Abstract
In the present study, we report the photochemical transformation of pyrazolo[1,2-a]pyrazolone substrates that reach an excited state upon irradiation with visible light to initiate the homolytic C–N bond cleavage process that yields the corresponding N1-substituted pyrazoles. Moreover, chemoselective heterolytic C–N bond cleavage is possible in the pyrazolo[1,2-a]pyrazole core in the presence of bromomalonate.
For organic substrates that do not absorb visible light, it is difficult to use direct energy transfer applying visible light to achieve transformation.1 In most cases, due to the poor visible light absorption of the reaction substrates, it is necessary to use photosensitizers to initiate the transformations.2 In the past decade, numerous efficient photocatalysts, including iridium, ruthenium, nickel, and copper complexes,3 as well as various organic dyes,4 have been investigated for visible light induced organic transformations to form carbon–carbon and carbon-heteroatom bonds under very mild reaction conditions. However, the high cost and sometimes complicated preparation of these photocatalysts limit their industrial application, especially for large-scale syntheses.5 A novel strategy for radical generation from catalytic visible-light-absorbing dithiocarbamates was recently presented by Melchiorre.6 The development of visible light-induced organic reactions using photoactive substrates without external photosensitizers or photocatalysts is considered a promising research direction, thus offering a cost-effective and more environmentally friendly approach to organic synthesis.7 Our group has explored the potential of some azomethine imines as 1,3-dipoles in [3 + 2]-annulation reactions.8 Recently, we have developed a visible-light-induced aerobic oxidation of N1-substituted pyrazolidin-3-ones to afford the corresponding azomethine imines, which can be further reacted in situ with ynones under copper-catalyzed [3 + 2] cycloaddition conditions to give the corresponding pyrazolo[1,2-a]pyrazoles.9N,N-Bicyclic pyrazolidin-3-ones, that is, pyrazolo[1,2-a]pyrazol-1-one derivatives exhibit pronounced bioactivity and have attracted much attention in drug development.10 Among them, pyrazole derivatives have been given special consideration in cancer therapy.11 Stoichiometric oxidation-ring opening (Br2, CAN, O2/Cu2+) of the corresponding pyrazolo[1,2-a]pyrazoles in the presence of water as a nucleophile (Scheme 1a) was previously explored by us and others and leads to N1-substituted pyrazoles.12 Since pyrazolo[1,2-a]pyrazoles absorb in the visible frequency range (up to 420 nm), we envisioned that visible-light induced transformations of the pyrazolo[1,2-a]pyrazole core could provide a mild and economical route to valuable N1-substituted pyrazole derivatives, as shown in Scheme 1b.
Scheme 1. Ring Scission of Pyrazolo[1,2-a]pyrazoles.
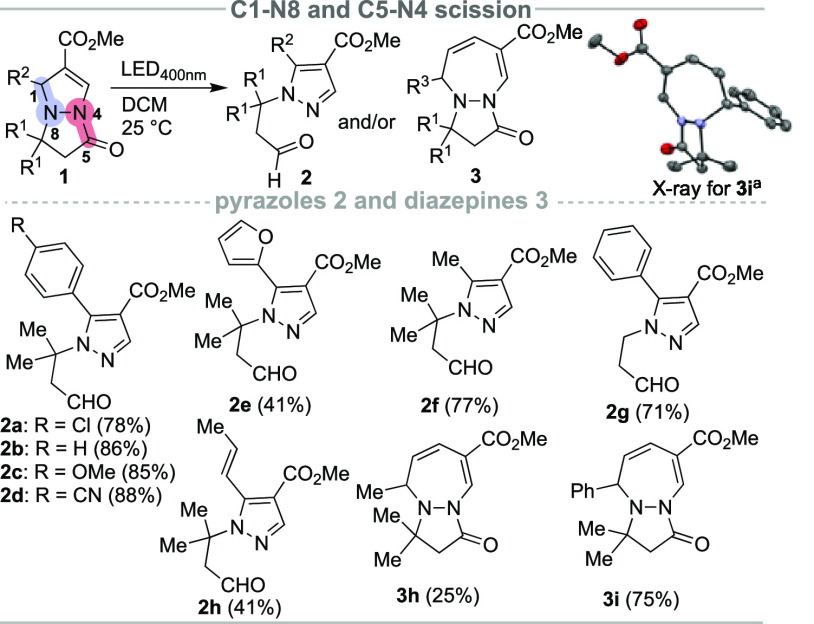
To investigate the feasibility of the proposed strategy, we chose 1a (R1 = Me, R2 = 4-Cl-C6H4) with the absorption maxima at 360 nm (εmax = 8400 M–1 cm–1, λem = 535 with Θf = 0.11)13 as a model substrate for visible-light-induced C–N bond cleavage in the pyrazolo[1,2-a]pyrazole core. Surprisingly, irradiation of 1a with 400 nm 3 W LEDs for 24 h in DCM at 25 °C led to the formation of the corresponding aldehyde 2a in 78% yield. Careful examination of different solvents (THF, EtOAc, MeCN, acetone, MeOH, and DMF) revealed that DCM performed best in this protocol. Moreover, a control experiment with longer wavelengths (450 nm LEDs) slowed the conversion. The reaction was additionally tested in dichloroethane at 50 °C for 12 and 24 h, resulting in lower yields of 2a with notable side reactions (for details, see Table SI1). Next, various substituted pyrazolo[1,2-a]pyrazoles 1 were investigated to evaluate the generality of the transformation. Here, C1 phenyl-, heteroaryl-, and alkyl-substituted substrates afforded the corresponding aldehydes 2a–g in moderate to good yields (Scheme 2). When vinyl-derived substituents were introduced onto the C1 position of the pyrazolo[1,2-a]pyrazole scaffold, the ring expansion products 3 were formed, together with the formation of products 2 (Scheme 2, examples 3h and 3i). The formation of ring-expansion products 3 commenced via C1–N8 homolytic bond cleavage followed by radical 7-endo-trig cyclization.13
Scheme 2. C1–N8 and C5–N4 Bond Cleavage of Pyrazolo[1,2-a]pyrazoles.
