Abstract
Recent reports suggest that glucocorticoids (GCs), which can be synthesized in the oral mucosa, play an important role in cancer development. Therefore, the objectives of this study were to characterize the role of the oral GC system in oral cancer, and determine the effect of black raspberry (BRB) administration on GC modulation during oral cancer chemoprevention. We determined the expression of GC enzymes in various oral cancer cell lines, and investigated the role of the GC inactivating enzyme HSD11B2 on CAL27 oral cancer cells using siRNA mediated knockdown approaches. Using two in vivo models of oral carcinogenesis with 4-nitroquinoline 1-oxide carcinogen on C57Bl/6 mice and F344 rats, we determined the effect of BRB on GC modulation during head and neck squamous cell carcinoma chemoprevention. Our results demonstrate that HSD11B2, which inactivates cortisol to cortisone, is downregulated during oral carcinogenesis in clinical and experimental models. Knockdown of HSD11B2 in oral cancer cells promotes cellular proliferation, invasion and expression of angiogenic biomarkers EGFR and VEGFA. An ethanol extract of BRB increased HSD11B2 expression on oral cancer cells. Dietary administration of 5% BRB increased Hsd11b2 gene and protein expression and reduced the active GC, corticosterone, in cancer-induced mouse tongues. Our results demonstrate that the oral GC system is modulated during oral carcinogenesis, and BRB administration upregulates Hsd11b2 during oral cancer chemoprevention. In conclusion, our findings challenge the use of synthetic GCs in head and neck cancer, and support the use of natural product alternatives that potentially modulate GC metabolism in a manner that supports oral cancer chemoprevention.
We show that the glucocorticoid activating enzyme, HSD11B2, is downregulated during oral carcinogenesis and black raspberry administration restores HSD11B2 expression and reduces active glucocorticoid levels in oral cancer cells.
Graphical Abstract
Graphical Abstract.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the 10 leading forms of cancer, with over 700 000 cases and 350 000 deaths worldwide (1). Typically, HNSCC is caused by tobacco use, alcohol consumption and infections from the human papillomavirus (2). Current treatments, which include surgery, radiotherapy and chemotherapy (3) remain challenging, especially when intervention is performed at late stages of the disease. Although progress has been made in its management, there remains a gap in understanding of the molecular basis of HNSCC. Identifying the underlying mechanisms of HNSCC establishment and growth is pivotal to the design of novel therapies against the disease (4,5).
Glucocorticoids (GCs) are lipid hormones involved in stress response regulation, metabolism and immune homeostasis. Cortisol is the principal GC in humans and is synthesized from cholesterol in the adrenal cortex upon activation by adrenocorticotropic hormone (ACTH). Cytochrome P450 enzymes convert cholesterol to cortisol in a multi-step process. 11β-Hydroxysteroid dehydrogenase 1 (HSD11B1) and 11β-hydroxysteroid dehydrogenase 2 (HSD11B2) catalyze the interconversion of cortisol and the biologically inactive cortisone (6). HSD11B1 is responsible for the conversion of inactive cortisone (11-dehydrocorticosterone in mice) to active cortisol (corticosterone in mice), while HSD11B2 inactivates cortisol to cortisone. Although the adrenal gland is the primary source of GC, other tissues—including the thymus, brain, prostate gland and skin—have also been shown to be involved in the modulation of GCs (7). Notably, reports show that the oral mucosa can produce and regulate the local concentration of GC (8). However, there is little information on how modulation of GC signaling affects HNSCC development, growth and metastasis.
A number of reports demonstrate that HSD11B2, which drives the conversion of cortisol to cortisone, is downregulated in various forms of cancers such as squamous cell carcinoma of the skin (9) and colorectal cancer (10). Such findings suggest that cortisol levels are elevated in these cancers. In malignant colon cancer cells, cortisol production correlates with downregulated antitumor T-cell activity (11). GCs have also been shown to promote chemoresistance in breast cancer cells (12). In HNSCC cells, GCs have been found to increase the resistance to 5-fluorouracil and doxorubicin (13). Additionally, a positive correlation has been observed between decreased HSD11B2 expression, increased GC levels and progression of HNSCC (8,9,14). These studies highlight the critical role of HSD11B2, the key enzyme in the inactivation of active GC, as an important regulator of HNSCC tumor progression.
In addition to tissue-specific de novo GC synthesis, synthetic GCs are routinely used in the treatment of diseases such as cancer, either as a palliative agent to reduce the common side effects of chemotherapy or in combination with other chemotherapeutic drugs as the main line of treatment. For example, dexamethasone is widely used for breast cancer, acute lymphoblastic leukemia, chronic lymphocytic leukemia, multiple myeloma, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma treatments (15). Additionally, topical GCs are used in the treatment of oral inflammatory diseases such as oral lichen planus, which is a risk factor for HNSCC (8,16). The activity of GCs is mediated by binding to the glucocorticoid receptor (GR), and subsequent translocation to the nucleus, where they modulate the expression of their target genes (17). Therefore, the intracellular concentration of active GCs and GR in the target tissues determines the potential activity of the GC system (6). The anti-inflammatory and immunosuppressive properties of GCs make them attractive candidates for treatment of certain hematologic cancers, cancers potentiated by chronic inflammation and mitigation of pain and other symptoms associated with cancer chemotherapy and associated cancer pathologies (18). However, the ability of GCs to inhibit antitumor CD8+ T-cell responses calls into question their utility in the management of cancers such as HNSCC which require a potent antitumor immune response for disease resolution (9). Consequently, there is a need for alternative anti-inflammatory agents that also reduce tumor promoting GC levels in the oral mucosa.
Preclinical studies have demonstrated the remarkable ability of black raspberry (BRB) (Rubus occidentalis) to inhibit cancers of the oral cavity, esophagus and colon (19–23). We and others have demonstrated that the chemopreventive properties of BRB are mediated in part by modulation of inflammatory, apoptotic, angiogenic and cell cycle related pathways which are critical for oral cancer initiation, promotion and progression (19,20,24,25). Extensive clinical studies further demonstrate the efficacy of BRB phytochemicals in HNSCC chemoprevention (19,26). BRBs possess a wide array of bioactive phytochemicals such as anthocyanins, ellagitannins, ellagic acid and others which may act in an additive or synergistic manner to inhibit cancer development. However, the mechanisms that mediate the chemopreventive properties of the wide array of bioactive phytochemicals in BRB remain to be fully explored. These studies are essential in order to fully exploit BRB phytochemicals in HNSCC chemoprevention.
The focus of the present study is to determine (i) the impact of local (oral) GC system on oral carcinogenesis and (ii) the effects of BRB on the GC system during oral cancer chemoprevention. Our studies reveal that the GC inactivating enzyme, HSD11B2, is downregulated during oral carcinogenesis and administration of BRB restores HSD11B2 expression and reduces active GC levels during oral cancer chemoprevention. Ultimately, our results indicate that regulation of HSD11B2 affects HNSCC progression, and the impact of BRB on the oral GC system demonstrates promise for future therapeutic applications against HNSCC.
