Abstract
Introduction
The rising burden of cancer in low- and middle-income countries (LMICs) has led to substantial efforts to improve access to chemotherapy. The present study’s objectives were to obtain an overview of the safe handling practices implemented in LMICs’ healthcare facilities when dealing with chemotherapy drugs and to prioritize opportunities for improving them.
Methods
We conducted an online survey, from June 2018 to April 2019, among LMIC healthcare facilities dealing with chemotherapy drugs. Facilities were asked to self-assess their chemotherapy handling processes using Cyto-SAT, a self-assessment tool incorporating 134 items organized into 10 domains (management, personnel, logistics, prescription, preparation, administration, incident management, waste management, cleaning, and patient counselling). Data were recorded on an online platform (www.datapharma.ch/cyto-SAT).
Results
The survey enrolled 53 healthcare facilities (15 from low-income, 26 from lower-middle-income, and 12 from upper-middle-income countries). The median level of implementation of safe practices was 63% (Q1:39%–Q3:77%). Facilities in low-income countries (LICs) reported lower median levels of safe practices than middle-income countries (MICs) [LICs: 32% (Q1:24%–Q3:62%), Lower-MICs: 63% (Q1:49%–Q3:70%), Upper-MICs: 85% (Q1:77%–Q3:93%)]. The biggest differences between country categories were observed in the domains related to personnel, preparation processes, and incident management.
Conclusion
This overview of practices highlighted a large variability and major gaps in the safe handling of chemotherapy drugs in LMICs. Improvement strategies are needed to increase patient and staff safety and limit environmental contamination, especially in LICs. Safe handling programs should be part of continuing efforts to improve access to quality cancer drugs and should be integrated into national cancer control programs.
Keywords: Safe handling practices, cytotoxic drugs, low- and middle-income countries, oncology, chemotherapy
Introduction
It is well known that handling chemotherapy drugs is a high-risk process for human and environmental health. These drugs have long been considered hazardous and require special precautions.1,2 Due to their inherent toxicities, their narrow therapeutic index, and the fragility of cancer patients, any incident resulting from a medication error can have dramatic consequences on patient health.3–5 Beyond patient safety, risks related to occupational exposure are also a major concern. In the 1970s and 1980s, acute, long-term toxic effects were reported among the personnel handling these products without specific precautions.6,7 Since then, the risks of occupational exposure have been widely discussed in the literature. 8 Professional associations, national authorities, insurance companies, and other organs have developed guidelines to protect workers as well as recommendations on safe handling practices.2,9–14 Protective measures should be applied not only to healthcare workers (e.g. physicians, nurses, and pharmacists) but also to other technicians involved in the transport, storage, cleaning, or disposal of chemotherapy drugs and related waste. Protection relies on a combination of three different levels of preventive measures and hazard controls: engineering measures, administrative and organizational measures, and personal protective equipment.2,9,10 Besides the risk of occupational exposure, improper cytotoxic waste management could also have dramatic, long-term ecological consequences and constitute a community-wide public health threat. 15 Careful planning in terms of the collection, separation, storage, transport, and final disposal of cytotoxic waste should not be overlooked. Efforts should be made to minimize the risks of contaminating water supplies and soil and facilitate the safe disposal of cytotoxic waste. Thus, implementing safe handling practices are of utmost importance to prevent occupational exposures, ensure patient safety, and limit environmental contamination.
Cancer was long considered as an issue reserved for wealthy countries. However, in recent years, the rising burden of cancer has become a great concern in low- and middle-income countries (LMICs). According to estimates from the International Agency for Research on Cancer (IARC), 10.6 million new cancer cases and 6.7 million cancer-related deaths occurred in LMICs in 2018. 16 To address this heavy economic burden and related human development issues, the World Health Organization (WHO) endorsed a “Global Action Plan for the Prevention and Control of Non-communicable Diseases 2013–2020.” 17 Reducing premature deaths from cancers and implementing cancer prevention initiatives were two objectives set out in both the WHO’s plan and the United Nations Sustainable Development Goals.17,18 In 2017, the desire to accelerate those initiatives and boost hopes of reaching the targets set for 2030 was reflected in the World Health Assembly resolution (WHA 70.12) entitled “Cancer prevention and control through an integrated approach.”19,20 As part of global monitoring strategies, great efforts were made to improve access to chemotherapy. More than 30 chemotherapy agents were included in the WHO’s model list of essential medicines. 21 In the coming years, the number of patients and the use of chemotherapy are both expected to increase significantly; therefore, the potential health hazards related to the handling of chemotherapy drugs must be promptly and fully addressed. To the best of our knowledge, the current literature on handling practices in LMICs settings remains scarce. The present study’s objectives were thus to obtain an overview of the safe handling practices implemented in LMICs’ healthcare facilities when dealing with cytotoxic medicines and to prioritize opportunities for improving them.