Hydrogens are omitted for clarity.
1 (0.5 mmol), DCM (2.5 mL), LED400nm, 25 °C, under N2 for 24–48 h.
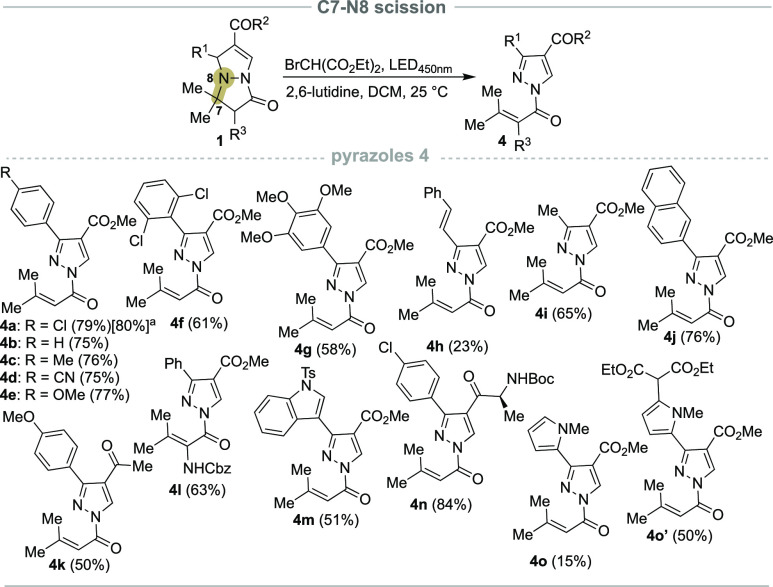
Considering the ability of 1a to act as a reducing agent in the excited state (Eox* ∼ – 1.8 V vs SCE),13 the photoreduction of activated alkyl bromides, such as diethyl bromomalonate (Ered = −0.62 V vs SCE) or 2-bromoacetophenone (Ered = −1.46 V vs SCE), would be possible.14 For details and discussion on the optimization studies, see Supporting Information. Detailed screening of the reaction conditions revealed that 1a could be converted to the N1-acryloyl-substituted pyrazole 4a and isolated in 78% yield when irradiated with blue light (450 nm) in the presence of diethyl bromomalonate (2.0 equiv) and 2,6-lutidine (1.5 equiv) as the base in DCM. To explore the substrate scope, the above optimized reaction conditions were applied to a variety of substituted pyrazolo[1,2-a]pyrazoles 1 (Scheme 3). Bicycles 1 with electron-withdrawing groups on the benzene ring, such as chloro and cyano, and electron-donating substituents, such as methoxy and methyl, were well-tolerated in the present transformation and showed no obvious difference in reactivity, as the corresponding products 4a–g were isolated in good yields. Moreover, the reaction result was not altered when a naphthalene unit (example 4j) was introduced. Notably, it was also possible to extend the substrate range to carbonyl, aminocarbonyl, and carbamate substituents on both pyrazole rings in bicyclic substrates, resulting in good yields of the corresponding products 4k, 4l, and 4n. Alkyl substitution was also tolerated as product 4i was isolated in a 65% yield. Unfortunately, the styryl-substituted pyrazolo[1,2-a]pyrazole 1 gave product 4h in a rather low 30% isolated yield. When the N-methylpyrrole substituted bicycle 1 was reacted under standard conditions, the corresponding N-acryloyl substituted pyrazole 4o was isolated in a 15% yield, together with the C5′ malonyl substituted derivative 4o′ in a 50% yield. The coupling of this electron-deficient malonate radical at the C2 position with electron-rich arenes, such as pyrroles, thiophenes, and furans under visible light-mediated conditions was documented by Stephenson,15 Trapp,16 Noël,17 and Wu.18 To demonstrate the scalability of the method, a gram-scale experiment was performed to give 4a with a comparable 80% product yield.13
Scheme 3. C7–N8 Bond Cleavage of Pyrazolo[1,2-a]pyrazoles.