Materials and methods
Animal handling
Six-week female C57BL/6 mice were purchased from Jackson Laboratories. Animals were kept in accordance with regulations maintained by University Laboratory Animal Resources and were approved by the Institutional Animal Care and Use Committee (Protocol #2018A00000054) and Institutional Biosafety Committee of The Ohio State University.
Food and chemicals
BRB used in this feed was purchased from the Stokes Berry Farm (Wilmington, OH) before being shipped to Van Drunen Farms (Momence, IL) for freeze drying. Standard AIN-76A and its special formulations were prepared by Dyets (Bethlehem, PA) and stored at −20°C. Nutrient analysis of the BRB diet used in this study has been published previously, and matches expected levels (21,24). The carcinogen 4-nitroquinoline 1-oxide (4NQO) was purchased from Sigma–Aldrich (St. Louis, MO; #N8141) and stored at room temperature away from light. Fresh 4NQO solution was prepared weekly.
BRB extract preparation
BRB powder was extracted three times with 80:19:1 ethanol/water/formic acid, sonicated for 10 min in an ice bath, and vacuum filtered. The extract resulting from this extraction was concentrated on a rotary evaporator then lyophilized to remove any remaining water. This procedure yielded 40 g extract/100 g BRB powder and an anthocyanin content of 57 mg cyanidin-3-glucoside equivalents/g of extract. Our analysis of the anthocyanin content in the BRB extract (BRB-E) was performed according to protocols described previously by Giusti et al. (27).
Oral carcinogenesis and chemoprevention
Rat oral carcinogenesis and chemoprevention was performed as described previously (24). For mouse oral carcinogenesis, mice were administered 4NQO (100 µg/ml in drinking water) for 16 weeks then regular water for 8 weeks (28). BRB chemoprevention mouse groups were placed on a 5% BRB supplemented diet at 1 week prior to 4NQO exposure and until terminal sacrifice.
Cell culture
Authenticated human oral cancer cell lines CAL27, CA83, SCC83, SCC15, UMSCC6, UMSCC22A and the normal tongue epithelial cell line TE1177 were kindly provided by Dr. Weghorst and Dr. Mitchell, and grown in Dulbecco’s modified Eagle’s medium/F12 media supplemented with 10% fetal bovine serum and 1% Pen Strep (Life Technologies, Waltham, MA) and specific growth factors. Cells were maintained at 5% CO2 at 37°C. Cells were seeded at a density of 0.6 × 105 cells per well in 6-well dishes. After overnight incubation, the cells were treated with indicated concentrations of agents in fresh growth medium (without fetal bovine serum) for different times.
HSD11B2 siRNA knockdown
Cells were reverse transfected with siRNA (10 nM) targeting HSD11B2 (ID-s531876) or scrambled vector (ID-4390843, Life Technologies, Waltham, MA). Lipofectamine RNAiMax (Life Technologies, Waltham, MA) was used to transfect 0.7 × 105 cells/ml/well for 48 h. Confirmation of siRNA knockdown was performed by RT–qPCR.
Western blotting
Extracted proteins were loaded on 10% Tris–HCl gels and then transferred onto polyvinylidene difluoride membranes (0.2 µm). Blots were blocked in 5% skim milk for 1 h and incubated in primary antibodies overnight followed by incubation with peroxidase conjugated secondary antibodies for 1 h. Chemiluminescence was detected by ECL Western blotting substrate (ThermoScientific, Waltham, MA). HSD11B2 Rabbit pAb (Proteintech, Rosemont, IL); HSD11B1 Rabbit pAb (Invitrogen, Carlsbad, CA) and β-actin Rabbit mAb (Cell Signaling Technologies, Danvers, MA) were used to determine expression of proteins.
Cortisol/corticosterone ELISA
Cortisol and corticosterone competitive ELISA were performed per manufacturer’s protocol (R&D Systems, Minneapolis, MN). The supernatant from the test samples were collected and added to a goat anti-mouse pAb precoated microtiter plate. 50 µl of cortisol or corticosterone conjugate and the primary antibody were added to the wells. Plates were washed and incubated with substrate solution. Readings were taken at 450 and 540 nm.
RT–qPCR
High Capacity cDNA Kit (Applied Biosystems, CA) was used for cDNA synthesis of extracted total RNA. PCR amplification was performed with PowerUp SYBR green PCR master mix (Bio-Rad, Hercules, CA) in the CFX384 Real-Time System (Bio-Rad). Primers were designed for BCL2, HSD11B2, HSD11B1, CYP11A1, CYP11B1, HSD3B, CYP21A2, VEGFA, EGFR, PCNA and CDKNA1. ACTB and GAPDH were used as reference genes.
Cell proliferation assay
Alamar Blue (Bio-Rad, Hercules, CA) was used to assess cell proliferation. A final concentration of 10% Alamar Blue solution was added to the cells post treatment. Absorbance was measured at 570 and 600 nm wavelength, respectively (Spectra MAX 190).
Scratch assay
Confluent monolayers of siRNA-treated CAL27 and CA83 (after 48 h) were linearly scratched with a 200 µl tip to create cell-free areas (29). Images were captured at 0 and 24 h to determine the migratory capacity of the treated cells. Images were captured under an inverted bright field microscope at ×10 magnification with the reference mark outside the field of view. Percentage of migration was quantified by CaptaVision+™ software.
Cytotoxicity assay
Cytotoxicity assay was conducted using the LDH Cytotoxicity kit in accordance with the manufacturer’s protocol (Takara, San Jose, CA).10 000 cells/well were seeded in triplicate in a 96 well plate and incubated overnight at 37°C. Cells were treated with the appropriate test or control agents and incubated for 24 h. LDH assay reagent was added to culture supernatants and incubated for 30 min in the dark, and absorbance was measured at 490 and 680 nm.
Immunohistochemistry
Animal tongue tissues were fixed in 10% neutral buffered formalin and paraffinized. Immunostaining was performed using HSD11B2 Rabbit pAb (Proteintech, Rosemont, IL); HSD11B1 Rabbit pAb (Invitrogen, Carlsbad, CA); β-actin Rabbit Ab (Cell Signaling Technologies, Danvers, MA). After secondary antibody incubation, DAB peroxidase kit (Vector Laboratories, Burlingame, CA) was used to detect for the presence of proteins. Analysis of HSD11B1 and HSD11B2 was performed using the FIJI package in ImageJ (http://rsbweb.nih.gov/ij/) to generate a digital histological score as described (30).
Statistical analysis
Statistical analysis was done using Prism (GraphPad software, San Diego, CA). The statistical comparisons were performed using either one-way analysis of variance or Student’s t-test. *P value ≤0.05; **P value <0.01; ***P value <0.005.