Methods
Instrument design and dissemination
We conducted a cross-sectional study among volunteer healthcare facilities dealing with cytotoxic medicines in LMICs designated as such by the World Bank. 22 Participating facilities were asked to form a small, multidisciplinary team and assess their chemotherapy drug handling practices by using the Cyto-SAT self-assessment tool. This free online tool consists of 134 items organized into 10 domains and 28 sub-domains (Table 1) covering all the steps of chemotherapy drug handling (e.g., receipt, storage, transport, prescription, preparation, administration, waste management, cleaning, and patient counseling). Cyto-SAT was validated using a two-round Delphi process involving a panel of 27 pharmaceutical experts in oncology practice from 13 LMICs and high-income countries. 23
Table 1.
Cyto-SAT domain and sub-domain classifications and their number of items.
| Domains | Sub-domains | Number of items accepted by the Delphi panel |
|---|---|---|
| 1. Management | 11 | |
| 2. Personnel | • Education and training | 4 |
| • Medical surveillance | 3 | |
| 3. Logistics | • Receipt | 5 |
| • Storage | 6 | |
| • Transport | 5 | |
| 4. Prescription | 5 | |
| 5. Preparation | • Management and organization | 4 |
| • Parenteral medicine preparation areas | 10 | |
| • Hygiene and personal protective equipment | 6 | |
| • Preparation process set-up | 4 | |
| • Preparation technique | 9 | |
| • Packaging and labeling | 3 | |
| • Checking procedure | 2 | |
| • Documentation | 3 | |
| • Maintenance | 2 | |
| • Non-sterile preparation | 1 | |
| 6. Administration | • Management | 2 |
| • Hygiene and safety measures | 5 | |
| • Documentation | 3 | |
| • Work practices | 4 | |
| 7. Incident management | • Surface contamination | 6 |
| • Staff contamination | 3 | |
| • Extravasation | 3 | |
| • Quality assurance | 1 | |
| 8. Waste management | • Waste disposal | 7 |
| • Patients’ excreta | 3 | |
| 9. Cleaning | • Management and organization | 2 |
| • Cleaning practices | 6 | |
| • Laundry | 2 | |
| 10. Patient counseling | 4 | |
| Total | 134 |
The survey was distributed internationally through social media, professional websites, professional associations’ membership lists (e.g., the International Society of Oncology Pharmacy Practitioners and Pharm-Ed 24 ), community of practice forums (e.g., e-med 25 and e-drugs), newsletters (e.g., Pharm-Ed and Union for International Cancer Control), and professional networking.
Healthcare facilities which decided to participate were provided with detailed written instructions about the survey and how to perform the self-assessment. Data were collected between June 2018 and April 2019. Participants were encouraged to enter their data directly into a web-based platform (www.datapharma.ch/cyto-SAT). However, for facilities with limited internet access, a Microsoft Excel® version of Cyto-SAT was sent out by email, and the principal investigator subsequently transcribed the results returned onto the online platform.
Scoring system
Participants assessed each item on the tool using a scoring system from 1 (no activity) to 4 (fully implemented). The scoring system (Table 2) was based on the one used by Institute for Safe Medication Practices (ISMP) tools. 26
Table 2.
Scoring system.
| Scoring system | |
|---|---|
| 1 | There has been no implementation activity for this item. |
| 2 | This item has been discussed and considered, but it has not been implemented yet. There may be a document, but there has been no implementation and only some staff awareness-raising. |
| 3 | The item has been partially implemented in the facility or implemented only in some areas, or for some patients and/or staff. |
| 4 | The item has been fully implemented throughout the facility for all patients, drugs, and/or staff. |
N.A.: not applicable. This item cannot be considered in the local context.
Note: Scores 3 and 4 can only be selected if there has been real implementation. Unapplied procedures or guidelines are not enough.
Analysis
Data were exported into Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) for the calculation of descriptive statistics. Items with the “not applicable” were not considered answers and were therefore not counted in the data analysis.
