Abstract
Despite all improvements in rheumatoid arthritis, we are still not able to prevent or cure the disease. Diagnostic delays due to lack of access to a specialist and costly therapies are still a major obstacle for many patients. Even in first-world countries, the treat-to-target principle and the goal of disease remission are often missed. Thus, rheumatoid arthritis (RA) is still the reason for disability and reduced quality of life for many patients. So, is it time to move the goalpost even further? Where are we heading next? And will we finally be able to cure the disease? These questions are addressed in our review article.
Keywords: P4 medicine, personalized medicine, digital health, Rheumatoid arthritis
The glory of medicine is that it is constantly moving forward, that there is always more to learn. (William James Mayo-physician and founder of the Mayo Clinic)
In the last decades, we have seen unprecedented changes in medicine and in particular in the field of rheumatology. From a microscopic to a macroscopic level, the knowledge about rheumatoid arthritis (RA) and the therapeutic options for the management of the disease have expanded significantly.
In recent years, we have gained deep insights into the etiology of the disease: 1 we have learned for instance that RA is a heterogeneous disease that, based on data combining genetic risk factors and autoantibodies, can be classified into anti-citrullinated protein antibodies (ACPAs)-positive and -negative RA. The presence of ACPA and rheumatoid factor (RF), as well as elevated C-reactive protein (CRP) levels in some patients’ years before the onset of clinical symptoms, indicate that the immune responses critical to the development of RA are initiated very early.2,3 Furthermore, important cytokine signaling pathways which are involved in the disease progression have been identified and synovial studies have shown that not only adaptive but also innate immune responses are important. 4 With increasing knowledge about the pathophysiology of the disease, specific treatment options have expanded tremendously. We now possess a wide armamentarium of therapeutic options ranging from old drugs such as steroids and disease-modifying anti-rheumatic drugs (DMARDs) to targeted therapies which aim to inhibit specific cells or cytokines.5,6
Also, international management recommendations have been developed that help to guide disease management which is based on new treatment principles such as tight control strategy and the treat-to-target approach with the ultimate goal of disease remission.7,8
Moreover, there have been great advances in the field of diagnostics and disease monitoring. Potential biomarkers or cytokine panels promise to allow earlier diagnosis and treatment monitoring. 9 With ever-improving high-resolution imaging techniques and the widespread availability of ultrasound, the disease can be detected at a very early stage, and even minor disease progression can be assessed. 10
Despite all these improvements, we are still not able to prevent or cure the disease. Diagnostic delays due to the lack of access to a specialist and costly therapies are still a major obstacle for many patients. Even in first-world countries, the treat-to-target principle and the goal of disease remission are often missed. 11 RA is still the reason for disability and reduced quality of life for many patients. 12
So, is it time to move the goalpost even further? Where are we heading next? And will we finally be able to cure the disease?
The good physician treats the disease; the great physician treats the patient who has the disease. (William Osler-Canadian physician and professor of medicine)
Due to the high complexity of the interplay between genetics and epigenetics, a genuinely individualized therapy for RA is currently not possible. At present, standard therapeutic algorithms are used, but they are not tailored to the individual characteristics of the patient.
This is where artificial intelligence (AI) and machine learning (ML) promises to be a game changer. Owing to the constantly growing data volumes from various digital data sources (e.g. biomarker and ‘omics’ data, electronic health records, radiological image sets, or wearable data), an increasingly complex picture is being assembled, which can be decoded with the help of AI. The technology is able to identify new patterns and correlations that can help us to diagnose and treat the disease. 13
Even though the technology is still evolving, there are already initial models that can be used to predict therapy response 14 and disease outcome. 15 AI can already detect arthritic changes in some radiological images with the precision of experts 16 and can be used in in silico trials to develop new therapies. 17 Recent studies in small cohorts showed that individualized flare risk could also be predicted with a high probability. 18 With the further increase in digitalisation in medicine and exponentially growing digital data, ML algorithms will steadily improve and thus allow accurate individualized predictions in the future. RA therapy guidelines will become significantly more complex and will be constantly updated with AI support at a much higher frequency level.