Results
De novo synthesis of GCs in HNSCC cells
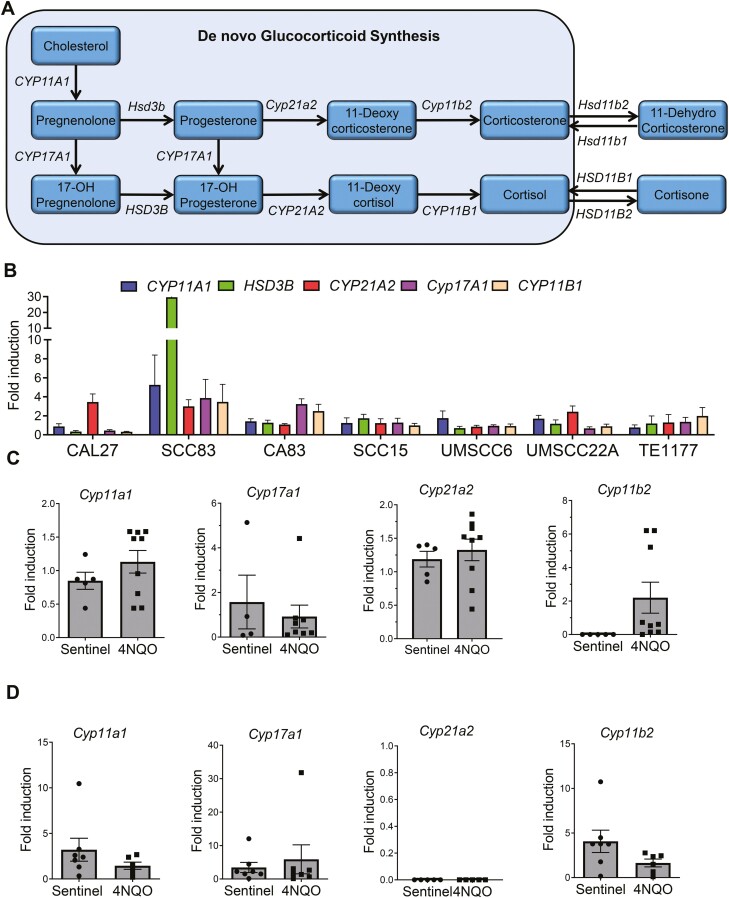
ACTH triggers GC synthesis de novo from cholesterol. The cytochrome P450 enzymes CYP11A1, CYP17A1, HSD3B, CYP21A2, CYP11B1 and CYP11B2 catalyze the synthesis of the active GC cortisol (humans) or corticosterone (mice) (Figure 1A). We therefore determined the ability of human HNSCC cells to synthesize cortisol de novo. The HNSCC cell lines CAL27, SCC83, CA83, SCC15, UMSCC6, UMSCC22A and the normal oral epithelial cell, TE1177 were tested for expression of these enzymes after exogenous administration of ACTH for 24 h. It was observed that all the cell lines were capable of expressing the various enzymes involved in the de novo pathway at variable levels (Figure 1B). Next, we investigated potential changes in enzymes involved in de novo GC synthesis during oral carcinogenesis. F344 rats and C57BL/6 mice were exposed to 4NQO using established oral carcinogenesis protocols (24). Expression of de novo GC synthesis enzymes was determined by RT–qPCR. As observed in vitro, no significant changes in the enzyme levels of Cyp11a1, Cyp17b1, Cyp11b2 and Cyp21a2 were observed in 4NQO-exposed groups (Figure 1C and D). Taken together these results suggests that although the de novo GC synthesis pathway is viable in oral cancers cells, the enzymes associated with this pathway (Cyp11a1, Cyp11b2, Cyp21a2, Cyp17b1 and Hsd3b) are not severely altered during HNSCC.
Figure 1.
De novo synthesis of GCs in HNSCC cells. (A) De novo biosynthesis of cortisol/corticosterone from cholesterol. (B) Gene expression analysis of CYP11A1, HSD3B, CYP21A2, CYP17A1 and CYP11B1 in CAL27, SCC83, CA83, SCC15, UMSCC6, UMSCC22A oral cancer cell lines and TE1177 normal oral epithelial cell line, induced with ACTH (10 nM) for 24 h (N = 4 per group each from two independent experiments). Gene expression profile of the enzymes from the de novo synthesis pathway—Cyp11a1, Cyp21a2, Cyp17a1 and Cyp11b1 from 4NQO-exposed or control (C) F344 rats (N = 5–9 per group) and (D) C57BL/6 mice (N = 5–7 per group). Data are presented as mean ± SE.
Characterization of hydroxysteroid dehydrogenases expression and GC interconversion in HNSCC cell lines
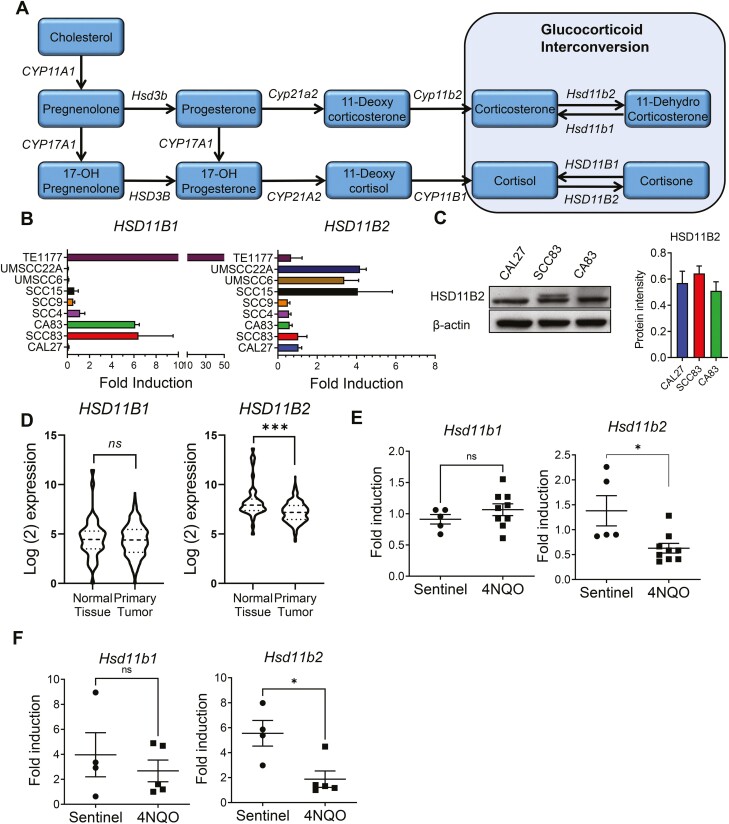
Given that we did not observe significant differences in the enzymes involved in the de novo pathway between normal and cancer oral epithelial cells, we focused on the local interconversion of GCs. Cortisone and 11-dehydrocorticosterone are the inactive forms of GC while cortisol and corticosterone are the active forms in humans and mice, respectively. The interconversion between the active cortisol to the inactive cortisone mediated by HSD11B2 while the reverse reaction from cortisone to cortisol is mediated by HSD11B1 (Figure 2A). We first analyzed gene expression of HSD11B2 and HSD11B1 in our panel of human HNSCC and normal oral epithelial cell lines. We found that the HNSCC cell lines expressed HSD11B1 in low amounts whereas HSD11B2 was present in relatively higher yet at varying levels (Figure 2B). Gene expression of HSD11B2 was corroborated by expression of the HSD11B2 protein in CAL27, SCC83 and CA83 cells (Figure 2C and Supplementary Figure 1A, available at Carcinogenesis Online). Given that CAL27 cells expressed sufficient and quantifiable amount of HSD11B2, we used this cell line for our further experiments. We next determined whether cortisol affects HSD11B2 levels in CAL27 cells. To do this, we incubated CAL27 cells with cortisol or cortisone for 24 and 48 h. We observed that the presence of cortisol or cortisone did not have significant effects on HSD11B2 gene and protein expression levels (Supplementary Figure 1B and C, available at Carcinogenesis Online).