Results
Characteristics of the participating facilities
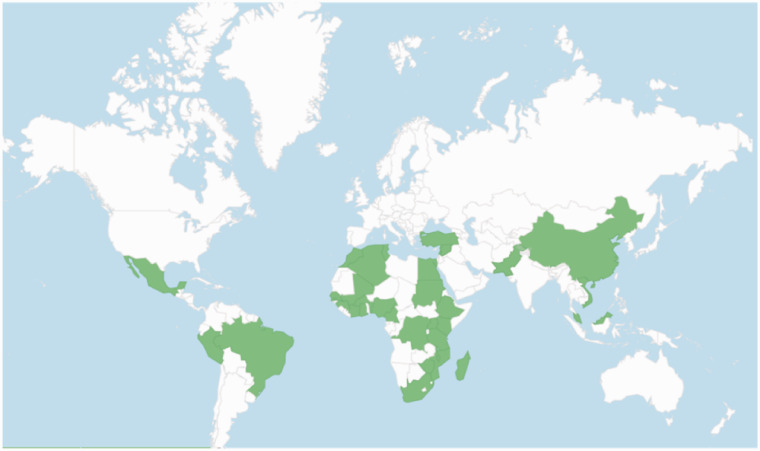
Of the 82 healthcare facilities that registered on the Cyto-SAT web platform, 53 (65%) facilities from 34 countries met the inclusion criteria (Figure 1) and 29 were excluded (26 facilities only completed the general information and 3 were from high-income countries). Among the 53 respondents, 15 (28%) were from low-income countries, 26 (49%) from lower-middle-income countries, and 12 (23%) from upper-middle-income countries (Figure 2).
Figure 1.
Study flow diagram.
Figure 2.
Geographical locations of included survey participants.
Different types of healthcare facilities participated in the survey, with the highest proportion of the respondents (51%) being university hospitals (Table 3). A median number of 300 chemotherapies were reported to be administered monthly, with a great variation among the respondents (Q1:87.5–Q3:950) 27 .
Table 3.
Characteristics of participating healthcare facilities.
| Characteristics of participating healthcare facilities | Number | (%) |
|---|---|---|
|
TOTAL respondents |
53 |
|
| By country income levela | ||
| Upper-middle-income | 12 | 23% |
| Lower-middle-income | 26 | 49% |
| Low-income | 15 | 28% |
| Types of healthcare facility | ||
| Academic/university hospital | 27 | 51% |
| Non-profit private healthcare facility | 3 | 6% |
| For-profit private healthcare facility | 3 | 6% |
| Regional hospital | 8 | 15% |
| District hospital | 2 | 4% |
| Healthcare center | 1 | 2% |
| Unknown | 9 | 17% |
|
|
Median (Q1–Q3) |
|
| Number of departments administering chemotherapies | 1 (1–4) | |
| Number of chemotherapies administered/month | 300 (87.5–950) | |
| Number of staff involved in the preparation and administration of chemotherapies | 6 (5–14.25) | |
aAccording to the World Bank classification for the 2021 fiscal year.
General findings
The median level of the implementation of safe practices was 63% (Q1:39%–Q3:77%) 27 . Facilities from LICs reported a lower level of implementation of safe practices than MICs [LICs: 32% (Q1:24%–Q3:62%), lower-MICs: 63% (Q1:49%–Q3:70%), upper-MICs: 85% (Q1:77%–Q3:93%)].
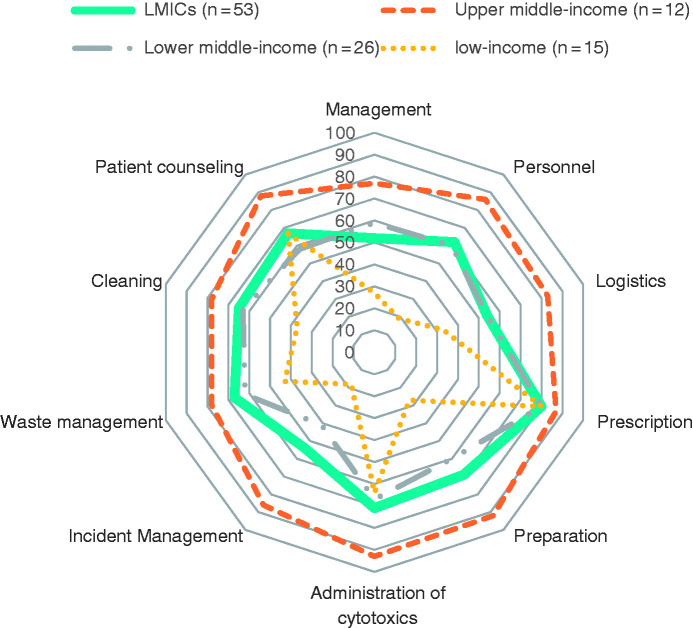
The greatest differences in median implementation levels between country categories were observed in the domains of personnel [LICs: 19% (Q1:14%–Q3:36%), upper-MICs: 86% (Q1:71%–Q3:92%)], preparation processes [LICs: 27% (Q1:17%–Q3:63%), upper-MICs: 92% (Q1:84%–Q3:98%)], and incident management [LICs: 18% (Q1:7%–Q3:65%), upper-MICs: 86% (Q1:82%–Q3:96%)] (Figure 3). Median results for all the domains and sub-domains are presented in Appendix 1.
