Abstract
Background:
Endocrine deficiencies are common following craniospinal irradiation (CSI) in children with brain tumors, but empirical data comparing outcomes following proton (PRT) and photon radiation therapy (XRT) are limited.
Methods:
This retrospective chart review compared the incidence of hypothyroidism, growth hormone deficiency (GHD), and adrenal insufficiency (AI) in patients with medulloblastoma treated with XRT and PRT between 1997 and 2016. All patients received CSI and had routine endocrine screening labs to evaluate for thyroid dysfunction, GHD, and AI. We used proportional hazards regression to calculate hazard ratios (HR) and 95% confidence intervals (CI) comparing the development of hypothyroidism, AI, and GHD between radiation modalities, adjusting for age at diagnosis, sex, race/ethnicity, and CSI dose.
Results:
We identified 118 patients with medulloblastoma who were followed for a median of 5.6 years from the end of radiotherapy. Thirty-five (31%) patients developed hypothyroidism, 71 (66%) GHD, and 20 (18%) AI. Compared to PRT, XRT was associated with a higher incidence of primary hypothyroidism (28% vs. 6%; HR=4.61, 95% CI 1.2–17.7, p=0.03). Central hypothyroidism, GHD, and AI incidence rates were similar between the groups.
Conclusions:
Primary hypothyroidism occurs less often after PRT CSI, compared to XRT CSI. This suggests that the thyroid and pituitary glands receive less radiation after spine and posterior fossa boost RT, respectively, using PRT.
Keywords: Hypothyroidism, Adrenal Insufficiency, Growth Hormone Deficiency, Cranial Radiotherapy, Pediatric Medulloblastoma, Proton radiation therapy
INTRODUCTION
Medulloblastoma is a malignant brain tumor diagnosed in approximately 400 to 500 children and adolescents annually in the United States [1]. Depending on individual patient factors, management often involves a multimodal treatment approach that includes surgery, radiotherapy, and chemotherapy [2]. Advances in treatment over recent decades have led to significant improvements in survival, with five-year survival rates ranging between 60% to 85% depending on risk group at diagnosis, calculated by the extent of residual disease after surgery, age, and presence or absence of metastatic spread [3].
Though mortality has improved, curative therapy remains a significant source of long-term morbidity. Survivors of medulloblastoma are at risk for secondary malignancies, neurocognitive impairment, and many chronic medical conditions [4–6]. Craniospinal irradiation (CSI) often causes direct injury to the hypothalamic pituitary axis (HPA) and can, depending on the type of radiation and dose administered, also affect non-CNS organs including the thyroid gland, heart, lungs, liver, pancreas, kidneys, gonads, and bones including spinal growth [7]. Endocrinopathies are some of the most common adverse effects of CSI and include growth hormone deficiency (GHD), primary and secondary hypothyroidism, adrenal insufficiency (AI), hypogonadism, and precocious puberty [2, 8–12].
Improvements in the delivery of radiotherapy have resulted in enhanced coverage of the target organ and decreased irradiation to the normal surrounding structures. Recent studies comparing photon therapy (XRT) to proton therapy (PRT) have demonstrated that surrounding non-target organs, such as the HPA and the thyroid gland, receive significantly less radiation with PRT than XRT [13–15]. Some have also reported a lower incidence of hypothyroidism and other late endocrine effects among patients treated with PRT [12, 16], highlighting the potential for newer technologies to result in fewer endocrine late effects. However, further research on the clinical effects of PRT relative to XRT is needed. In a recent analysis from our group, Bielamowicz et al reported a lower incidence of primary hypothyroidism among medulloblastoma patients treated with PRT compared to XRT; however, the difference did not reach statistical significance [16]. Here, we report on an expansion of this cohort with extended follow up time. In addition to reexamining hypothyroidism, this study compares rates of GHD and AI among patients treated with both XRT and PRT.
METHODS
Patient Selection and Data Collection
We identified 127 patients diagnosed with medulloblastoma between 1997 and 2016 at Texas Children’s Hospital. This observational medical record review was approved by the institutional review board at Baylor College of Medicine. Demographic variables, including age at diagnosis, gender, race and ethnicity, and clinical information, including diagnosis and treatment protocol, were abstracted from electronic medical records. All patients underwent maximal safe resection of the primary tumor followed by CSI, posterior fossa/tumor bed boost, and multi-agent chemotherapy. Before 2007, all patients were treated with 3D photons to the craniospinal axis followed by intensity-modulated radiation therapy (IMRT) for the boost volume. All PRT patients with records available received passive scatter proton therapy. One PRT patient was treated at an outside institution and detailed radiation exposure information was unavailable. Standard/low-risk patients received 15–23.4 Gy while high-risk patients received 36–39.6 Gy CSI. Cumulative radiation dose to the tumor bed ranged from 54 and 55.8 Gy. Two patients with incomplete radiation exposure information and two patients treated with both IMRT and PRT were excluded from the analysis. Five additional patients were also excluded because they were lost to follow up or did not have sufficient endocrinology evaluations to determine if a diagnosis was present or absent. The remaining 118 individuals were evaluable and included in the analysis.