Figure 2.
Characterization of hydroxysteroid dehydrogenases expression and GC interconversion during oral carcinogenesis. (A) Representation of the interconversion between cortisol and cortisone by HSD11B1 and HSD11B2. (B) RNA level expression of HSD11B1 and HSD11B2 in CAL27, SCC83, CA83, SCC4, SCC9, SCC15, UMSCC6, UMSCC22A oral cancer cell lines and TE1177 normal oral epithelial cell line (N = 4 per group each from two independent experiments). (C) Representative western blot showing the expression of HSD11B2 in CAL27, SCC83 and CA83 oral cancer cells along with quantification of the bands for HSD11B2 (N = 3 per group from two independent experiments). (D) mRNA normalized counts of HSD11B1 and HSD11B2 in tumors and adjacent normal tissues of 550 clinical oral cancer patients from TCGA data. Gene expression of Hsd11b1 and Hsd11b2 in tongues of 4NQO-exposed (E) F344 rats (N = 5–9 per group) and (F) C57BL/6 mice (N = 5–7 per group). Data are presented as mean ± SE *P < 0.05 and ***P<0.001, respectively.
HSD11B2 is downregulated during oral carcinogenesis in vivo
The expression of HSD11B2 is diminished in various cancers such as squamous cell carcinoma of the skin (9), colorectal cancer (10) and ovarian cancer (31). Therefore, we analyzed HNSCC patients from The Cancer Genome Atlas (TCGA) database. Our analysis of 550 oral cancer patients from TCGA demonstrates that unlike HSD11B1 which has comparable expression between tumors and adjacent normal tissues (P = 0.565, Welch’s t-test), HSD11B2 expression is significantly reduced in tumors compared with adjacent normal tissues (P = 0.0001, Welch’s t-test) (Figure 2D). These data corroborate recent findings from Cirillo et al. (9) demonstrating significantly reduced HSD11B2 in patients with oral squamous cell carcinoma. Similarly, during experimental 4NQO-induced oral cancer, gene expression of Hsd11b2 was significantly downregulated in tongue tissue of carcinogen-exposed rats and mice, compared with non-carcinogen-induced control animals (Figure 2E and F). No notable difference was present in the relative expression of Hsd11b1 (Figure 2E and F). Together, these results suggest that Hsd11b2 expression levels are down regulated during HNSCC in vivo.
Attenuation of HSD11B2 promotes tumor growth and invasion
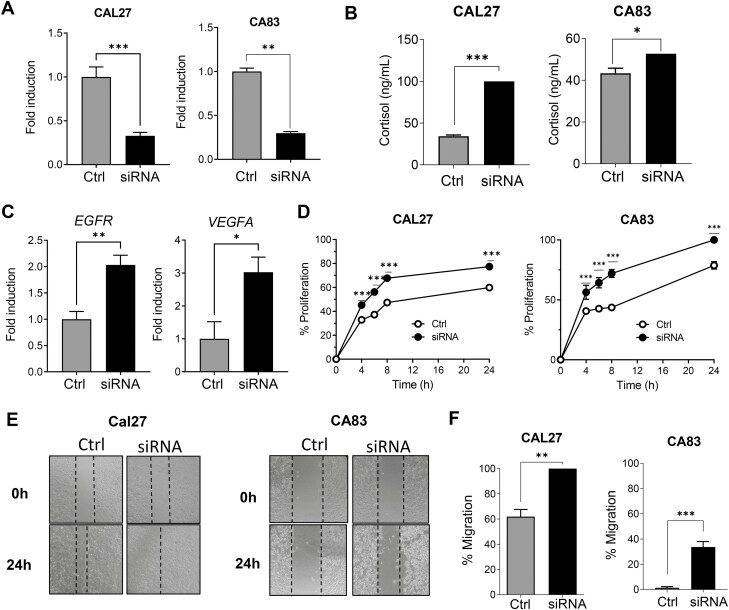
In order to confirm the role of HSD11B2 on tumor cell malignancy, we transiently silenced the HSD11B2 gene in CAL27 and CA83 cells using HSD11B2 siRNA. siRNA mediated silencing was confirmed by RNA expression (Figure 3A) A second siRNA construct was used to confirm efficient HSD11B2 knockdown (Supplementary Figure 2A, available at Carcinogenesis Online). Additionally, cortisol levels in the siRNA-treated HNSCC cells were significantly increased compared with cells treated with scrambled vector, confirming that knockdown of HSD11B2 results in increased cortisol levels in HNSCC cells (Figure 3B). We found that the genes EGFR and VEGFA which play a role in the proliferation and angiogenesis of cancer cells, respectively, were significantly upregulated in the siRNA-treated CAL27 cells when compared with the cells treated with scrambled vector (Figure 3C and Supplementary Figure 2B, available at Carcinogenesis Online). We next investigated the functional effects of HSD11B2 knockdown on key hallmark features of HNSCC cancer cells CAL27 and CA83 such as proliferation and migration. Analysis of cell proliferation by Alamar Blue, indicated that HSD11B2 knockdown increased the rates of proliferation over time compared with CAL27 and CA83 cells treated with scrambled vector (Figure 3D and Supplementary Figure 2C, available at Carcinogenesis Online). After 48 h of HSD11B2 knockdown, a scratch assay was used to assess the migratory capacity of CAL27 and CA83 HNSCC cells. We observed that knockdown of HSD11B2 increased HNSCC cell migration than the scramble mRNA-treated cells (Figure 3E and F and Supplementary Figure 2D, available at Carcinogenesis Online). Taken together these results show that inhibition of HSD11B2 increases cortisol levels and promotes the tumorigenic abilities of cancer cells.
Figure 3.
Attenuation of HSD11B2 promotes tumor growth and invasion. (A) Confirmation of HSD11B2 knockdown in siRNA-treated CAL27 and CA83 cells by RT–qPCR. (B) Quantification of cortisol in siRNA-treated CAL27 and CA83 cells by ELISA. (C) Gene expression analyses of Egfr, VegfA in scrambled control or siRNA-treated CAL27 cells. (D) Percentage of proliferation of the siRNA-treated CAL27 and CA83 cells as assessed by Alamar Blue assay. (E) Representative images of in vitro scratch assay upon treatment of CAL27 and CA83 cells with siRNA showing time dependent gap closure. (F) Plot of percent migration. For all experiments, error bars represent standard error of the mean (SEM) from n = 5 biological samples per group each from two independent experiments; *P < 0.05, **P < 0.01 and ***P < 0.005 for comparisons between control and siRNA-treated cells using Student’s t-test.