Figure 3.
Median percentage level of implementation of safe practices, by domain.
The five highest-scored items in the survey were in the domains of prescription (2 items), management (2), and personnel (1), for which 64%–87% of participating facilities self-scored 4 (fully implemented). The five lowest-scored items were in the domains of cleaning (2 items), management (2), and personnel (1), for which 47%–55% of participating facilities self-scored 1 (no activity). Details of these item scores are presented in Table 4.
Table 4.
Top five highest and lowest self-scored items in the survey.
| Top 5 of the highest scored items | |||||
|---|---|---|---|---|---|
|
Item n° |
Domain |
Description |
Number of answers |
% of 4 s |
Median score (Q1–Q3) |
| 35 | Prescription | Only authorized healthcare practitioners can prescribe chemotherapy treatments. | 53 | 87% | 4 (4–4) |
| 7 | Management | Smoking, drinking, and eating are forbidden in areas where cytotoxic medicines are prepared, stored, and administered. | 51 | 71% | 4 (3–4) |
| 17 | Personnel | No pregnant or breastfeeding women are involved in the handling of cytotoxic medicines. | 53 | 66% | 4 (3–4) |
| 6 | Management | A list of the cytotoxic medicines used in the facility is available and regularly updated. | 53 | 66% | 4 (3–4) |
| 36 | Prescription | Prescriptions are based on standard, pre-prepared chemotherapy treatment protocols dependent on the diagnosis and available in the facility (these have either been developed in-house or with reference to an external review board or nationally approved clinical research protocols or guidelines). | 53 | 64% | 4 (3–4) |
| Top 5 of the lowest scored items | |||||
|
Item n° |
Domain |
Description |
Number of answers |
% of 1 s |
Median score (Q1–Q3) |
| 130 | Cleaning | Laundry staff and patients’ relatives have received instructions and know the procedures for handling contaminated linen and clothing, and they wear adequate personal protective equipment. | 46 | 54% | 1 (1–2.75) |
| 52 | Preparation | Pressure gradients between the different rooms in the preparation zone are maintained and monitored continuously. | 52 | 54% | 1 (1–3) |
| 4 | Management | A self-assessment of compliance with safety guidelines regarding the safe handling of cytotoxic medicines is carried out regularly. | 51 | 51% | 1 (1–3) |
| 129 | Cleaning | Contaminated, reusable protective clothing (gowns) and linen soiled with patients’ excreta are placed in clearly labelled laundry bags and are washed separately from other clothing. | 44 | 48% | 2 (1–3) |
| 50 | Preparation | Access to the preparation room is through airlocks only, with adequate procedures to prevent simultaneous door-opening (doors to the cytotoxic preparation room and the external environment). | 53 | 47% | 2 (1–3) |
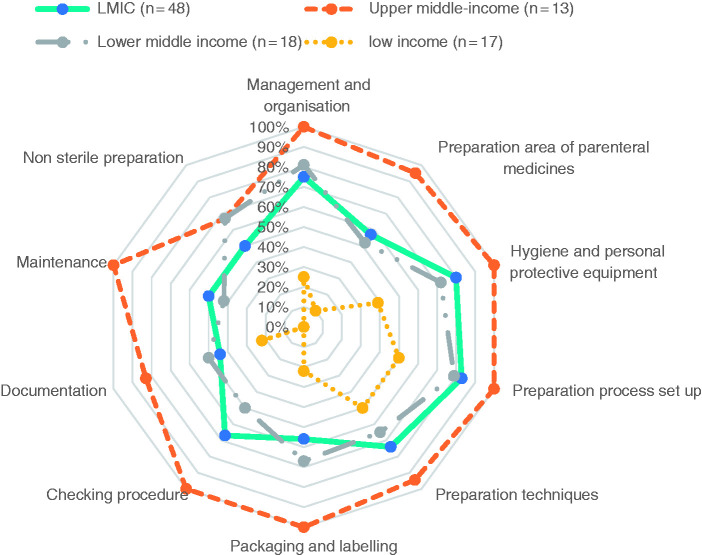
Focus on preparation sub-domains
As pharmacists, we were particularly interested in the results from the items in the preparation domain. Figure 4 presents the median percentages of the level of implementation of safe handling practices in the ten preparation sub-domains. Large variations in the levels of safe handling practices were observed between country’s income classification categories for all sub-domains. Of the 53 participating facilities, 21 reported having no centralized preparation area (11 in LICs, 8 in lower-MICs, and 2 in upper-MICs). In 20 of the 53 facilities (12 in LICs, 8 in lower-MICs), chemotherapies were prepared without biosafety cabinets or isolators. The use of inappropriate protective personal equipment (PPE) was reported by 13 facilities (8 in LICs, 5 in lower-MICs), and 12 facilities (4 in LICs, 8 in lower-MICs) reported that the use of appropriate PPE was only partially implemented. Twenty-five of 53 facilities (10 in LICs, 15 in lower-MICs) had no in-process controls to ensure that the right cytotoxic agent had been selected or to verify its volume and dosage during the preparation of the chemotherapy, and they made no analytical checks on the final preparation. In more than half of the participating facilities (32 of 53: 12 in LICs, 16 in lower-MICs, 4 in upper-MICs), no production worksheet was completed to ensure the preparation’s traceability.
