Abstract
The industrial mussel processing generates significant quantities of waste. Nearly 30% of one metric tonne of processed mussel is finally destined for human consumption. Regardless of the mussel commodities, an important quantity of waste is concentrated at several sub-processes, such as input reception, washing and declumping shells, and mussel meat extraction stages, or by means of the rejection of mussels only due to a size characteristic criterion established by the target market. Despite the main segregated waste comprising shells, byssus threads, residual meat and wastewater, a heterogeneous composition must be taken into account, since much of the solid waste is commonly gathered and compacted for landfill transportation purposes. This paper reviews the sustainable management strategies for mussel by-products, addressing their limitations for an industrial implementation to obtain value-added products. It is concluded that, although there is a well-known diversity of waste sustainable management alternatives, several proposed products (e.g., collagen, bio-adhesives, biopolymer, and adsorbent for pollutants) still remain in a potential framework, circumscribed into laboratory results, subject to an optimization process, to a validation by industrial pre-scale trials, or even limited by the associated production costs. Future researches should focus on reducing the uncertainties linked with their technical–economic feasibility for an industrial scale development.
Keywords: Industrial mussel processing, waste management, mussel processing waste, mussel by-products, mussel wastes, mussel waste valorization
Introduction
According to the Food and Agriculture Organization of the United Nations (FAO), food loss is defined as a decline in the quantity or quality of food, as a result of decisions or actions by the majority of collectors, producers and suppliers in the food supply chain, excluding minor food service providers and consumers (Food and Agriculture Organization of the United Nations, 2019). In contrast, food waste, as distinguished by the FAO, refers to the decrease in the quantity or quality of food as consequences of decisions or actions attributed to small-scale food service providers and final consumers (Food and Agriculture Organization of the United Nations, 2019). In any case, the reduction of both food loss and food waste, has become a priority objective. The actions aimed at achieving this are oriented towards reducing production costs, increasing efficiency in the food production and consumption system, and improving nutrition and food security in a sustainable context.
In numbers, according to the State of Food and Agriculture report issued by the FAO in 2019 (Food and Agriculture Organization of the United Nations, 2019), it is estimated that, on average, about 14% of the world’s food is lost at the harvesting phase, or “harvesting”, and before it reaches the retailer in the food supply chain. However, the loss of food may be higher than this average value, since the loss quantification must be stratified by food group, region, and also it depends on the metric established to quantify it.
By 2018 the global fish production was estimated in 179 million metric tonnes (Mt), pointing out that this figure refers to fish, crustaceans, molluscs and other aquatic animals, excluding aquatic mammals, reptiles, seaweeds and other aquatic plants (Food and Agriculture Organization of the United Nations, 2020b). About 88% of the aforementioned global production has been estimated as human consumption, meaning an annual supply of 20.5 kg per capita, while the remaining 12% was destined for non-food products, mostly for the manufacture of fishmeal and fish oil (Food and Agriculture Organization of the United Nations, 2020b).
In 2018 the estimated worldwide shelled molluscs production – essentially bivalves (i.e., mussels, clams, scallops and oysters) – was about 17.7 Mt (live weight); representing a value of USD 34.6 billion (Food and Agriculture Organization of the United Nations, 2020b). Since molluscs’ inland aquaculture is less than 2%, this worldwide figure is mainly attributed to the combined production carried out in the sea (i.e., mariculture) and in areas adjacent to the sea, such as costal ponds and gated lagoons (i.e., coastal aquaculture) (Food and Agriculture Organization of the United Nations, 2020b).
Recent statistical figures, collected and published by the FAO, indicates that the worldwide bivalves production is broadly dominated by the People’s Republic of China, with 13.4∙103 Mt (live weight), followed by the Republic of Korea, with 3.9∙102 Mt, and Chile with 3.8∙102 Mt (Food and Agriculture Organization of the United Nations, 2020b).
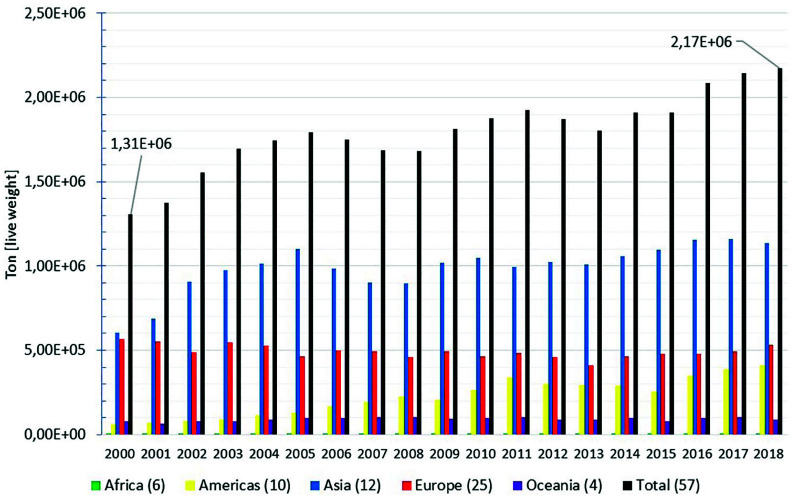
Furthermore, by means of the FAO Fisheries Division (NFI) website (Food and Agriculture Organization of the United Nations, 2020a), an estimation of worldwide mussel production can be retrieved from the global fishery and aquaculture dataset (Food and Agriculture Organization of the United Nations Fisheries Division, 2020a), using FishStatJ (Food and Agriculture Organization of the United Nations Fisheries Division, 2020), a software for measuring fishery and aquaculture statistical time series. This software, among others at the NFI website, is available to anyone interested in accessing the FAO’s fisheries and aquaculture datasets. Figure 1 depicts the global aquaculture production time series, distributed by continent, after filtering in order to show the estimated worldwide mussel production. Notice that this FAO dataset refers to the production reported by 57 producer countries, distributed as pointed out at the bottom legend of the figure. According to the dataset, this worldwide production counts for a total of 14 mussel species. As the FAO advises, some countries’ production figures have been estimated by the organization, based on the information available when certain production figures are not reported (Food and Agriculture Organization of the United Nations, Fisheries Division, 2020a). For instance, this is the case for the low annual figures attributed to African producer countries during the 2000–2018 period. By 2018, the African countries had produced less than 3000 Mt (live weight).
Figure 1.
Estimated global mussel production collected from the Food and Agriculture Organization global fishery and aquaculture production dataset. Adapted from dataset available at Food and Agriculture Organization of the United Nations Fisheries Division (2020a).
The 2000–2018 time series tendency indicates that the estimated worldwide mussel production has experienced a significant growth. The estimated global production in 2018, that is, 2.17 megatonnes (live weight), was more than 200% higher in relation to the production at the beginning of the current century. Despite the worldwide production has been permanently dominated by Asian producers, it is also worthy to notice how the Americas’ production has increased. In 2018, the mussel production due to the American producer countries was more than 690% higher in relation to the estimated production for the Americas in 2000, that is, 59.4 kilotonnes. In contrast, the Europe and Oceania figures suggest less variability over the 2000–2018 period.
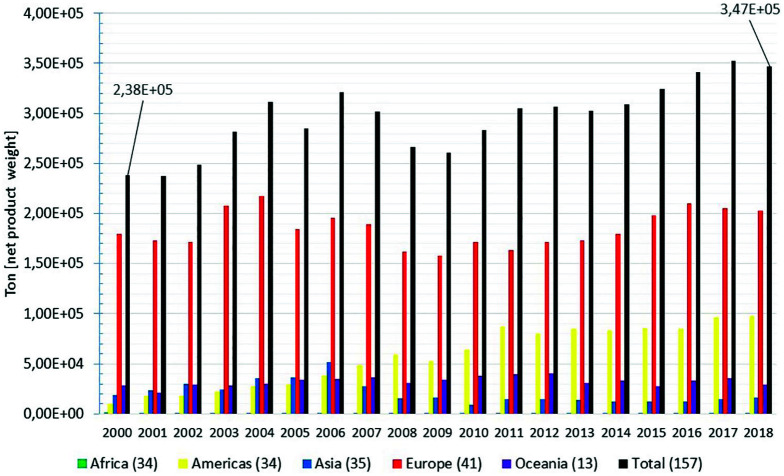
Global mussels’ commodities exportation quantities can also be retrieved from the FAO’s commodities production and trade dataset (Food and Agriculture Organization of the United Nations Fisheries Division, 2020b). In this dataset, the exported commodities are, but not limited to, mussel meat prepared or preserved, frozen, in airtight containers, in brine as well as fresh, chilled or frozen mussels, among other commodities. Figure 2 depicts the retrieved time series for the 2000–2018 period, and distributed along five continents.
Figure 2.
Estimated global mussel’s commodities exportation collected from the corresponding FAO dataset. Adapted from dataset available at Food and Agriculture Organization of the United Nations Fisheries Division (2020b).
From 2000 to 2018, the European countries reported the highest exportation figures. By 2018, 59% of the estimated global exports came from European producers, followed by the export figures reported by American producers (28%), which have shown an increased tendency during the 2000–2018 period.
Although there are no consolidated data on loss estimates in the industrial processing of bivalve molluscs, background information suggests that about 27% of one ton (live weight) of harvested mussels is rejected as by-products, for example, undersized, broken or dead mussels (Vareltzis and Undeland, 2012). Consequently, sustainability in this industrial process is required, in accordance with the current trend in the industrial aquaculture activity (Rustad, 2003; Shahidi et al., 2019). In fact, there is a wide diversity of application for by-products derived from the aquaculture industry (Jayathilakan et al., 2012; Ravindran and Jaiswal, 2016; Rustad, 2003; Shahidi et al., 2019). In this context, by-products are defined as raw materials discarded from the mussel industrial processing, but which can be recycled after treatment (Rustad et al., 2011). Despite that several alternatives for those by-products have been identified (Naik and Hayes, 2019; Suplicy, 2018), as it will be argued in this review, a gap still persists between the development and commercial availability of the required technologies to transform such by-products on an industrial scale.
