.0005 0.025 |
0.005 0.25 |
0.0005 0.025 |
0.0005 0.025 |
0.05 2.5 |
– – |
– – |
0.005 0.25 |
0.0005 0.025 |
0.0005 0.025 |
– – |
– – |
0.0005 0.025 |
– – |
0.05 2.5 |
0.05 2.5 |
0.0005 0.025 |
0.0005 0.025 |
‘Normal use’ is defined as the average of reported usages and ‘maximum use’ is defined as the 95th percentile of reported usages.
Additional food category 05.3 (chewing gum as per Annex II part D of Reg. (EC) 1333/2008) for which EFFA submitted use levels (Documentation provided to EFSA n. 1 and 3). These data have been considered in the calculation of mTAMDI.
C.2. mTAMDI calculations
The method for calculation of modified Theoretical Added Maximum Daily Intake (mTAMDI) values is based on the approach used by the SCF up to 1995 (SCF, 1995). The assumption is that a person may consume the amount of flavourable foods and beverages listed in Table C.2. These consumption estimates are then multiplied by the reported use levels in the different food categories and summed up.
Table C.2.
Estimated amount of flavourable foods, beverages and exceptions assumed to be consumed per person per day (SCF, 1995)
| Class of product category | Intake estimate (g/day) |
|---|---|
| Beverages (non‐alcoholic) | 324.0 |
| Foods | 133.4 |
| Exception a: Candy, confectionery | 27.0 |
| Exception b: Condiments, seasonings | 20.0 |
| Exception c: Alcoholic beverages | 20.0 |
| Exception d: Soups, savouries | 20.0 |
| Exception e: Others, e.g. chewing gum | e.g. 2.0 (chewing gum) |
The mTAMDI calculations are based on the normal use levels reported by Industry. The seven food categories used in the SCF TAMDI approach (SCF, 1995) correspond to the 18 food categories as outlined in Commission Regulation (EC) No 1565/2000 and reported by the Flavour Industry in the following way (see Table C.3)
Beverages (SCF, 1995) correspond to food Table C.3: category 14.1
Foods (SCF, 1995) correspond to the food categories 1, 2, 3, 4.1, 4.2, 6, 7, 8, 9, 10, 13, and/or 16
Exception a (SCF, 1995) corresponds to food categories 5 and 11
Exception b (SCF, 1995) corresponds to food category 15
Exception c (SCF, 1995) corresponds to food category 14.2
Exception d (SCF, 1995) corresponds to food category 12
Exception e (SCF, 1995) corresponds to others, e.g. chewing gum.
Table C.3.
Distribution of the 18 food categories listed in Commission Regulation (EC) No 1565/2000 into the seven SCF food categories used for mTAMDI calculations (SCF, 1995)
| Key | Food categories according to Commission Regulation 1565/2000 | Distribution of the seven SCF food categories | ||
|---|---|---|---|---|
| Food category | Foods | Beverages | Exceptions | |
| 01.0 | Dairy products, excluding products of category 02.0 | Foods | ||
| 02.0 | Fats and oils, and fat emulsions (type water‐in‐oil) | Foods | ||
| 03.0 | Edible ices, including sherbet and sorbet | Foods | ||
| 04.1 | Processed fruit | Foods | ||
| 04.2 | Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds | Foods | ||
| 05.0 | Confectionery | Exception a | ||
| 06.0 | Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery | Foods | ||
| 07.0 | Bakery wares | Foods | ||
| 08.0 | Meat and meat products, including poultry and game | Foods | ||
| 09.0 | Fish and fish products, including molluscs, crustaceans and echinoderms | Foods | ||
| 10.0 | Eggs and egg products | Foods | ||
| 11.0 | Sweeteners, including honey | Exception a | ||
| 12.0 | Salts, spices, soups, sauces, salads, protein products, etc. | Exception d | ||
| 13.0 | Foodstuffs intended for particular nutritional uses | Foods | ||
| 14.1 | Non‐alcoholic (‘soft’) beverages, excl. dairy products | Beverages | ||
| 14.2 | Alcoholic beverages, incl. alcohol‐free and low‐alcoholic counterparts | Exception c | ||
| 15.0 | Ready‐to‐eat savouries | Exception b | ||
| 16.0 | Composite foods (e.g. casseroles, meat pies, mincemeat) – foods that could not be placed in categories 01.0–15.0 | Foods | ||
Appendix D – Summary of safety evaluations
Table D.1.