Figure 4.
Median percentage of implementation of safe handling practices for the preparation sub-domain.
Discussion
This survey gives a snapshot of the level of safe handling practices implemented in LMICs. Although the median level of safe handling practices was quite good (63%), the survey revealed great disparities in practices between healthcare facilities depending on their country’s World Bank level of income categorization or whether they were supported by an NGO or international collaboration. One major gap was observed in the domain of preparation, which is one of the chemotherapy process’s riskiest steps.28,29 Any calculation, dosing, or sampling error made during the chemotherapy preparation process could have potentially dramatic consequences for the patient. Furthermore, the risk of occupational exposure is particularly high as it involves handling concentrated cytotoxic drugs. Improvements in this domain should be facilities’ top priority. Major opportunities for improvement were also highlighted in essential cross-cutting domains such as personnel and incident management. Initial and continuous staff education about safe handling, with proper knowledge checks and supervision, is a core element of safety and the quality of care—it should never be neglected. In case of a spill, contamination of personnel, or extravasation, the lack of clear written procedures and the unavailability of emergency management kits in many facilities from lower-middle and low-income countries also revealed important weaknesses in procedures. When such an incident occurs, staff should always be able to act safely and rapidly. Thus, standard operating procedures and regular simulation exercises are essential to ensuring appropriate incident management.
Study strengths and limitations
The broad geographical distribution of the survey’s participants, the different types of facilities, and the variety of contexts in which they worked enriched the study’s results. The survey material’s availability in French and English allowed us to reach countries on different continents, but the absence of a Spanish version may have limited the participation of facilities in South America.
The survey’s convenience sampling methodology (with no information on non-respondents) and sample size do not allow for the generalization of its results across LMICs. In addition, the data was collected based on hospital self-assessments; therefore, the recorded data’s validity cannot be measured. We did not test how cultural differences may have influenced how self-assessments were conducted, nor did we test respondents’ reliability (such as test–retest or inter-rater reliability). For all these reasons, any broad interpretations of the present results should be made with caution.
Comparison with other studies
To the best of our knowledge, no similar international surveys have been conducted in LMICs. However, several studies conducted locally in resource-poor settings have previously shown unsatisfactory levels of knowledge and unsafe practices regarding the preparation and use of chemotherapy drugs.30–33 In particular, weaknesses were revealed during the preparation and administration of chemotherapy. Although the present survey did not examine the reasons or challenges behind inappropriate practices, other studies have identified insufficient knowledge, unsuitable infrastructure, the unavailability of materials, multitasking, work pressures, and high patient loads as barriers to safety.29,30,32 Other studies have reported that improper work practices were due to a lack of training, a lack of awareness, and false beliefs. In India, for example, the lack of national-level guidelines or recommendations and the lack of administrative support or regulations were considered as major difficulties in the implementation of safety standards for chemotherapy.