But let’s move even further from the disease to the patient himself. In recent years, a new approach to modern medicine has emerged, shifting the focus away from the disease toward the promotion of a state of wellness defined as a state of optimal health. This approach is called P4 medicine and follows the four Ps: predictive, preventive, personalized, and participatory. 19 The concept aims at preserving health and at early detection of the transition from wellness to disease to enable early intervention and thus the prevention of disease. Let us take a closer look at each P and its relevance in RA (Figure 1).
Figure 1.
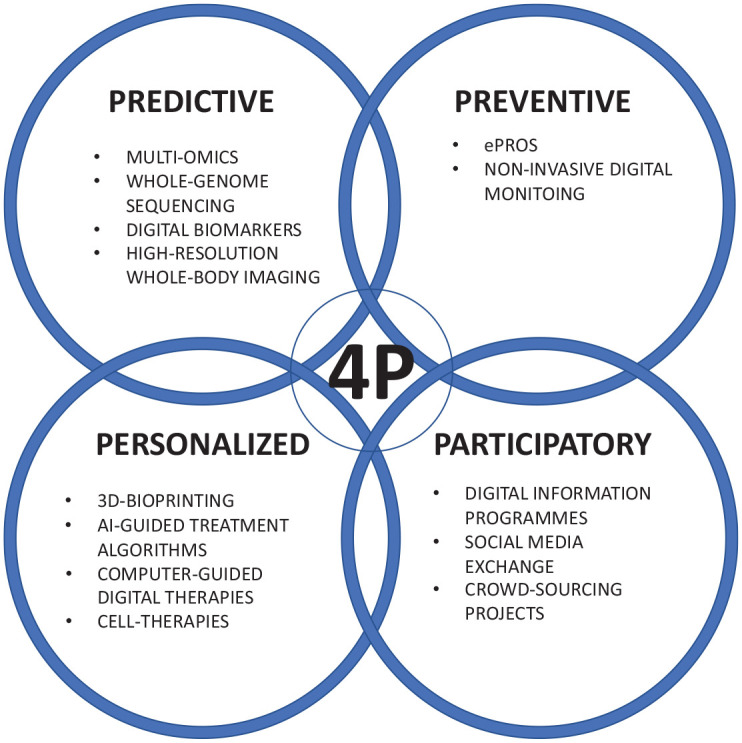
Four P-concept for rheumatoid arthritis.
Predictive
Prediction aims at early identification of healthy individuals at risk for RA and to monitor them closely, if necessary. In a few years, whole genome sequencing will presumably be available and affordable for everybody. New-borns will receive a ‘multi-omics’-testing integrating information obtained from each ‘omic’ discipline (in particular transcriptomics, proteomics, metabolomics, genomics, epigenomics, cytomics and environmentomics).
First steps have been taken to identify patients with arthralgia at risk for the development of RA through the integrative analysis of epigenetic markers and alternative splicing. 20 Differences were observed for 59 genes with the most significant difference in methylation in the promoter regions MIRLET7B and microRNA10B (MIR10B). MIR10B was found to regulate Th17 cells in ankylosing spondylitis, while its role in RA has not been clarified. A different approach is the development of a polygenic risk score for RA that can measure individual genetic risks for certain diseases. So far, polygenic risk scores have been studied in other diseases such as cardiovascular disease, diabetes, and breast cancer, 21 but its potential in rheumatic diseases is evident. 22
The omic data will be combined with digital biomarkers that play a role in the development and disease course of RA. In keeping with the new principle that ‘data is the new blood’, medical data collected through digital tools, such as fitness trackers, health apps, sleep sensors, pocket ECG, and blood pressure measuring devices, will be used to identify patterns in different populations and to develop models that explain, influence, or predict health-related outcomes. In contrast to invasive biomarkers, digital data have the benefit of being continuously collected and thus allow for a longitudinal context-associated evaluation. 23
‘Omics’ data and digital biomarkers will be stored in clouds and on a microchip inserted under the skin. These chips about a size of a rice grain are already being used in Sweden by more than 4000 people as digital keys to swipe into their homes, offices, gyms, and even to buy train tickets and access social media. 24 Based on these data, each individual will receive elaborated risk profiled and tailored prevention. Since most ‘omics’ are subject to changes over time, a single screening at baseline (birth) will be insufficient, and the individual risk profile will be constantly updated to integrate new insights from the emerging field of digital biomarkers.