Endocrine Assessments
The primary endpoints for this study were incidence of primary and central hypothyroidism, GHD, and AI. After patients completed radiotherapy and chemotherapy, they continued regular follow up with oncology, long term survivor clinic, and/or endocrinology. Routine laboratory studies included thyroid stimulating hormone (TSH), free thyroxine (FT4), insulin-like growth factor-1 (IGF-1) and IGF binding protein-3. Total thyroxine (T4) and FT4 by equilibrium dialysis (FT4 by ED) were also utilized if available. If growth velocity or IGF-1 levels were suggestive of GHD, then growth hormone (GH) stimulation testing was often performed. Annual screening of cortisol function included evaluation for clinical symptoms suggestive of AI, and if clinically indicated, patients underwent 1 μg cosyntropin stimulation testing. In addition to the above studies, medication lists were reviewed to assess for hormone replacement therapy, and all notes from endocrine clinic visits were closely examined to ensure accurate diagnoses.
For the purposes of this study, primary hypothyroidism was defined as TSH > 10 mIU/L. Central hypothyroidism was defined as FT4 by ED lower than the normal range, FT4 <80% of the lower limit of normal, or T4 <80% of the lower limit of normal with normal or low TSH. Thyroid dysfunction not otherwise specified (NOS) was defined as a clinical diagnosis without the appropriate supporting laboratory evidence available for analysis or lab criteria that do not fit one of the above diagnoses. Patients were excluded if they did not have thyroid studies at least one year from completion of radiation. None of the patients included in the analysis had documentation of hypothyroidism prior to diagnosis. However, thyroid studies were not routinely obtained prior to initiation of radiotherapy or chemotherapy.
GHD was defined as a peak human GH level during GH stimulation tests with two different stimuli of less than 10 ng/mL. Alternatively, if a patient did not have formal stimulation testing but had low IGF-1 levels with decreased growth velocity or was started on recombinant GH therapy by an endocrinologist, they were considered to have GHD. Eleven patients were excluded from the GH analysis due to lack of GH stimulation test, IGF-1 levels, or sufficient growth data for appropriate evaluation as the authors felt the diagnosis could be neither ruled in nor ruled out. Specific growth data reviewed included serial measurements of height and weight plotted on standard growth curves. Bone age was not assessed prior to radiation.
AI was defined as a peak cortisol level during a 1μg cosyntropin stimulation test of less than 18 μg/dL. We also considered the relative diagnosis as supplied by a pediatric endocrinologist based on clinical data, which in some cases included patients who were empirically started on chronic hormone replacement therapy in the absence of formal stimulation testing.
We did not have sufficient data to assign a diagnosis of hypothyroidism, GHD, or AI in all patients. Patients were included in the analyses of a particular endocrine outcome when sufficient information was available for that endpoint, which resulted in slight differences in sample sizes between groups. All patients included in the study were followed at minimum for one year from completion of radiation therapy.
Statistical Methods
The descriptive characteristics of the study sample were compared between radiation modalities (PRT and XRT) using standard methods (t-tests for continuous variables and Fisher’s exact tests for categorical variables). Similarly, clinical and demographic characteristics of the cohort were compared between individuals with and without each endocrine outcome (hypothyroidism, AI, GHD). The cumulative incidence of each endocrinopathy was estimated with Kaplan-Meier method. Time-to-event analyses accounted for time from completion of RT until the date the individual developed a particular endocrinopathy, last follow up, or censored at 10-years post-radiotherapy, whichever came first. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) comparing time from the end of radiation therapy to the development of each endocrine outcome between PRT and XRT. Associations between radiation modality and each endocrine outcome were adjusted for potential confounding variables, selected using a backwards stepwise approach to identify covariates associated (p<0.2) with at least one of the endocrine outcomes. Final models accounted for age, sex, and CSI radiation dose category. Secondary analyses further evaluated the associations between radiation modality and central or primary hypothyroidism. Patients with hypothyroidism NOS were excluded from this analysis. Finally, because temporal changes in the frequency of radiation modalities strongly correlated with treatment protocol, we performed a sensitivity analysis restricted to protocols (COG AA9961 and SJMB03) that included individuals treated with both XRT and PRT. Specifically, restricting the analysis to individuals treated according to these protocols, we calculated the probability of exposure to PRT (vs XRT) given the observed set of covariates (age, sex, diagnosis, CSI radiation dose category, and treatment protocol), and propensity score 1:1 matched PRT-treated to XRT-treated patients. Within the propensity-score matched sample, we evaluated the association between radiation modality and each endocrine outcome using Kaplan-Meier survival curves and log-rank p-values. All statistical comparisons were conducted applying a two-sided p-value <0.05 to define statistical significance.
RESULTS
Patient clinical and demographic characteristics are presented in Table 1. Overall, 118 eligible patients were included in the analysis, consisting of mostly males (n=85, 72%) of non-Hispanic white (n=48, 40.7%) or Hispanic (n=42, 35.6%) race/ethnicity. The mean age at diagnosis was 7.6 years (range: 2.0 – 18.0 years). The majority of patients had average risk disease at presentation (n=77, 65.2%) and received mean CSI dose of 24.2 Gy ± 4.4 Gy. The remainder had high-risk disease at presentation and received mean CSI dose of 34.0 Gy ± 5.2 Gy. As expected, compared to patients treated with XRT, those treated with PRT were more often treated during the more recent treatment era and according to more recent protocols (i.e., SJMB03, SJMB12) (p <0.001).