Implications for practice
The present survey shows that there remain many safety deficiencies in chemotherapy handling practices, particularly in countries with limited resources. There are thus many potential health hazards which will have to be fully addressed as patient numbers are expected to significantly increase in LMICs, as will the use of and exposure to chemotherapy drugs. The WHO’s endorsement of safe handling guidelines and the integration of safe handling practices recommendations into National Cancer Control Plan models could help raise standards through advocacy and encourage the allocation of resources for the improvement of practices. There is a great need for financial, managerial, organizational, and human resources.
Each cancer care facility has a mission to provide safe, high-quality care. To design appropriate risk management strategies, every institution administering chemotherapies should conduct a comprehensive risk assessment. As part of this process, the Cyto-SAT tool could be a useful one with which to assess handling practices and help design action plans to address gaps and improve safety.
Future research
To pursue our work on the safe handling of chemotherapy in LMICs, we recently developed an online training package on this subject. Eleven e-learning lessons covering the ten domains addressed by the Cyto-SAT tool are available for free on our www.Pharm-Ed.net platform. A set of practical tools has also been developed to support the implementation of safe practices (e.g., videos, checklists, procedures, etc.). In the near future, we hope to evaluate this program’s impact on facilities in LMICs.
Conclusion
The present study’s overview of safe handling practices for chemotherapy showed that unsafe practices remain a significant risk issue in low- and middle-income countries. Strategies to remedy and improve this situation are needed in order to increase patient and staff safety and limit the risks of environmental contamination, especially in lower-income countries. The promotion of safe handling programs should be part of the efforts to improve access to quality cancer drugs and must be integrated into National Cancer Control Plans.
Acknowledgements
We deeply thank all the people and associations (ISOPP, UICC, e-med, Pharm-Ed) who agreed to share the survey with their networks as well as all participating facilities which completed the survey.
Appendix 1
Table 5.
Median percentages of the implementation of safe practices in the different domains and sub-domains by country income level.
| Domains | Sub-domains | LMICs | LICs | Lower-MICs | Upper-MICs |
|---|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | Median (Q1–Q3) | ||
| (%) | (%) | (%) | (%) | ||
| Management | 52 (30–79) | 27 (23–42) | 59 (33–79) | 77 (69–95) | |
| Personnel | 62 (24–76) | 19 (14–36) | 60 (44–70) | 86 (71–92) | |
| Education and training | 50 (25–67) | 17 (8–29) | 50 (27–58) | 79 (67–94) | |
| Medical surveillance | 67 (44–89) | 33 (22–56) | 67 (58–89) | 94 (75–100) | |
| Logistics | 54 (38–77) | 33 (18–53) | 54 (43–70) | 83 (72–94) | |
| Receipt | 70 (37–93) | 33 (13–63) | 67 (53–93) | 90 (80–100) | |
| Storage | 67 (33–89) | 33 (31–46) | 72 (61–89) | 83 (64–90) | |
| Transport | 40 (17–80) | 13 (7–53) | 33 (23–63) | 83 (65–100) | |
| Prescription | 80 (60–93) | 80 (53–87) | 80 (73–93) | 87 (79–93) | |
| Preparation | 69 (29–84) | 27 (17–63) | 60 (45–76) | 92 (84–98) | |
| Management and organization | 75 (25–100) | 25 (10–46) | 81 (44–83) | 100 (98–100) | |
| Parenteral chemotherapy preparation areas | 57 (17–80) | 10 (3–42) | 52 (23–70) | 95 (85–100) | |
| Hygiene and personal protective equipment | 80 (39–100) | 39 (22–69) | 72 (44–88) | 100 (97–100) | |
| Preparation process set-up | 83 (50–100) | 50 (29–71) | 7 (58–92) | 100 (100–100) | |
| Preparation techniques | 74 (48–93) | 50 (27–78) | 65 (49–88) | 94 (87–97) | |
| Packaging and labelling | 56 (22–100) | 22 (0–44) | 67 (36–97) | 100 (81–100) | |
| Checking procedures | 67 (0–100) | 0 (0–83) | 50 (4–79) | 100 (96–100) | |
| Documentation | 44 (22–78) | 22 (0–50) | 50 (33–78) | 83 (58–100) | |
| Maintenance | 50 (33–100) | 0 (0–92) | 42 (33–67) | 100 (92–100) | |
| Non-sterile preparation | 50 (0–67) | 0 (0–33) | 67 (33–100) | 67 (67–100) | |