Summary of safety evaluations performed by JECFA (JECFA, 2002b, 2005) and EFSA conclusions on flavouring substances in FGE.63 and its revisions
| JECFA conclusions | EFSA conclusions | |||
|---|---|---|---|---|
|
FL‐no JECFA‐no |
EU Union List chemical name | Structural formula |
Class(a) Evaluation procedure path(b) Outcome on the named compound based on the MSDI(c) approach |
Procedural path if different from JECFA Conclusion based on the MSDI(d) approach on the named compound and on the material of commerce |
|
02.102 1140 |
Oct‐3‐en‐2‐ol |

|
Class I A3: Intake below threshold No safety concern |
Class II A3: Intake below threshold No safety concern. The chemical name should be changed to Oct‐(3E)‐en‐2‐ol and the CAS number to 57648‐55‐2 Concluded in FGE.63Rev4 |
|
02.193 1141 |
Oct‐2‐en‐4‐ol |

|
Class I A3: Intake below threshold No safety concern |
Class II A3: Intake below threshold No safety concern. The chemical name should be changed to Oct‐(2E)‐en‐4‐ol and the CAS number to 20125‐81‐9 Concluded in FGE.63Rev4 |
|
02.252 1841 |
4,8‐Dimethyl‐3,7‐nonadien‐2‐ol |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
07.044 1124 |
Pent‐3‐en‐2‐one |

|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. The chemical name should be changed to Pent‐(3E)‐en‐2‐one and the CAS number to 3102‐33‐8 Concluded in FGE.63Rev4 |
|
07.048 1125 |
4‐Hexen‐3‐one |
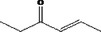
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. The chemical name should be changed to Hex‐(4E)‐en‐3‐one and the CAS number to 50396‐87‐7 Concluded in FGE.63Rev4 |
|
07.104 1126 |
Hept‐2‐en‐4‐one |
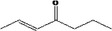
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. The chemical name should be changed to Hept‐(2E)‐en‐4‐one and the CAS number to 22286‐99‐3. Concluded in FGE.63Rev4 |
|
07.139 1133 |
5‐Methylhept‐2‐en‐4‐one |
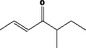
|
Class I A3: Intake below threshold No safety concern |
Class II A3: Intake below threshold No safety concern. The chemical name should be changed to 5‐Methylhept‐(2E)‐en‐4‐one and the CAS number to 102322‐83‐8. Concluded in FGE.63Rev4 |
|
09.657 1146 |
1‐Methylbutyl acetate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
09.658 1142 |
1‐Methylbutyl butyrate |
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
09.923 1144 |
Hept‐2‐yl butyrate |
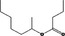
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
09.924 1143 |
3‐Heptyl acetate (mixture of R and S) |
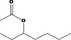
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
09.925 1145 |
Nonan‐3‐yl acetate |
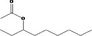
|
Class I A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
02.023 1152 |
Oct‐1‐en‐3‐ol |
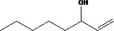
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
02.099 1150 |
Pent‐1‐en‐3‐ol |
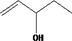
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
02.104 1151 |
Hex‐1‐en‐3‐ol |
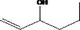
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
02.136 1153 |
Dec‐1‐en‐3‐ol |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
02.155 1842 |
1‐Hepten‐3‐ol |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
07.015 1120 |
6‐Methylhept‐5‐en‐2‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.069 1121 |
Tetrahydro‐pseudo‐ionone |
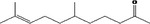
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.081 1148 |
Oct‐1‐en‐3‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
07.082 1129 |
Oct‐2‐en‐4‐one |
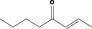
|
Class II A3: Intake below threshold No safety concern |
Class II B3: intake below the threshold B4: Adequate NOAEL exists No safety concern. The chemical name should be changed to oct‐(2E)‐en‐4‐one and the CAS number to 22286‐99‐3 to reflect the stereochemical configuration. Concluded in FGE.63Rev4 |