Gram scale yield.
1 (0.5 mmol), diethyl bromomalonate (2.0 equiv), 2,6-lutidine (1.5 equiv), DCM (2.5 mL), N2, 18 h.
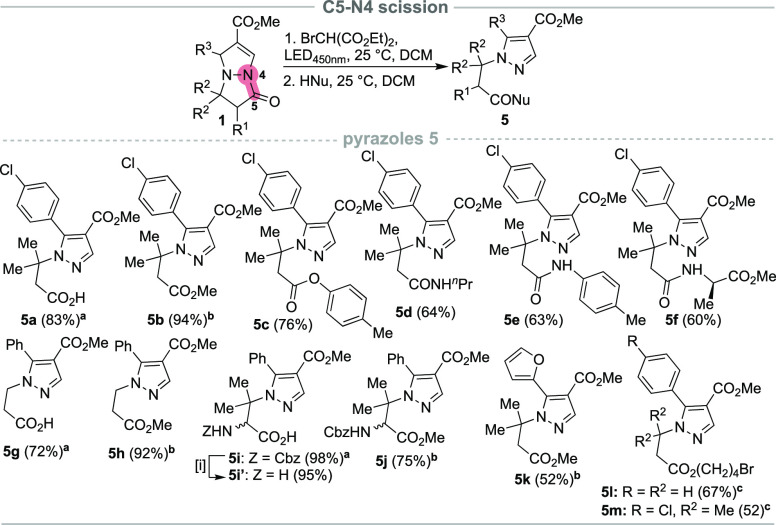
Optimization studies revealed that the presence of nucleophiles, such as water (Table SI2, entry 10)13 in the reaction mixture favored the formation of 5a as the major product. This suggests the possibility of regioselective C5–N4 photoinduced nucleophilic ring opening of pyrazolo[1,2-a]pyrazoles 1. To explore the substrate and nucleophile range for these types of transformations, we investigated the reaction of 1a with various nucleophiles (Scheme 4). In the presence of diethyl bromomalonate (2.0 equiv) in DCM under LED450nm irradiation for 19 h at 25 °C, followed by the addition of water (10 equiv), substrate 1a was successfully transformed to the corresponding acid 5a in an excellent 97% yield after isolation (Scheme 4). It is worth noting that in this case no additional base was required. In addition to water (Scheme 4, examples 5a, 5g, and 5i), other nucleophiles were also introduced. The reaction proved to be equally successful in the presence of methanol and p-cresol, obtaining the corresponding esters 5b, 5c, 5h, 5j, and 5k in good to excellent yields. Aliphatic amines and anilines were also tolerated in this protocol, giving the desired amides 5d and 5e in reasonable 64% and 63% yields, respectively. Moreover, l-alanine methyl ester was successfully coupled under the developed protocol, obtaining product 5f in a 60% yield. In addition, the reaction result was not significantly altered by the substitution pattern on the pyrazolo[1,2-a]pyrazole core, as exemplified by products 5g–k. Interestingly, when bicycles 1 were reacted in THF as the chosen solvent, the corresponding terminal halohydrin esters 5l and 5m formed in reasonable yields as a result of tetrahydrofuran ring opening induced by the pyrazolium intermediate I2 (Scheme 5).
Scheme 4. C5–N4 Bond Cleavage of Pyrazolo[1,2-a]pyrazoles.
Degassed Me2CO (H2O, 10 equiv).
MeOH (anhydrous, degassed, 2.5 mL).
THF (anhydrous, degassed, 2.5 mL). [i] H2 (2 bar), 10% Pd(C), 6 h.
1 (0.5 mmol), diethyl bromomalonate (2.0 equiv), DCM (anhydrous, degassed, 2.5 mL), N2, 18 h, HNu (2.0 equiv).
Scheme 5. Mechanistic Insight.