| Administration | 71 (48–83) | 64 (32–78) | 67 (49–75) | 93 (81–98) | |
| Management | 83 (50–83) | 67 (33–83) | 75 (50–83) | 92 (79–100) | |
| Hygiene and safety measures | 80 (60–93) | 73 (43–87) | 73 (60–87) | 97 (85–100) | |
| Documentation | 67 (67–100) | 67 (39–78) | 67 (67–89) | 100 (67–100) | |
| Work practices | 67 (33–92) | 42 (19–83) | 63 (42–75) | 100 (79-100) | |
| Incident Management | 54 (23–82) | 18 (7–65) | 41 (24–59) | 86 (82–96) | |
| Surface contamination | 50 (22–89) | 22 (6–61) | 39 (24–65) | 100 (93–100) | |
| Staff contamination | 56 (11–78) | 22 (0–72) | 39 (3–67) | 89 (78–100) | |
| Extravasations | 56 (22–78) | 11 (6–67) | 44 (36–67) | 83 (67–100) | |
| Quality assurance | 33 (0–67) | 0 (0–50) | 33 (0–67) | 100 (67–100) | |
| Waste management | 67 (33–79) | 43 (23–70) | 62 (33–73) | 78 (69–90) | |
| Waste disposal | 67 (33–86) | 38 (24–79) | 62 (31–80) | 90 (83–100) | |
| Patients’ excreta | 44 (33–67) | 44 (6–67) | 53 (33–67) | 56 (28–92) | |
| Cleaning | 65 (37–80) | 37 (22–63) | 63 (44–77) | 78 (70–92) | |
| Management and organization | 67 (33–92) | 33 (0–67) | 50 (33–67) | 83 (58–100) | |
| Cleaning practices | 78 (44–90) | 44 (33–75) | 72 (58–83) | 97 (88–100) | |
| Laundry | 17 (0–54) | 0 (0–25) | 33 (0–50) | 42 (0–88) | |
| Patient counseling | 67 (48–83) | 67 (46–83) | 58 (42–75) | 88 (67–100) | |
| Total | 63 (39–77) | 32 (24–62) | 63 (49–70) | 85 (77–93) |
According to the World Bank classification of countries LMICs: low- and middle-income countries; LICs: low-income countries; lower-MICs: lower-middle-income countries; upper-MICs: upper-middle-income countries; Q1: first quartile; Q3: third quartile.Bold values summarize results for the domains.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sandrine von Grünigen https://orcid.org/0000-0002-5501-2522
References
- 1.Connor T, MacKenzie B, DeBord D, et al. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings. National Institute for Occupational Safety and Health, Department of Health and Human Services, Centers for Disease Control and Prevention, www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf (2016, accessed 8 February 2017).
- 2.Connor TH, Burroughs G, McDiarmid M, et al. NIOSH alert: preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. Atlanta, GA: DHHS NIOSH, 2004, pp.1–50.
- 3.Phillips J, Beam S, Brinker A, et al. Retrospective analysis of mortalities associated with medication errors. Am J Health Syst Pharm 2001; 58: 2131–2131. [DOI] [PubMed] [Google Scholar]
- 4.Greenall J, Shastay A, Vaida AJ, et al. Establishing an international baseline for medication safety in oncology: findings from the 2012 ISMP international medication safety self assessment® for oncology. J Oncol Pharm Pract 2015; 21: 26–35. [DOI] [PubMed] [Google Scholar]
- 5.ASHP Council on Professional Affairs. ASHP guidelines on preventing medication errors with antineoplastic agents. Am J Health-Syst Pharm 2002; 59: 1648–1668. [DOI] [PubMed] [Google Scholar]
- 6.Falck K, Gröhn P, Sorsa M, et al. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet Lond Engl 1979; 313: 1250–1251. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Occupational Safety and Health. Hazardous drug exposure in healthcare, effects of occupational exposure. Workplace safety and health topics hazardous drug exposures in health care antineoplastic agents, www.cdc.gov/niosh/topics/hazdrug/effects.html (2019, accessed 26 January 2021).
- 8.Pan American Health Organization. Safe Handling of Hazardous Chemotherapy Drugs in Limited-Resource Settings, https://iris.paho.org/handle/10665.2/28554 (2013, accessed 24 June 2020).
- 9.Power LA, Coyne JW. ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm 2018; 75: 1996–2031. [DOI] [PubMed] [Google Scholar]
- 10.International Society of Oncology Pharmacy Practicioners Standards Committee. ISOPP standards of practice. Safe handling of cytotoxics. J Oncol Pharm Pract 2007; 13: 1–81. [DOI] [PubMed] [Google Scholar]
- 11.SUVA C nationale suisse d’assurance en cas d’accidents. Mesures de protection relatives à la manipulation de médicaments (Protective measures related to the handling of medicines), https://www.suva.ch/fr-CH/materiel/documentation/mesures-de-protection-relatives-e-la-manipulation-des-medicaments (2018, accessed 19 November 2020).