|
07.099 1134 |
6‐Methylhepta‐3,5‐dien‐2‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
07.100 1119 |
5‐Methylhex‐5‐en‐2‐one |
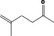
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.101 1131 |
4‐Methylpent‐3‐en‐2‐one |
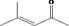
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev2 |
|
07.102 1147 |
Pent‐1‐en‐3‐one |
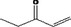
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
07.105 1127 |
Hept‐3‐en‐2‐one |
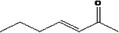
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to hept‐(3E)‐en‐2‐one and the CAS number to 5609‐09‐6. Concluded in FGE.63Rev4 |
|
07.106 1132 |
5‐Methylhex‐3‐en‐2‐one |
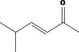
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to 5‐Methylhex‐(3E)‐en‐2‐one and the CAS number to 1821‐29‐0. Concluded in FGE.63Rev4 |
|
07.107 1128 |
Oct‐3‐en‐2‐one |
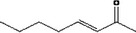
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to Oct‐(3E)‐en‐2‐one and the CAS number to 18402‐82‐9. Concluded in FGE.63Rev4 |
|
07.114 1123 |
6,10,14‐Trimethylpentadeca‐5,9,13‐trien‐2‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.121 1130 |
Dec‐3‐en‐2‐one |
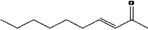
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to Dec‐(3E)‐en‐2‐one and the CAS number to 18402‐84‐1. Concluded in FGE.63Rev4 |
|
07.123 1122 |
Geranylacetone |
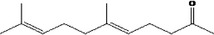
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. The chemical change should be changed to (E)‐geranylacetone Concluded in FGE.63 |
|
07.151 1118 |
Decan‐3‐one |
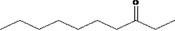
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.177 1135 |
7‐Methyl‐3‐octenone‐2 |
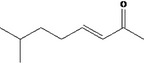
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to 7‐Methyl‐oct‐(3E)‐en‐2‐one and the CAS number to 1004754‐77‐1. Concluded in FGE.63Rev4 |
|
07.188 1136 |
Non‐3‐en‐2‐one |
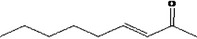
|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. The chemical name should be changed to Non‐(3E)‐en‐2‐one and the CAS number to 18402‐83‐0. Concluded in FGE.63Rev4 |
|
07.190 1848 |
Octa‐1,5‐dien‐3‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
07.240 1156 |
2‐Methylheptan‐3‐one |
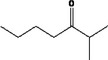
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
07.244 1138 |
(6E)‐Methyl‐3‐hepten‐2‐one |

|
Class II A3: Intake below threshold No safety concern |
Class I A3: Intake below threshold No safety concern. Concluded in FGE.63Rev4 |
|
07.247 1139 |
(E,E)‐3,5‐Octadien‐2‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
07.249 1155 |
Undecan‐6‐one |

|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63 |
|
07.256 1137 |
(E) & (Z)‐4,8‐Dimethyl‐3,7‐nonadiene‐2‐ one |
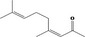
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
|
09.281 1836 |
Oct‐1‐en‐3‐yl acetate |
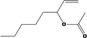
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
09.282 1837 |
Oct‐1‐en‐3‐yl butyrate |
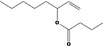
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev3 |
|
09.936 1847 |
4,8‐Dimethyl‐3,7‐nonadien‐2‐yl acetate |
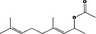
|
Class II A3: Intake below threshold No safety concern |
No safety concern at the estimated level of intake. Concluded in FGE.63Rev1 |
Thresholds of concern: Class I = 1,800 µg/person per day, Class II = 540 µg/person per day, Class III = 90 µg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
EU MSDI: Amount added to food as flavour in (kg/year) × 109/(0.1 × population in Europe (= 375 × 106) × 0.6 × 365) = µg/capita per day.
Refer to Appendix C for MSDI values considered by EFSA based on EU production figures submitted by industry (Documentation provided to EFSA n.: 1 and 3).