To gain more insight into the photoinduced transformation of pyrazolo[1,2-a]pyrazoles 1, several control experiments were conducted. First, an experiment was performed with on/off irradiation with visible light. As shown in Figure SI2,13 continuous irradiation with visible light is essential for successful transformation. A reaction with deuterated solvent (CD2Cl2) was also performed in which no deuterated aldehyde 2D was detected. However, when the C1-deuterated substrate 1D (Scheme 5a) was reacted under the standard reaction conditions, only deuterated aldehyde 2D was obtained. Furthermore, a crossover experiment with equimolar mixture of 1d (100% H on C1) and 1D (>98% D on C1) under the standard reaction conditions resulted in no crossover product being detected in the crude reaction mixture (Scheme 5b). To clarify from which excited state of substrates 1 do the aldehyde products 2 originate from, the reaction of 1a was caried out in the presence of a triplet-annihilator, trans-stilbene (ET = 49.3 kcal/mol). It is noteworthy that 1 equiv of trans-stilbene inhibits the reaction (Figure SI1),13 and a significant amount of cis-stilbene (70%) is produced during the reaction. The above experiments are consistent with visible light excitation of bicycle 1 to the S1 excited state, followed by ISC to the T1 excited state, which derives the corresponding biradical intermediate after C5–N4 bond cleavage. Subsequently, 1,5-hydrogen shift and aromatization gives the desired products, pyrazoles 2 (Scheme 5a). In the case of C1-vinyl derived substrates 1, homolytic C1–N8 bond cleavage becomes the competitive reaction process yielding diazepine products 3 upon 7-endo-trig cyclization (Scheme SI9).13 In addition, the reaction of 1a with diethyl bromomalonate was also studied more in detail. The reaction of 1a with diethyl bromomalonate was tested under standard reaction conditions in the presence of TEMPO as a radical scavenging reagent (Scheme 5c). The formation of the TEMPO-malonate product can be clearly identified by HRMS analysis (exact mass: 316.2118 [C16H30NO5]) in the crude reaction mixture.13 This result and the formation of diethyl malonate adduct 4o′ (Scheme 3) suggest that the malonyl radical is most likely involved in this transformation. The Stern–Volmer plot suggests that the interaction between the excited pyrazolo[1,2-a]pyrazoles 1 and diethyl bromomalonate (Figure SI9)13 exists. Following the reaction progress by 1H NMR shows the formation of intermediate I2 (Figure SI3),13 which upon the addition of water converts to a corresponding carboxylic acid 5 or into product 4 upon addition of 2,6-lutidine as the base. On the basis of these experiments and related literature precedents,15−18 a possible reaction mechanism is shown in Scheme 5c. The pyrazolo[1,2-a]pyrazole 1 was first excited with visible light to form the excited 1*, which underwent single electron transfer (SET) with diethyl bromomalonate to generate the corresponding radical cation of 1. The mesolytic loss of a bromide ion from the bromomalonate radical anion would then provide the malonate radical. Deprotonation of the radical cation intermediate of 1 yields the corresponding radical intermediate I1, which in turn reacts with the malonyl radical by SET to furnish the cationic intermediate I2. The pyrazolium intermediate I2 can provide products 4 under basic reaction conditions or alternatively give products 5 in the presence of nucleophiles.
In summary, we have demonstrated a novel visible-light-induced transformation of substituted pyrazolo[1,2-a]pyrazoles 1. Excitation leads to chemoselective C–N bond cleavage of the pyrazolo[1,2-a]pyrazole scaffold, resulting in densely substituted pyrazoles. The reaction outcome depends on the nature of the substrates and the reaction protocol, thus providing a versatile approach for functionalized pyrazole derivatives originating from readily available starting materials. Further investigations into applications of this methodology are currently ongoing.
Acknowledgments
Financial support from the Slovenian Research Agency through grant P1-0179 is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c01376.