Once we are aware of the individual risk, imaging techniques like high-resolution whole-body imaging can help to detect signs of inflammation before being clinically relevant. Vitamin and other deficiencies as well as metabolic disorders can be identified early by screening of blood and urine samples and countermeasures can be taken to maintain health. Ideally, the risk for RA-associated comorbidities will be known for each individual due to the broad screening process and can be minimized by individual lifestyle adjustments and other supportive measures.
This enables early actions to minimize risk for RA and preserve the state of perfect health as long as possible. With reliable invasive and non-invasive biomarkers, we will also be able to predict how each person will respond to therapy, diet, exercise, and supplements so that therapy decisions can be made accordingly. Large studies have already investigated the efficiency of personalized diets based on genetic metabolic analyses. 25
Preventive
Knowing the individual risk of RA, special emphasis must be placed on prevention to promote the state of well-being. Since the healthcare system will not be able to cover tight controls neither in terms of personnel nor financial resources, individuals at risk can be handed tools to monitor their health independently and to be vigilant for changes that indicate an early and potentially reversible disease. Among these tools, electronic patient reported outcomes (ePROs) have already or will soon find their way into rheumatology. The assessment of ePROS allows for continuous, patient-centered monitoring, and rheumatologists see great potential in their use. 26 Other non-invasive passive monitoring tools encompass wearables but also non-invasive analysis devices that can analyze urine, stool, 27 and sweat 28 for anomalies.
Smart gloves can track morning stiffness and changes in range of motion in patients with RA. 29 The SkinBot might be crawling the body of individuals at risk to detect inflamed joints at very early stage. 30 Furthermore, vocal pattern recognition has the potential to process voice patterns, such as pitch, tone, rhythm, and frequency, as well as breathing or coughing by AI and thus to develop vocal biomarkers for diseases. 31 In RA, this could help to detect early interstitial lung disease as well as pain and fatigue. To further expand the home-based diagnostic arsenal, an automated AI stethoscope will be available to identify signs of interstitial lung disease and enable the differentiation of ILD and pneumonia. 32
All these tools will lead to continuous passive monitoring of patients at risk or already diseased, and in case of changes, patient-specific measures can be taken at an early stage.
Furthermore, vaccinations may allow the early prevention of RA. Recently, passive immunization with the protein 14-3-3zeta (ζ), that is involved in T-cell polarization and IL-17A signal transduction has been shown to suppress arthritis in 14-3-3ζ knockout inflammatory arthritis rat-models by the suppression of IL-1beta levels and increased collagen production. 33
Another approach is the polarization of T helper cell 2 (Th2) by the peptide vaccine CEL-4000 which utilizes an MHC class-II specific ligand to activate regulatory responses. 34
DEN-181 uses liposomal technology to inject collagen II as a liposome-encapsulated antigen together with calcitriol in patients with ACPA-positive RA. 35 A phase-1 trial has shown good safety and an effect on antigen-specific T-cells in RA patients, while specific effects on the prevention of RA have to be studied in future trials. 35
Prevention, however, should not be limited to the disease itself but include comorbidities. Since cardiovascular disease due to accelerated atherogenesis is a common cause of premature mortality in RA, the search for genetic, serological, and non-invasive markers of cardiovascular disease in the early stages of this condition will be a goal to be reached. 36
Personalized
The entire process of risk stratification aims at a personalized approach. If the transition from health to sickness occurs despite all preventive efforts, personalized medicine yields tailored approaches for the patients in the future: 3D bioprinting will produce personalized pills. With knowledge of the needs and exact pharmacogenetics of the person concerned, pills can be individually designed and provided with the required active ingredients and dosages. These polypills or ‘smart’ pills can then be printed rapidly and cost-effectively. 37 Current therapy regimes such as methotrexate (MTX) as the cornerstone of RA could be replaced by, for example, tsDMARDs according to the individual risk profile calculated by ML algorithms.