TABLE 1.
Clinical and demographic characteristics of patients with pediatric medulloblastoma, 1997–2016
| XRT (n=54) |
PRT (n=64) |
P-value | |
|---|---|---|---|
| Mean age at diagnosis, year (SD) | 8.47 (4.04) | 6.83 (3.20) | 0.02 |
| Sex, n(%) | 0.42 | ||
| Male | 41 (75.9) | 44 (68.8) | |
| Female | 13 (24.1) | 20 (31.3) | |
| Race/Ethnicity, n(%) | 0.37 | ||
| Non-Hispanic White | 19 (35.2) | 29 (45.3) | |
| Hispanic | 19 (35.2) | 23 (35.9) | |
| Non-Hispanic Black | 11 (20.4) | 6 (9.4) | |
| Non-Hispanic Other | 5 (9.3) | 6 (9.4) | |
| CSI radiation dose, n(%)* | 0.24 | ||
| <30 Gy | 40 (74.1) | 40 (63.5) | |
| ≥30 Gy | 14 (25.9) | 23 (36.5) | |
| Treatment Protocol, n(%)* | <0.001 | ||
| SJMB 961 | 22 (40.7) | 0 (0.0) | |
| SJMB 032 | 15 (27.8) | 38 (60.3) | |
| COG AA99613 | 14 (25.9) | 7 (11.1) | |
| SJMB 124 | 0 (0.0) | 9 (14.3) | |
| Other | 3 (5.6) | 9 (14.3) | |
| Diagnosis Year, n(%) | <0.001 | ||
| 1997–2005 | 44 (81.5) | 0 (0.0) | |
| 2006–2016 | 10 (18.5) | 64 (100.0) |
XRT, photon radiation therapy; PRT, proton radiation therapy; SD, standard deviation; CSI, craniospinal irradiation; SJMB, St. Jude Medulloblastoma; COG, Children’s Oncology Group
Data incomplete for CSI radiation dose (n=1) and treatment protocol (n=1).
Per protocol, treated with 23.4 Gy for average-risk disease and 36.0–39.6 Gy for high-risk disease
Per protocol, treated with 23.4 Gy for average-risk disease and 36.0–39.6 Gy for high-risk disease
Per protocol, treated with 23.4 Gy CSI and 55.8 Gy total dose to PF
Radiation dose dependent upon molecular subtype and further risk stratification related to residual disease, histology, and presence of metastasis
Patients were followed for a median of 5.6 years (range: 1.0 – 10.0 years) for the incidence of hypothyroidism, AI, and GHD. By five-years post-radiotherapy, the estimated cumulative incidence of hypothyroidism was 35.8% (95% CI: 26.7 – 47.1), compared to 15.9% for AI (95% CI: 9.8 – 25.3) and 63.2% for GHD (95% CI: 53.6 – 72.8) (Fig. 1). The median time from end of radiotherapy to the development of each endocrinopathy among cases treated with PRT was: 2.1 years (range: 0.1 – 7.1 years) for hypothyroidism, 2.1 years (range: 0.1 – 7.1 years) for AI, and 2.4 years (range: 0.7 – 6.8 years) for GHD. For those treated with XRT, the median time to diagnosis of hypothyroidism, AI, and GHD was 2.9 years (range: 0.8 – 7.2 years), 3.9 years (range: 0.7 – 6.8 years), and 2.6 years (range: 1.4 – 9.6 years), respectively.
Figure 1.