The material flow and waste generation in the industrial mussel processing have been a research object of several authors. Concurrently, researchers have tackled the material flow and waste generation in this industry from the life cycle analysis (LCA) perspective, for example by Bugallo et al. (2012), Iribarren et al. (2010a, 2010b, 2010c) and Lourguioui et al. (2017). For the industry served by the aquaculture, the LCA methodology has been addressed in a sustained way, since the beginning of 2000 (Bohnes and Laurent, 2019).
Iribarren et al. (2010a, 2010b, 2010c) have applied LCA to evaluate the mussel processing industry in Galicia, Spain; one of the industries with the highest production and strong presence in the international market. Mussel LCA has been studied by discriminating the production process in three main sectors, namely: the mussel culture sector; the fresh mussel for human consumption or as input to industrial plants; and the industrial processing sector – represented by the canning plants and the mussel cooking plants (Iribarren et al., 2010c). The same authors carried out the shells and other organic remains valorizations from the LCA (Iribarren et al., 2010a). These researchers evaluated, by means of environmental indicators, the energy efficiency and environmental impact of the transformation process to obtain calcium carbonate (CaCO3) from mussel shells. The shells transformation into CaCO3 is tackled by the aforementioned researchers, and compared with two options, in order to manage this waste, that is, (a) through incineration and (b) through disposal of in landfills. The incineration option is ruled out, due to its adverse environmental impact and energy expenses. In contrast, disposal of in landfills, although according to those authors it would have less adverse environmental impact, also depends on other variables that constrained this option, making the alternative of producing CaCO3 a best suitable option for the mussel shells, in terms of its environmental impact (Iribarren et al., 2010a). Although, Iribarren et al. reported results derived from the analysis at a local industrial environment, the general conclusion that emerged from their research can be extended: the sustainable waste transformation is not only feasible, but necessary. This is consistent with what has been reported by other researchers, who have performed similar studies in other regions, for example by Lourguioui et al. (2017).
The present review revisits sustainable options for the by-products derived from industrial mussel processing, highlighting the limitations associated with those alternatives for a development on an industrial scale. The remaining sections in this review have been organized as follows: the section “Industrial mussel processing” depicts a general scope of basic and common stages at the industrial processing to produce mussel commodities; the “Mussel waste characterization” section outlines a classification for wastes derived from mussel industrial processing; a case study section comprises a chemical and physical characterization of mussel waste, which is based on the processing of waste samples supplied by a local mussel industry. That section pursues an overview to put into the context of main mussel wastes, generated at basic and common sub-processes in the process line; the next section refers succinctly to current handling and disposal strategies at the mussel industry; subsequently, current sustainability alternatives for mussel waste are discussed, according to the main waste types identified in the previous section. While alternatives are addressed, their limitations and challenges to industry scale-up are also tackled in this section. Finally, conclusions are drawn, as well as suggestions for future study topics.
To conduct the current review, a sample collection was performed through the search and selection of peer reviewed articles. Papers were collected from recognized academic databases. The research method can be summarized as follows: the database set used for this investigation comprises Web of Science Core Collection (ISI Web of Science), SCOPUS, Science Direct, EBSCOHost and Google Scholar.
The review was conducted following the recommended phases for a systematic review (Moher et al., 2009): (a) identification; (b) screening; and (b) eligibility – as preliminary steps to include the studies in the present review article. Eligibility criteria address the research’s specificity, research type, research outcomes, period, number of citations, as well as a full text review to meet the main objective of this critical study. Since the review is also an iterative process (Moher et al., 2009), the final included works were organized and gathered according to the by-products identified in the industrial mussel processing.
Search terms such as “mussel waste”, “mussel by-products”, “mussel organic waste”, “mussel waste management”, “mussel processing waste” were used to search through the aforementioned database set. Although, initially, the selected period for this review was intended to be from 2010 to 2020, several references from outside of this period were also taken into account due to their relevance for the main objective of present research. At least, a total of 104 articles were identified and, therefore, referenced for this research.
Examination of state of the art articles revealed previous works reviewing mussel processing wastes valorization (Barnaby, 2004; Hart, 2020; Mo et al., 2018; Naik and Hayes, 2019; Shahidi et al., 2019), some of them focused on a specific by-product valorization. The current review intends to address a discussion, covering the main alternatives for mussel wastes valorization, taking into account their limitations for an industrial implementation, supported by the research findings.
Industrial mussel processing: A general scope
A high percentage of the mussel’s weight is waste. Mussel shells alone can constitute between 70% and 80% (dry weight) of the waste mass resulting from the industrial mussel processing (Naik and Hayes, 2019). Different waste types, both organic and inorganic in origin, make up the industrial wastes, generated from raw material reception up to final process stages.
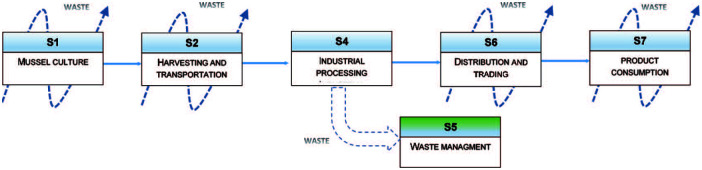
The diagram shown in Figure 2 summarizes the mussel industrial processing chain by identifying major systems or processes (azti tecnalia, 2017; Bugallo et al., 2012; Iribarren et al., 2010a, 2010b, 2010c).
The diagram in Figure 2 also depicts that each life cycle system is associated with a waste generation. This review is oriented to the waste management derived from the industrial mussels processing. Therefore, the focus is on the system labelled as S5 in Figure 3, dealing with by-products coming from system S4 (Industrial Processing).
Figure 3.
A general mussel commodity life cycle diagram. Adapted from azti tecnalia (2017), Bugallo et al. (2012), and Iribarren et al. (2010a, 2010b, 2010c).
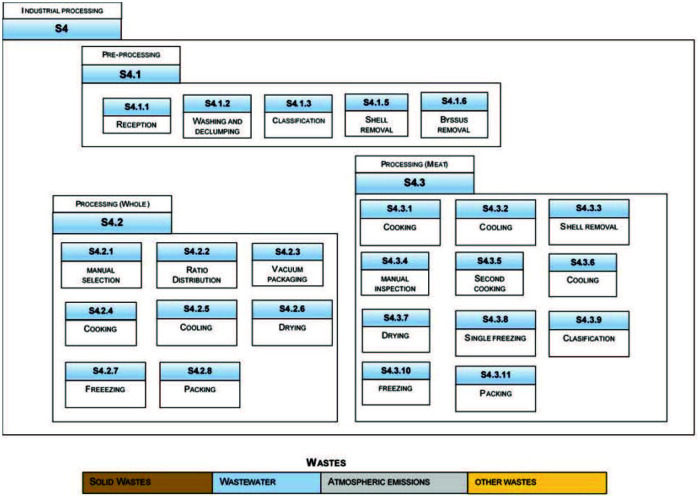
Despite the fact that the industrial line of sub-processes depends on the mussel commodity (e.g., canned or frozen), common sub-processes can be recognized as the groups shown in Figure 4.
Figure 4.
An overview for mussel industrial processing and related wastes. Adapted from azti tecnalia (2017), Bugallo et al. (2012), and Iribarren et al. (2010a, 2010b, 2010c).
Figure 4 illustrates the main sub-processes defined for the S4 system, according to the common types of product mussels, identified in Figure 4 as WHOLE (S4.2) and MEAT (S4.3) or mussels without shells. In addition, the diagram in Figure 4 also shows a common and basic sub-process: preprocessing (S4.1). Such sub-processes are basic when the mussel product is packaged and hermetically sealed. When the final mussel commodity is canned or tinned, other sub-processes must be added, as it has been described in Barros et al. (2009b). A detailed description of material flow for each sub-process has been referred by several authors, for example by Bugallo et al. (2012) and Iribarren et al. (2010b, 2010c).
Figure 4 also illustrates a general waste classification for the industrial mussel processing (Barnaby, 2004; Bugallo et al., 2012; Iribarren et al., 2010a), which is succinctly addressed in the following section.
Mussel waste characterization
Solid wastes
This category defines solid remains obtained through different sub-processes. Those wastes can be heterogeneous, comprising crushed solid remains of shells, mussels’ tissue, even whole mussels rejected due to dimensional quality criteria (i.e., mussels’ size is smaller than the approved one by quality control), and byssus (i.e., mussels’ filaments that uses to attach itself to a substrate such as rocks, or to the cultured hangings). They even comprise other molluscs, and algae being part of the raw material.
It must be noticed that several waste solid residues are commonly gathered at early processing stages (i.e., preprocessing system shown in Figure 4) for transportation purposes to landfill sites (Iribarren et al., 2010a). This gives a heterogeneous composition for some of the solid wastes to deal with and, therefore, a complex condition for an effective sustainable management, as it will be discussed later in detail.
Wastewater (WW)
It refers to the WW involved in different sub-processes, such as in the whole mussel cooking sub-process and mussel meat cooking sub-process. Through the industrial mussel processing, the WW has organic-origin residues (Amado and Vázquez, 2015). In fact, in this manufacturing process the WW varies its composition depending on the sub-process carried out. Low organic load (LOL) WW is generated at early stages as washing and declumping (i.e., S4.1.2 in Figure 4), as well as during others auxiliary operations (Barros et al., 2009b). In contrast, high organic load (HOL) WW is derived from cooking sub-processes (e.g., S4.3.1 in Figure 4) (Barros et al., 2009b; Bugallo et al., 2012).