Data for the ML algorithms is currently being collected in studies investigating the potential of multi-omics for the prediction of treatment response. 38 Furthermore, cytokine signatures might allow the identification and stratification of patients with an increased risk for relapse from long-term remission. 39 Precision medicine with tailored treatments to subgroups of patients will become reality.
In addition, genome-engineered bioartificial implants which regulate anti-cytokine drug delivery hold great promise for a tailored intra-articular treatment approach. Choi et al. 40 were able to use CRISPR-Cas9 and genome editing of induced pluripotent stem cells in order to create a synthetic gene circuit that senses changing levels of endogenous inflammatory cytokines to trigger a proportional therapeutic response. These therapeutic implants completely prevented increased pain sensitivity and bone erosions. Custom-designed cells that express therapeutic transgenes in response to dynamically changing biological signals promises a range of potential applications for treating RA.
In 2020, Schett et al. 41 addressed the question: ‘Why remission in RA is not enough?’. In routine clinical practice, remission is often not achieved or disease-related problems such as fatigue 42 or fibromyalgia persist despite numerical remission, as they are not assessed by the standard remission scores. This is another important aspect where individualized therapy concepts with a focus on disease-related comorbidities could become more important. In particular, personalized computer-based physiotherapy programs 43 or digital psychological support is already available today. AI-based rehabilitation and nutrition programmes with a preventive character are also feasible.
Nevertheless, current therapy concepts focus solely on symptom control and the prevention of disease progression. The downside of this concept is the need for potentially lifelong therapy. But what if the disease were truly curable? In the future, the cure of rheumatic diseases is in fact conceivable, by applying new genetic engineering methods or cell-based therapies, such as autologous stem cell transplantation 44 and CAR-T-cell therapy. 45 Even if these therapy concepts are currently still risky and expensive, it can be assumed that the method for the therapy of autoimmune diseases could definitely gain in importance. In the future, the cure and not the remission of the disease may become the new target.
Participatory
Participatory decision-making has become an important pillar of medicine, especially in chronic diseases. The goal is to move away from a paternalistic top–down approach toward shared decision–making, an approach that requires a more precise knowledge of the health literacy of those affected. 46 With the focus shifting away from sick care to healthcare and prevention of diseases being the highest goal in medicine, patients need to be extensively informed about their health and risk profiles and possibilities to prevent disease occurrence. 47 With virtual reality (VR) and augmented reality (AR), two interactive technologies are currently being introduced in patient-centered healthcare. VR and AR can be used in patient education 48 and other settings such as medication management and adherence improvement. 49 Supporting patient decision aids via these emerging technologies has the potential to increase health literacy and enable patients to form an opinion and take decisions in terms of shared decision-making.
Together with VR and AR, adaptive learning could sustainably change patient education. Adaptive learning platforms use computer algorithms and AI to deliver tailored information and learning activities and address the unique needs of each patient regarding his interests, level, and preferences. This way, learning is more efficient while providing great variability making learners more likely to stick to the program.
Further challenges and requirements
In addition to the mentioned developments and paradigm shifts required to establish P4 medicine in rheumatology, there are other important aspects that need to be addressed. In the long term, the goal is to use the P4 approach to conduct a more patient-oriented, efficient and cost-effective medicine that provides the best possible care for each individual. Therefore, costs for ‘omics’-analyses need to be reduced; knowledge must exist that enables the right interpretation of the data. Regulations for storage, processing, and distribution of data are already highly needed. 50
Prediction also entails the danger of discrimination of individuals at risk by, for example, insurance companies and employers. Legislation that prevents this discrimination is a prerequisite for realization of P4 medicine.
