Abstract
Background:
Tumor necrosis factor (TNF) inhibitors have improved treatment of ulcerative colitis (UC), but loss of response remains a frequent problem. The anti-TNF agent, golimumab, was approved in Switzerland for the treatment of UC in 2014. This study aims to summarize the experience of golimumab in a real-world setting in Switzerland.
Methods:
We analyzed real-world data from 1769 UC patients from the Swiss Inflammatory Bowel Disease Cohort (SIBDC) study and performed a chart review of golimumab-treated patients. We extracted the partial Mayo score at t0 (baseline), t1 (2–16 weeks), t2 (17–35 weeks), and t3 (36–89 weeks). The primary endpoint was clinical response at t1, defined as marked improvement in partial Mayo score and objective parameters. Clinical remission was defined as resolution of symptoms and normalization of objective parameters.
Results:
Our chart review included 103 UC patients with golimumab treatment (5.8% of all SIBDC UC patients); only 16 (15.5%) were anti-TNF naïve. Sixty-three patients remained on golimumab (61.2%) after 180 days, 51 (44.7%) after 365 days, and 34 (33%) after 630 days after the start of treatment. Upon golimumab treatment, the partial Mayo score decreased from 4 [interquartile range (IQR): 2–6] at t0 to 2 (IQR: 0–4) at t1, 1 (IQR: 0–3.5) at t2, and 1 (IQR: 0–3) at t3 (p < 0.001 for all comparisons with t0). The primary endpoint, clinical response at t1, could be evaluated in 52 patients and was met in 15 individuals (28.8%). Clinical remission at t1 was observed in 8 out of 52 patients (15.4%). Golimumab was generally well tolerated, one patient developed meningitis. The most frequent reasons to stop treatment were primary and secondary non-response.
Conclusion:
Golimumab was used in 5.8% of Swiss UC patients, mainly in biologic-experienced individuals. Golimumab treatment was associated with a sustained reduction of symptoms and clinical response in approximately 30% of patients.
[ClinicalTrials.gov identifier: NCT00488631]
Keywords: golimumab, inflammatory bowel disease, TNF inhibitor, ulcerative colitis
Introduction
Anti-tumor necrosis factor (TNF) therapies are a cornerstone for the treatment of inflammatory bowel diseases (IBD) including ulcerative colitis (UC) with moderate to severe disease activity.1–4 TNF inhibitors have been associated with clinical improvement, healing of mucosal lesions, and reduction in hospitalization and surgery rates in UC.4–6 However, a significant fraction of patients fail to respond to anti-TNF therapies (primary non-response rates of up to 46% in clinical trials and 10–20% in clinical series) or will experience secondary loss of response (23–46% at 12 months after anti-TNF initiation). 4 Thus, either new treatment options or more information/ algorithms relating to the optimal use of available drugs are needed.7–9 After failure or loss of effectiveness of one TNF inhibitor, switching to another TNF inhibitor can be effective. 10
In Switzerland, three anti-TNF therapies are available for UC: infliximab, adalimumab, and golimumab. 11 Golimumab was introduced for the treatment of moderate to severe UC after success of pivotal studies in February 2014.12,13 In the induction phase of the PURSUIT trial, golimumab improved rates for clinical remission and mucosal healing, and health-related quality of life at week 6 in anti-TNF naïve patients with moderate to severe UC. 13 In the maintenance phase, approximately 50% of patients in the golimumab group maintained clinical response through week 54 compared with 31% in the golimumab withdrawal group (placebo). 12 Furthermore, in the prospective, single-arm Go-Colitis study in anti-TNF naïve patients, 68.8% of patients had a clinical response and 38.5% of patients achieved clinical remission following treatment with golimumab. 14
Real-world data remain important to assess performance of a drug under non-ideal conditions in clinical practice, when treatment cannot be limited to patients fulfilling narrow inclusion criteria, 15 with only about one in four real-life UC patients qualifying for participation in randomized controlled trials. 16 Some real-world data and post-marketing studies for golimumab in UC are available,14,17–26 these generally confirmed effectiveness and safety of golimumab treatment. In some of these studies, clinical response and remission,18,22,23,25 and persistence rates, 17 were higher in anti-TNF naïve patients compared with non-naïve patients. However, as clinical conditions can change over time and/ or from country to country, it is important to continuously explore how golimumab is applied in clinical practice.
The current study aims to summarize the real-life experience with golimumab in patients with UC from the Swiss Inflammatory Bowel Disease Cohort (SIBDC) study.
Patients and methods
SIBDC study design and characteristics
We used prospectively obtained data from the patients’ baseline and annual follow-up questionnaires and physicians’ medical records from patients included in the SIBDC from 2006 to 2019.27,28 SIBDC is a nation-wide cohort study enrolling IBD patients in Switzerland since 2006 and is supported by the Swiss National Science Foundation. Data are collected in secondary and tertiary referral centers, as well as private practices. The SIBDC has been approved by the respective ethics committees in Switzerland (EK-1316, BASEC 2018-02068). All participants provided written informed consent.
Golimumab-related data from SIBDC
All analyses were restricted to patients with UC or IBD unclassified. The following data were retrieved from the SIBDC database:
General patient characteristics: age at diagnosis, disease duration until golimumab treatment, and gender.
IBD characteristics: maximal extent of disease (proctitis, left-sided colitis, pancolitis); disease activity, assessed by the modified Truelove and Witts activity index (MTWAI), extraintestinal manifestations (EIM; peripheral arthritis, uveitis/iritis, pyoderma gangrenosum, erythema nodosum, aphthous oral ulcers, stomatitis, ankylosing spondylitis, primary sclerosing cholangitis), intestinal surgery or surgery for fistulae and abscesses, and complications (defined as in Schreiner et al. 29 ).
Use of biologics: TNF inhibitors (either infliximab, golimumab, adalimumab, or certolizumab) and vedolizumab.
To describe global patterns of biologic usage in Switzerland in UC, we retrieved the number of UC patients treated with each TNF inhibitor approved for treatment of UC in Switzerland (infliximab, adalimumab, and golimumab), the number treated with vedolizumab and the total number of UC patients from 2006 to 2019. We also compared baseline epidemiological characteristics of patients treated with golimumab and other TNF inhibitors with anti-TNF naïve patients.
Chart review
We performed a retrospective chart review of all UC patients treated with golimumab registered in the SIBDC. If applicable, we identified the start and the end of the golimumab treatment for each patient. We confirmed previous and concomitant conventional medication (steroids, 5-aminosalicylic acid, and immunosuppressants) and biologic therapies (infliximab, adalimumab, vedolizumab, and certolizumab).
We identified key clinical symptoms of all patients (stool frequency, blood in stool, physician assessed general well-being) to complete the partial Mayo Score for UC patients. We also collected additional information to assess disease activity: lab work including C-reactive protein (CRP), calprotectin, hemoglobin (CRP values were left-censored at 5 mg/l, calprotectin values were left-censored at 30 µg/g and right censored at 1000 µg/g); endoscopy, imaging including ultrasound, and computed tomography scan. Additional clinical signs and symptoms (e.g. EIM) and need for surgery were also noted. We also assessed/confirmed basic epidemiological and clinical characteristics [smoking, 30 family history (i.e. ⩾1 first degree relative with IBD), extent of disease, and disease activity according to MTWAI].
We checked for potential side effects of golimumab. In case golimumab had been stopped, we noted the reasons (side effects, primary non-response, loss of response, reimbursement, patient preference, etc.).