Other wastes
These comprise wastes not included in the other categories. They may refer to those ones derived from additional raw materials supplied depending on the final mussel commodity. For instance, those ones that are rejected due to quality criteria applied to the final product (commodity) such as deteriorated cans or packages. Odours and noise also comprise this category (Bugallo et al., 2012), as well as residual fluids derived from additional industrial sub-process (e.g., residual brine) and those derived from the equipment maintenance.
Atmospheric emissions
These comprise emissions derived from different sub-processes, such as exhaust gases related to power generation and vapour emanated from the cooking sub-process, for instance as described in Bugallo et al. (2012) and Iribarren et al. (2010b, 2010c).
Mussel waste physical–chemical characterization: A case study
The chemical composition of mussel byproducts has been analysed by several researchers, for example by Abdulkarim et al. (2013), Beaulieu et al. (2013), Cros et al. (2004), Pastrana et al. (1993), Prieto et al. (2015), Vareltzis and Undeland (2012), Waldenstedt and Jönsson (2006), and Zhang et al. (2013). Most of these investigations can be gathered according to the mussel byproduct object of study. For instance, there have been reported chemical characterizations obtained by: (a) processing mussel shells (Abdulkarim et al., 2013; Paradelo et al., 2016; Zhang et al., 2013); (b) processing WW (Cros et al., 2004; Pastrana et al., 1993; Prieto et al., 2015); (c) processing byssus threads (Qin et al., 2016); and (d) processing mussel meat (Vareltzis and Undeland, 2012; Waldenstedt and Jönsson, 2006; Wang et al., 2013). In most of these studies, it is assumed that the specific mussel byproduct is available due to a prior waste segregation activity, whereas in others studies, laboratory samples have been prepared from mussels purchased at a local market or supplier, for example by Vareltzis and Undeland (2012) and Wang et al. (2013). However, as it is discussed later, heterogeneity is also a typical composition for wastes derived from initial operations in industrial mussel processing. Consequently, to put into context, the following case study is presented to illustrate basic chemical characteristics of heterogeneous waste samples collected at a local mussel industry. In addition, a physical characterization for rejected mussel samples is next developed.
A set of waste samples have been analysed for chemical and physical characterization purposes. The waste samples were provided by a local industry, which processes Mytilus chilensis, a variety of Chilean mussel.
Figure 5 depicts photograph of a compacted waste sample, resulting from pre-processing stages (namely, S4.1 in Figure 4).
Figure 5.

Heterogeneous waste sample provided by a local mussel processing plant.
This industrial waste sample was properly refrigerated, prior to being moved into the laboratory facilities for its characterization. Chemical composition results were obtained from this heterogeneous residual industrial liquid (RIL). The determination of protein, calcium (Ca), iron (Fe), ash, fat and lipid profile were conducted by processing two fraction types, and after centrifugation and filtration steps. These fractions were prepared and analysed by the Services Laboratory of the Institute of Food Science and Technology (ICYTAL), at the Universidad Austral de Chile (Laboratorio de Servicios ICYTAL, 2019a, 2019b).
The first fraction type was obtained from the soluble part of the industrial waste provided, identified in the following tables as soluble fraction. In contrast, the second fraction type was obtained from the insoluble part of this RIL, and it was identified as filtered fraction. The chemical characterization comprises protein, Ca, Fe, ash, fat and fatty acids content determination, addressed by standardized methods recommended by the Association of Official Analytical Chemists (Laboratorio de Servicios ICYTAL, 2019a, 2019b).
Furthermore, mussel samples, rejected due to the size criterion, were also available for a physical characterization. Those samples came from the mussel processing line, after cleaning and shelling stages (or first shell removal step, i.e., S4.1.2 in Figure 4) and from the mussel classification stage, based on their shell size (i.e., S4.1.3 in Figure 4). This classification sub-process generates the rejection of mussels that, usually, do not present physical deterioration. Similarly, subsequent quality control phases (i.e., visual inspections or classification stage assisted by automated operation) may produce additional whole mussels’ rejection (Bugallo et al., 2012).
Protein, Ca, Fe and ash determination
Table 1 depicts crude protein (N × 6.25), Ca, Fe and ash determination in the two laboratory samples. Three samples per fraction type were processed to perform a chemical analysis.
Table 1.
Protein, calcium (Ca), iron (Fe) and ash estimation for soluble and filtered fractions.
| Fraction type | Protein (N × 6.25) * (g/100 g) | Ca (mg/100 g) | Fe (mg/100 g) | Ash (g/100 g) |
|---|---|---|---|---|
| Soluble | 2.4 ± 0.16 | 122.8 ± 30.9 | 6.9 ± 1.82 | 2.2 ± 0.13 |
| Filtered | 3.2 ± 0.59 | 359.0 ± 62.2 | 17.7 ± 1.6 | 1.75 ± 0.52 |
*, crude protein (N × 6.25) determination by semi-micro Kjeldhan method (International Organization for Standardization 8968-3; 2004); Ca and Fe determination according to the Journal of Association of Official Analytical Chemists, Vol. 83, No. 5, 2000; and ash determination according to Association of Official Analytical Chemists 930.30: 2007.
As expected, due to its heterogeneous composition, filtered samples exhibit more protein, Ca, and Fe content than the samples derived from the soluble residues. It is noticeable that the crude protein content in the non-soluble residue is 33% higher than the one found in soluble residues. In addition, Ca content in filtered samples is 190% higher compared to soluble samples’ Ca content. Similarly, Fe content detected in filtered samples is about 150% higher than the one estimated in soluble samples. Notice that major content of ash measured in the soluble fraction may be salt (NaCl).
Lipidic profile
Table 2 presents the corresponding lipid profile from the processed samples. According to these results, similar content in the two sample types is observed in saturated, monounsaturated and polyunsaturated acids, while fatty matter content is higher in samples derived from non-soluble residues.
Table 2.
Lipidic profile for soluble and filtered fractions.
| Fraction type | Fatty matter * (g/100 g) | Acids (g/100 g) | |||
|---|---|---|---|---|---|
| Saturated | Monounsaturated | Polyunsaturated | Trans-fatty | ||
| Soluble | 0.2 ± 0.04 | 0.1 ± 0.01 | 0.1 ± 0.04 | 0.05 ± 0.01 | 0 |
| Filtered | 0.4 ± 0.0 | 0.17 ± 0.0 | 0.18 ± 0.0 | 0.06 ± 0.02 | 0 |
Notes: *, fatty matter determination by Association of Official Analytical Chemists (AOAC) 95.1.02: 2005; saturated, monounsaturated and polyunsaturated acids determination by AOAC 963.22 1995; and trans-fatty acid determination according to AOAC 963.21 1995.
No significant trans-fat content has been detected in any of the processed samples.
Rejected mussels’ dimensional characterization
Figure 6 and Table 3 illustrate the dimensional characterization of the rejected mussels’ samples available in three size sets. Sizes 1 and 2 correspond to samples of mussels rejected from the process line by vibration-induced size segregation. In addition, Size 3 represents samples obtained from mussels removed from the process line when applying a dimensional quality criterion. This quality criterion responds to the requirement of the target market for the final product (frozen whole and half-shell mussels).
Figure 6.

Mussel samples rejected due to size criterion (samples provided by a local mussel processing plant).
Table 3.
Mussel samples rejected due to dimensional criterion.
| Size 1 | Size 2 | Size 3 |
|---|---|---|

|

|

|
| Range (cm) | ||
| (1.5,4.8) | (4.5,6.8) | (6.0,8.2) |
| Average yield (%) | ||
| 14.5% | 19.6% | 26.3% |
The ranges linked to each sample size is depicted in Table 3. The average yield refers to the weight fraction of the meat extracted from the discarded mussel in relation to the mussel total weight.
As it is observed, the average yield value varies from 15% to 26% (dry basis), approximately. The latter value is similar to the one expected for those mussels that meet the dimensional quality criterion. This suggests an immediate opportunity for the valorization of the meat recovered manually from the discarded mussels. In fact, such an opportunity may be implemented by relying on the current installed capacity of the production line. Additional alternatives are examined in the “Current sustainability strategies for mussel wastes” section.
Current handling and disposal of mussel wastes
Recalling from Figure 3, the main solid wastes, that is, shells, undersized mussels, byssus, and other molluscs, are derived from initial stages as reception, washing, declumping, and byssus removal. The solid residues are usually gathered, crushed and milled, reducing their size for disposal purposes (i.e., to facilitate the transportation to landfills or incineration) (Bugallo et al., 2012; Iribarren et al., 2010b). Despite this common practice for the solid waste management, references indicates that some producers have already adopted a waste segregation alternative, specifically, to provide the removed shells to a third party for their valorization (Iribarren et al., 2010a). Typically, liquid effluents, derived from several sub-processes, are collected in a homogenization container where they are treated (Bugallo et al., 2012). However, before a common WW treatment, and due to organic load variation, as it has been pointed out in the “Mussel waste characterization” section, specific treatment for HOL and LOL effluents should be performed for a better removal of the polluting load (Barros et al., 2009b).
Current sustainability strategies for mussel wastes
In a general context, the sustainable management of waste, at the industry served by aquaculture, ranges from its transformation, based on its nutritional value, to its use as biomass or fertilizer (Lopes et al., 2015).
Although there are different recognized alternatives for the transformation into by-products, it must be noted that several options remains as “potential” or are limited by the technology required for the implementation in an industry context. In contrast, the alternative of mussel shells processing to obtain CaCO3, the available evidence confirms that the required technology is fully established at the industry level (Barnaby, 2004; Bugallo et al., 2012; Iribarren et al., 2010a, 2010b; Yao et al., 2014).