Materials and methods, experimental procedures, mechanistic and optimization studies, characterization data, and 1H NMR and 13C NMR spectra (PDF)
Accession Codes
CCDC 2077375–2077377 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Zeitler K. Photoredox Catalysis with Visible Light. Angew. Chem., Int. Ed. 2009, 48, 9785–9789. 10.1002/anie.200904056. [DOI] [PubMed] [Google Scholar]; b Michelin C.; Hoffmann N. Photosensitization and Photocatalysis–Perspectives in Organic Synthesis. ACS Catal. 2018, 8, 12046. 10.1021/acscatal.8b03050. [DOI] [Google Scholar]
- For recent reviews on visible light-induced photoredox catalysis, see:; a Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]; c Ravelli D.; Protti S.; Fagnoni M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850. 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]; d Lang X.; Zhao J.; Chen X. Cooperative Photoredox Catalysis. Chem. Soc. Rev. 2016, 45, 3026. 10.1039/C5CS00659G. [DOI] [PubMed] [Google Scholar]; e Kärkäs M. D.; Porco J. A.; Stephenson C. R. J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683. 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Corrigan N.; Shanmugam S.; Xu J.; Boyer C. Photocatalysis in Organic and Polymer Synthesis. Chem. Soc. Rev. 2016, 45, 6165. 10.1039/C6CS00185H. [DOI] [PubMed] [Google Scholar]; g Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J. Visible Light Photoredox-Controlled Reactions of N-Radicals and Radical Ions. Chem. Soc. Rev. 2016, 45, 2044. 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- For selected examples using metal-based photocatalysts, see:; a Pirnot M. T.; Rankic D. A.; Martin D. B. C.; MacMillan D. W. C. Photoredox Activation for the Direct β-Arylation of Ketones and Aldehydes. Science 2013, 339, 1593. 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Du J.; Skubi K. L.; Schultz D. M.; Yoon T. P. A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392. 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dai C.; Narayanam J. M. R.; Stephenson C. R. J. Visible-Light-Mediated Conversion of Alcohols to Halides. Nat. Chem. 2011, 3, 140. 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]; e Xuan J.; Zeng T.-T.; Feng Z.-J.; Deng Q.-H.; Chen J.-R.; Lu L.-Q.; Xiao W.-J.; Alper H. Redox-Neutral α-Allylation of Amines by Combining Palladium Catalysis and Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2015, 54, 1625. 10.1002/anie.201409999. [DOI] [PubMed] [Google Scholar]; f Huang H.; Jia K.; Chen Y. Hypervalent Iodine Reagents Enable Chemoselective Deboronative/Decarboxylative Alkenylation by Photoredox Catalysis. Angew. Chem., Int. Ed. 2015, 54, 1881. 10.1002/anie.201410176. [DOI] [PubMed] [Google Scholar]; g Musacchio A. J.; Nguyen L. Q.; Beard G. H.; Knowles R. R. Catalytic Olefin Hydroamination with Aminium Radical Cations: A Photoredox Method for Direct C–N Bond Formation. J. Am. Chem. Soc. 2014, 136, 12217. 10.1021/ja5056774. [DOI] [PubMed] [Google Scholar]; h Zoller J.; Fabry D. C.; Ronge M. A.; Rueping M. Synthesis of Indoles Using Visible Light: Photoredox Catalysis for Palladium-Catalyzed C–H Activation. Angew. Chem., Int. Ed. 2014, 53, 13264. 10.1002/anie.201405478. [DOI] [PubMed] [Google Scholar]; i Tomita R.; Yasu Y.; Koike T.; Akita M. Combining Photoredox-Catalyzed Trifluoromethylation and Oxidation with DMSO: Facile Synthesis of α-Trifluoromethylated Ketones from Aromatic Alkenes. Angew. Chem., Int. Ed. 2014, 53, 7144. 10.1002/anie.201403590. [DOI] [PubMed] [Google Scholar]