BRB-E enhances HSD11B2 expression and decreases tumorigenic properties in HNSCC cells
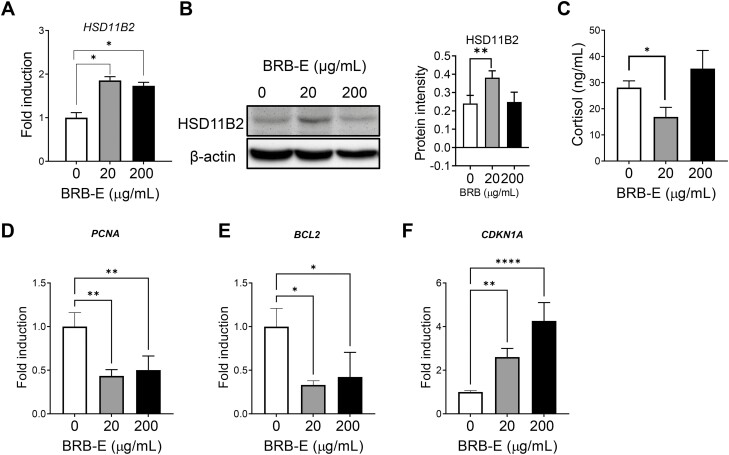
Previous studies by our group and others demonstrate the ability of BRB in inhibiting lesion growth in HNSCC (24). In order to determine if BRB exerts its anticancer effects by modulating the GC system in cancer cells, we tested its effect on the HSD11B2 expression. We tested an ethanol BRB-E on CAL27 cells to determine the optimal non-lethal range. Non-lethal concentrations of 20 and 200 µg/ml (Supplementary Figure 3A, available at Carcinogenesis Online) were chosen for further analysis, which was also shown by previous studies (32,33). After 24 h of treatment with the chosen concentrations of BRB-E, we found that BRB-E could effectively increase the HSD11B2 mRNA levels at 20 and 200 µg/ml concentrations (Figure 4A). We further confirmed increased HSD11B2 protein expression by BRB-E at 20 µg/ml (Figure 4B). BRB-E also slightly decreased the cortisol levels in CAL27 cells (Figure 4C). These results suggest that BRB-E modulates the GC system by upregulating HSD11B2 and subsequent inhibition of cortisol in HNSCC cells. Next, we investigated if the increase in HSD11B2 is associated with modulation of proliferation and apoptosis in HNSCC cells. We found that BRB-E treatment resulted in a significant decrease in the expression of proliferating cell nuclear antigen (PCNA) which plays a pivotal role in oral cancer proliferation (34) (Figure 4D). BRB-E also significantly decreased the antiapoptotic gene BCL2 thereby contributing to the promotion of cell death in CAL27 HNSCC cells (Figure 4E). There was also a significant increase in the expression of the tumor suppressor gene, CDKN1A in CAL27 cells treated with BRB-E (Figure 4F). These results thus suggest that HNSCC chemoprevention by BRB-E is associated with increased HSD11B2 and subsequent reduction in cortisol levels.
Figure 4.
BRB-E enhances HSD11B2 expression and decreases tumorigenic properties in HNSCC cells. (A) RT–PCR analysis of HSD11B2 in control or BRB-treated samples. (B) Representative western blot image depicting the HSD11B2 protein levels in CAL27 cells treated with BRB-E—20 and 200 µg/ml, along with quantification of HSD11B2 band intensity. (C) Cortisol levels in conditioned media of control or BRB-E-treated CAL27 cells for 24 h as assessed by ELISA. Gene expression analysis of (D) PCNA, (E) BCL2 and (F) CDK1A in CAL27 cells treated with different concentrations of BRB-E for 24 h. All data are presented as mean ± SE from two independent experiments (N = 6 samples per group). *P < 0.05, **P < 0.01 and ****P < 0.0001 for comparisons between control, 20 and 200 µg/ml of BRB-E.
BRB regulates the GC pathway in 4NQO-induced oral carcinogenesis in vivo
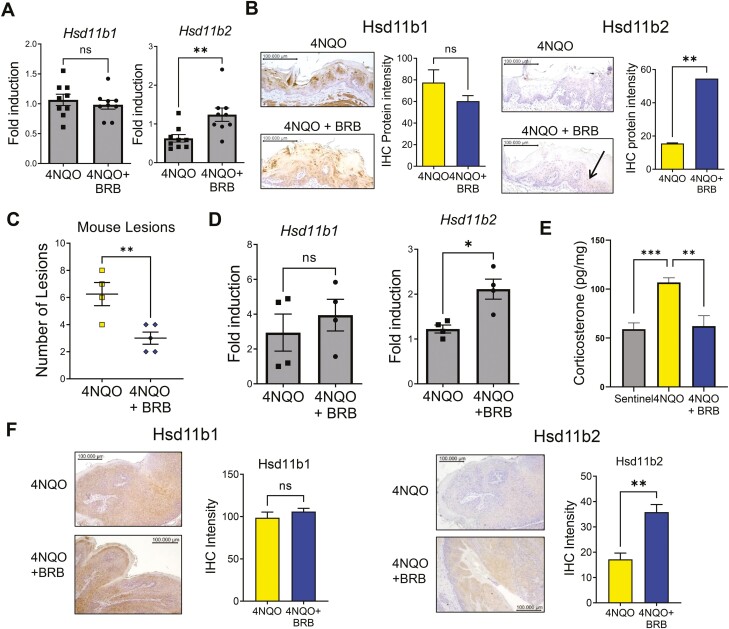
Previous studies from our laboratory analyzed urine samples from carcinogen-exposed rats fed either control diet, or diets supplemented with 5% BRB. Predicted pathway activity profiles based on the pathway analysis of our LC–MS data as determined by Mummichog (MetaboAnalyst) revealed that steroid hormone biosynthesis was the most significantly modulated metabolic pathway (P < 0.0005) (20). These data further support our hypothesis that BRB modulates active GC levels during oral carcinogenesis. To confirm our in vitro data and more clearly define the impact of BRB administration in in vivo oral cancer models, we further analyzed tongue samples from control or 4NQO-induced rats fed with regular diet or BRB supplemented diet in our previously conducted chemoprevention study (24). First, we analyzed the expression of genes involved in GC biosynthesis and interconversion in tongue tissues of the various rat groups. Gene expression levels of Cyp11a1, Cyp11b1 and Cyp11b2 were not significantly altered by administration of BRB (Supplementary Figure 3C, available at Carcinogenesis Online). Expression of Hsd11b1 was unchanged, but Hsd11b2, which was downregulated in 4NQO-exposed rats fed normal diet, were restored to physiological levels in 4NQO-exposed rats fed 5% BRB supplemented diets (Figure 5A). Immunohistochemical analysis of the tongue lesions further showed that BRB diets significantly increased the protein expression of Hsd11b2 but not Hsd11b1 in the 4NQO-exposed rats fed BRB diet compared with 4NQO-exposed rats fed normal diet (Figure 5B).
Figure 5.