Strategies for shells’ valorization
In Table 4 to Table 8, the main by-products from mussel industry processing are summarized, addressing their corresponding valorizations, pointing out reference sets as examples linked to each by-product item. Each identified mussel by-product (i.e., shells, byssus, residual meat, heterogeneous waste, and WW) may be derived from one or more sub-processes.
Table 4.
Main sustainable alternatives for shells derived from industrial mussel processing.
Table 8.
Main sustainable alternatives for WW derived from industrial mussel processing.
| Mussel by-product | Main valorizations | References |
|---|---|---|
| Wastewater | Concentrate for food industry | Cros et al. (2004, 2005), Song et al. (2019) |
| Substrate for bioproduction of astaxanthin, gibberellins, amylases, glucose oxidase, citric acid, pediocin, hyaluronic acid, and single cell proteins | González et al. (1987), Mirón et al. (2010), Murado et al. (1997), Pastrana et al. (1993), Pintado et al. (1993), Prieto et al. (2015), Rodríguez Amado and Vázquez (2015), Vázquez et al. (2004, 2010a) |
As it is discussed next, excepting the removed shells, the rest of the mussel waste is usually collected and compacted. Table 4 shows the current sustainability strategies for mussel shells.
The mussel shells by-product as an aggregate for cement, constitutes the most established organic waste recovery for sustainability purposes in the industrial processing of bivalve molluscs (Hart, 2020; Mo et al., 2018).
The shells are mainly composed of naturally formed CaCO3, the mineral phase being calcite with some argonite. The main chemical composition of seashells is similar to limestone, consisting mainly of CaCO3, with small fractions of other carbonates and oxides.
In general, the technology required for the transformation of this waste consists of three fundamental processes: washing, calcining and crushing to the required size (Mo et al., 2018). During the washing process, the NaCl content of the seashell waste is removed, while heating or calcination is performed to remove water and organic materials (Ballester et al., 2007). When seashell wastes are used as aggregate, crushing is required to ensure that smaller fractions are obtained, and a more rounded shape of the seashell is required to improve adhesion to the cement, as well as to reduce voids (Yoon et al., 2004).
Iribarren et al. (2010a), based on data collected from a canned mussel processing plant, indicate that from 100 tonnes of waste, consisting of shells and other organic remains, 56% of this waste is shells, while the rest (44%) represents various organic remains. The same authors report that 65 tonnes of CaCO3 can be produced from processing 100 tonnes of waste.
In contrast to the alternative to produce CaCO3 (Barros et al., 2009a; Hamester et al., 2012; Lu et al., 2018), other options have emerged for shells’ valorization, such as bio-absorbent for pollutants, which results from transformation into a powder through a conventional process of fine grinding (Paradelo et al., 2016; Peña-Rodríguez et al., 2013; Quintáns-Fondo et al., 2016; Romar-Gasalla et al., 2018; Seco-Reigosa et al., 2013, 2014). In this case, its performance has been proven to mitigate soil contamination from soluble fluoride, which, in significant quantities, can be attributed to industrial activities such as aluminium production or phosphate-containing fertilizers (Quintáns-Fondo et al., 2016). For instance, Quintáns-Fondo et al. (2016) examined the fluoride absorption and desorption performances in forest, vineyard, pyrite material, granitic material and finely ground mussel shells (<1 mm.), as well as in soil types, adding to each 48 T/ha of finely ground shell residues. Although the results indicate that the absorption of soluble fluoride by powdered shells alone is the lowest (i.e., 34%, according to the reported results), adding them to pyritic material not only results in the best absorption levels, above 80%, but also the addition does not decrease the original desorption of the pyritic material from the fluoride (Quintáns-Fondo et al., 2016). Though it is concluded, on the basis of the experimental results reported in Quintáns-Fondo et al. (2016), that fluoride retention can be increased by adding pulverized mussel shell residue, additional tests are required to evaluate fluoride absorption by pH and temperature analysis.
Similarly, the potential use of this residue to filter highly contaminating metals present in aqueous solutions has been evaluated, for example by Craggs et al. (2010), Paradelo et al. (2016), Peña-Rodríguez et al. (2013), and Romar-Gasalla et al. (2018). As an example, in Seco-Reigosa et al. (2014) three mixtures of different industrial wastes are examined for the retention of arsenic, chromium and mercury. This study focuses on the waste from the incineration of the shells, which, due to its physical and chemical characteristics, is complex to recycle (Seco-Reigosa et al., 2014). According to the reported results, two mixtures, with different percentages of: ash from calcined shells, from mud and shells, and from wood ash, showed significant retention of mercury (98%) and arsenic (over 90%). This percentage is lower for chromium (32%) (Seco-Reigosa et al., 2014). The findings in Seco-Reigosa et al. (2014) endorse the potential application of this organic waste to form mixtures, in order to absorb residual contaminating elements derived from high-contaminated industries such as, for instance, mining. Comparing this valorization to the production of CaCO3, the transformation of the mussel shells into bio-absorbent material, despite the availability of industrial technology, has not achieved a similar diffusion.
A promising approach to shells’ valorization is based on chitin and chitosan extraction (Abdulkarim et al., 2013; Alabaraoye et al., 2018; Hamed et al., 2016; Vakili et al., 2014). Chitin (C8H13O5N)n is a natural polysaccharide, contained in the shell of the shrimps and crabs, cartilage of the squid, and in the exoskeleton of arthropods (Abdulkarim et al., 2013; Hamed et al., 2016). Chitosan is also a natural polysaccharide, derived from chitin. Both have important biological activities such as anti-cancer, antioxidant, and immune-enhancing, and they can be used in diverse industrial applications (e.g., medical, cosmetic, food, and textile) (Hamed et al., 2016). Chitin can be industrially extracted from marine food processing industries; mainly from crustacean shells, such as shrimp and crab shells or krill (Hamed et al., 2016; Kaur and Dhillon, 2015). It has been estimated that sources from marine ecosystems provided 1328 megatonnes of chitin per year (Cauchie, 2002).
The industrial extraction process for chitin comprises various chemical sub-processes, such as demineralization, deproteination, bleaching and decoloration, and deacetylation (Abdulkarim et al., 2013; Hamed et al., 2016). The chitin extraction is performed by acid and alkali solutions at high temperature, during a prolonged incubation time. This extraction method affects the physico-chemical properties of chitin, increasing the cost of the chitin purification process and, paradoxically, producing effluent WW (Dhillon et al., 2013). As a result of those disadvantages, biological extraction techniques are gaining wider attention (Kaur and Dhillon, 2015). Although crustaceans, crab and shrimp shells are recognized as the main industrial sources for chitin, it is worthy to notice that authors have reported a 23.25% chitin content in mussel shells (Abdulkarim et al., 2013), a similar percentage content estimated in some of the aforementioned sources (El Knidri et al., 2018).
Strategies for byssus valorization
The byssus has the function of fixing the mussel to an underwater substrate. The byssal threads provide to mussel a robust adhesion to wet, NaCl-encrusted, corroded and slimy surfaces (Lee et al., 2011). Together with the shells, it is a distinctive waste in the industrial mussels processing. In the industrial process line, this residue comes typically from the shell washing and debyssing sub-processes (S4.1.6 in Figure 3). Table 5 presents the sustainable options for this waste.
Table 5.
Main sustainable alternatives for byssus waste derived from industrial mussel processing.
| Mussel by-product | Main valorizations | References |
|---|---|---|
| Byssus | Collagen | Byette et al. (2014), Coyne et al. (1997), Montroni et al. (2018), Renner-Rao et al. (2019), Suhre et al. (2014) |
| Bio-adhesives | Cha et al. (2008), Harrington et al. (2018), Kaushik et al. (2015), Lee et al. (2011), Naik and Hayes (2019), Wiegemann (2005), Yu et al. (1999) | |
| Bio-absorbent for dye pollution | Montroni et al. (2017) |
Given its physical–chemical characteristics, byssus can be used to obtain collagen and bio-adhesives, both of which are used in the pharmaceutical, cosmetic or food industries (Coyne et al., 1997; Harrington et al., 2018; Kaushik et al., 2015; Renner-Rao et al., 2019; Yu et al., 1999). However, obtaining collagen from byssus processing remains in development, at the experimental context (Renner-Rao et al., 2019; Suhre et al., 2014). The required technology has not been extended to an industrial level (Naik and Hayes, 2019). The same applies to the technology needed for the industrial production of bio-adhesives. The technological resources for this transformation on an industrial scale are still limited by the associated production costs and, therefore, remain in research and development phases (Naik and Hayes, 2019). Due to this, the current valorization of this organic waste is more likely to come from alternatives to manage the heterogeneous waste, which is described later in this article.
Strategies for residual meat
Table 6 outlines several sustainable options to deal with residual mussel meat. An immediate use of residual mussel meat can come from the yield obtained from the discarded mussels due to dimensional criteria. This alternative, although in a lesser contribution, may be also for the remains of meat attached to the shells, even after the cooking sub-processes.
Table 6.
Main sustainable alternatives for residual meat waste derived from industrial mussel processing.
| Mussel by-product | Main valorizations | References |
|---|---|---|
| Residual meat | Fertilizer | Barnaby (2004), Kuo et al. (2004), Spångberg et al. (2013) |
| Additive for fish meal, mussel meal | Iribarren et al. (2010b), Langeland et al. (2016), Muminović (2011), Waldenstedt and Jönsson (2006), Weiss and Buck (2017) | |
| Emulsions | Iribarren et al. (2010a) | |
| Food flavouring agent | Breternitz et al. (2017) | |
| Isolate proteins | Vareltzis and Undeland (2012), Wang et al. (2013) | |
| Peptides | Beaulieu et al. (2013) |
A frequent option is to supply this waste to a third party to use it as a raw material for fishmeal processing (Iribarren et al., 2010b; Langeland et al., 2016; Weiss and Buck, 2017). In addition, and as another alternative to reduce the volume of wastes going to the landfills, the residual meat has been suggested and investigated for composting purposes (Barnaby, 2004; Kuo et al., 2004; Spångberg et al., 2013).