Hypothesis and primary outcome
We aimed to test the following hypothesis: Patients with moderate to severe UC, refractory to conventional therapy (bionaïve), or biologic-experienced patients can be effectively treated with golimumab in a real-world setting in Switzerland, with clear improvement of symptoms, and control of the disease, measured by patient reported outcomes (stool frequency and blood in stools), physician assessment, and objective measures.
The primary outcome of our study is clinical response rate at t1 (2–16 weeks). This large time range was chosen to avoid bias by excluding patients due to timing of follow-up. Secondary outcomes are response rates at 6 months (t2: 17–35 weeks), 12 months (t3: 36–89 weeks), and clinical remission at t1, t2, and t3. To overcome limitations of the non-standardized clinical assessment in the real-world setting, we pre-defined the following composite endpoints:
The composite primary endpoint, clinical response at t1 (2–16 weeks), was met if the following two criteria were fulfilled:
Marked improvement in partial Mayo score, defined as: decrease in partial Mayo score ⩾ 2 points and ⩾ 30% from baseline, and a decrease in rectal bleeding subscore ⩾ 1 point or absolute rectal bleeding score ⩽ 1 and
Improvement in one or more of the following parameters: CRP, anemia resolution, calprotectin (cutoff 100 µg/g), endoscopy, and/or intestinal ultrasound. Improvement in lab work was defined as a reduction of the difference between baseline values and the next limit of normal by ⩾30%. Improvement in endoscopy/ultrasound was defined as a reduction of colitis in the same technique compared with baseline substantiated by images (endoscopy) or measurements of diameter of the bowel wall.
Clinical remission was met when the following two criteria were fulfilled:
Normalization of Mayo score, defined as: partial Mayo score ⩽ 2 and no individual Mayo subscore > 1
No evidence of residual disease activity in all of the following parameters: Endoscopy data (Mayo score ⩽ 1), ultrasound, CRP, calprotectin (cutoff 100 µg/g), hemoglobin (anemia).
Data analysis
In general, categorical data are presented as raw numbers and percentages. Continuous or ordinal data are provided as median with interquartile range, minimum, and maximum. Three types of analysis will be conducted. We start with some descriptive analysis to compare some groups of patients, defined by their anti-TNF treatment. Differences in categorical variables were assessed using the Fisher’s exact test or the chi-square test. Differences in continuous or ordinal data were analyzed using the Mann–Whitney test. Second, we use Kaplan–Meier survival techniques to analyze patients remaining on golimumab treatment, and to calculate an attrition curve. Finally, we longitudinally analyze biomarkers of UC disease activity (hemoglobin, calprotectin, and CRP), endoscopy results (macroscopic and histologic scoring), and clinical disease activity (MTWAI, partial Mayo score, and the three individual subscores for diarrhea, bleeding, and physician general assessment) by building a linear mixed-effects model for each outcome. Each model considered fixed effects of time (in days) as a continuous variable and random effects (regarding trend and intercept) for the patient ID. The p value for the time trend is indicated, uncorrected and after Bonferroni correction, adjusting for 10 tests.
Post hoc power analysis
To calculate the statistical power for potential additional subanalysis, we performed a post hoc power analysis with the following assumptions: In the phase 3 study for golimumab in UC, for the 200 mg/100 mg dosage, response rates of 20.9% over placebo were observed (51% versus 30.1%). 13 We assume that differences between response rates in subgroups will not be greater than the response rates of treated patients over placebo. Our power analysis indicated that a total sample size of 148 (74 patients in each group) would be necessary to detect such a difference with a one-sided p value of <0.05 and a power of 80%. Likewise, an identical difference of 20.9% over placebo with lower absolute response rates (i.e. 31% versus 30.1%) needed a total patient number of 106 for a one-sided p value of <0.05 and a power of 80%. Since the total number of patients in our analysis was 103 of whom only 52 could be evaluated for the primary endpoint, no subgroup analysis was attempted. Specifically, our predefined analysis according to line of therapy (naïve, second line, third, and subsequent) was not attempted, and we limited this secondary analysis to a report of response rates of anti-TNF experienced versus anti-TNF naïve patients. Advanced statistical analyses (for instance multivariate analysis) were also not feasible due to the small number of patients.
Linear mixed-effects models were calculated using the fitlme command of Matlab 2019b; the power analysis was done using G*Power 3.1, 31 all other data were analyzed using Graphpad Prism version 8.4.3.
Our study was registered at www.clinicaltrials.org [ClinicalTrials.gov identifier: NCT00488631].
Results
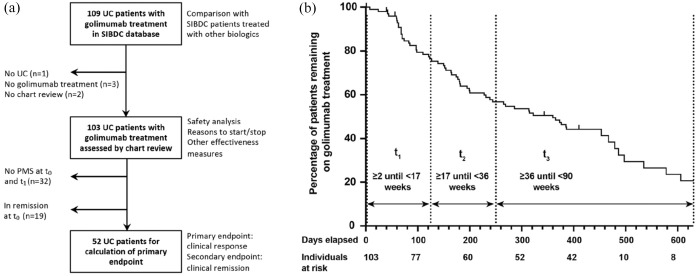
Out of 1769 SIBDC patients with UC, exposure to golimumab was documented for 109 individuals at least once in the SIBDC database (Figure 1(a)). Patients treated with golimumab showed similar clinical characteristics as patients treated with other TNF inhibitors (Table 1). As expected, patients ever treated with biologics showed signs of more severe disease (e.g. higher MTWAI, and more EIM) than patients without such a treatment (Table 1).
Figure 1.
Flow chart and time course of patients treated with golimumab during follow-up.
PMS, partial Mayo Score; SIBDC, Swiss Inflammatory Bowel Disease Cohort; UC, ulcerative colitis.
Table 1.
Clinical characteristics of SIBDC patients with ulcerative colitis (UC), treated with golimumab, other anti-TNF medication, or without any anti-TNF treatment.
| Golimumab | Other anti-TNF | No anti-TNF | p value chi2 or Kruskal–Wallis test | |
|---|---|---|---|---|
| Number of patients | 109 (6.2%) | 572 (32.3%) | 1088 (61.5%) | — |
| Gender | ||||
| Male | 52 (47.7%) | 317 (55.4%) | 569 (52.3%) | 0.248 |
| Female | 57 (52.3%) | 255 (44.6%) | 519 (47.7%) | |
| Age at diagnosis | ||||
| Median, IQR, | 27.0, 20.6 – 35.5, | 26.5, 18.9 – 37.1, | 29.4, 19.7 – 40.2, | 0.025 |
| Min–max | 10.3 – 70.5 | 2.5 – 72.2 | 0.8 – 85.5 | |
| Disease duration | ||||
| Median, IQR, | 10.7, 8.2 – 18.1, | 11.0, 6.4 – 17.1, | 11.7, 5.9 – 19.7, | 0.343 |
| Min–max | 0.2 – 41.5 | 0.3 – 44.2 | 0.1 – 54.2 | |
| Maximal MTWAI | ||||
| Median, IQR, | 7, 4 – 10, | 6, 3 – 9, | 3, 2 – 6, | < 0.001 |
| Min–max | 0 – 19 | 0 – 18 | 0 – 18 | |
| Initial location | ||||
| Proctitis | 20 (20.0%) | 68 (13.1%) | 268 (27.1%) | < 0.001 |
| Left-sided colitis | 36 (36.0%) | 179 (34.4%) | 317 (32.0%) | |
| Pancolitis | 44 (44.0%) | 273 (52.5%) | 405 (40.9%) | |
| Unknown | 9 | 52 | 98 | |
| Maximal extent | ||||
| Proctitis | 4 (3.7%) | 21 (3.7%) | 141 (13.3%) | < 0.001 |
| Left-sided colitis | 31 (28.4%) | 130 (22.9%) | 334 (31.4%) | |
| Pancolitis | 74 (67.9%) | 417 (73.4%) | 587 (55.3%) | |
| Unknown | 0 | 4 | 26 | |
| Occurrence of | ||||
| EIM | 61 (56.0%) | 276 (48.3%) | 363 (33.4%) | < 0.001 |
| Complication | 73 (67.0%) | 372 (65.0%) | 494 (45.4%) | < 0.001 |
| Intestinal surgery | 16 (14.7%) | 80 (14.0%) | 83 (7.6%) | < 0.001 |
| Any surgery | 30 (27.5%) | 150 (26.2%) | 204 (18.8%) | 0.001 |
Source: SIBDC database. Please note that our chart review confirmed UC diagnosis and golimumab treatment only in 103 of 109 patients.