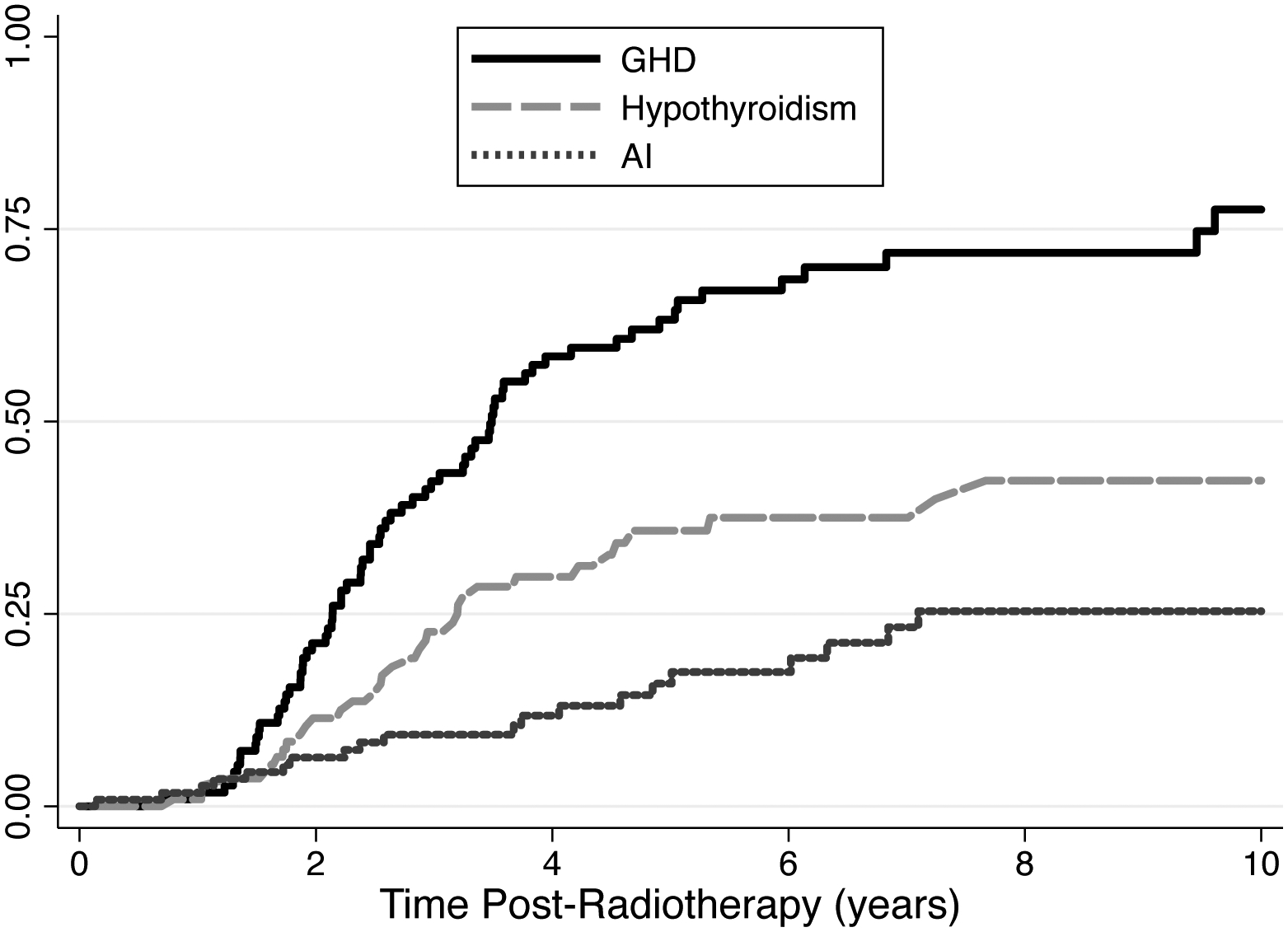
Kaplan-Meier cumulative incidence plots from hypothyroidism, adrenal insufficiency, and growth hormone deficiency
In univariate analyses, no clinical or demographic factors were associated with the prevalence of any endocrinopathy, with the exception of moderate differences in GHD across treatment protocols (Table 2). There was lower incidence of hypothyroidism, AI, and GHD among patients who received <30 Gy compared to ≥30 Gy CSI, though none of these differences reached statistical significance.
TABLE 2.
Association between clinical and demographic characteristics and endocrine outcomes in patients with pediatric medulloblastoma, 1997–2016
| Hypothyroidism | Adrenal Insufficiency | Growth Hormone Deficiency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Present (n=35) * |
Absent (n=78) * |
P-value | Present (n=20) * |
Absent (n=94) * |
P-value | Present (n=71) * |
Absent (n=36) * |
P-value | |
| Mean age at diagnosis, yr (SD) | 8.22 (4.29) | 7.40 (3.54) | 0.29 | 7.76 (3.82) | 7.36 (3.58) | 0.66 | 7.00 (3.17) | 7.95 (4.18) | 0.19 |
| Sex, n(%) | 0.82 | 0.17 | 0.96 | ||||||
| Male | 24 (68.6) | 56 (71.8) | 17 (85.0) | 65 (69.2) | 51 (71.8) | 26 (72.2) | |||
| Female | 11 (31.4) | 22 (28.2) | 3 (15.0) | 29 (30.9) | 20 (28.2) | 10 (27.8) | |||
| Race/Ethnicity, n(%) | 0.18 | 0.28 | 0.08 | ||||||
| Non-Hispanic White | 18 (51.4) | 27 (34.6) | 5 (25.0) | 42 (44.7) | 33 (46.5) | 13 (36.1) | |||
| Hispanic | 13 (37.1) | 28 (35.9) | 10 (50.0) | 31 (33.0) | 26 (36.6) | 11 (30.6) | |||
| Non-Hispanic Black | 3 (8.6) | 14 (18.0) | 4 (20.0) | 12 (12.8) | 8 (11.3) | 5 (13.9) | |||
| Non-Hispanic Other | 1 (2.9) | 9 (11.5) | 1 (5.0) | 9 (9.6) | 4 (5.6) | 7 (19.4) | |||
| CSI radiation dose, n(%) | 0.19 | 0.29 | 0.82 | ||||||
| <30 Gy | 21 (60.0) | 58 (74.4) | 12 (60.0) | 68 (72.3) | 48 (67.6) | 25 (71.4) | |||
| ≥30 Gy | 14 (40.0) | 20 (25.6) | 8 (40.0) | 26 (27.7) | 23 (32.4) | 10 (28.6) | |||
| Treatment Protocol, n(%) | 0.35 | 0.30 | 0.04 | ||||||
| SJMB 96 | 7 (20.0) | 14 (18.0) | 2 (10.0) | 20 (21.3) | 11 (15.5) | 4 (11.4) | |||
| SJMB 03 | 17 (48.6) | 35 (44.9) | 9 (45.0) | 43 (45.7) | 37 (52.1) | 14 (40.0) | |||
| COG AA9961 | 8 (22.9) | 13 (16.7) | 7 (35.0) | 14 (14.9) | 14 (19.7) | 5 (14.3) | |||
| SJMB 12 | 0 (0.0) | 8 (10.3) | 1 (5.0) | 7 (7.5) | 6 (8.5) | 3 (8.6) | |||
| Other | 3 (8.6) | 8 (10.3) | 1 (5.0) | 10 (10.6) | 3 (4.2) | 9 (25.7) | |||
Number of cases and controls may not combine to a total of 118 patients since patients were excluded if there was insufficient laboratory data to determine their endocrinopathy status.