Another potential option is using residual meat as an input to prepare emulsions. The technology required for this transformation is similar to the one employed in other industries, that are also served by aquaculture activity (Iribarren et al., 2010a), such as the tuna and salmon industries, in which it is well established (Aquerreta et al., 2002). Despite its technical feasibility on an industrial scale, this alternative has not been fully exploited. Based on the application of LCA, other researchers have suggested the potential of this alternative by conducting a hypothetical exercise using production and process data from a canned mussel production plant (Iribarren et al., 2010a). According to this hypothetical exercise, 100 Mt of mussel meat residue could be used as input to produce, approximately, 278 Mt of emulsion or spreadable mussel meat (Iribarren et al., 2010a).
The lipid and protein content in mussel meat has also been a subject of interest, in order to study its use in the preparation of nutritional supplements or functional foods (Vareltzis and Undeland, 2012; Wang et al., 2013). However, this option has not been fully developed, and it still remains in the area of research and development (Naik and Hayes, 2019).
Furthermore, flavouring agents obtained from mussel meat by the microencapsulation technique (Breternitz et al., 2017), is another evaluated option for managing this waste, which may be taken into account for mussels discarded only on the basis of dimensional criteria. In the food industry, the production of flavourings is widespread. In fact, the microencapsulation spray drying technique is one of the most widely used techniques for obtaining flavourings (Breternitz et al., 2017). In this regard, consumer acceptance of this option has been evaluated by producing a flavouring, based on an experimental test, applied to a frozen sample of Perna perna mussel meat (Breternitz et al., 2017). The flavouring has been obtained by microencapsulation of meat protein, which has been synthesized through enzymatic hydrolysis. The results of the flavouring’ sensory acceptance suggest the potential of this alternative. In fact, revised references confirm the commercial availability of this alternative (e.g., Carnad A/S (n.d.).
Strategies for heterogeneous waste
The sustainable management alternatives discussed in the aforementioned paragraphs require the implementation of waste segregation practices. Nevertheless, as a result of waste compaction, the waste in the industrial mussel processing is mainly heterogeneous. Even, from the shell removal sub-process (S4.3.3 in Figure 3), the shells present tissue residues, which is a limitation to obtain CaCO3 efficiently. In fact, during the pre-processing phases (S4.1 in Figure 3), a large amount of heterogeneous waste is generated. Although this type of waste is still common at the industrialized mussel processing, it has not been widely examined in the technical literature as it occurs for the other mentioned waste categories, which require waste segregation processes. Consequently, the efficient management of this organic debris on an industrial scale is still the major focus of interest for mussel processing plants.
Table 7 depicts the alternatives for this type of waste, proposed by several identified references.
Table 7.
Main sustainable alternatives for heterogeneous waste derived from industrial mussel processing.
| Mussel by-product | Main valorizations | References |
|---|---|---|
| Heterogeneous waste | Fertilizer | Barnaby (2004), Messiga et al. (2016), Spångberg et al. (2013) |
| Biomass | Ammenberg and Feiz (2017), Feiz and Ammenberg (2017), Nkemka and Murto (2013), Rezaei et al. (2013), Wollak et al. (2018) | |
| Peptides | Beaulieu et al. (2013) |
The most common alternatives for the valorization of the heterogeneous organic waste are those linked to its use as fertilizer and as biomass (e.g., Messiga et al., 2016; Wollak et al., 2018).
The controlled decomposition of this organic waste is the means of transforming the waste into fertilizer. The decomposition must be such that the nutrients for the plants can be assimilated. Fertilizer efficiency is usually associated with the presence of nitrogen (N), phosphorus (P) and potassium (K), which are basic and necessary elements in general application fertilizers. As a fertilizer, experimental evidence (e.g., Barnaby, 2004) suggests that mussels’ tissue, while it can be used for this purpose, is less effective than a general-purpose commercial fertilizer (Barnaby, 2004). Moreover, this effectiveness is reduced when the fertilizer is obtained from residual tissue or meat with presence of shell remains. The high CaCO3 content of the shell will buffer the pH of the mix at a level closer to 8.0 making pH control difficult. In addition, while the levels of N and P are similar to those found in a commercial fertilizer of general application, the lack of K content represents a limitation, which can be mitigated by mixing with other organic waste (Spångberg et al., 2013). This mixture also improves its odour characteristic. Furthermore, such potassium deficiency can be compensated for by adding potassium permanganate, or potassium hydroxide (Barnaby, 2004). More recently, sediments derived from blue mussel (Mytilus edulis) industrial processing, have been tested, and compared with synthetic fertilizer, as a sustainable option to improve annual ryegrass and tomato plants’ growth (Messiga et al., 2016). However, mussel sediments have shown lesser effectivity as fertilizer when compared with synthetic fertilizer. Related to its limited performance is that sediments analysis has revealed some variability on N, P, K and Ca contents, which depends on time and harvest location, showing also a better performance as fertilizer for acid soils (Messiga et al., 2016) – this by-product has been successfully tested. Nevertheless, this organic waste is a proved source of micronutrients for crops, and despite its limited efficiency, it may help to reduce the synthetic fertilizer use. However, evidence has also been found that mussels concentrate some dangerous heavy metals such as cadmium, much of which will be partitioned into the waste stream (Bias and Karbe, 1985). Therefore, some of these heavy metals will also concentrate in plants, implying a potential issue with fertilizer.
On the other hand, the capacity of heterogeneous waste to constitute biomass, or to generate biofuels, has also been investigated (e.g., Nkemka and Murto, 2013; Rezaei et al., 2013). Anaerobic digestion of waste, consisting of mussel shells and meat, has been experimentally evaluated to identify its efficiency in biogas production. According to Nkemka and Murto (2013), the results indicate that the efficiency of methane production is lower when the biomass is made up of meat and mussel shells, compared to the efficiency obtained when the biomass is made up of only mussel meat. In addition to questioning the use of this residue, consisting of meat and shells, as a biomass, the authors also warned of a risk of ammonia. To overcome this issue, they proposed to add low N substrates to aerobic digestion (Nkemka and Murto, 2013). It also worthy to notice that, under anaerobic condition, mussels’ shell is inert, but it will buffer the solution against acid instability.
Also, in an experimental context, the production of calcium oxide (CaO) from the process of calcination of the shells has been examined, as CaO is a catalyst for the production of biodiesel. Using response surface methodology, researchers Rezaei et al. (2013) explored the effects of calcination temperature, catalyst concentration and molar ratio of methanol to oil used in the investigation (soybean oil). The results indicate that using a calcination temperature of 1050°C, a catalyst concentration of 12% and a molar ratio of 24:1, it is possible to produce biodiesel with the highest purity value (Rezaei et al., 2013). Despite these promising results, it has to be taken into account that most lime is still made from limestone that is an abundant, common and inexpensive mineral.
Those references coincide in that the efficiency of the waste, for an energetic use, is weakened due to its heterogeneous condition. Particularly, an issue of technological origin arises when dealing with the presence of shells in this organic waste. The shells must be crushed, a condition that favours sedimentation and makes the operation of the digester difficult. This can be overcome by using multi-stage digesters (Ammenberg and Feiz, 2017). However, such a solution is still being explored in pilot schemes (e.g., Nkemka and Murto 2013), without having plenty of evidence for its availability at the industrial level.
An interesting potential solution has been explored by Beaulieu et al. (2013) at pilot scale. They processed 100 kg of heterogeneous waste (i.e., damaged shells and non-commercial size mussels), coming from a production line of blue mussel (M. edulis), in order to obtain protein hydrolysates (peptides) by a pilot scale enzymatic hydrolysis process. Although this work focuses on the recovered protein hydrolysates’ anti-proliferative activity (i.e., inhibition growth), tested and validated on four immortalized cancerous cell lines, it must be also highlighted that the proposed process for the heterogeneous waste is as an alternative, in relation to conventional strategies, such as those discussed before. The authors recognized the necessity to improve this still experimental process, to generate bioactive peptides of interest, suggesting, among other factors, that the removal of mussels’ shells is required to enhance the recovery of bioactive peptides, since presence of CaCO3 affects the enzymatic hydrolysis (Beaulieu et al., 2013).
Strategies for WW
The WW derived from cooking the whole mussel or its meat, has an organic load that, instead of being treated regularly as an effluent, can become a by-product, given its nutritional value (Cros et al., 2004). Several alternatives have been explored for WW, as it indicated in Table 8. For example, research carried out by Cros et al. (2004) concluded that it is technically feasible to treat the WW from mussel cooking, in order to produce a concentrate for the food or animal feed industries. By means of well-established industrial techniques such as centrifugation, electrodialysis and reverse osmosis processes, applied to WW samples recovered from the industrial cooking of M. edulis, the aforementioned researchers reported obtaining a concentrate that retains the original aroma characteristics of the water used for cooking. Although their paper warns that an economic feasibility study should be carried out, the results of the concentrate’s chemical characterization, as well as a sensory evaluation by a panel of experts, support this potential option. In fact, the commercial availability of this by-product is already known (e.g., Carnad A/S, n.d.).
The mussel processing WW has also been explored as a natural source to synthesize astaxanthin (Rodríguez Amado and Vázquez, 2015), a broad-spectrum carotenoid used in the pharmaceutical, food and cosmetic industries (Guerin et al., 2003). Currently, the natural source for astaxanthin is the green freshwater algae (Haematococcus pluvialis). In fact, its production is higher than yeasts, the preferred source for astaxanthin bioproduction on an industrial scale, due to their higher growth rates and easier cultivation conditions (Bumbak et al., 2011). Nevertheless, the growing demand for this carotenoid has also contributed to examining other alternatives, employing low-cost substrates such as those derived from food waste (Gervasi et al., 2019).