EIM, extraintestinal manifestation; IQR, interquartile range; MTWAI, modified Truelove and Witts activity index; SIBDC, Swiss Inflammatory Bowel Disease Cohort; TNF, tumor necrosis factor.
Shifting patterns of biologic use for ulcerative colitis treatment in Switzerland
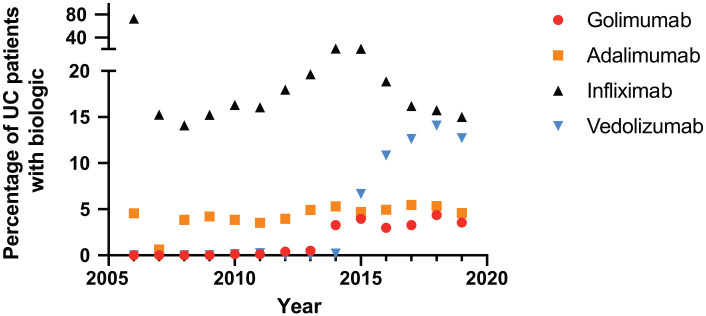
Our data demonstrate a shift of choice of biologics used for IBD treatment: While from 2006 to 2015 infliximab was used the most (in 14–21% of patients each year), rates of vedolizumab treatment increased from 2015 (Figure 2, Table 2). Golimumab was initially used only infrequently, though rates increased in 2014. However, golimumab consistently remained the least frequently used anti-TNF drug (among the biologics approved for UC) throughout the observation period (3.3–4.4%). In comparison with infliximab, golimumab and vedolizumab were used more frequently as a second- or third-line treatment (Table 3, p < 0.0001 for each comparison of another biologic with golimumab).
Figure 2.
Shifting patterns of treatment with biologics in patients of the Swiss IBD cohort (SIBDC) study with ulcerative colitis (UC).
Table 2.
Numbers of ulcerative colitis patients treated with the indicated biologic. For comparison, the total number of ulcerative colitis patients with a clinical visit at the indicated year is also provided.
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulcerative colitis | 22 | 321 | 546 | 663 | 778 | 853 | 985 | 1018 | 1038 | 1111 | 1070 | 1006 | 845 | 480 |
| Golimumab | 0 | 0 | 0 | 0 | 1 | 1 | 4 | 5 | 34 | 44 | 32 | 33 | 37 | 17 |
| Adalimumab | 1 | 2 | 21 | 28 | 30 | 30 | 39 | 50 | 55 | 52 | 53 | 55 | 45 | 22 |
| Infliximab | 16 | 49 | 77 | 101 | 127 | 137 | 177 | 200 | 217 | 228 | 202 | 163 | 133 | 72 |
| Vedolizumab | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 74 | 116 | 127 | 119 | 61 |
Source: Swiss Inflammatory Bowel Disease Cohort Study database.
Table 3.
Number of ulcerative colitis patients for which the indicated biologic remained second, third, or fourth line of treatment.
| Ever treated with | No previous biologic |
One previous biologic |
Two previous biologics |
Three previous biologics |
Comparison with infliximab |
|---|---|---|---|---|---|
| Infliximab; n = 572 | 554 (96.9%) | 17 (2%) | 0 | 1 (0.2%) | |
| Golimumab; n = 109 | 76 (69.7%) | 15 (13.8%) | 16 (14.7%) | 2 (1.8%) | <0.0001 |
| Adalimumab; n = 188 | 145 (77.1%) | 33 (17.6%) | 9 (4.8%) | 1 (0.5%) | <0.0001 |
| Vedolizumab; n = 207 | 125 (60.4%) | 50 (24.2%) | 24 (11.6%) | 8 (3.9%) | <0.0001 |
Source: Swiss Inflammatory Bowel Disease Cohort Study database. Statistical test: chi-square test.
Chart review of SIBDC patients treated with golimumab
To analyze golimumab treatment at a higher time resolution, we performed a chart review in all UC patients with golimumab treatment recorded in the SIBDC database. With this strategy, we aimed to overcome the limitation of only yearly data entry into the SIBDC database. A total of 109 patients had golimumab treatment recorded in the database. However, for one patient, UC diagnosis was not confirmed, for three patients, no golimumab treatment was documented, and for two patients, the charts could not be accessed. Therefore, 103 patients were included into our data analysis (Figure 1(a)).
Clinical characteristics of patients treated with golimumab at t0 (baseline)
Most golimumab-treated patients had long-standing disease (median disease duration: 7 years) with documented pancolitis in almost half of patients (Table 4). We also noted clinically active disease (median MTWAI 7, median partial Mayo 4) and high calprotectin values in most patients at the start of golimumab treatment (t0). Most patients had been treated with biologics before. Of note, three patients had been exposed to three anti-TNF agents prior to golimumab (i.e. infliximab, adalimumab, and off-label certolizumab). For only 16 patients (15.5%), golimumab was the first biologic used.
Table 4.
Clinical and epidemiological characteristics of patients with golimumab treatment.