As we are constantly moving forward, the future for RA patients will be bright. With the availability of digital and biological biomarkers, we will be able to detect and monitor the disease even more accurately. 51 AI will help us to predict the disease course even better, and therefore, prevention of disease damage or even the onset will be much easier. Prevent-to-target may become a new treatment approach. By combining genetic and epigenetic data and applying ML algorithms, truly personalized treatment will be possible. As genetic and cell-based therapies are evolving the cure of RA might even be within reach. Continuous treatment adaptions, also focusing on comorbidities and lifestyle requirements, will be possible. As the focus of the disease management will shift from sick care to healthcare, our role as rheumatologists will change. 52
Finally, patient empowerment and participatory decision-making will truly put the RA patient and not the disease in the center just as William Osler suggested more than a century ago.
Footnotes
Author contributions: Johanna Mucke: Conceptualization; resources; writing – original draft.
Martin Krusche: Conceptualization; supervision; writing – review & editing.
Gerd Burmester: Conceptualization; formal analysis; resources; supervision; writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Johanna Mucke  https://orcid.org/0000-0001-8915-7837
https://orcid.org/0000-0001-8915-7837
Gerd R. Burmester  https://orcid.org/0000-0001-7518-1131
https://orcid.org/0000-0001-7518-1131
Contributor Information
Johanna Mucke, Policlinic and Hiller Research Unit for Rheumatology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany.
Martin Krusche, Division of Rheumatology and Inflammatory Rheumatic Diseases, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
Gerd R. Burmester, Department of Rheumatology and Clinical Immunology, Charité –Universitätsmedizin Berlin, Charitéplatz 1, D-10117 Berlin, Germany.
References
- 1. Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun 2020; 110: 102400. [DOI] [PubMed] [Google Scholar]
- 2. Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50: 380–386. [DOI] [PubMed] [Google Scholar]
- 3. ten Brinck RM, van Steenbergen HW, van Delft MAM, et al. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017; 56: 2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018; 4: 18001. [DOI] [PubMed] [Google Scholar]
- 5. Burmester GR, Bijlsma JWJ, Cutolo M, et al. Managing rheumatic and musculoskeletal diseases – past, present and future. Nat Rev Rheumatol 2017; 13: 443–448. [DOI] [PubMed] [Google Scholar]
- 6. Shams S, Martinez JM, Dawson JRD, et al. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol 2021; 12: 680043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 8. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2021; 73: 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mun S, Lee J, Park M, et al. Serum biomarker panel for the diagnosis of rheumatoid arthritis. Arthritis Res Ther 2021; 23: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carstensen SMD, Terslev L, Jensen MP, et al. Future use of musculoskeletal ultrasonography and magnetic resonance imaging in rheumatoid arthritis. Curr Opin Rheumatol 2020; 32: 264–272. [DOI] [PubMed] [Google Scholar]
- 11. Einarsson JT, Willim M, Ernestam S, et al. Prevalence of sustained remission in rheumatoid arthritis: impact of criteria sets and disease duration, a Nationwide Study in Sweden. Rheumatology 2019; 58: 227–236. [DOI] [PubMed] [Google Scholar]
- 12. Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44: 123–130. [DOI] [PubMed] [Google Scholar]
- 13. Hügle M, Omoumi P, van Laar JM, et al. Applied machine learning and artificial intelligence in rheumatology. Rheumatol Adv Pract 2020; 4: rkaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tao W, Concepcion AN, Vianen M, et al. Multiomics and machine learning accurately predict clinical response to adalimumab and etanercept therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2021; 73: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norgeot B, Glicksberg BS, Trupin L, et al. Assessment of a deep learning model based on electronic health record data to forecast clinical outcomes in patients with rheumatoid arthritis. JAMA Netw Open 2019; 2: e190606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bressem