BRB regulates the GC pathway in 4NQO-induced oral carcinogenesis in vivo: (A) Gene expression profiles of Hsd11b1 and Hsd11b2 in 4NQO-exposed rats fed normal diet or 5% BRB diet. (B) Representative histological images of tongue lesions showing HSD11B1 and HSD11B2 from tumor bearing rats, fed control diet or BRB supplemented diet. Graph showing intensity of HSD11B1 and HSD11B2 staining is also shown. (C) Lesion multiplicity in 4NQO-exposed mice fed normal diet or BRB supplemented diet. (D) Gene expression profiles of Hsd11b1 and Hsd11b2 in 4NQO-exposed mice fed normal diet or 5% BRB supplemented diet. (E) Analysis of corticosterone levels from non-4NQO-exposed or 4NQO-exposed mice fed normal diet or BRB supplemented diet. (F) Representative histological images of tongue lesions showing HSD11B1 and HSD11B2 from tumor bearing mice, treated with control diet or BRB diet. Graph showing intensity of HSD11B1 and HSD11B2 staining is also shown. All images were taken at ×200 magnification. Quantification of graphical images were taken from at least three fields with N = 3–5 animals per group. *P < 0.05, **P < 0.01 and ***P < 0.005 for comparisons between 4NQO and 4NQO + BRB-treated group.
Next, to confirm results with our rat model, we analyzed effects of BRB administration in a mouse model of oral cancer chemoprevention. Administration of a 5% BRB diet to 4NQO carcinogen-induced mice resulted in a moderate but significant reduction in tumor lesion incidence and multiplicity (Figure 5C) Gene expression and immunohistochemical analysis demonstrated a significant increase in Hsd11b2 but not Hsd11b1 levels of 4NQO-exposed mice fed BRB supplemented diet compared with 4NQO-exposed mice fed control diet (Figure 5D and E). Similar to our rat chemoprevention model, gene expression levels of Cyp11a1, Cyp11b1 and Cyp11b2 remained unchanged (Supplementary Figure 3D, available at Carcinogenesis Online). In further support of BRB mediated modulation of Hsd11b2, the increased corticosterone levels observed in 4NQO-exposed mice fed control diet was reduced in 4NQO-exposed mice fed 5% BRB diet (Figure 5F). In summary, our data suggest that BRB chemoprevention of HNSCC is associated with a reduction in active GC levels in the oral mucosa, mediated by increased levels of HSD11B2.
Discussion
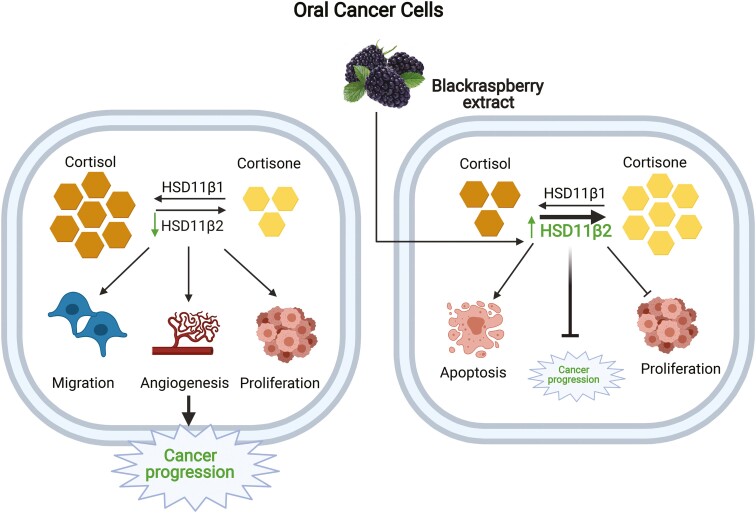
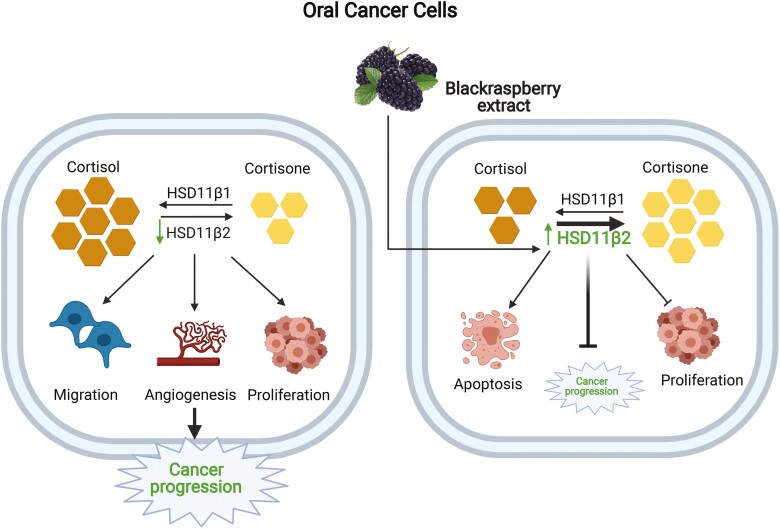
Our study demonstrates the role of oral GC system in the progression of HNSCC malignancy. In particular, it reveals how the expression of HSD11B2 is altered in HNSCC tumor cells, and is linked to the enhancement of tumorigenic properties. The key observations made in this study are (i) HSD11B2 is downregulated during oral cancer progression, resulting in increased cortisol levels; (ii) knockdown of HSD11B2 promotes increased tumorigenic properties of HNSCC cancer cells and (iii) BRB mediated chemoprevention of HNSCC is associated with increased HSD11B2 levels and concomitant reduction of active GC levels in vitro and in vivo. Our proposed model based on our data on the effects of active GC on oral carcinogenesis and mechanisms underlying BRB mediated modulation of GC during oral cancer chemoprevention is shown in Figure 6.
Figure 6.
Graphical illustration of the effect of BRB on GC system during oral carcinogenesis: Low expression of HSD11B2 results in increased cortisol levels during oral carcinogenesis, which drives cancer cell proliferation and invasive potential. BRB administration promotes the upregulation of HSD11B2 which inactivates active cortisol to cortisone, thereby diminishing oral cancer progression.
Our finding of downregulation HSD11B2 in HNSCC cells is supported by previous studies on reports (31,35). We observed that different HNSCC cell lines displayed different levels of HSD11B2 expression, suggesting that the involvement of HSD11B2 and the GC pathway on tumor development might vary, depending on the molecular landscape and mutational characteristics of the specific HNSCC. Together with our analysis of HSD11B2 expression in clinical HNSCC patients from TCGA data, we conclude that Hsd11b2 expression is inversely correlated with tumor development in preclinical and clinical HNSCC.
To clearly delineate the impact of reduced levels of HSD11B2 in HNSCC cells, we transiently silenced the gene using siRNA. CAL27 cells deficient in HSD11B2 demonstrate increased ability to proliferate and migrate which could be prompted by the elevated expression of EGFR and VEGFA. siRNA mediated silencing of CA83 cells showed similar effects as in CAL27 cells. Additionally, the cortisol levels were notably higher following HSD11B2 silencing in CAL27 and CA83 cells confirming that low levels of HSD11B2 drives cancer malignancy. Taken together, our results demonstrate that HSD11B2 plays a critical role in modulating active GC levels, and subsequent tumorigenic properties in HNSCC cells. This also suggests that chemopreventive or therapeutic approaches that increase HSD11B2 levels and reduce active GC in the HNSCC tumor microenvironment can potentially improve HNSCC tumor outcomes.