| All patients with golimumab treatment; n = 103 | Patients for primary endpoint with partial Mayo score available at t0 + t1; not in remission; n = 52 |
Comparison | |
|---|---|---|---|
| Epidemiological characteristics | |||
| Age: median (IQR), range | 38.2 (28.6–53.1), 12.4–72.4 | 33.6 (26.7–44.7), 12.4–66.5 | n.s. |
| Gender: female/male (% female) | 49/54 (47.6%) | 26/26 (50%) | n.s. |
| Family history yes/no (% yes) | 15/88 (14.6%) | 11/41 (11.2%) | n.s. |
| Disease duration | 7.7 (4.3–14.6), 0–42.9 | 7.7 (4–13.6), 0.5–42.9 | n.s. |
| Extent of disease | n.s. | ||
| Pancolitis | 50 (48.5%) | 25 (48.1) | |
| Left-sided colitis | 43 (41.7%) | 20 (38.5%) | |
| Proctitis | 7 (6.8%) | 5 (9.6%) | |
| Pouchitis | 2 (1.9%) | 2 (3.8%) | |
| Unknown | 1 (1%) | ||
| EIM | n.s. | ||
| None | 72 (69.9%) | 36 (69.2%) | |
| 1 EIM | 24 (23.3%) | 12 (23.1%) | |
| 2 EIM | 7 (6.8%) | 4 (7.7%) | |
| Smoking | n.s. | ||
| No | 83 (80.6%) | 43 (82.7%) | |
| Yes | 8 (7.8%) | 3 (5.8%) | |
| Ex-smoker | 10 (9.7%) | 5 (9.6%) | |
| Unknown | 2 (1.9%) | 1 (1.9%) | |
| Previous anti-TNF | n.s. | ||
| No | 18 (17.5%) | 9 (17.3%) | |
| 1 anti-TNF | 51 (49.5%) | 28 (53.8%) | |
| 2 anti-TNF | 27 (26.2%) | 13 (25%) | |
| 3 anti-TNF | 3 (2.9%) | 2 (3.8% | |
| Unknown | 4 (3.9%) | 0 | |
| Previous vedolizumab | n.s. | ||
| Yes | 13 (12.6%) | 6 (11.5%) | |
| No | 90 (87.4%) | 46 (88.5%) | |
| Number biologics | |||
| None | 16 (15.5%) | 8 (15.4%) | |
| 1 | 45 (43.7%) | 24 (46.2%) | |
| 2 | 32 (31.1%) | 18 (34.6%) | |
| 3 | 6 (5.8%) | 2 (3.8%) | |
| Unknown | 4 (3.9%) | 0 | |
| Co-medication at t0 | |||
| Prednison | 44 (42.7%) | 29 (55.8%) | n.s. |
| Budesnoid (p.o. or local) | 15 (14.6%) | 9 (17.3%) | n.s. |
| 5-ASA (p.o. or local) | 32 (31.1%) | 21 (40.4%) | n.s. |
| Immune-modulator | 20 (19.4%) | 11 (21.2%) | n.s. |
| MTWAI at t0 | 7 (4–10.5), 0–15 (n = 85) | 8 (6–11.25), 2–14 (n = 50) | p = 0.0055 |
| Partial Mayo at t0 | 4 (2–6), 0–9 (n = 84) | 5 (4–9), 3–6 (n = 52) | p = 0.0027 |
| Stool frequency at t0 | 2 (0–3), 0–3 (n = 84) | 2 (2–3), 2–3 (n = 52) | p = 0.004 |
| Bloody stool at t0 | 1 (0–2), 0–3 (n = 84) | 1 (0.25–2), 0–3 (n = 52) | p = 0.03 |
| Physician’s general assessment at t0 | 1 (1–2), 0–3 (n = 84) | 2 (1–2), 0–3 (n = 52) | p = 0.021 |
| Lab values | |||
| Hemoglobin (g/l) at t0 | 136.5 (127.8–150), 79–167 (n = 86) | 134 (127–149), 79–167 (n = 49) | n.s. |
| CRP (mg/l) at t0 | 5 (5–10), 5–104 (n = 82) | 6.5 (5–11.8), 5–99 (n = 48) | n.s. |
| Calprotectin (µg/g) at t0 | 828 (366–1000), 30–1000 (n = 63) | 1000 (424–1000), 128–1000 (n = 38) | n.s. |
| Endoscopy | |||
| Macroscopic score at t0 | 2 (1–3), 0–3, n = 80 | 2.5 (2–3), 0–3, n = 46 | n.s. |
| Histological score at t0 | 2 (1–3), 0–3, n = 71 | 2 (1.25–3), 0–3, n = 41 | n.s. |
Data for the complete group (n = 103) and the subgroup of patients used to calculate the primary endpoint with available partial Mayo score at t0 + t1 and not fulfilling criteria for clinical remission (n = 52) are provided. The median, IQR, and range are indicated. CRP was left-censored at 5 mg/l; calprotectin was left-censored at 30 mg/g and right-censored at 1000 mg/g. Statistical analysis: Mann–Whitney U test, Fisher’s exact test, chi-square test, whatever appropriate. 5-ASA, 5-aminosalicylic acid; anti-TNF, anti-tumor necrosis factor medication; CRP, C-reactive protein; EIM, extraintestinal manifestation; IQR, interquartile range; MTWAI, modified Truelove and Witts activity index.
Due to our retrospective study design, some clinical data were missing and for 71 (68.9%) patients, the partial Mayo score was available at time points t0 and t1 for subsequent calculation of the primary endpoint. In 19 patients, our criteria for clinical remission were already fulfilled at t0, consequently no improvement could have been achieved (Table 5). Therefore, the analysis was restricted to 52 patients (Figure 1(a)), which had slightly higher scores of disease activity but otherwise showed similar characteristics (Table 4). Timing of follow-up was heterogeneous for t1, reflecting variability in clinical practice (2 patients < 30 day, 23 patients 30–49 days, 12 patients 50–69 days, 8 patients 10–89 days, and 7 patients 90–117 days).
Table 5.
Reasons for missing data. The number of patients for which the partial Mayo has been assessed is indicated, along with reasons for missing values. The numbers in each column add up to 103.
| t1 | t2 | t3 | |
|---|---|---|---|
| Partial Mayo score assessed | 80 a | 61 | 45 |
| Follow-up without assessment of partial Mayo | 8 | 8 | 5 |
| No follow-up in this time period (but later) | 7 | 4 | 1 |
| No follow-up under continuous golimumab therapy | 3 | 3 | 3 |
| Golimumab stopped | 3 | 21 | 43 |
| Lost to follow-up | 2 | 6 | 6 |
Please note that the primary endpoint could only be assessed for 52 of these patients since patients without a partial Mayo at t0 or already fulfilling criteria for clinical response/remission needed to be excluded (see text).
In the majority of patients (78 of 103), active UC was the main reason for initiating golimumab treatment (72 patients with intestinal activity, 6 patients with EIM; Table 7). In 33 patients, start of golimumab was immediately preceded by primary or secondary non-response to previous treatments (TNF inhibitors in 27, vedolizumab in 2, and 1 each for methotrexate, 6-mercaptopurine, tacrolimus, and the combination of TNF inhibitor and azathioprine).
Table 7.
Primary/secondary endpoint of the study – clinical response/remission at t1, stratified according to prior anti-TNF experience. Calculation of the composite primary/secondary endpoint is indicated with the number patients meeting and not meeting the specific requirement (true/false).