KK, Vahldiek JL, Adams L, et al. Deep learning for detection of radiographic sacroiliitis: achieving expert-level performance. Arthritis Res Ther 2021; 23: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappalardo F, Russo G, Tshinanu FM, et al. In silico clinical trials: concepts and early adoptions. Brief Bioinform 2019; 20: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 18. Vodencarevic A, Tascilar K, Hartmann F, et al. Advanced machine learning for predicting individual risk of flares in rheumatoid arthritis patients tapering biologic drugs. Arthritis Res Ther 2021; 23: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sagner M, McNeil A, Puska P, et al. The P4 health spectrum – a predictive, preventive, personalized and participatory continuum for promoting healthspan. Prog Cardiovasc Dis 2017; 59: 506–521. [DOI] [PubMed] [Google Scholar]
- 20. Li Yim AYF, Ferrero E, Maratou K, et al. Novel insights into rheumatoid arthritis through characterization of concordant changes in DNA methylation and gene expression in synovial biopsies of patients with differing numbers of swollen joints. Front Immunol 2021; 12: 651475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet 2019; 28: R133–R142. [DOI] [PubMed] [Google Scholar]
- 22. Brown MA, Li Z. Polygenic risk scores and rheumatic diseases. Chin Med J 2021; 134: 2521–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodarte C. Pharmaceutical perspective: how digital biomarkers and contextual data will enable therapeutic environments. Digit Biomark 2017; 1: 73–81, https://www.karger.com/Article/FullText/479951 (accessed 30 September 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thousands of Swedes are getting microchip IDs inserted into their hands to swipe into homes, offices, concerts and even to access social media, https://www.dailymail.co.uk/sciencetech/article-6306569/Thousands-Swedes-getting-microchip-IDs-inserted-hands.html (accessed 6 October 2021).
- 25. Celis-Morales C, Marsaux CF, Livingstone KM, et al. Can genetic-based advice help you lose weight? Findings from the Food4Me European randomized controlled trial. Am J Clin Nutr 2017; 105: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 26. Krusche M, Klemm P, Grahammer M, et al. Electronic patient-reported outcomes: a survey about acceptance, usage and barriers among German Rheumatologists. JMIR Mhealth Uhealth 2020; 8: e18117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toi Labs, https://www.toilabs.com/trueloo/ (accessed 6 October 2021).
- 28. Song Y, Min J, Yu Y, et al. Wireless battery-free wearable sweat sensor powered by human motion. Sci Adv 2020; 6: eaay9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henderson J, Condell J, Connolly J, et al. Review of wearable sensor-based health monitoring glove devices for rheumatoid arthritis. Sensors 2021; 21: 1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dementyev A, Hernandez J, Follmer S, et al. SkinBot. In: Gajos K. (ed.) Adjunct publication of the 30th annual ACM symposium on user interface software and technology. New York: ACM, 2017, pp. 5–6. [Google Scholar]
- 31. The Lancet Digital Health. Do I sound sick? Lancet Digit Health 2021; 3: e534. [DOI] [PubMed] [Google Scholar]
- 32.https://www.m3dicine.com (accessed 30 January 2022).
- 33. Kim J, Chun K, McGowan J, et al. 14-3-3ζ: a suppressor of inflammatory arthritis. Proc Natl Acad Sci USA 2021; 118: e2025257118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zimmerman DH, Mikecz K, Markovics A, et al. Vaccination by two DerG LEAPS conjugates incorporating distinct proteoglycan (PG, aggrecan) epitopes provides therapy by different immune mechanisms in a mouse model of rheumatoid arthritis. Vaccines 2021; 9: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. A phase I, randomized, double-blind, placebo-controlled, single center, single-dose escalation to investigate the safety, tolerability, and pharmacodynamics of subcutaneously administered DEN-181 in adult patients with ACPA+ rheumatoid arthritis on stable methotrexate, https://acrabstracts.org/abstract/a-phase-i-randomized-double-blind-placebo-controlled-single-center-single-dose-escalation-to-investigate-the-safety-tolerability-and-pharmacodynamics-of-subcutaneously-administered-den-181-in-a/ (accessed 28 December 2021).