Antitumor T-cell mediated responses play a vital role in restricting tumor growth in HNSCC (36,37). Previous work showed that tumor-derived GCs could suppress T-cell activation which in turn could lead to tumor progression (9,11). Another recent report further showed that active GC in the tumor microenvironment negatively regulates CD8+ T-cell effector differentiation and leads to the development of dysfunctional CD8+ tumor infiltrating lymphocytes (38). Mechanistically, GC signaling in CD8+ T cells promotes the expression of checkpoint receptor expression including PD-1, TIM3 and LAG3, thereby promoting T-cell exhaustion. This suggests that increasing localized cortisol levels in the tumor microenvironment by downregulation of HSD11B2 is an immune evasion mechanism exploited by HNSCC cells to inhibit antitumor immune responses. Given that T cells express the receptor for active GC (NR3C1), future work will determine whether blocking this interaction will inhibit the immunosuppressive effect exerted by HNSCC cells on antitumor infiltrating CD8+ T cells. Further, the use of inhibitors of GC signaling could potentiate the efficacy of checkpoint inhibitors in HNSCC treatment.
It should be noted that some reports demonstrate an opposite effect of HSD11B2 in carcinogenesis, where the increased levels of HSD11B2 promote proliferation and tumor progression as a result of reduced GCs (31,39). In ovarian cancer, the anti-inflammatory effect of cortisol in patients with low HSD11B2 activity was demonstrated to have a cancer inhibitory effect, while decreased cortisol levels due to increased HSD11B2 activity was suggested to play a role in tumor proliferation (31). Another recent report suggests that HSD11B2 expression promotes the invasiveness and metastasis of colorectal cancer cells in vitro and in vivo (39). These inconsistencies with other studies could be due to the distinct molecular mechanisms that govern malignant transformation in various types of cancer. It should be noted that while Chen et al. (39) overexpressed HSD11B2 in their cells while we knocked down HSD11B2 using siRNA approaches. However, these studies highlight the importance of additional work that clearly defines the role of GC enzymes in cancer cell development, metastasis and antitumor immune responses.
The use of GC in patients with oral premalignancies is common practice (15,40). Indeed, topical corticosteroids (such as the potent and long-acting GC dexamethasone) are considered as first-line therapy in the treatment of oral premalignant lesions for symptomatic control. Specifically, GC is used in (i) patients with the inflammatory oral disease oral lichen planus (which affects 1–2% of the world population and has a propensity to progress to fully evolved oral squamous cell carcinoma in up to 5% of cases) (41,42), (ii) oral cancer patients that have been treated with chemotherapeutic drugs to address side effects such as fatigue, weight loss and vomiting (15,43) or (iii) the mitigation of pain and other symptoms associated with oral cancer pathology. Results from this study and other recent reports, challenge the existing paradigm on the use of synthetic GCs in the management of HNSCC and other solid tumors (44,45).
Previous studies by our group have successfully established the chemopreventive effect of BRB in the rat 4NQO oral cancer model (24). It was shown that reduction of oral lesions in rats fed BRB correlated with a decrease in proinflammatory and antiapoptotic markers. Further, LC–MS analysis of urine samples from this cohort indicated that BRB administration significantly modulated the steroid metabolism pathway during HNSCC chemoprevention (20). This led us to further investigate the effect of BRB on enzymes and metabolites involved in GC synthesis and interconversion during HNSCC chemoprevention. Our results reveal specific modulation of the GC inactivating enzyme HSD11B2, by BRB phytochemicals. Specifically, BRB significantly increased the HSD11B2 levels in CAL27 HNSCC cells, with a concomitant reduction in cortisol levels. In these cells, we also observed a significant decrease in the expression of the proliferation and antiapoptotic biomarkers. While these results do not necessarily indicate that anticancer effects of BRB phytochemicals are mediated by modulation of HSD11B2, it does present that possibility. We are currently investigating whether modulation of HSD11B2 is a major mechanism of BRB mediated HNSCC chemoprevention.
Our in vitro results on BRB mediated modulation of the GC pathway were further corroborated in vivo by dietary BRB administration on 4NQO carcinogen-induced HNSCC animal models. In our previously conducted rat study (24), our immunohistochemical analyses of tongue sections show that dietary BRB administration significantly increases the Hsd11b2 levels in the carcinogen-induced animals. These results can be correlated to the reduction in antiapoptotic and cell cycle associated markers with BRB administration (24). We also observed significant increases in Hsd11b2 protein in tongues of carcinogen-induced mice fed with a BRB supplemented diet compared with carcinogen-induced mice fed control diet as detected by immunohistochemistry. There was also a concomitant decrease in the corticosterone levels in our mouse oral cancer chemoprevention models.
A number of questions remain regarding the involvement of HSD11B2 in BRB mediated chemoprevention of HNSCC, and the specific bioactive phytochemicals in BRB that modulate the GC pathway. These ongoing studies are essential to the development and application of BRB-derived bioactive phytochemicals for use in HNSCC chemoprevention strategies. The use of natural alternatives that not only reduce inflammation, but also modulate GC metabolism in a manner that reduces tumor development can potentially be applied to clinical HNSCC chemoprevention and treatment. These phytochemicals that upregulate HSD11B2 levels in cancer tissues can potentially improve patient tumor outcomes (35).
In summary, our study provides a comprehensive understanding of the effect of the oral GC system and the impact of its dysregulation in HNSCC. This is the first report showing the effect of BRB phytochemicals on the oral GC system, and HSD11B2 during HNSCC (Figure 6). Potential applications of HSD11B2 as a biomarker for HNSCC malignancy, and chemopreventive efficacy are supported by the results of this work. These findings also challenge the use of synthetic GCs in HNSCC or other cancer management, and support the use of natural product alternatives that potentially modulate GC metabolism in a manner that supports HNSCC chemoprevention.
Supplementary Material
Acknowledgements
We thank Christopher Weghorst and Thomas Knobloch for providing the black raspberry (BRB) powder used for these experiments, and Darrion Mitchell for providing HNSCC cell lines.
Glossary
Abbreviations:
- 4NQO
4-nitroquinoline 1-oxide
- ACTH
adrenocorticotropic hormone;
- BRB
black raspberry
- BRB-E
black raspberry extract
- GC
glucocorticoid
- GR
glucocorticoid receptor
- HSD11B
11β-hydroxysteroid dehydrogenases
Funding
This research was funded by the National Institutes of Health grant numbers K01CA207599 (NCI/NIH) and R56DE030093 (NIDCR/NIH) as well as the American Cancer Society (ACS), grant number RSG-19-079-01-TBG awarded to S.O.