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Castle L, Engel K‐H, Fowler PJ, Frutos Fernandez MJ, Fürst P, Gundert‐Remy U, Gürtler R, Husøy T, Manco M, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wölfle D, Wright M, Benigni R, Bolognesi C, Chipman K, Cordelli E, Degen G, Marzin D, Nørby KK, Svendsen C, Vianello G and Mennes W, 2022. Scientific Opinion on Flavouring Group Evaluation 63, Revision 4 (FGE.63Rev4): consideration of aliphatic secondary saturated and unsaturated alcohols, ketones and related esters evaluated by JECFA (59th and 69th meetings) structurally related to flavouring substances evaluated by EFSA in FGE.07Rev6. EFSA Journal 2022;20(2):7102, 46 pp. 10.2903/j.efsa.2022.7102
Requestor: European Commission
Question numbers: EFSA‐Q‐2019‐00478, EFSA‐Q‐2019‐00477, EFSA‐Q‐2019‐00476, EFSA‐Q‐2019‐00475, EFSA‐Q‐2019‐00465, EFSA‐Q‐2019‐00464, EFSA‐Q‐2019‐00463, EFSA‐Q‐2019‐00460, EFSA‐Q‐2019‐00458, EFSA‐Q‐2019‐00456, EFSA‐Q‐2019‐00455, EFSA‐Q‐2019‐00454, EFSA‐Q‐2019‐00453, EFSA‐Q‐2019‐00401
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul J Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle, Matthew Wright and Maged Younes.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch
Acknowledgments: The Panel wishes to thank Vibe Meister Beltoft and Alkiviadis Stagkos‐Georgiadis for the support provided to this scientific output.
*: Member of the EFSA Working Group on Flavourings of the Panel on Food Additives and Flavourings (FAF) until 31 September 2021.
Adopted: 15 December 2021
Notes
This substance, dec‐3‐en‐2‐one, is currently under evaluation as active substance under Regulation (EC) 1107/2009 (EFSA‐Q‐2017‐00813).
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC)No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
As foreseen by the EFSA's Catalogue of support initiatives during the lifecycle of applications for regulated products.
The FAF Panel is aware that a revised Procedure for the Safety Evaluation of Flavouring agents has been agreed by JECFA (JECFA, 2016). The EFSA Scientific Committee has developed a modified procedure for evaluation of substances based on the TTC approach (EFSA Scientific Committee, 2019). However, these developments have no impact on the present evaluation, which should follow the requirements as set out in Commission Regulation (EC) No 1565/2000.
Innocuous products: products that are known or readily predicted to be harmless to humans at the estimated intake of the flavouring agent (JECFA, 1997).
Endogenous substances: intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included (JECFA, 1997).
EU MSDI: Amount added to food as flavour in (kg/year) 9 109/(0.1 9 population in Europe (= 375 9 106) 9 0.6 9 365) = µg/capita per day.
Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
References
- Couri D and Milks M, 1982. Toxicity and metabolism of the neurotoxic hexacarbons n‐hexane, 2‐hexanone, and 2,5‐ hexanedione. Annual Review of Pharmacology and Toxicology, 22, 145–166. [DOI] [PubMed] [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard – a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- EFSA AFC Panel (EFSA panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food) , 2008. Scientific opinion on a request from the Commission related to Flavouring Group Evaluation 63 (FGE.63): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th meeting) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids evaluated by EFSA in FGE.07 (2005).