SD, standard deviation; CSI, craniospinal irradiation; SJMB, St. Jude Medulloblastoma; COG, Children’s Oncology Group
Multivariable Cox proportional hazards models were used to assess the association between radiation modality and each endocrine outcome while accounting for age at diagnosis, sex, and CSI radiation dose (Table 3). Compared to patients receiving PRT, patients receiving XRT had higher rates of hypothyroidism (HR = 2.36, 95% CI: 1.11–5.02). Differences in hypothyroidism risk by radiation modality were largely attributed to higher rates of primary hypothyroidism following XRT (HR = 4.61, 95% CI: 1.20–17.66). The difference in the incidence of central hypothyroidism between PRT and XRT did not achieve statistical significance (HR = 2.35, 95% CI: 0.81–6.82). The rates of AI (HR = 1.07, 95% CI: 0.41–2.81) and GHD (HR = 0.71, 95% CI: 0.43–1.17) were similar between the two radiation groups (Table 3).
TABLE 3.
Adjusted associations between cranial radiotherapy modality and incidence of endocrine outcomes
| Events | Total | HR (95% CI) | |
|---|---|---|---|
| Endocrine outcome | |||
| Any hypothyroidism | |||
| PRT | 10 | 60 | Ref. |
| XRT | 25 | 53 | 2.36 (1.11–5.02) |
| Central hypothyroidism | |||
| PRT | 5* | 55 | Ref. |
| XRT | 14 | 42 | 2.35 (0.81–6.82) |
| Primary hypothyroidism | |||
| PRT | 3* | 53 | Ref. |
| XRT | 11 | 39 | 4.61 (1.20–17.66) |
| Adrenal insufficiency | |||
| PRT | 8 | 60 | Ref. |
| XRT | 12 | 54 | 1.07 (0.41–2.81) |
| Growth hormone deficiency | |||
| PRT | 39 | 62 | Ref. |
| XRT | 22 | 45 | 0.71 (0.43–1.17) |
Adjusted for age at diagnosis, sex, race/ethnicity, and CSI radiation dose
The sum of central and primary hypothyroidism events does not combine to equal the hypothyroidism events as two patients receiving PRT were diagnosed with hypothyroidism not otherwise specified (NOS).
HR, hazard ratio; CI, confidence interval; XRT, photon radiation therapy; PRT, proton radiation therapy
In order to evaluate whether differences in endocrinopathies could be attributed to temporal or protocol-specific differences detection, we conducted a propensity score-matched comparison restricted to patients treated on AA9961 and SJMB03 (Fig. 2). The propensity score-matched sensitivity analysis resulted in similar findings as the overall analysis, indicating PRT was associated with a statistically significant lower incidence of primary hypothyroidism, a non-statistically significant lower incidence of central hypothyroidism, and similar rates of AI and GHD. These results suggest variation in chemotherapy and/or temporal changes in the screening of endocrine function are unlikely to explain the observed benefits of PRT.
Figure 2.