| Anti-TNF experienced True/all |
Anti-TNF naïve True/all |
|
|---|---|---|
| Primary endpoint | ||
| Decrease in partial Mayo score ⩾ 2 (y/ n) | 23/43 (53.5%); N = 43 | 8/9 (88.9%) |
| Decrease in partial Mayo score ⩾ 30% (y/ n) | 24/43 (55.8%); N = 43 | 8/9 (88.9%) |
| Decrease in rectal bleeding by | 33/43 (76.7%); N = 43 | 8/9 (88.9%) |
| ⩾1 or t1 score ⩽ 1 point (y/ n) | ||
| Marked clinical improvement (y/ n) | 22/43 (51.2%); N = 43 | 8/9 (88.9%) |
| Resolution of anemia | 0/31 (0%); no anemia at t0 in 22 | 0/5 (0%); no anemia at t0 in 4 |
| Improvement of CRP | 14/30 (46.7%); normal CRP at t0 in 13 | 2/5 (40%); normal CRP at t0 in 2 |
| Improvement of calprotectin | 6/15 (40%) | 2/2 (100%); normal at t0 in 2 |
| Improvement of endoscopic findings | 1/11 (9.1%); normal at t0 in 1 | 0/1 (0%); normal at t0 in 1 |
| Improvement of histology | 2/10 (20%); normal at t0 in 1 | 0/0 (0%) |
| Any improvement | 17/35 (48.6%) | 3/7 (42.9%) |
| Primary outcome | 12/43 (27.9%) | 3/9 (33.3%) |
| Outcome tested, persistent normal + untested | 13/43 (30.2%) | 7/9 (77.8%) |
| Secondary endpoint | N = 43 | N = 9 |
| t1 normal partial Mayo ⩽ 2 | 18/43 (41.9%) | 6/9 (66.7%) |
| t1 no subscore > 1 | 19/43 (44.2%) | 7/9 (77.8%) |
| t1 clinical remission | 17/43 (39.5%) | 6/9 (66.7%) |
| No anemia | 24/33 (72.7%) | 4/5 (80.0%) |
| Normal CRP ⩽ 5 | 21/33 (63.6%) | 5/5 (100%) |
| Normal calprotectin ⩽ 100 | 2/15 (13.3%) | 1/4 (25%) |
| Endoscopy ⩽ 1 | 1/13 (7.7%) | 0/1 (0%) |
| Histology ⩽ 1 | 1/12 (8.3%) | 0/0 (0%) |
| No evidence residual disease | 9/37 (24.3%) | 3/7 (42.9%) |
| New remission tested | 5/38 (11.6%) | 3/9 (33.3%) |
| New remission tested + not tested | 9/34 (20.9%) | 4/9 (44.4%) |
CRP, C-reactive protein; TNF, tumor necrosis factor.
Follow-up after start of golimumab treatment
After the first injection, golimumab was still used by 63 individuals (61.2%) after 180 days, by 51 individuals (44.7%) after 365 days, and by 34 individuals (33%) after 630 days; declining rates of golimumab treatment are illustrated by the attrition curve (Figure 1(b)). Golimumab state during follow-up after the first injection was unknown for 6 patients (5.8%). In the chart review, we found follow-up data (clinical data and/ or lab work) for t1 for 86 individuals, for t2 for 64 individuals, and for t3 for 45 individuals. The partial Mayo score was known for 84, 80, 61, and 45 patients at t0, t1, t2, and t3, respectively. Reasons for missing data include lack of follow-up in the respective time period, lack of data for calculation of the partial Mayo score, stop of golimumab (mainly at later time points) and loss of follow-up in a few cases (Table 5).
Effectiveness of golimumab treatment
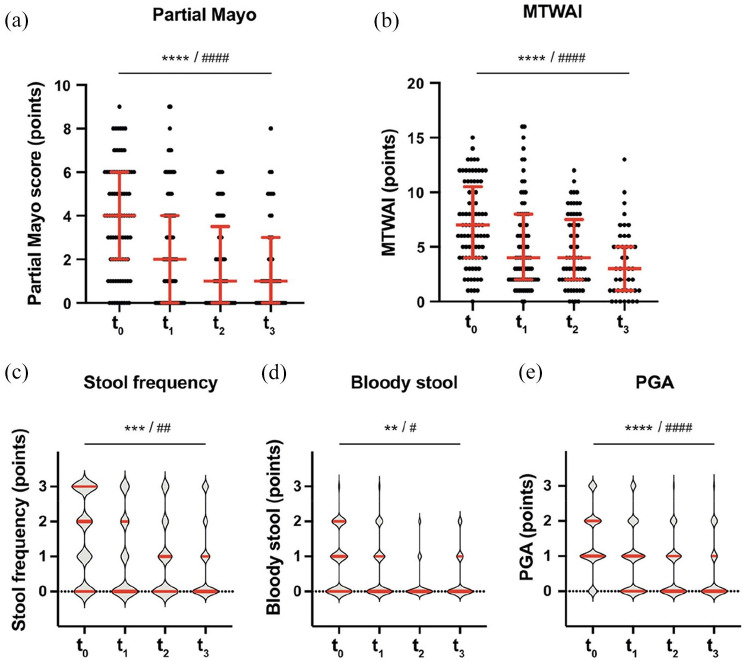
Upon golimumab treatment, the partial Mayo score decreased from 4 (IQR: 2–6) at t0 to 2 (IQR: 0–4) at t1, 1 (IQR: 0–3.5) at t2, and 1 (IQR: 0–3) at t3 (q < 0.0001 for the time trend in a mixed effects model, Figure 3(a)). We also observed a significant decrease of each subscore of the Mayo score (diarrhea, bloody stool, and PGA, Figure 3(c)–(e)); even after multiple test correction. We also found a highly significant decrease of the disease activity score MTWAI over time (Figure 3(b)).
Figure 3.
Clinical efficacy of golimumab treatment. The medians and interquartile ranges are indicated by bars. Statistical analysis: linear mixed-effects model calculating fixed effects of time. Significance for the time trend are indicated without multiple test correction: (*)p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, and after Bonferroni-correction for 10 independent tests in Figures 3 and 4: #q < 0.05; ##q < 0.01; ###q < 0.001; ####q < 0.0001. Mann–Whitney U test.
MTWAI, modified Truelove and Witts activity index; PGA, physician’s general assessment.
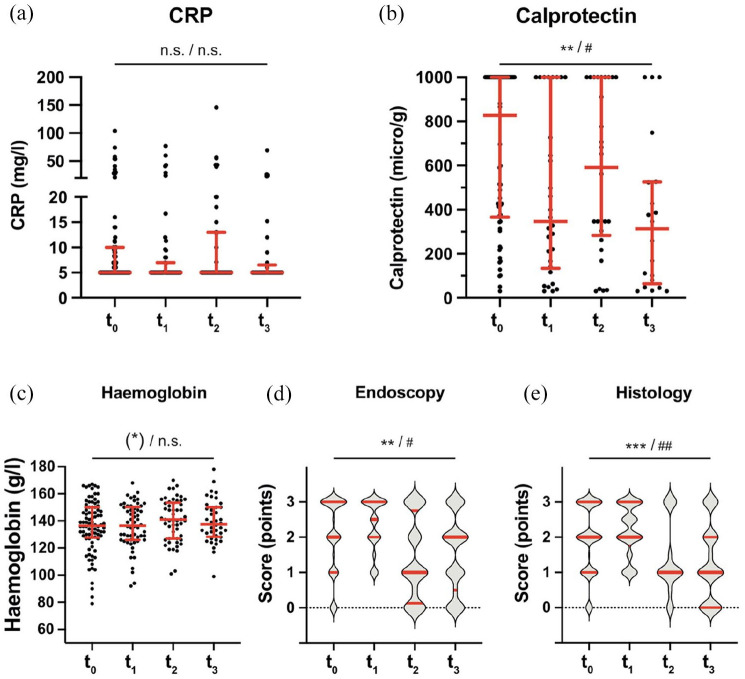
Clinical improvement was accompanied by improvements in calprotectin values (q < 0.05 for the time trend, as well as endoscopic and histological scores (Figure 4(b), (d), and (e)). In contrast, improvements in hemoglobin values and CRP did not reach significance, likely due to the small number of observations (Figure 4(a) and (c)).
Figure 4.
Changes in laboratory parameters upon golimumab treatment. The medians and interquartile ranges are indicated by bars. Statistical analysis: linear mixed-effects model calculating fixed effects of time. Significance for the time trend are indicated without multiple test correction: (*)p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, and after Bonferroni-correction for 10 independent tests in Figures 3 and 4: #q < 0.05; ##q < 0.01; ###q < 0.001; ####q < 0.0001.
CRP, C-reactive protein.