Despite the reported results (Rodríguez Amado and Vázquez, 2015) suggesting that mussel processing WW is a promising nutritive medium for astaxanthin, even higher than a synthetic industrial media (i.e., yeast peptone dextrose), those results are kept in an experimental framework, emphasizing the potential of this option, but without contrasting results with the other natural sources already used for astaxanthin bioproduction.
In a similar context, mussel processing WW has been successfully evaluated as a carbon substrate for a diverse number of microbial bioproducts, such as gibberellic acid (Pastrana et al., 1993), single cell proteins (González et al., 1987), glucose oxidase (Mirón et al., 2010), citric acid (Pintado et al., 1993), pediocin (Vázquez et al., 2004), hyaluronic acid (Vázquez et al., 2010b), and amylases (Murado et al., 1997). Though there are promising laboratory results, reported by several studies, the outcomes also confirm the necessity to optimize the corresponding procedures, as well as industrial-scale trials development for validation purposes (Prieto et al., 2015).
Conclusion and perspectives
There are several, and well recognized, alternatives towards to a sustainable management of organic waste derived from the mytiliculture industry. Nevertheless, a vast majority of them still remain in an experimental context, in a development phase, or are limited by uncertainties linked with their technical–economic feasibility for an industrial scale development. Consequently, future research should be oriented towards technology development for the commercial availability of the majority of the above sustainable options revisited, in conjunction with the implementation of human health and environmental safety polices.
In the light of the conducted review, the following conclusions can be stated:
(a) Mussels shells’ valorization alternatives are the most implemented waste sustainable strategies on an industrial scale. There is plenty of evidence for their industrial waste valorization. Mussels shells constitute a reliable source of CaCO3, calcium hydroxide and CaO.
(b) Byssus threads’ valorization remains as a potential alternative in an industrial context. They are mainly an object of interest due to their biological adhesion capacity and ability to attach to wet fouled surfaces, receiving, therefore, attention for biomedicine applications. However, there is still a gap between the results confined in a laboratory environment and a functional extraction of their adhesive proteins. Restrictions are on limited yields of these proteins as well as on costly extraction procedures.
(c) Employing residual meat as additive for fishmeal or as a source to produce mussel meal are the most common industrial valorizations. It is worthy to notice that, even though the required industrial technology is well established, other sustainable options such as emulsions for human consumption or as food flavouring agents are not quite extended in an industrial context. Furthermore, the physical characterization practised on rejected mussels’ samples suggests that it is feasible to obtain up to a similar yield to those mussels that meet the dimensional criterion. Therefore, the recovery meat could be either supplied to a third party or reprocessed for a production oriented to another market.
(d) Production of aromatic mussel concentrates from WW derived from a cooking process is also a feasible industrial valorization. In fact, this valorization is already commercially available. Plenty of experimental evidence points out that mussel processing WW can be also used as a growth medium for bioproduction.
(e) The heterogeneous mussel waste constitutes a significant limitation for an efficient development of the sustainable strategies revisited above. As it has been mentioned, commonly, heterogeneous waste is generated when solid mussel waste is compacted due to disposal purposes. While waste segregation is not fully implemented, the challenge will be dealing with this waste configuration. Even the efficiency of well-established options, such as obtaining CaCO3 from shells or transforming it into fertilizers or biomass, are affected by the waste’s heterogeneity.
Furthermore, the outcomes from this review also give rise to the following perspectives:
(a) Mussels shells can be valorized as a bio-absorbent for pollutants, or even as a soil amendment. A promising approach, for future research on the valorization of this specific waste, is the development of feasible industrial techniques for the extraction of chitin and chitosan.
(b) Because of limitations for a functional extraction of byssus proteins, research efforts have been oriented to develop hybrid types of mussels’ byssus-inspired adhesive proteins.
(c) Development of research to improve the production efficiency when mussel WW is used as a growth medium for bioproduction is still required to reduce the gap between these alternatives and their industrial implementations.
Interventions in the process line, aimed at early implementation of waste segregation sub-processes, from stages such as raw material pre-processing and quality control stages, are short-term strategies, with low difficulty and low associated cost, to facilitate the incorporation and improvement of sustainable management in this industry.
Acknowledgments
The authors acknowledge the reviewers for their insightful comments and recommendations to improve this article.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We thank the Institute of Design and Industrial Methods, from the Faculty of Engineering Sciences and the Institute of Anatomy, Histology and Pathology, from the Faculty of Medicine, at Universidad Austral de Chile, for the financial support granted to develop this research.
ORCID iD: Luis U Medina Uzcátegui  https://orcid.org/0000-0001-9681-0590
https://orcid.org/0000-0001-9681-0590
References
- Abdulkarim A, Isa MT, Abdulsalam S, et al. (2013) Extraction and characterisation of chitin and chitosan from mussel shell. Extraction 3: 108–114. [Google Scholar]
- Abeynaike A, Wang L, Jones MI, et al. (2011) Pyrolysed powdered mussel shells for eutrophication control: Effect of particle size and powder concentration on the mechanism and extent of phosphate removal. Asia-Pacific Journal of Chemical Engineering 6: 231–243. [Google Scholar]
- Alabaraoye E, Achilonu M, Hester R. (2018) Biopolymer (Chitin) from various marine seashell wastes: Isolation and characterization. Journal of Polymers and the Environment 26: 2207–2218. [Google Scholar]
- Álvarez E, Fernández-Sanjurjo MJ, Seco N, et al. (2012) Use of mussel shells as a soil amendment: Effects on bulk and rhizosphere soil and pasture production. Pedosphere 22: 152–164. [Google Scholar]
- Amado IR, Vázquez JA. (2015) Mussel processing wastewater: A low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous. Microbial Cell Factories 14: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammenberg J, Feiz R. (2017) Assessment of feedstocks for biogas production, part II—Results for strategic decision making. Resources, Conservation and Recycling 122: 388–404. [Google Scholar]
- Aquerreta Y, Astiasarán I, Mohino A, et al. (2002) Composition of pâtés elaborated with mackerel flesh (Scomber scombrus) and tuna liver (Thunnus thynnus): Comparison with commercial fish pâtés. Food Chemistry 77: 147–153. [Google Scholar]
- azti tecnalia (2017) Guía de Minimización de Subproductos y residuos de la acuicultura [By-products and waste minimization guide from aquaculture] (p. 76). Fundación Biodiversidad. Available at: https://www.observatorio-acuicultura.es/sites/default/files/images/adjuntos/libros/guia_mininimizacion_residuos_acuicultura_web.pdf (accessed 20 February 2021). [In Spanish.]
- Ballester P, Mármol I, Morales J, et al. (2007) Use of limestone obtained from waste of the mussel cannery industry for the production of mortars. Cement and Concrete Research 37: 559–564. [Google Scholar]
- Barnaby C. (2004) An investigation into the reuse of organic waste produced by the New Zealand Mussel Industry. PhD Thesis, Auckland University of Technology. [Google Scholar]
- Barros MC, Bello PM, Bao M, et al. (2009. a) From waste to commodity: Transforming shells into high purity calcium carbonate. Journal of Cleaner Production 3: 400–407. [Google Scholar]
- Barros MC, Magán A, Valiño S, et al. (2009. b) Identification of best available techniques in the seafood industry: A case study. Journal of Cleaner Production 17: 391–399. [Google Scholar]
- Beaulieu L, Thibodeau J, Bonnet C, et al. (2013) Evidence of anti-proliferative activities in blue mussel (Mytilus edulis) by-products. Marine Drugs 11: 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee S-L, Hamid ZA. (2020) Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceramics International 46: 17149–17175. [Google Scholar]
- Bias R, Karbe L. (1985) Bioaccumulation and partitioning of cadmium within the freshwater mussel Dreissena polymorpha Pallas. Internationale Revue der gesamten Hydrobiologie und Hydrographie 70: 113–125. [Google Scholar]
- Bohnes FA, Laurent A. (2019) LCA of aquaculture systems: Methodological issues and potential improvements. The International Journal of Life Cycle Assessment 24: 324–337. [Google Scholar]
- Breternitz NR, Bolini HMA, Hubinger MD. (2017) Sensory acceptance evaluation of a new food flavoring produced by microencapsulation of a mussel (Perna perna) protein hydrolysate. LWT-Food Science and Technology 83: 141–149. [Google Scholar]
- Buasri A, Chaiyut N, Loryuenyong V, et al. (2013) Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. The Scientific World Journal 2013: 460923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugallo PMB, Stupak A, Andrade LC, et al. (2012) Material flow analysis in a cooked mussel processing industry. Journal of Food Engineering 113: 100–117. [Google Scholar]
- Bumbak F, Cook S, Zachleder V, et al. (2011) Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Applied Microbiology and Biotechnology 91: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byette F, Pellerin C, Marcotte I. (2014) Self-assembled pH-responsive films prepared from mussel anchoring threads. Journal of Materials Chemistry B 2: 6378–6386. [DOI] [PubMed] [Google Scholar]
- Carnad A/S. (n.d.) Carnad Mussel Extract/Juice and Powder. Recuperado 1 de agosto de 2019, de Available at: http://www.carnad.dk/mussel.asp (accessed 20 February 2021).