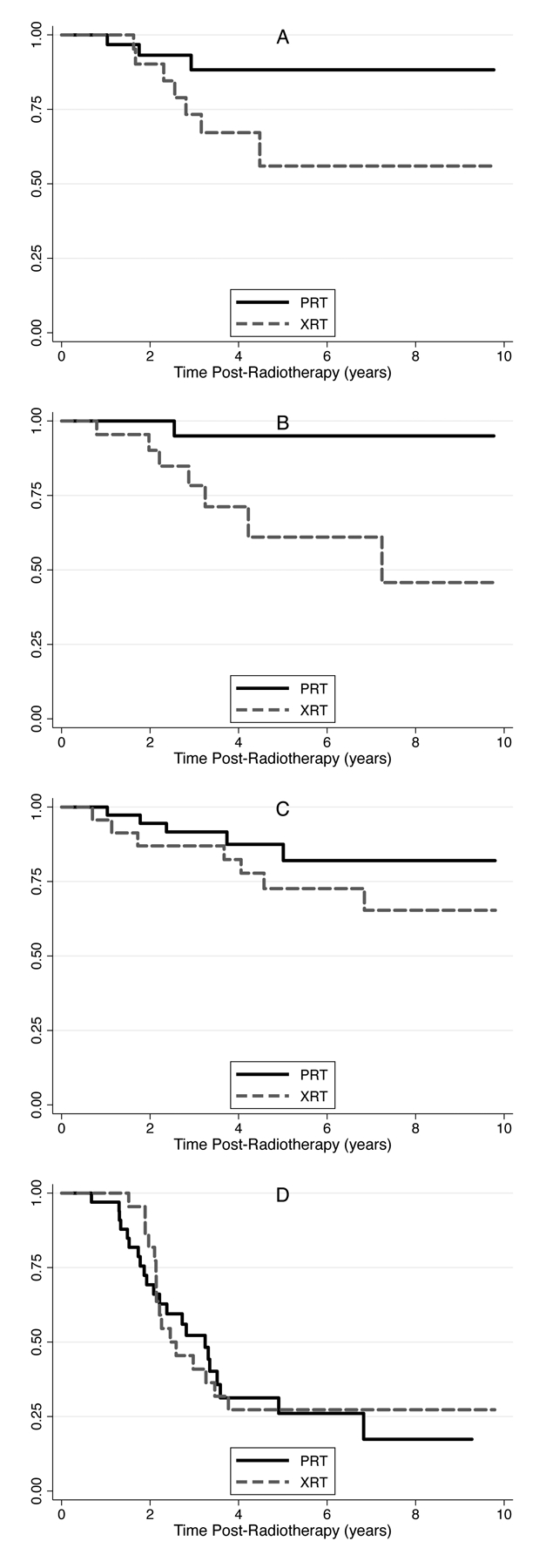
Kaplan-Meier plots comparing incidence of primary hypothyroidism (A, p=0.06), central hypothyroidism (B, p=0.01), adrenal insufficiency (C, p=0.62), and growth hormone deficiency (D, p=0.61) between XRT and PRT, propensity-score matched on age at diagnosis, sex, CSI radiation dose, and treatment protocol (COG AA9961 or SJMB03)
DISCUSSION
Treatment of pediatric brain tumors is an evolving field. With improvements in mortality, more attention has been focused on maximizing quality of life (QOL) and minimizing effects of therapy. There are efforts to tailor treatment protocols and reduce radiation doses in patients with more favorable molecular subtypes [17]. Advances in radiation therapy, including PRT, promise to reduce radiation doses delivered to normal tissues. Data is needed to further evaluate whether PRT is superior to XRT in terms of reducing clinically meaningful long-term sequelae of radiation and whether dose reductions can achieve similar survival rates.
Two prior studies done by Eaton et al. and Bielamowicz et al. directly examined the differences between PRT and XRT [12, 16]. Eaton et al. found that PRT was associated with lower incidence of hypothyroidism (23% vs. 69%, P<0.001) and sex hormone deficiency (3% vs. 19%, P=0.025), reduced need for endocrine replacement therapy (55% vs. 78%, P=0.03), and greater height SDS at follow up. Bielamowicz et al. focused on hypothyroidism, specifically looking at central and primary hypothyroidism, and while not statistically significant, the data suggested a trend towards decreased risk of both primary hypothyroidism (7.3% after PRT, 22% after XRT) and central hypothyroidism (9.8% after PRT, 24.0% after XRT).
This study extends the work of Bielamowicz et al. to examine the incidence of primary and central hypothyroidism, GHD, and AI at a single institution among patients who have undergone PRT or XRT with an expanded cohort and longer follow up time. There was a higher incidence of hypothyroidism among patients who underwent XRT even after accounting for age at diagnosis, sex, race/ethnicity, and CSI radiation dose. More specifically there was significantly higher incidence of primary hypothyroidism among XRT vs. PRT patients. Difference in the incidence of primary hypothyroidism supports prior hypotheses that spinal RT using protons may spare normal healthy tissues such as the thyroid, heart, and lungs that are distant from the target volume. The difference in the incidence of central hypothyroidism, while higher among patients who received XRT, was not statistically significant. This suggests that pituitary gland may have less radiation exposure after posterior fossa boost in patients treated with PRT CSI compared to XRT CSI. There was no significant difference between the incidences of AI or GHD between the two groups. Thus, the overall risk of HPA dysfunction remains similar despite use of PRT, and shows that the relative sensitivity of the growth hormone and ACTH producing pituitary tissue to RT is high even with lower overall dosage. This is consistent with the study done by Merchant et al., which suggested that the dose to the HPA was large enough with both PRT and XRT to cause GHD [18].