- For selected examples using organic dyes as the photocatalysts, see:; a Romero N. A.; Margrey K. A.; Tay N. E.; Nicewicz D. A. Site-Selective Arene C–H Amination via Photoredox Catalysis. Science 2015, 349, 1326. 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]; b Perkowski A. J.; Cruz C. L.; Nicewicz D. A. Ambient-Temperature Newman–Kwart Rearrangement Mediated by Organic Photoredox Catalysis. J. Am. Chem. Soc. 2015, 137, 15684. 10.1021/jacs.5b11800. [DOI] [PubMed] [Google Scholar]; c Tröster A.; Alonso R.; Bauer A.; Bach T. Enantioselective Intermolecular [2 + 2] Photocycloaddition Reactions of 2(1H)-Quinolones Induced by Visible Light Irradiation. J. Am. Chem. Soc. 2016, 138, 7808. 10.1021/jacs.6b03221. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Hari D. P.; Schroll P.; König B. Metal-Free, Visible-Light-Mediated Direct C–H Arylation of Heteroarenes with Aryl Diazonium Salts. J. Am. Chem. Soc. 2012, 134, 2958. 10.1021/ja212099r. [DOI] [PubMed] [Google Scholar]; e Meng Q.-Y.; Zhong J.-J.; Liu Q.; Gao X.-W.; Zhang H.-H.; Lei T.; Li Z.-J.; Feng K.; Chen B.; Tung C.-H.; Wu L.-Z. A Cascade Cross-Coupling Hydrogen Evolution Reaction by Visible Light Catalysis. J. Am. Chem. Soc. 2013, 135, 19052. 10.1021/ja408486v. [DOI] [PubMed] [Google Scholar]; f Guo W.; Lu L.-Q.; Wang Y.; Wang Y.-N.; Chen J.-R.; Xiao W.-J. Metal-Free, Room-Temperature, Radical Alkoxycarbonylation of Aryldiazonium Salts through Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2015, 54, 2265. 10.1002/anie.201408837. [DOI] [PubMed] [Google Scholar]; g Xiao T.; Li L.; Lin G.; Wang Q.; Zhang P.; Mao Z.; Zhou L. Synthesis of 6-Substituted Phenanthridines by Metal-Free, Visible-Light Induced Aerobic Oxidative Cyclization of 2-Isocyanobiphenyls with Hydrazines. Green Chem. 2014, 16, 2418. 10.1039/C3GC42517G. [DOI] [Google Scholar]
- a Fu M.-C.; Shang R.; Zhao B.; Wang B.; Fu Y. Photocatalytic Decarboxylative Alkylations Mediated by Triphenylphosphine and Sodium Iodide. Science 2019, 363, 1429. 10.1126/science.aav3200. [DOI] [PubMed] [Google Scholar]; b Liu Q.; Liu F.; Yue H.; Zhao X.; Li J.; Wei W. Photocatalyst-Free Visible Light-Induced Synthesis of β-Oxo Sulfones via Oxysulfonylation of Alkenes with Arylazo Sulfones and Dioxygen in Air. Adv. Synth. Catal. 2019, 361, 5277. 10.1002/adsc.201900984. [DOI] [Google Scholar]; c Guillemard L.; Colobert F.; Wencel-Delord J. Visible-Light-Triggered, Metal- and Photocatalyst-Free Acylation of N-Heterocycles. Adv. Synth. Catal. 2018, 360, 4184. 10.1002/adsc.201800692. [DOI] [Google Scholar]
- a Schweitzer-Chaput B.; Horwitz M. A.; de Pedro Beato E.; Melchiorre P. Photochemical Generation of Radicals from Alkyl Electrophiles Using a Nucleophilic Organic Catalyst. Nat. Chem. 2019, 11, 129. 10.1038/s41557-018-0173-x. [DOI] [PubMed] [Google Scholar]; b Spinnato D.; Schweitzer-Chaput B.; Goti G.; Ošeka M.; Melchiorre P. A. Photochemical Organocatalytic Strategy for the α-Alkylation of Ketones by using Radicals. Angew. Chem., Int. Ed. 2020, 59, 9485. 10.1002/anie.201915814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 9572. 10.1002/ange.201915814
- For selected recent examples, see:; a Sahoo B.; Hopkinson M. N.; Glorius F. External-Photocatalyst-Free Visible-Light-Mediated Synthesis of Indolizines. Angew. Chem., Int. Ed. 2015, 54, 15545. 10.1002/anie.201506868. [DOI] [PubMed] [Google Scholar]; b Liu P.; Liu W.; Li C.-J. Catalyst-Free and Redox-Neutral Innate Trifluoromethylation and Alkylation of Aromatics Enabled by Light. J. Am. Chem. Soc. 2017, 139, 14315. 10.1021/jacs.7b08685. [DOI] [PubMed] [Google Scholar]; c Dossena A.; Sampaolesi S.; Palmieri A.; Protti S.; Fagnoni M. Visible Light Promoted Metal- and Photocatalyst-Free Synthesis of Allylarenes. J. Org. Chem. 2017, 82, 10687. 10.1021/acs.joc.7b01532. [DOI] [PubMed] [Google Scholar]; d Li J.; Zhu D.; Lv L.; Li C.-J. Radical Difluoromethylthiolation of Aromatics Enabled by Visible Light. Chem. Sci. 2018, 9, 5781. 10.1039/C8SC01669K. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu W.; Li J.; Querard P.; Li C.-J. Transition-Metal-Free C–C, C–O, and C–N Cross-Couplings Enabled by Light. J. Am. Chem. Soc. 2019, 141, 6755. 10.1021/jacs.9b02684. [DOI] [PubMed] [Google Scholar]; f Zhang Y.; Chen W.; Jia X.; Wang L.; Li P. A Visible-Light-Induced Oxidative Cyclization of N-Propargylanilines with Sulfinic Acids to 3-Sulfonated Quinoline Derivatives Without External Photocatalysts. Chem. Commun. 2019, 55, 2785. 10.1039/C8CC10235J. [DOI] [PubMed] [Google Scholar]; g Zhao L.; Li P.; Zhang H.; Wang L. Photoinduced Synthesis of α-Trifluoromethylated Ketones Through the Oxidative Trifluoromethylation of Styrenes Using CF3SO2Na as a Trifluoromethyl Reagent Without an External Photoredox Catalyst. Org. Chem. Front. 