- 36. López-Mejías R, Castañeda S, González-Juanatey C, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun Rev 2016; 15: 1013–1030. [DOI] [PubMed] [Google Scholar]
- 37. Kotta S, Nair A, Alsabeelah N. 3D printing technology in drug delivery: recent progress and application. Curr Pharm Des 2019; 24: 5039–5048. [DOI] [PubMed] [Google Scholar]
- 38. Puentes-Osorio Y, Amariles P, Calleja MÁ, et al. Potential clinical biomarkers in rheumatoid arthritis with an omic approach. Auto Immun Highlights 2021; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagatani K, Sakashita E, Endo H, et al. A novel multi-biomarker combination predicting relapse from long-term remission after discontinuation of biological drugs in rheumatoid arthritis. Sci Rep 2021; 11: 20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi Y-R, Collins KH, Springer LE, et al. A genome-engineered bioartificial implant for autoregulated anticytokine drug delivery. Sci Adv 2021; 7: eabj1414, https://www.science.org/doi/10.1126/sciadv.abj1414 (accessed 22 September 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schett G, Tanaka Y, Isaacs JD. Why remission is not enough: underlying disease mechanisms in RA that prevent cure. Nat Rev Rheumatol 2021; 17: 135–144. [DOI] [PubMed] [Google Scholar]
- 42. Pollard LC, Choy EH, Gonzalez J, et al. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology 2006; 45: 885–889. [DOI] [PubMed] [Google Scholar]
- 43. Griffiths AJ, White CM, Thain PK, et al. The effect of interactive digital interventions on physical activity in people with inflammatory arthritis: a systematic review. Rheumatol Int 2018; 38: 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alexander T, Thiel A, Rosen O, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 2009; 113: 214–223. [DOI] [PubMed] [Google Scholar]
- 45. Orvain C, Boulch M, Bousso P, et al. Is there a place for chimeric antigen receptor–T cells in the treatment of chronic autoimmune rheumatic diseases? Arthritis Rheumatol 2021; 73: 1954–1965. [DOI] [PubMed] [Google Scholar]
- 46. Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ 1999; 319: 780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koller-Smith L, Mehdi AM, March L, et al. Rheumatoid arthritis is a preventable disease: 11 ways to reduce your patients’ risk. Intern Med J. Epub ahead of print 23 September 2021. DOI: 10.1111/imj.15537. [DOI] [PubMed] [Google Scholar]
- 48. Hsieh MC, Lee JJ. Preliminary study of VR and AR applications in medical and healthcare education. J Nurs Health Stud 2018; 3: 1, http://www.imedpub.Com/articles/preliminary-study-of-vr-and-ar-applications-in-medical-and-healthcare-education.php?aid=21861 (accessed 30 September 2021). [Google Scholar]
- 49. Ingeson M, Blusi M, Nieves JC. Microsoft Hololens – a mHealth solution for medication adherence. In: Koch F, Koster A, Riaño D, et al. (eds) Artificial intelligence in health (Lecture notes in computer science), vol. 11326. Cham: Springer International Publishing, 2019, pp. 99–115, http://link.Springer.com/10.1007/978-3-030-12738-1_8 (accessed 30 September 2021). [Google Scholar]
- 50. Mucke J, Sewerin P, Schneider M. Rheumatology in 2049: the age of all data. Ann Rheum Dis 2021; 80: 825–827. [DOI] [PubMed] [Google Scholar]
- 51. Burmester GR. Rheumatology 4.0: big data, wearables and diagnosis by computer. Ann Rheum Dis 2018; 77: 963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krusche M, Mucke J, Burmester G-R. What will be the job of the rheumatologist in 2030? Joint Bone Spine 2020; 87: 525–527. [DOI] [PubMed] [Google Scholar]