Clinical response after golimumab treatment
Out of 52 patients, a fraction (47.6%) showed marked clinical and objective improvement. The primary endpoint was reached in 15 patients (28.8%, Table 6).
Table 6.
Primary/secondary endpoint of the study – clinical response/remission at t1. Calculation of the composite primary/secondary endpoint is indicated with the number patients meeting and not meeting the specific requirement (true/all).
| True/all (%) | |
|---|---|
| Primary endpoint | |
| Decrease partial Mayo sore ⩾ 30% (y/n) | 32/52 (61.5%) |
| Decrease rectal bleeding by ⩾ 1 or t1 score ⩽ 1 point (y/n) | 41/52 (78.8%) |
| Marked clinical improvement (y/n) | 30/52 (57.7%) |
| Resolution of anemia | 0/36 (0%) a |
| Improvement of CRP | 16/35 (45.7%) b |
| Improvement of calprotectin | 8/17 (47.1%) c |
| Improvement of endoscopic findings | 1/12 (8.3%) d |
| Improvement of histology | 2/10 (20%) e |
| Any improvement | 20/42 (47.6%) |
| Primary outcome | 15/52 (28.8%) |
| Primary outcome tested + untested | 20/52 (38.5%) |
| Secondary endpoint | |
| t1 normal partial Mayo ⩽ 2 | 24/52 (46.2%) |
| t1 no subscore > 1 | 26/52 (50.0%) |
| t1 clinical remission | 23/52 (44.2%) |
| t1 steroid free remission | 17/52 (32.7%) |
| No anemia | 28/38 (73.7%) |
| Normal CRP ⩽ 5 mg/l | 26/38 (68.4%) |
| Normal calprotectin ⩽ 100 µg/g | 3/19 (15.8%) |
| Endoscopy ⩽ 1 | 1/14 (7.1%) |
| Histology ⩽ 1 | 1/12 (8.3%) |
| No evidence residual disease | 12/44 (27.2%) |
| New remission tested | 8/52 (15.4%) |
| New remission tested + not tested | 13/52 (25.0%) |
CRP, C-reactive protein.
No anemia at t0 in 26.
Normal CRP at t0 in 16.
Normal at t0 in 0.
Normal at t0 in 2.
Normal at t0 in 1.
When patients were stratified according to prior anti-TNF exposure, we noted a similar clinical response in the 43 anti-TNF experienced patients (27.9%) compared with the 9 anti-TNF naïve patients (33.3%, Table 7, n.s.).
Clinical remission after golimumab treatment
For 52 patients, the Mayo score was available at t0 and t1, and criteria of remission were not met at t0. Clinical criteria of remission at time point t1 were met in 23 out of 52 patients (44.2%) (Table 6). Assessment of clinical activity either by lab work or endoscopy was attempted in 44 patients and in 12 of those (27.2%), normal results were obtained. Therefore, and according to our stringent definition, 8 out of 52 patients (15.4%) reached UC remission at t1.
Our post hoc power analysis indicated insufficient power for a subgroup analysis (see Methods), and we limited subgroups to patient with and without previous TNF usage: In TNF experienced patients, a non-significant trend for lower remission rates were observed in anti-TNF experienced patients compared with anti-TNF naïve patients (Table 7, statistical analysis not shown).
Side effects of golimumab treatment
Various reasons were named for stopping golimumab, the most frequent were primary non-response or loss of response (Table 8).
Table 8.
Reasons to start/stop therapy with golimumab and adverse events upon treatment with golimumab.
| Reasons to start golimumab a | N = 103 |
|---|---|
| Active disease | 78 (75.7%) |
| Intestinal activity | 72 (69.9%) |
| EIMs | 6 (5.8%) |
| Side effect of previous drugs | 9 (8.7%) |
| Antibodies against previous TNF inhibitors | 9 b (8.7%) |
| Prevention of flare | 1 (1%) |
| Unknown | 14 (13.6%) |
| Reasons to stop golimumab | N = 103 |
| Primary non-response | 24 (23.3%) |
| Loss of response | 16 (15.5%) |
| Side effects | 8 c (7.8%) |
| Patients’ preference | 2 (1.9%) |
| Pregnancy | 1 (1%) |
| Unknown | 25 (24.3%) |
| Ongoing at the time of chart review | 27 (26.2%) |
| Adverse events a | |
| Dizziness | 4 |
| Fever | 2 |
| Psoriasis | 2 |
| Hair loss | 2 |
| Common cold or cough | 2 |
| Combination of symptoms | 4 |
| Problems breathing, fatigue, dizziness, dry eyes | 1 |
| Dizziness, malaise, weakness, fever | 1 |
| Dizziness, bloating, nausea, headache | 1 |
| Dizziness, visual problems, swollen legs, and hair loss | 1 |
| Meningitis | 1 |
EIM, extraintestinal manifestation; TNF, tumor necrosis factor.
More than one could be mentioned.
Eight against infliximab, one against adalimumab.
Four combined with primary non-response and one with secondary non-response.
Sixteen patients reported non-severe side effects (Table 8). One severe side effect (infectious meningitis with need for hospitalization but an overall favorable outcome) was observed in one patient. In this patient, golimumab was stopped during hospitalization and not started again after discharge.
Discussion
In this study, we performed a real-life analysis of usage patterns and clinical success of the TNF inhibitor golimumab in UC patients in Switzerland. We would like to highlight the following key observations: (1) We observed meaningful and significant improvements in clinical scores of disease activity (partial Mayo score, MTWAI) in patients remaining on golimumab treatment at all time points. (2) The primary endpoint of our study, clinical response according to a pre-defined composite endpoint of clinical measures and objective parameters, was met in 15 out of 52 patients (28.8%) at time point t1 (2 until < 17 weeks). (3) Clinical remission, according to a stringent pre-defined endpoint, was observed in 8 out of 52 patients (15.4%). (4) Golimumab was generally well tolerated; reported side effects included dizziness, fever, psoriasis, hair loss, and a severe infectious adverse event (meningitis) in one patient. (5) While the TNF inhibitor infliximab was the most frequently used biologic in UC from 2009 to 2019, vedolizumab and also golimumab were increasingly administered after 2015 in Switzerland. (6) Golimumab was mainly used in biologic-experienced patients (85.5%) with severe disease. (7) In a majority of patients, golimumab was stopped within the first 12 months of treatment but 45% of patients continued treatment beyond the first year.
In 2014 in Switzerland, golimumab had been introduced as the third TNF inhibitor for the treatment of UC. Golimumab and vedolizumab (introduced in 2015) improved the therapeutic options for UC patients. We showed that after 2015, infliximab and vedolizumab remained the most frequently used biologics in UC, while usage of golimumab plateaued at <5% of all UC patients and was typically used as a second, third, or even fourth line of treatment. Therefore, for SIBDC patients, golimumab was the least frequently applied biologic, used as a treatment/ biologic of last resort in many cases. Preferences for long-established therapies (such as infliximab and adalimumab) and consideration regarding 32–34 and safety (in case of vedolizumab) 35 partially explain choices of physicians and patients.