- Cauchie H-M. (2002) Chitin production by arthropods in the hydrosphere. Hydrobiologia 470: 63–95. [Google Scholar]
- Cha HJ, Hwang DS, Lim S. (2008) Development of bioadhesives from marine mussels. Biotechnology Journal: Healthcare Nutrition Technology 3(5): 631–638. [DOI] [PubMed] [Google Scholar]
- Chiou IJ, Chen CH, Li YH. (2014) Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Construction and Building Materials 64: 480–487. [Google Scholar]
- Coyne KJ, Qin X-X, Waite JH. (1997) Extensible collagen in mussel byssus: A natural block copolymer. Science 277: 1830–1832. [DOI] [PubMed] [Google Scholar]
- Craggs R, Cooke J, Mathieson T, et al. (2010) Potential of mussel shell as a biosorbent for stormwater treatment. Auckland Regional Council Technical Report No.046. 57pp. [Google Scholar]
- Cros S, Ignot BL, Razafintsalama C, et al. (2004) Electrodialysis desalination and reverse osmosis concentration of an industrial mussel cooking juice: Process impact on pollution reduction and on aroma quality. Journal of Food Science 69: C435–C442. [Google Scholar]
- Cros S, Lignot B, Bourseau P, et al. (2005) Desalination of mussel cooking juices by electrodialysis: Effect on the aroma profile. Journal of Food Engineering 69: 425–436. [Google Scholar]
- Dhillon GS, Kaur S, Brar SK, et al. (2013) Green synthesis approach: Extraction of chitosan from fungus mycelia. Critical Reviews in Biotechnology 33: 379–403. [DOI] [PubMed] [Google Scholar]
- Edralin EJM, Garcia JL, dela Rosa FM, et al. (2017) Sonochemical synthesis, characterization and photocatalytic properties of hydroxyapatite nano-rods derived from mussel shells. Materials Letters 196: 33–36. [Google Scholar]
- El Knidri H, Belaabed R, Addaou A, et al. (2018) Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules 120: 1181–1189. [DOI] [PubMed] [Google Scholar]
- FAO Fisheries Division, Statistics and Information Branch. 2020. FishStatJ: Universal software for fishery statistical time series. Available at: www.fao.org/fishery/statistics/software/fishstatj/en (accessed 20 February 2021).
- Feiz R, Ammenberg J. (2017) Assessment of feedstocks for biogas production, part I—A multi-criteria approach. Resources, Conservation and Recycling 122: 373–387. [Google Scholar]
- Felipe-Sesé M, Eliche-Quesada D, Corpas-Iglesias FA. (2011) The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceramics International 37: 3019–3028. [Google Scholar]
- Ferraz E, Gamelas JA, Coroado J, et al. (2019) Recycling waste seashells to produce calcitic lime: Characterization and wet slaking reactivity. Waste and Biomass Valorization 10: 2397–2414. [Google Scholar]
- Food and Agriculture Organization of the United Nations (2019) State of Food and Agriculture 2019 (Sofa): Moving forward on food loss and waste reduction. Available at: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed 20 February 2021).
- Food and Agriculture Organization of the United Nations (2020. a) FAO Fisheries & Aquaculture—Statistics—Introduction. Available at: http://www.fao.org/fishery/statistics/en (accessed 20 February 2021).
- Food and Agriculture Organization of the United Nations (2020. b) The State of World Fisheries and Aquaculture 2020: Sustainabilty in Action. Available at: http://www.fao.org/3/ca9229en/ca9229en.pdf (accessed 20 February 2021).
- Food and Agriculture Organization of the United Nations Fisheries Division (2020. a) Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2018 (FishstatJ). Updated 2020. Available at: www.fao.org/fishery/statistics/software/fishstatj/en (accessed 20 February 2021).
- Food and Agriculture Organization of the United Nations Fisheries Division (2020. b) Fishery and Aquaculture Statistics. Global Fisheries commodities production and trade 1976–2018 (FishstatJ). Updated 2020. Available at: www.fao.org/fishery/statistics/software/fishstatj/en (accessed 20 February 2021).
- Gervasi T, Santini A, Daliu P, et al. (2019) Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agroforestry Systems 1–6. [Google Scholar]
- González M, Mirón J, Murado MA. (1987) Culture of Endomyces fibuliger in mussel processing wastes and precipitation with pegs of its extracellular amylolytic system. Biotechnology Letters 9: 281–286. [Google Scholar]
- Guerin M, Huntley ME, Olaizola M. (2003) Haematococcus astaxanthin: Applications for human health and nutrition. TRENDS in Biotechnology 21: 210–216. [DOI] [PubMed] [Google Scholar]
- Hamed I, Özogul F, Regenstein JM. (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends in Food Science & Technology 48: 40–50. [Google Scholar]
- Hamester MRR, Balzer PS, Becker D. (2012) Characterization of calcium carbonate obtained from oyster and mussel shells and incorporation in polypropylene. Materials Research 15: 204–208. [Google Scholar]
- Harrington MJ, Jehle F, Priemel T. (2018) Mussel byssus structure–function and fabrication as inspiration for biotechnological production of advanced materials. Biotechnology Journal 13: 1800133. [DOI] [PubMed] [Google Scholar]
- Hart A. (2020) Mini-review of waste shell-derived materials’ applications. Waste Management & Research 38: 514–527. [DOI] [PubMed] [Google Scholar]
- Iribarren D, Moreira MT, Feijoo G. (2010. a) Implementing by-product management into the life cycle assessment of the mussel sector. Resources, Conservation and Recycling 54: 1219–1230. [Google Scholar]
- Iribarren D, Moreira MT, Feijoo G. (2010. b) Life cycle assessment of fresh and canned mussel processing and consumption in Galicia (NW Spain). Resources, Conservation & Recycling 2: 106–117. [Google Scholar]
- Iribarren D, Moreira MT, Feijoo G. (2010. c) Revisiting the life cycle assessment of mussels from a sectorial perspective. Journal of Cleaner Production 18: 101–111. [Google Scholar]
- Jayathilakan K, Sultana K, Radhakrishna K, et al. (2012) Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. Journal of Food Science and Technology 49: 278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Dhillon GS. (2015) Recent trends in biological extraction of chitin from marine shell wastes: A review. Critical Reviews in Biotechnology 35: 44–61. [DOI] [PubMed] [Google Scholar]
- Kaushik NK, Kaushik N, Pardeshi S, et al. (2015) Biomedical and clinical importance of mussel-inspired polymers and materials. Marine Drugs 13: 6792–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MD, Ahn JW, Nam G. (2018) Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. Journal of Environmental Management 223: 947–951. [DOI] [PubMed] [Google Scholar]
- Kumar GS, Girija EK, Venkatesh M, et al. (2017) One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceramics International 43: 3457–3461. [Google Scholar]
- Kuo S, Ortiz-Escobar ME, Hue NV, et al. (2004) Composting and compost utilization for agronomic and container crops. Recent Research Development Environmental Biology 1: 451–513. [Google Scholar]
- Laboratorio de Servicios ICYTAL (2019. a) Informe de Resultados (muestras de residuo triturado) [Results Report (shredded waste samples)] (N.o 7168; p. 1). Instituto de Ciencia y Tecnología de los Alimentos. Universidad Austral de Chile. [In Spanish.] [Google Scholar]
- Laboratorio de Servicios ICYTAL (2019. b) Informe de Resultados (muestras de residuo compactado) [Results Report (compacted residue samples)] (N.o 7168; p. 1). Instituto de Ciencia y Tecnología de los Alimentos. Universidad Austral de Chile. [In Spanish.] [Google Scholar]
- Langeland M, Vidakovic A, Vielma J, et al. (2016) Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis). Aquaculture Nutrition 22: 485–495. [Google Scholar]
- Lee BP, Messersmith PB, Israelachvili JN, et al. (2011) Mussel-inspired adhesives and coatings. Annual Review of Materials Research 41: 99–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertwattanaruk P, Makul N, Siripattarapravat C. (2012) Utilization of ground waste seashells in cement mortars for masonry and plastering. Journal of Environmental Management 111: 133–141. [DOI] [PubMed] [Google Scholar]
- Li M, Yao ZT, Chen T, et al. (2014) The antibacterial activity and mechanism of mussel shell waste derived material. Powder Technology 264: 577–582. [Google Scholar]
- Li X, Gao G, Sun C, et al. (2015) Preparation and antibacterial performance testing of Ag nanoparticles embedded biological materials. Applied Surface Science 330: 237–244. [Google Scholar]
- Lopes C, Antelo LT, Franco-Uría A, et al. (2015) Valorisation of fish by-products against waste management treatments–Comparison of environmental impacts. Waste Management 46: 103–112. [DOI] [PubMed] [Google Scholar]
- Lourguioui H, Brigolin D, Boulahdid M, et al. (2017) A perspective for reducing environmental impacts of mussel culture in Algeria. The International Journal of Life Cycle Assessment 22: 1266–1277. [Google Scholar]
- Lu J, Cong X, Li Y, et al. (2018) Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. Journal of Cleaner Production 172: 1978–1985. [Google Scholar]
- Luo H, Huang G, Fu X, et al. (2013). Waste oyster shell as a kind of active filler to treat the combined wastewater at an estuary. Journal of Environmental Sciences 25: 2047–2055. [DOI] [PubMed] [Google Scholar]
- Macha IJ, Ozyegin L, Chou J, et al. (2013) An alternative synthesis method for di calcium phosphate (Monetite) powders from Mediterranean mussel ( Mytilus galloprovincialis) shells. Journal of the Australian Ceramic Society 49: 122–128. [Google Scholar]
- Martínez-García C, González-Fonteboa B, Carro-López D, et al. (2019) Design and properties of cement coating with mussel shell fine aggregate. Construction and Building Materials 215: 494–507. [Google Scholar]
- Messiga AJ, Sharifi M, McVicar K, et al. (2016) Mussel’s post-harvest washing sediments consistency over time, and contribution to plant growth and nutrient uptake. Journal of Cleaner Production 113: 216–223. [Google Scholar]
- Mirón J, Vázquez JA, González P, et al. (2010) Enhancement glucose oxidase production by solid-state fermentation of Aspergillus niger on polyurethane foams using mussel processing wastewaters. Enzyme and Microbial Technology 46: 21–27. [Google Scholar]
- Mo KH, Alengaram UJ, Jumaat MZ, et al. (2018) Recycling of seashell waste in concrete: A review. Construction and Building Materials 162: 751–764. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montroni D, Piccinetti C, Fermani S, et al. (2017) Exploitation of mussel byssus mariculture waste as a water remediation material. RSC Advances 7: 36605–36611. [Google Scholar]
- Montroni D, Valle F, Rapino S, et al. (2018) Functional biocompatible matrices from mussel byssus waste. ACS Biomaterials Science & Engineering 4: 57–65. [DOI] [PubMed] [Google Scholar]
- Muminović M. (2011) Domestic Feed Sources to Farmed Arctic Charr (Salvelinus alpinus). MSc Thesis. Department of Animal and Aquacultural Sciences, Norwegian University of Life Sciences. Available at: https://core.ac.uk/download/pdf/52079524.pdf (accessed 20 February 2021).