- 12.Sessing P, Sewell G, Vandenbroucke J. Preventing occupational exposure to cytotoxic and other hazardous drugs, european policy recommendations, www.europeanbiosafetynetwork.eu/wp-content/uploads/2016/05/Exposure-to-Cytotoxic-Drugs_Recommendation_DINA4_10-03-16.pdf (2016, accessed 19 November 2020).
- 13.Neuss MN, Gilmore TR, Belderson KM, et al. 2016 Updated American society of clinical oncology/oncology nursing society chemotherapy administration safety standards, including standards for pediatric oncology. ONF 2016; 12: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 14.Occupational Safety and Health Administration. Controlling occupational exposure to hazardous drugs. United States Department of Labor, www.osha.gov/SLTC/hazardousdrugs/controlling_occex_hazardousdrugs.html (accessed 7 February 2019).
- 15.Chartier Y. Safe management of wastes from health-care activities. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 16.Cancer today, http://gco.iarc.fr/today/home (2018, accessed 2 November 2020).
- 17.World Health Organisation. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 18.dpicampaigns. About the sustainable development goals. United nations sustainable development, www.un.org/sustainabledevelopment/sustainable-development-goals/ (2018, accessed 20 February 2019).
- 19.World Health Organisation. Cancer fact sheets, www.who.int/news-room/fact-sheets/detail/cancer (2018, accessed 19 February 2018).
- 20.World Health Organization. Seventieth World Health Assembly, http://apps.who.int/gb/ebwha/pdf_files/WHA70-REC1/A70_2017_REC1-en.pdf#page=27 (2017, accessed 20 February 2019).
- 21.World Health Organization. World Health Organization Model List of Essential Medicines, 21st List, https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1 (2019, accessed 20 May 2020).
- 22.World Bank Country and Lending Groups – World Bank Data Help Desk, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (2019, accessed 29 October 2020).
- 23.von Grünigen S, Geissbühler A, Bonnabry P. Cyto-SAT: a self-assessment tool for the safe handling of cytotoxic drugs adapted for use in low- and middle-income countries. J Oncol Pharm Pract. Epub ahead of print 17 September 2020. DOI: 1078155220956687. [DOI] [PubMed]
- 24.Pharmacie des Hôpitaux Universitaires de Genève. Pharm-Ed: plateforme éducative et collaborative pour une gestion efficiente, sûre et responsable des médicaments dans les hôpitaux des pays en développement, www.Pharm-Ed.net (2015, accessed 15 June 2020).
- 25.ReMed. E-med, http://remed.org/e-med/ (accessed 29 December 2020).
- 26.Self Assessments. Institute for Safe Medication Practices, www.ismp.org/self-assessments (2020, accessed 30 June 2020).
- 27.Wikipedia. Quartile, https://en.wikipedia.org/wiki/Quartile (2020, accessed 29 December 2020).
- 28.Connor TH, McDiarmid MA. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J Clin 2006; 56: 354–365. [DOI] [PubMed] [Google Scholar]
- 29.Turk M, Davas A, Ciceklioglu M, et al. Knowledge, attitude and safe behaviour of nurses handling cytotoxic anticancer drugs in Ege university hospital. Asian Pac J Cancer Prev 2004; 5: 164–168. [PubMed] [Google Scholar]
- 30.Simegn W, Dagnew B, Dagne H. Knowledge and associated factors towards cytotoxic drug handling among university of Gondar comprehensive specialized hospital health professionals, institutional-based cross-sectional study. Environ Health Prev Med 2020; 25: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan N, Khowaja KZA, Ali TS. Assessment of knowledge, skill and attitude of oncology nurses in chemotherapy administration in tertiary hospital Pakistan. OJN 2012; 02: 97–103. [Google Scholar]
- 32.Chaudhary R, Karn BK. Chemotherapy-knowledge and handling practice of nurses working in a medical university of Nepal. JCT 2012; 03: 110–114. [Google Scholar]
- 33.Keat CH, Sooaid NS, Yun CY, et al. Improving safety-related knowledge, attitude and practices of nurses handling cytotoxic anticancer drug: pharmacists’ experience in a general hospital. Malaysia Asian Pac J Cancer Prev 2013; 14: 69–73. [DOI] [PubMed] [Google Scholar]