Our real-life analysis, which included many ‘difficult to treat’ biologic-experienced patients, confirms efficacy of golimumab in patients with moderate to severe UC. We observed a pronounced decrease in diarrhea, rectal bleeding, and improvement in general well-being (assessed by the physician general assessment, PGA score) at time points t1, t2, and t3, that is, within the first year of golimumab treatment. Our predefined stringent composite primary endpoint of clinical response was met by 28.8% of patients. In contrast, in the seminal PURSUIT trial, clinical response was observed in 51–54.5% of patients versus 30.3% in patients receiving placebo.13 We would like to highlight several potential explanations for these discrepancies: (1) previous usage of TNF inhibitors or integrin inhibitors was excluded in the PURSUIT trial, while approximately 85% of patients in our study had previous experience with biologics. (2) Clinical activity in the PURSUIT study was high (Mayo score 6–12), while in our study, 50% of patients had moderate clinical activity (median partial Mayo score of 5, partial Mayo score ranges from 0 to 9). (3) While the definition of the clinical endpoint was similar in both trials, in our study, a decrease in objective measures of disease activity was additionally required. The clinical endpoint was met in 50% of all patients (similar to the PURSUIT study), while the objective confirmation reduced the rate of clinical response to 28.8%.
Effectiveness of golimumab has already been confirmed in other real-world studies. In most studies, response ranged from 60-70% of patients,14,18,19,22,25 considerably higher than the 28.8% observed in our study. However, similar to the PURSUIT trial, the above-mentioned real-world studies only use a clinical endpoint, while in our study, objective confirmation of improvement was required. The rate of marked clinical improvement in our study (57.7%) corresponds to the primary endpoint of the other studies with similar results. Moreover, in our study, 84% of patients were anti-TNF experienced, higher than in the other studies, with 0%, 14 11%, 19 36%, 18 60%, 25 and 73%. 22 One small real-world study with 23 patients had a high clinical response rate in anti-TNF naïve and experienced patients (85% and 70%, respectively) 23 ; in another study with 17 evaluated patients, clinical remission was 47% and 50% in biological naïve and experienced patients, respectively. 36 Similar to previous studies, our work confirmed the overall safety of golimumab; however, one severe adverse event related to infection was observed in this cohort of 103 patients.
Our study has several strengths and limitations: Strengths include usage of data from the SIBDC, a large cohort of well-characterized IBD patients. Furthermore, we performed a careful and comprehensive chart review in 27 study sites including private practices and large secondary and tertiary hospitals. All analyses were performed according to a pre-specified study protocol. Limitations include the observational nature of our study and lack of a protocolled follow-up. Therefore, follow-up intervals for t1 to t3 were wide, and the Mayo score could not be deduced from physician notes in all cases. Moreover, 19 (18.4%) of our patients were in remission at t0, resulting in 52 evaluated patients. Similarly, acquisition of lab work, including calprotectin was not complete in all study patients which further limited assessment of our composite endpoints.
In conclusion, golimumab was used between 2014 and 2019 in 103 UC patients from the SIBDC, mainly biologic-experienced patients. Overall, golimumab was generally well tolerated; one severe infectious adverse event was observed. Golimumab was used beyond 1 year in less than 50% of patients. In those remaining under golimumab treatment, highly significant improvements in clinical parameters were noted. Pre-defined composite endpoints of clinical response and remission were met in 28.8% and 15.4% of patients, respectively.
Acknowledgments
The authors thank all SIBDC patients for their participation and commitment and the SIBDC study nurses and physicians for their cooperation.
Footnotes
Author contributions: Kathrin Perrig: Data curation; Investigation; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Niklas Krupka: Writing – review & editing.
Sebastian Bruno Ulrich Jordi: Formal analysis; Writing – original draft; Writing – review & editing.
Jean-Benoît Rossel: Writing – review & editing.
Luc Biedermann: Writing – review & editing.
Thomas Greuter: Writing – review & editing.
Philipp Schreiner: Writing – review & editing.
Stephan R. Vavricka: Writing – review & editing.
Pascal Juillerat: Writing – review & editing.
Emanuel Burri: Data curation; Writing – review & editing.
Dorothee Zimmermann: Data curation; Writing – review & editing.
Michel H. Maillard: Data curation; Writing – review & editing.
Michael Christian Sulz: Writing – review & editing.
Stephan Brand: Writing – review & editing.
Gerhard Rogler: Writing – review & editing.
Benjamin Misselwitz: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KP has nothing to disclose. NK has nothing to disclose. SBUJ has nothing to disclose. JBR has nothing to disclose. LB reports fees for consulting/advisory board from Abbvie, MSD, Vifor, Falk, Esocap, Calypso, Ferring, Pfizer, Shire, Takeda, Janssen, and Ewopharma. TG has consulting contracts with Sanofi-Regeneron and Falk Pharma GmbH, received travel grants from Falk Pharma GmbH and Vifor, and an unrestricted research grant from Novartis. PS has consulted to Pfizer, Takeda, Abbvie, and Janssen-Cilag and received travel support from Falk and UCB. SRV has received consulting fees, speakers honorary, and unrestricted research grants from Abbott, Alfasigma, Amgen, Arenapharm, Falk Pharma GmbH, Ferring Pharmaceuticals, Gilead, iQuone, Janssen, MSD, Permamed, Pfizer Inc, Sanofi-Aventis, Takeda, Tillotts, UCB, and Vifor. PJ has received a research grant from Vifor unrelated to this work. EB received consultant and/or speaker fees from Abbvie, Janssen, MSD, Norgine, Pfizer, Sandoz, Takeda, and Vifor. DZ has nothing to disclose. MHM has received consultant fees from Vifor, Abbvie, UCB, MSD, Lilly, Janssen, and Takeda. He also received grants from UCB, Abbvie, Vifor, MSD, Takeda. He received speaker fess from Vifor, Janssen, Abbvie, MSD, Pfizer, UCB, and Takeda. MCS has received consultant and/or speaker fees from Abbvie, Ferring, MSD, Janssen, Pfizer, Takeda, UCB. SB has consulted to Abbvie, Celgene, Ferring, Gilead, Janssen, MSD, Pfizer, Roche, UCB, and Takeda, Vifor; SB has received speaker’s honoraria from Abbvie, FALK, Ferring, MSD, Takeda, UCB, and Vifor; SB has received an educational grant from Takeda. GR has consulted to Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions, and Zeller; GR has received speaker’s honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor, and Zeller; GR has received educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB, and Zeller. BM has received a research grant from MSD for this work. BM has served at an advisory board for Gilead and Novigenix. He has received speaking fees from Vifor, MSD, and Takeda and traveling fees from Vifor, Novartis, Gilead, and Takeda.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research grant from MSD and by the Swiss National Science Foundation (SNF) to the Swiss IBD Cohort (Grant No. 33CS30-148422).
Role of the funder: MSD funded this study. The study protocol was prepared by the authors and reviewed and approved by the funder. Data were collected, analyzed, and interpreted by the authors, without involvement of the funder. The funder reviewed the manuscript and provided suggestions. The final decision to publish was not influenced by the funder.
ORCID iDs: Kathrin Perrig  https://orcid.org/0000-0002-8730-954X
https://orcid.org/0000-0002-8730-954X
Luc Biedermann  https://orcid.org/0000-0003-0824-4125
https://orcid.org/0000-0003-0824-4125
Data availability: Original data will be available upon request to the corresponding author. To protect patient privacy, some restrictions apply according to Swiss law. Sharing of SIBDC data need to be approved by the scientific committee of the SIBDC.
Contributor Information
Kathrin Perrig, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Niklas Krupka, Department of Visceral Surgery and Medicine, Inselspital Bern University Hospital, University of Bern, Bern, Switzerland.
Sebastian Bruno Ulrich Jordi, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, SwitzerlandDepartment of Visceral Surgery and Medicine, Inselspital Bern University Hospital, University of Bern, Bern, Switzerland.