This single-site, retrospective cohort study evaluated a large cohort of pediatric patients with medulloblastoma, where follow up laboratory studies and endocrinology referrals have become more standardized. The criteria used to diagnose hypothyroidism, were strictly defined, similar to the study done by Bielamowicz et al., and did not include cases of subclinical hypothyroidism unless started on thyroid replacement therapy. With PRT now in use at our institution for over ten years, there are patients who have had significant follow up time to allow for the development of endocrine sequelae. Prior studies have shown that the majority of endocrinopathies may occur in the subsequent 6 years from tumor therapy [24, 25], but endocrine complications have been reported decades later [26, 27], so re-assessing this cohort in the future may still be beneficial and may clarify what impact, if any, length time bias has on these outcomes. While this study’s median follow up was 5.6 years and likely reports a majority of outcomes for those individuals with >5 years of follow up, it is possible that for patients with follow up time <5 years and particularly <2 years (all patients treated with PRT), the incidence of endocrine dysfunction may be underreported [18, 28, 29]. Heterogeneity in treatment protocols may have further confounded our results, although we attempted to control for this with propensity matching and multivariable models. We also compared the associations between radiation modality and each endocrinopathy between average risk and high-risk disease, but did not identify any statistically significant differences in this cohort (results not shown). Future studies are warranted to confirm the benefits of PRT in more contemporary populations.
An additional limitation relates to the stimulation testing for GHD and AI. In most situations, stimulation testing was only performed if clinically indicated (obvious abnormalities in growth patterns, abnormal IGF testing, abnormal cortisol testing or symptoms of AI), which could explain lower incidence of GHD in our study. However, in later years more standardized referral to endocrinology and serial testing was adopted, and thus closer monitoring of endocrine function may have improved the findings in this study. In fact, rates of AI in our study were higher than the published literature, which could be related to more proactive screening protocols in recent years.
Ultimately, the results of this study add to the evidence that PRT results in less harm to non-target organs such as the thyroid, leading to decreased risk of primary hypothyroidism with PRT in comparison to XRT. Differences in central hypothyroidism between radiation modalities were not statistically significant, and we observed similar rates of GHD and AI with PRT and XRT. However, further studies are needed to investigate longer-term effects of PRT and verify whether this finding remains statistically significant in a cohort followed decades after completion of therapy. Further studies must also be performed to assess whether lower radiation doses achieved with PRT will impact the rate of secondary malignancy and reduce the risk of late effects on the heart and lungs.
Funding:
This work was supported in part by the National Institutes of Health National Cancer Institute (K07CA218362). The funding sources had no involvement in the study design; collection, analysis, or interpretation of data; report writing; or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose. The authors have no relevant financial or non-financial interests to disclose.
Availability of data and material:
All data was obtained upon retrospective review from patient medical records and is not publicly available.
REFERENCES
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A (2014) Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64: 83–103 doi: 10.3322/caac.21219 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, Krull KR, Robison LL, Packer RJ (2009) Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 101: 946–958 doi: 10.1093/jnci/djp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer RJ, Vezina G (2008) Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol 65: 1419–1424 doi: 10.1001/archneur.65.11.1419 [DOI] [PubMed] [Google Scholar]
- 4.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP (2010) Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102: 1083–1095 doi: 10.1093/jnci/djq238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller S, Fullerton HJ, Stratton K, Leisenring W, Weathers RE, Stovall M, Armstrong GT, Goldsby RE, Packer RJ, Sklar CA, Bowers DC, Robison LL, Krull KR (2013) Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 86: 649–655 doi: 10.1016/j.ijrobp.2013.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE (2004) Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 5: 399–408 doi: 10.1016/S1470-2045(04)01507-4 [DOI] [PubMed] [Google Scholar]
- 7.Brodin NP, Munck Af Rosenschöld P, Aznar MC, Kiil-Berthelsen A, Vogelius IR, Nilsson P, Lannering B, Björk-Eriksson T (2011) Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol 50: 806–816 doi: 10.3109/0284186X.2011.582514 [DOI] [PubMed] [Google Scholar]
- 8.Pasqualini T, Diez B, Domene H, Escobar ME, Gruñeiro L, Heinrich JJ, Martinez A, Iorcansky S, Sackmann-Muriel F, Rivarola M (1987) Long-term endocrine sequelae after surgery, radiotherapy, and chemotherapy in children with medulloblastoma. Cancer 59: 801–806 doi: [DOI] [PubMed] [Google Scholar]