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2012a. Scientific Opinion on Flavouring Group Evaluation 63, Revision 1 (FGE.63Rev1): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th and 69th meetings) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids evaluated by EFSA in FGE.07Rev4. EFSA Journal 2012;10(10):2900, 37 pp. 10.2903/j.efsa.2012.2900 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2012b. Scientific Opinion on Flavouring Group Evaluation 205, (FGE.205): Consideration of genotoxicity data on representatives for 13 α, βunsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup 1.2.2 of FGE.19 by EFSA. EFSA Journal 2012;10(10):2902, 22 pp. 10.2903/j.efsa.2012.2902 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2013. Scientific Opinion on Flavouring Group Evaluation 63, Revision 2 (FGE.63Rev2): Consideration of aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th and 69th meetings) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids evaluated by EFSA in FGE.07Rev4. EFSA Journal 2013;11(4):3188, 45 pp. 10.2903/j.efsa.2013.3188 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015. Scientific opinion on Flavouring Group Evaluation 25, Revision 3 (FGE.25Rev3): Aliphatic hydrocarbons from chemical group 311. EFSA Journal 2015;13(4):4069, 45 pp. 10.2903/j.efsa.2015.4069 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2016a. Scientific Opinion on Flavouring Group Evaluation 63, Revision 3 (FGE.63Rev3): aliphatic secondary alcohols, ketones and related esters evaluated by JECFA (59th and 69th meetings) structurally related to saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids evaluated by EFSA in FGE.07Rev4. EFSA Journal 2017;15(1):4662, 41 pp. 10.2903/j.efsa.2017.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2016b. Scientific opinion of Flavouring Group Evaluation 205 Revision 1 (FGE.205Rev1): consideration of genotoxicity data on representatives for 13 a,b‐unsaturated aliphatic ketones with terminal double bonds and precursors from chemical subgroup1.2.2 of FGE.19. EFSA Journal 2016;14(7):4535, 23 pp. 10.2903/j.efsa.2016.4535 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2017. Scientific Opinion on Flavouring Group Evaluation 7, Revision 5 (FGE.07Rev5): saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched‐chain carboxylic acids from chemical group 5. EFSA Journal 2017;15(3):4725, 81 pp. 10.2903/j.efsa.2017.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , 2019. Scientific Opinion on Flavouring Group Evaluation 204 Revision 1 (FGE.204Rev1): consideration of genotoxicity data on representatives for 17 monounsaturated, aliphatic, a,b‐unsaturated ketones and precursors from chemical subgroup 1.2.1 of FGE.19. EFSA Journal 2019;17(7):5750, 26 pp. 10.2903/j.efsa.2019.5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , 2022. Scientific Opinion on Flavouring Group Evaluation 7, Revision 6 (FGE.07Rev6): saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5. EFSA Journal 2022;20(1):7090, 63 pp. 10.2903/j.efsa.2022.7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2019. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO, WHO Expert Committee on Food Additives) , 1996. Toxicological evaluation of certain food additives. The forty‐fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17‐26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2005. Evaluation of certain food additives and flavouring agents. Sixty‐fifth report of the Joint FAO/WHO Expert committee on Food Additives, WHO Technical Report Series, no. 56, 2005, Geneva, Switzerland.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2002a. Compendium of food additive specifications. Addendum 10. Joint FAO/WHO Expert Committee of Food Additives 59th session. Geneva, 4-13 June 2002. FAO Food and Nutrition paper 52 Addition 10.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 2002b. Evaluation of certain food additives. Fifty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 913. Geneva, 4‐13 June 2002.
- Sanz P, Flores CI, Soriano T, Repetto G and Repetto M, 1995. In vitro quantitative structure‐activity relationship assessment of pyrrole adducts production by γ‐diketone‐forming neurotoxic solvents. National Institute of Toxicology, Seville, Spain. Toxicology in Vitro, 9, 783–787. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Shearson CM, Wongmongkolrit T, Medori R and Gambetti P, 1986. Structural basis of gamma‐diketone neurotoxicity: Non‐neurotoxicity of 3,3‐dimethyl‐2,5‐hexanedione, a gamma‐diketone incapable of pyrrole formation. Toxicology Applied Pharmacology, 84, 36–44. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) , 1995. First annual report on chemically defined flavouring substances. May 1995, 2nd draft prepared by the SCF Working Group on Flavouring Substances (Submitted by the SCF Secretariat, 17 May 1995). CS/FLAV/FL/140‐Rev2. Annex 6 to Document III/5611/95, European Commission, Directorate‐General III, Industry.
- SCF (Scientific Committee on Food) , 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- Topping DC, Morgott DA, David RM and O’Donoghue JL, 1994. Ketones. In: Clayton GD and Clayton FE (eds.), Patty’s Industrial Hygiene and Toxicology, 4th Edition. vol. 2C, John Wiley & Sons Inc, New York, pp. 1739–1878. [Google Scholar]