2019, 6, 87. 10.1039/C8QO01079J. [DOI] [Google Scholar]; h Han Y.; Jin Y.; Jiang M.; Yang H.; Fu H. Photocatalyst-Free Visible-Light Photoredox Dearomatization of Phenol Derivatives Containing Ketoximes: An Easy Access to Spiropyrrolines. Org. Lett. 2019, 21, 1799. 10.1021/acs.orglett.9b00372. [DOI] [PubMed] [Google Scholar]; i Zhang X.-Y.; Weng W.-Z.; Liang H.; Yang H.; Zhang B. Visible-Light-Initiated, Photocatalyst-Free Decarboxylative Coupling of Carboxylic Acids with N-Heterocycles. Org. Lett. 2018, 20, 4686. 10.1021/acs.orglett.8b02016. [DOI] [PubMed] [Google Scholar]; j Quint V.; Chouchène N.; Askri M.; Lalevée J.; Gaumont A.-C.; Lakhdar S. Visible-Light-Mediated α-Phosphorylation of N-Aryl Tertiary Amines Through the Formation of Electron-Donor-Acceptor Complexes: Synthetic and Mechanistic Studies. Org. Chem. Front. 2019, 6, 41. 10.1039/C8QO00985F. [DOI] [Google Scholar]; k Franz J. F.; Kraus W. B.; Zeitler K. No Photocatalyst Required-Versatile, Visible Light Mediated Transformations with Polyhalomethanes. Chem. Commun. 2015, 51, 8280. 10.1039/C4CC10270C. [DOI] [PubMed] [Google Scholar]
- For review see:; a Grošelj U.; Požgan F.; Štefane B.; Svete J. Copper-Catalyzed Azomethine Imine–Alkyne Cycloadditions (CuAIAC). Synthesis 2018, 50, 4501. 10.1055/s-0037-1610284. [DOI] [Google Scholar]; For examples see:; b Pušavec Kirar E.; Grošelj U.; Mirri G.; Požgan F.; Strle G.; Štefane B.; Jovanovski V.; Svete J. Click” Chemistry: Application of Copper Metal in Cu-Catalyzed Azomethine Imine–Alkyne Cycloadditions. J. Org. Chem. 2016, 81, 5988. 10.1021/acs.joc.6b00945. [DOI] [PubMed] [Google Scholar]; c Pušavec Kirar E.; Grošelj U.; Golobič A.; Požgan F.; Pusch S.; Weber C.; Andernach L.; Štefane B.; Opatz T.; Svete J. Absolute Configuration Determination of 2,3-Dihydro-1H,5H-pyrazolo[1,2-a]pyrazoles Using Chiroptical Methods at Different Wavelengths. J. Org. Chem. 2016, 81, 11802. 10.1021/acs.joc.6b02270. [DOI] [PubMed] [Google Scholar]; d Pušavec Kirar E.; Drev M.; Mirnik J.; Golobič A.; Dahmann G.; Požgan F.; Štefane B.; Svete J.; et al. Synthesis of 3D-Rich Heterocycles: Hexahydropyrazolo[1,5-a]pyridin-2(1H)-ones and Octahydro-2H-2a,2a1-diazacyclopenta[cd]inden-2-ones. J. Org. Chem. 2016, 81, 8920. 10.1021/acs.joc.6b01608. [DOI] [PubMed] [Google Scholar]
- Petek N.; Grošelj U.; Svete J.; Požgan F.; Kočar D.; Štefane B. Eosin Y-Catalyzed Visible-Light-Mediated Aerobic Transformation of Pyrazolidine-3-one Derivatives. Catalysts 2020, 10, 981. 10.3390/catal10090981. [DOI] [Google Scholar]
- a Eicher T.; Hauptmann S.. The Chemistry of Heterocycles, 2nd ed.; Wiley-VHC: Weinheim, 2003. [Google Scholar]; b Claramunt R. M.; Elguero J. The Chemistry of Pyrazolidinones. A Review. Org. Prep. Proced. Int. 1991, 23, 273. 10.1080/00304949109458208. [DOI] [Google Scholar]; c Jungheim L. N; Sigmund S. K; Fisher J. W Bicyclic Pyrazolidinones, a New Class of Antibacterial Agent Based on the β-Lactam Model. Tetrahedron Lett. 1987, 28, 285. 10.1016/S0040-4039(00)95708-3. [DOI] [Google Scholar]; d Jungheim L. N.; Sigmund S. K. 1,3-Dipolar Cycloaddition Reactions of Pyrazolidinium Ylides with Acetylenes. Synthesis of a New Class of Antibacterial Agents. J. Org. Chem. 1987, 52, 4007. 10.1021/jo00227a013. [DOI] [Google Scholar]; e Ternansky R. J.; Draheim S. E. Structure-Activity Relationship Within a Series of Pyrazolidinone Antibacterial Agents. 1. Effect of Nuclear Modification on in Vitro Activity. J. Med. Chem. 1993, 36, 3219. 10.1021/jm00074a001. [DOI] [PubMed] [Google Scholar]; f Panfil I.; Urbańczyk-Lipkowska Z.; Suwinska K.; Solecka J.; Chmielewski M. Synthesis of Pyrazolidinone Analogs of β-Lactam Antibiotics. Tetrahedron 2002, 58, 1199. 10.1016/S0040-4020(01)01195-4. [DOI] [Google Scholar]