- 9.Heikens J, Michiels EM, Behrendt H, Endert E, Bakker PJ, Fliers E (1998) Long-term neuro-endocrine sequelae after treatment for childhood medulloblastoma. Eur J Cancer 34: 1592–1597 doi: 10.1016/s0959-8049(98)00212-3 [DOI] [PubMed] [Google Scholar]
- 10.Ramanauskienė E, Labanauskas L, Verkauskienė R, Sileikienė R (2014) Early development of endocrine and metabolic consequences after treatment of central nervous system tumors in children. Medicina (Kaunas) 50: 275–280 doi: 10.1016/j.medici.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Clement SC, Schouten-van Meeteren AY, Boot AM, Claahsen-van der Grinten HL, Granzen B, Sen Han K, Janssens GO, Michiels EM, van Trotsenburg AS, Vandertop WP, van Vuurden DG, Kremer LC, Caron HN, van Santen HM (2016) Prevalence and Risk Factors of Early Endocrine Disorders in Childhood Brain Tumor Survivors: A Nationwide, Multicenter Study. J Clin Oncol 34: 4362–4370 doi: 10.1200/JCO.2016.67.5025 [DOI] [PubMed] [Google Scholar]
- 12.Eaton BR, Esiashvili N, Kim S, Patterson B, Weyman EA, Thornton LT, Mazewski C, MacDonald TJ, Ebb D, MacDonald SM, Tarbell NJ, Yock TI (2016) Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol 18: 881–887 doi: 10.1093/neuonc/nov302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, Chang EL, Maor MH, Woo SY, Cox JD, Smith AR (2005) Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys 63: 362–372 doi: 10.1016/j.ijrobp.2005.01.060 [DOI] [PubMed] [Google Scholar]
- 14.Miralbell R, Lomax A, Russo M (1997) Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: spinal theca irradiation. Int J Radiat Oncol Biol Phys 38: 805–811 doi: 10.1016/s0360-3016(97)00005-9 [DOI] [PubMed] [Google Scholar]
- 15.St Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, Loeffler JS, Tarbell NJ (2004) Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys 58: 727–734 doi: 10.1016/S0360-3016(03)01574-8 [DOI] [PubMed] [Google Scholar]
- 16.Bielamowicz K, Okcu MF, Sonabend R, Paulino AC, Hilsenbeck SG, Dreyer Z, Suzawa H, Bryant R, Adesina A, Dauser R, Mahajan A, Chintagumpala M (2018) Hypothyroidism after craniospinal irradiation with proton or photon therapy in patients with medulloblastoma. Pediatr Hematol Oncol 35: 257–267 doi: 10.1080/08880018.2018.1471111 [DOI] [PubMed] [Google Scholar]
- 17.Gajjar AJ, Robinson GW (2014) Medulloblastoma-translating discoveries from the bench to the bedside. Nat Rev Clin Oncol 11: 714–722 doi: 10.1038/nrclinonc.2014.181 [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U (2008) Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 51: 110–117 doi: 10.1002/pbc.21530 [DOI] [PubMed] [Google Scholar]
- 19.Taylor AJ, Croft AP, Palace AM, Winter DL, Reulen RC, Stiller CA, Stevens MC, Hawkins MM (2009) Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer 125: 2400–2405 doi: 10.1002/ijc.24581 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein AM, Yuen J, Tucker MA (1997) Second cancers after medulloblastoma: population-based results from the United States and Sweden. Cancer Causes Control 8: 865–871 doi: 10.1023/a:1018464328836 [DOI] [PubMed] [Google Scholar]
- 21.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, Vlassov O, Bouville A, Goulko G, Hoshi M, Abrosimov A, Anoshko J, Astakhova L, Chekin S, Demidchik E, Galanti R, Ito M, Korobova E, Lushnikov E, Maksioutov M, Masyakin V, Nerovnia A, Parshin V, Parshkov E, Piliptsevich N, Pinchera A, Polyakov S, Shabeka N, Suonio E, Tenet V, Tsyb A, Yamashita S, Williams D (2005) Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 97: 724–732 doi: 10.1093/jnci/dji129 [DOI] [PubMed] [Google Scholar]
- 22.Nikiforov YE (2006) Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol 17: 307–317 doi: 10.1007/s12022-006-0001-5 [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD (2013) Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol 58: 807–823 doi: 10.1088/0031-9155/58/4/807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement SC, Meeteren AY, Kremer LC, van Trotsenburg AS, Caron HN, van Santen HM (2014) High prevalence of early hypothalamic-pituitary damage in childhood brain tumor survivors: need for standardized follow-up programs. Pediatr Blood Cancer 61: 2285–2289 doi: 10.1002/pbc.25176 [DOI] [PubMed] [Google Scholar]
- 25.Lawson SA, Horne VE, Golekoh MC, Hornung L, Burns KC, Fouladi M, Rose SR (2019) Hypothalamic-pituitary function following childhood brain tumors: Analysis of prospective annual endocrine screening. Pediatr Blood Cancer 66: e27631 doi: 10.1002/pbc.27631 [DOI] [PubMed] [Google Scholar]
- 26.Ricardi U, Corrias A, Einaudi S, Genitori L, Sandri A, di Montezemolo LC, Besenzon L, Madon E, Urgesi A (2001) Thyroid dysfunction as a late effect in childhood medulloblastoma: a comparison of hyperfractionated versus conventionally fractionated craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 50: 1287–1294 doi: 10.1016/s0360-3016(01)01519-x [DOI] [PubMed] [Google Scholar]
- 27.Waguespack SG (2019) Thyroid Sequelae of Pediatric Cancer Therapy. Horm Res Paediatr 91: 104–117 doi: 10.1159/000495040 [DOI] [PubMed] [Google Scholar]
- 28.Laughton SJ, Merchant TE, Sklar CA, Kun LE, Fouladi M, Broniscer A, Morris EB, Sanders RP, Krasin MJ, Shelso J, Xiong Z, Wallace D, Gajjar A (2008) Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol 26: 1112–1118 doi: 10.1200/JCO.2008.13.5293 [DOI] [PubMed] [Google Scholar]
- 29.Rose SR, Horne VE, Howell J, Lawson SA, Rutter MM, Trotman GE, Corathers SD (2016) Late endocrine effects of childhood cancer. Nat Rev Endocrinol 12: 319–336 doi: 10.1038/nrendo.2016.45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data was obtained upon retrospective review from patient medical records and is not publicly available.