Conflict of Interest Statement
The authors declare no conflict of interest. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors’ contributions
Design of the study: S.O. Experiments: D.N., N.R., K.A., F.L., H.I., P.J. and S.O. Data analysis: D.N., N.R., K.A., H.I., P.J. and S.O. Manuscript writing: D.N., N.R., K.A., F.L., P.J., H.I. and S.O. All authors critically revised and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Bray, F., et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Sinha, P., et al. (2012) Human papillomavirus, smoking, and head and neck cancer. Am. J. Otolaryngol., 33, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang, S.H., et al. (2013) Oral cancer: current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal, 18, e233–e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan, N., et al. (2020) STAT1 inhibits T-cell exhaustion and myeloid derived suppressor cell accumulation to promote antitumor immune responses in head and neck squamous cell carcinoma. Int. J. Cancer, 146, 1717–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson, K., et al. (2019) Immune suppression mediated by STAT4 deficiency promotes lymphatic metastasis in HNSCC. Front. Immunol., 10, 3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomlinson, J.W., et al. (2004) 11beta-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev., 25, 831–866. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed, A., et al. (2019) Extra-adrenal glucocorticoid synthesis in the intestinal mucosa: between immune homeostasis and immune escape. Front. Immunol., 10, 1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cirillo, N., et al. (2012) Characterization of a novel oral glucocorticoid system and its possible role in disease. J. Dent. Res., 91, 97–103. [DOI] [PubMed] [Google Scholar]
- 9. Cirillo, N., et al. (2017) Characterisation of the cancer-associated glucocorticoid system: key role of 11β-hydroxysteroid dehydrogenase type 2. Br. J. Cancer, 117, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zbánková, S., et al. (2004) Expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 in colorectal cancer. Cancer Lett., 210, 95–100. [DOI] [PubMed] [Google Scholar]
- 11. Sidler, D., et al. (2012) Colon cancer cells produce immunoregulatory glucocorticoids. Oncoimmunology, 1, 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu, W., et al. (2004) Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res., 64, 1757–1764. [DOI] [PubMed] [Google Scholar]
- 13. Celentano, A., et al. (2019) Glucocorticoids reduce chemotherapeutic effectiveness on OSCC cells via glucose-dependent mechanisms. J. Cell. Physiol., 234, 2013–2020. [DOI] [PubMed] [Google Scholar]
- 14. Xie, H., et al. (2015) Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol., 51, 991–997. [DOI] [PubMed] [Google Scholar]
- 15. Lin, K.T., et al. (2016) New dimension of glucocorticoids in cancer treatment. Steroids, 111, 84–88. [DOI] [PubMed] [Google Scholar]
- 16. Duffey, D.C., et al. (1996) Oral lichen planus and its association with squamous cell carcinoma: an update on pathogenesis and treatment implications. Laryngoscope, 106(3 Pt 1), 357–362. [DOI] [PubMed] [Google Scholar]
- 17. Schoneveld, O.J., et al. (2004) Mechanisms of glucocorticoid signalling. Biochim. Biophys. Acta, 1680, 114–128. [DOI] [PubMed] [Google Scholar]
- 18. Clark, A.R., et al. (2012) Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol. Ther., 134, 54–67. [DOI] [PubMed] [Google Scholar]
- 19. Kresty, L.A., et al. (2016) Black raspberries in cancer clinical trials: past, present and future. J. Berry Res., 6, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knobloch, T.J., et al. (2019) Metabolic regulation of glycolysis and AMP activated protein kinase pathways during black raspberry-mediated oral cancer chemoprevention. Metabolites, 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson, K., et al. (2020) Black raspberries and protocatechuic acid mitigate DNFB-induced contact hypersensitivity by down-regulating dendritic cell activation and inhibiting mediators of effector responses. Nutrients, 12, 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knobloch, T.J., et al. (2016) Suppression of proinflammatory and prosurvival biomarkers in oral cancer patients consuming a black raspberry phytochemical-rich troche. Cancer Prev. Res. (Phila)., 9, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guttenplan, J.B., et al. (2016) Effects of black raspberry extract and protocatechuic acid on carcinogen-DNA adducts and mutagenesis, and oxidative stress in rat and human oral cells. Cancer Prev. Res. (Phila)., 9, 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oghumu, S., et al. (2017) Inhibition of pro-inflammatory and anti-apoptotic biomarkers during experimental oral cancer chemoprevention by dietary black raspberries. Front. Immunol., 8, 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peiffer, D.S., et al. (2016) Dietary consumption of black raspberries or their anthocyanin constituents alters innate immune cell trafficking in esophageal cancer. Cancer Immunol. Res., 4, 72–82. [DOI] [PubMed] [Google Scholar]
- 26. Stoner, G.D. (2009) Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev. Res. (Phila)., 2, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giusti, M.M., et al. (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem., F1.2.1–F1.2.13. [Google Scholar]
- 28. Oghumu, S., et al. (2016) Deletion of macrophage migration inhibitory factor inhibits murine oral carcinogenesis: potential role for chronic pro-inflammatory immune mediators. Int. J. Cancer, 139, 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Omanakuttan, A., et al. (2016) Nitric oxide and ERK mediates regulation of cellular processes by Ecdysterone. Exp. Cell Res., 346, 167–175. [DOI] [PubMed] [Google Scholar]
- 30. Savaris, R., et al. (2000) Expression of alpha4beta1 and alphavbeta3 integrins in the endometrium of women using the T200 copper intrauterine device. Fertil. Steril., 74, 1102–1107. [DOI] [PubMed] [Google Scholar]
- 31. Temkin, S., et al. (2006) Type 2 11beta-hydroxysteroid dehydrogenase activity in human ovarian cancer. Steroids, 71, 1019–1023. [DOI] [PubMed] [Google Scholar]
- 32. Han, C., et al. (2005) Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr. Cancer, 51, 207–217. [DOI] [PubMed] [Google Scholar]
- 33. Zhang, Z., et al. (2011) A black raspberry extract inhibits proliferation and regulates apoptosis in cervical cancer cells. Gynecol. Oncol., 123, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poosarla, C., et al. (2015) Proliferating cell nuclear antigen in premalignancy and oral squamous cell carcinoma. J. Clin. Diagn. Res., 9, ZC39–ZC41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qi, L., et al. (2020) Chinese herbal medicine promote tissue differentiation in colorectal cancer by activating HSD11B2. Arch. Biochem. Biophys., 695, 108644. [DOI] [PubMed] [Google Scholar]
- 36. Knutson, K.L., et al. (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother., 54, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ward, M.J., et al. (2014) Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer, 110, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Acharya, N., et al. (2020) Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. Immunity, 53, 658–671.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen, J., et al. (2020) Type-2 11β-hydroxysteroid dehydrogenase promotes the metastasis of colorectal cancer via the Fgfbp1-AKT pathway. Am. J. Cancer Res., 10, 662–673. [PMC free article] [PubMed] [Google Scholar]
- 40. Gensler, L.S. (2013) Glucocorticoids: complications to anticipate and prevent. Neurohospitalist, 3, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez-Moles, M.A., et al. (2018) Outcomes of oral lichen planus and oral lichenoid lesions treated with topical corticosteroid. Oral Dis., 24, 573–579. [DOI] [PubMed] [Google Scholar]
- 42. Ramos-García, P., et al. (2021) Oral cancer development in lichen planus and related conditions—3.0 evidence level: a systematic review of systematic reviews. Oral Dis., 27, 1919–1935. [DOI] [PubMed] [Google Scholar]
- 43. Coleman, R.E. (1992) Glucocorticoids in cancer therapy. Biotherapy, 4, 37–44. [DOI] [PubMed] [Google Scholar]
- 44. Herr, I., et al. (2005) Glucocorticoids and progression of breast cancer. Cancer Biol. Ther., 4, 1415–1416. [DOI] [PubMed] [Google Scholar]
- 45. Tian, D., et al. (2019) Increased glucocorticoid receptor activity and proliferation in metastatic colon cancer. Sci. Rep., 9, 11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.