- For examples see:; a Rahmouni A.; Souiei S.; Belkacem M. A.; Romdhane A.; Bouajila J.; Ben Jannet H. B. Synthesis and Biological Evaluation of Novel Pyrazolopyrimidines Derivatives as Anticancer and Anti-5-lipoxygenase Agents. Bioorg. Chem. 2016, 66, 160. 10.1016/j.bioorg.2016.05.001. [DOI] [PubMed] [Google Scholar]; b Thangarasu P.; Manikandan A.; Thamaraiselvi S. Discovery, Synthesis and Molecular Corroborations of Medicinally Important Novel Pyrazoles; Drug Efficacy Determinations Through in silico, in vitro and Cytotoxicity Validations. Bioorg. Chem. 2019, 86, 410. 10.1016/j.bioorg.2019.02.003. [DOI] [PubMed] [Google Scholar]; c Faria J. V.; Vegi P. F.; Miguita A. G. C.; dos Santos M. S.; Boechat N.; Bernardino A. M. R. Recently Reported Biological Activities of Pyrazole Compounds. Bioorg. Med. Chem. 2017, 25, 5891. 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]; d Khan M. F.; Anwer T.; Bakht A.; Verma G.; Akhtar W.; Alam M. M.; Rizvi M. A.; Akhter M.; Shaquiquzzaman M. Unveiling Novel Diphenyl-1H-pyrazole Based Acrylates Tethered to 1,2,3-Triazole as Promising Apoptosis Inducing Cytotoxic and Anti-inflammatory Agents. Bioorg. Chem. 2019, 87, 667. 10.1016/j.bioorg.2019.03.071. [DOI] [PubMed] [Google Scholar]; e Omran D. M.; Ghaly M. A.; El-Messery S. M.; Badria F. A.; Abdel-Latif E.; Shehata I. A. Targeting Hepatocellular Carcinoma: Synthesis of New Pyrazole-based Derivatives, Biological Evaluation, DNA Binding, and Molecular Modeling Studies. Bioorg. Chem. 2019, 88, 102917. 10.1016/j.bioorg.2019.04.011. [DOI] [PubMed] [Google Scholar]; f Husseiny E. M. Synthesis, Cytotoxicity of Some Pyrazoles and Pyrazolo[1,5-a]pyrimidines Bearing Benzothiazole Moiety and Investigation of their Mechanism of Action. Bioorg. Chem. 2020, 102, 104053. 10.1016/j.bioorg.2020.104053. [DOI] [PubMed] [Google Scholar]; g Forde P. M.; Rudin C. M. Crizotinib in the Treatment of Non-Small-Cell Lung Cancer. Expert Opin. Pharmacother. 2012, 13, 1195. 10.1517/14656566.2012.688029. [DOI] [PubMed] [Google Scholar]
- a Prešeren A.; Svete J.; Stanovnik B. Oxidative Ring-opening of rel-(2R,3R,5S)-5-Aryl-2-benzoylamino-6,7-bis(methoxycarbonyl)-2,3-dihydro-1-oxo-3-phenyl-1H,5H-pyrazolo[1,2-a]-pyrazoles. Synthesis of rel-(2R,3R)-3-Phenyl-3-[5-aryl-3,4-bis(methoxycarbonyl)pyrazolyl-1]alanine Esters. J. Heterocycl. Chem. 1999, 36, 799. 10.1002/jhet.5570360337. [DOI] [Google Scholar]; b Luo N.; Zheng Z.; Yu Z. Highly Regioselective [3 + 2] Annulation of Azomethine Imines with 1-Alkynyl Fischer Carbene Complexes to Functionalized N,N-Bicyclic Pyrazolidin-3-ones. Org. Lett. 2011, 13, 3384. 10.1021/ol201139w. [DOI] [PubMed] [Google Scholar]; c Yang Z.-W.; Wang J.-F.; Peng L.-J.; You X.-L.; Cui H.-L. Thermal 1,3-Dipolar Cycloaddition of Azomethine Imines with Alkynes Affording N,N-Bicyclic Pyrazolidinones Under Microwave Irradiation. Tetrahedron Lett. 2016, 57, 5219. 10.1016/j.tetlet.2016.10.030. [DOI] [Google Scholar]
- For details see Supporting Infomation.
- Roth H. G.; Romero N. A.; Nicewicz D. A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714. 10.1055/s-0035-1561297. [DOI] [Google Scholar]
- a Furst L.; Matsuura B. S.; Narayanam J. M. R.; Tucker J. W.; Stephenson C. R. J. Visible Light-Mediated Intermolecular C–H Functionalization of Electron-Rich Heterocycles with Malonates. Org. Lett. 2010, 12, 3104. 10.1021/ol101146f. [DOI] [PubMed] [Google Scholar]; b Wallentin C.-J.; Nguyen J. D.; Finkbeiner P.; Stephenson C. R. J. Visible Light-Mediated Atom Transfer Radical Addition via Oxidative and Reductive Quenching of Photocatalysts. J. Am. Chem. Soc. 2012, 134, 8875. 10.1021/ja300798k. [DOI] [PubMed] [Google Scholar]
- Fuks E.; Huber L.; Schinkel T.; Trapp O. Investigation of Straightforward, Photoinduced Alkylations of Electron-Rich Heterocompounds with Electron-Deficient Alkyl Bromides in the Sole Presence of 2,6-Lutidine. Eur. J. Org. Chem. 2020, 2020, 6192. 10.1002/ejoc.202001003. [DOI] [Google Scholar]
- Bottecchia C.; Martín R.; Abdiaj I.; Crovini E.; Alcazar J.; Orduna J.; Blesa M. J.; Carrillo J. R.; Prieto P.; Noël T. De novo Design of Organic Photocatalysts: Bithiophene Derivatives for the Visible-light Induced C–H Functionalization of Heteroarenes. Adv. Synth. Catal. 2019, 361, 945. 10.1002/adsc.201801571. [DOI] [Google Scholar]
- Gu G.; Huang M.; Kim J. K.; Zhang J.; Li Y.; Wu Y. Visible-Light-Induced Photocatalyst-Free C-3 Functionalization of Indoles with Diethyl Bromomalonate. Green Chem. 2020, 22, 2543. 10.1039/D0GC00292E. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.