Jean-Benoît Rossel, Center for Primary Care and Public Health (Unisanté), University of Lausanne, Lausanne, Switzerland.
Luc Biedermann, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Thomas Greuter, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Philipp Schreiner, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Stephan R. Vavricka, Center of Gastroenterology and Hepatology, Zurich, Switzerland
Pascal Juillerat, Department of Visceral Surgery and Medicine, Inselspital Bern University Hospital, University of Bern, Bern, Switzerland.
Emanuel Burri, Department of Gastroenterology and Hepatology, University Medical Clinic, Kantonsspital Baselland, Liestal, Switzerland.
Dorothee Zimmermann, Department of Gastroenterology, Kantonsspital Nidwalden, Stans, Switzerland.
Michel H. Maillard, Service of Gastroenterology and Hepatology, Lausanne University Hospital, Lausanne, Switzerland; Crohn and Colitis Center, Gastroentérologie Beaulieu SA, Lausanne, Switzerland
Michael Christian Sulz, Department of Gastroenterology, Kantonsspital Münsterlingen, Münsterlingen, Switzerland.
Stephan Brand, Department of Gastroenterology and Hepatology, Kantonsspital St. Gallen, St. Gallen, Switzerland.
Gerhard Rogler, Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Benjamin Misselwitz, Department of Visceral Surgery and Medicine, Inselspital Bern University Hospital, University of Bern, Freiburgstr. 18, 3010 Bern, Switzerland; Department of Gastroenterology and Hepatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
References
- 1. Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 162–175. [DOI] [PubMed] [Google Scholar]
- 3. Misselwitz B, Juillerat P, Sulz MC, et al. Emerging treatment options in inflammatory bowel disease: Janus kinases, stem cells, and more. Digestion 2020; 101(Suppl. 1): 69–82. [DOI] [PubMed] [Google Scholar]
- 4. Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014; 13: 24–30. [DOI] [PubMed] [Google Scholar]
- 5. Kopylov U, Seidman E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Therap Adv Gastroenterol 2016; 9: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parragi L, Fournier N, Zeitz J, et al. Colectomy rates in ulcerative colitis are low and decreasing: 10-year follow-up data from the Swiss IBD Cohort Study. J Crohns Colitis 2018; 12: 811–818. [DOI] [PubMed] [Google Scholar]
- 7. Sofia MA, Rubin DT. Current approaches for optimizing the benefit of biologic therapy in ulcerative colitis. Therap Adv Gastroenterol 2016; 9: 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pouillon L, Bossuyt P, Peyrin-Biroulet L. Considerations, challenges and future of anti-TNF therapy in treating inflammatory bowel disease. Expert Opin Biol Ther 2016; 16: 1277–1290. [DOI] [PubMed] [Google Scholar]
- 9. Rogler G. Where are we heading to in pharmacological IBD therapy? Pharmacol Res 2015; 100: 220–227. [DOI] [PubMed] [Google Scholar]
- 10. Singh S, George J, Boland B, et al. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis 2018; 12: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.www.swissmedicinfo.ch.
- 12. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96–109.e1. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95; quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 14. Probert CS, Sebastian S, Gaya DR, et al. Golimumab induction and maintenance for moderate to severe ulcerative colitis: results from GO-COLITIS (Golimumab: a Phase 4, UK, open label, single arm study on its utilization and impact in ulcerative Colitis). BMJ Open Gastroenterol 2018; 5: e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 2018; 35: 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012; 10: 1002–1007; quiz e78. [DOI] [PubMed] [Google Scholar]
- 17. Bressler B, Williamson M, Sattin B, et al. Real world effectiveness of golimumab therapy in ulcerative colitis regardless of prior TNF exposure. J Can Assoc Gastroenterol 2018; 1: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bossa F, Biscaglia G, Valvano MR, et al. Real-life effectiveness and safety of golimumab and its predictors of response in patients with ulcerative colitis. Dig Dis Sci 2020; 65: 1767–1776. [DOI] [PubMed] [Google Scholar]
- 19. Tursi A, Allegretta L, Della Valle N, et al. Effectiveness of golimumab in inducing remission and clinical response in outpatient ulcerative colitis. Clin Res Hepatol Gastroenterol 2016; 40: e61–e63. [DOI] [PubMed] [Google Scholar]
- 20. Iborra M, García-Morales N, Rubio S, et al. Real-life experience with 4 years of golimumab persistence in ulcerative colitis patients. Sci Rep 2020; 10: 17774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samaan MA, Cunningham G, Tamilarasan AG, et al. Therapeutic thresholds for golimumab serum concentrations during induction and maintenance therapy in ulcerative colitis: results from the GO-LEVEL study. Aliment Pharmacol Ther 2020; 52: 292–302. [DOI] [PubMed] [Google Scholar]
- 22. Bosca-Watts MM, Cortes X, Iborra M, et al. Short-term effectiveness of golimumab for ulcerative colitis: observational multicenter study. World J Gastroenterol 2016; 22: 10432–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castro-Laria L, Argüelles-Arias F, García-Sánchez V, et al. Initial experience with golimumab in clinical practice for ulcerative colitis. Rev Esp Enferm Dig 2016; 108: 129–132. [DOI] [PubMed] [Google Scholar]
- 24. Detrez I, Dreesen E, Van Stappen T, et al. Variability in golimumab exposure: a ‘real-life’ observational study in active ulcerative colitis. J Crohns Colitis 2016; 10: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taxonera C, Rodríguez C, Bertoletti F, et al. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis 2017; 23: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 26. Stefanovic S, Detrez I, Compernolle G, et al. Endoscopic remission can be predicted by golimumab concentrations in patients with ulcerative colitis treated with the changed label. Eur J Gastroenterol Hepatol 2021; 33: 54–61. [DOI] [PubMed] [Google Scholar]
- 27. Pittet V, Juillerat P, Mottet C, et al. Cohort profile: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol 2009; 38: 922–931. [DOI] [PubMed] [Google Scholar]
- 28. Pittet V, Michetti P, Mueller C, et al. Cohort profile update: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol 2019; 48: 385–386f. [DOI] [PubMed] [Google Scholar]
- 29. Schreiner P, Rossel JB, Biedermann L, et al. Fatigue in inflammatory bowel disease and its impact on daily activities. Aliment Pharmacol Ther 2021; 53: 138–149. [DOI] [PubMed] [Google Scholar]
- 30. Biedermann L, Fournier N, Misselwitz B, et al. High rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation--data from the Swiss IBD Cohort Study. J Crohns Colitis 2015; 9: 819–829. [DOI] [PubMed] [Google Scholar]
- 31. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 32. Bonovas S, Lytras T, Nikolopoulos G, et al. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 454–465. [DOI] [PubMed] [Google Scholar]
- 33. Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One 2016; 11: e0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thorlund K, Druyts E, Toor K, et al. Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expert Rev Gastroenterol Hepatol 2015; 9: 693–700. [DOI] [PubMed] [Google Scholar]
- 35. Lukin D, Faleck D, Xu R, et al. Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis. Clin Gastroenterol Hepatol 2022; 20: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orlandini B, Dragoni G, Variola A, et al. Clinical efficacy and safety of golimumab in biologically experienced and naive patients with active ulcerative colitis: a real-life experience from two Italian IBD centers. J Dig Dis 2018; 19: 468–474. [DOI] [PubMed] [Google Scholar]