- Murado MA, González M, Torrado A, et al. (1997) Amylase production by solid state culture of Aspergillus oryzae on polyurethane foams. Some mechanistic approaches from an empirical model. Process Biochemistry 32: 35–42. [Google Scholar]
- Naik AS, Hayes M. (2019) Bioprocessing of mussel by-products for value added ingredients. Trends in Food Science & Technology 92: 111–121 [Google Scholar]
- Naqi A, Siddique S, Kim H-K, et al. (2020) Examining the potential of calcined oyster shell waste as additive in high volume slag cement. Construction and Building Materials 230: 116973. [Google Scholar]
- Nkemka VN, Murto M. (2013) Two-stage anaerobic dry digestion of blue mussel and reed. Renewable Energy 50: 359–364. [Google Scholar]
- Paradelo R, Conde-Cid M, Cutillas-Barreiro L, et al. (2016) Phosphorus removal from wastewater using mussel shell: Investigation on retention mechanisms. Ecological Engineering 97: 558–566. [Google Scholar]
- Pastrana LM, Gonzalez MP, Murado MA. (1993) Production of gibberellic acid from mussel processing wastes in submerged batch culture. Bioresource Technology 45: 213–221. [Google Scholar]
- Paz-Ferreiro J, Baez-Bernal D, Insúa JC, et al. (2012) Effects of mussel shell addition on the chemical and biological properties of a Cambisol. Chemosphere 86: 1117–1121. [DOI] [PubMed] [Google Scholar]
- Peña-Rodríguez S, Bermúdez-Couso A, Nóvoa-Muñoz JC, et al. (2013) Mercury removal using ground and calcined mussel shell. Journal of Environmental Sciences 25: 2476–2486. [DOI] [PubMed] [Google Scholar]
- Pintado J, Murado MA, González M, et al. (1993) Joint effect of nitrogen and phosphorus concentrations on citric acid production by different strains of Aspergillus niger grown on an effluent. Biotechnology Letters 15: 1157–1162. [Google Scholar]
- Prieto MA, Prieto I, Vázquez JA, et al. (2015) An environmental management industrial solution for the treatment and reuse of mussel wastewaters. Science of the Total Environment 538: 117–128. [DOI] [PubMed] [Google Scholar]
- Qin C, Pan Q, Qi Q, et al. (2016) In-depth proteomic analysis of the byssus from marine mussel Mytilus coruscus. Journal of Proteomics 144: 87–98. [DOI] [PubMed] [Google Scholar]
- Quintáns-Fondo A, Ferreira-Coelho G, Paradelo-Núñez R, et al. (2016) Promoting sustainability in the mussel industry: Mussel shell recycling to fight fluoride pollution. Journal of Cleaner Production 131: 485–490. [Google Scholar]
- Ravindran R, Jaiswal AK. (2016) Exploitation of food industry waste for high-value products. Trends in Biotechnology 34: 58–69. [DOI] [PubMed] [Google Scholar]
- Renner-Rao M, Clark M, Harrington MJ. (2019) Fiber formation from liquid crystalline collagen vesicles isolated from mussels. Langmuir 35: 15992–16001. [DOI] [PubMed] [Google Scholar]
- Rezaei R, Mohadesi M, Moradi GR. (2013) Optimization of biodiesel production using waste mussel shell catalyst. Fuel 109: 534–541. [Google Scholar]
- Rodríguez Amado I, Vázquez JA. (2015) Mussel processing wastewater: A low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous. Microbial Cell Factories 14: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romar-Gasalla A, Santás-Miguel V, Nóvoa-Muñoz JC, et al. (2018) Chromium and fluoride sorption/desorption on un-amended and waste-amended forest and vineyard soils and pyritic material. Journal of Environmental Management 222: 3–11. [DOI] [PubMed] [Google Scholar]
- Rustad T. (2003) Utilisation of marine by-products. Electronic Journal of Environmental Agricultural and Food Chemistry 2: 458–463. [Google Scholar]
- Rustad T, Storrø I, Slizyte R. (2011) Possibilities for the utilisation of marine by-products. International Journal of Food Science & Technology 46: 2001–2014. [Google Scholar]
- Seco-Reigosa N, Cutillas-Barreiro L, Nóvoa-Muñoz JC, et al. (2014) Mixtures including wastes from the mussel shell processing industry: Retention of arsenic, chromium and mercury. Journal of Cleaner Production 84: 680–690. [Google Scholar]
- Seco-Reigosa N, Peña-Rodríguez S, Nóvoa-Muñoz JC, et al. (2013) Arsenic, chromium and mercury removal using mussel shell ash or a sludge/ashes waste mixture. Environmental Science and Pollution Research 20: 2670–2678. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Varatharajan V, Peng H, et al. (2019) Utilization of marine by-products for the recovery of value-added products. Journal of Food Bioactives 6: 10–61. [Google Scholar]
- Song G, Zhang M, Zhou X, et al. (2019) Analysis of flavor change in the industrial production of fungal fermentation based mussel (Mytilus edulis) cooking liquor using a laser irradiation desorption based GC/MS method. LWT 112. [Google Scholar]
- Spångberg J, Jönsson H, Tidåker P. (2013) Bringing nutrients from sea to land – mussels as fertiliser from a life cycle perspective. Journal of Cleaner Production 51: 234–244. [Google Scholar]
- Suhre MH, Gertz M, Steegborn C, et al. (2014) Structural and functional features of a collagen-binding matrix protein from the mussel byssus. Nature Communications 5: 1–11. [DOI] [PubMed] [Google Scholar]
- Suplicy FM. (2018) A review of the multiple benefits of mussel farming. Reviews in Aquaculture 12(1): 204–223. [Google Scholar]
- Tayeh BA, Hasaniyah MW, Zeyad AM, et al. (2020) Durability and mechanical properties of seashell partially-replaced cement. Journal of Building Engineering 31: 101328. [Google Scholar]
- Vakili M, Rafatullah M, Salamatinia B, et al. (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydrate Polymers 113: 115–130. [DOI] [PubMed] [Google Scholar]
- Vareltzis PK, Undeland I. (2012) Protein isolation from blue mussels (Mytilus edulis) using an acid and alkaline solubilisation technique—Process characteristics and functionality of the isolates. Journal of the Science of Food and Agriculture 92: 3055–3064. [DOI] [PubMed] [Google Scholar]
- Vázquez JA, González MP, Murado MA. (2004) Pediocin production by Pediococcus acidilactici in solid state culture on a waste medium: Process simulation and experimental results. Biotechnology and Bioengineering 85: 676–682. [DOI] [PubMed] [Google Scholar]
- Vázquez JA, Montemayor MI, Fraguas J, et al. (2010) Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microbial Cell Factories 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenstedt L, Jönsson L. (2006) Mussel meal as a high quality protein source for broiler chickens. In: Proceedings of 12th European Poultry Conference, Verona, Italy, 10–14 September 2006. World’s Poultry Science Association. [Google Scholar]
- Wang B, Li L, Chi C-F, et al. (2013) Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chemistry 138: 1713–1719. [DOI] [PubMed] [Google Scholar]
- Weiss M, Buck BH. (2017) Partial replacement of fishmeal in diets for turbot (Scophthalmus maximus, Linnaeus, 1758) culture using blue mussel (Mytilus edulis, Linnaeus, 1758) meat. Journal of Applied Ichthyology 33: 354–360. [Google Scholar]
- Wiegemann M. (2005) Adhesion in blue mussels (Mytilus edulis) and barnacles (genus Balanus): Mechanisms and technical applications. Aquatic Sciences 67: 166–176. [Google Scholar]
- Wollak B, Forss J, Welander U. (2018) Evaluation of blue mussels (Mytilus edulis) as substrate for biogas production in Kalmar County (Sweden). Biomass and Bioenergy 111: 96–102. [Google Scholar]
- Yao Z, Xia M, Li H, et al. (2014) Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Critical Reviews in Environmental Science and Technology 44: 2502–2530. [Google Scholar]
- Yoon H, Park S, Lee K, et al. (2004) Oyster shell as substitute for aggregate in mortar. Waste Management & Research 22: 158–170. [DOI] [PubMed] [Google Scholar]
- Yu M, Hwang J, Deming TJ. (1999) Role of L-3, 4-dihydroxyphenylalanine in mussel adhesive proteins. Journal of the American Chemical Society 121: 5825–5826. [Google Scholar]
- Zhang Y, Liu S, Chen P. (2013) Study on the properties of calcined waste mussel shell. Nature Environment and Pollution Technology 12: 435. [Google Scholar]