Abstract
Background:
Presoaking anterior cruciate ligament (ACL) grafts in vancomycin has been reported to reduce the occurrence of septic arthritis (SA). However, strong recommendations for its universal use have been precluded by concerns regarding the fragility of previous meta-analyses.
Purpose:
The primary objective was to investigate whether presoaking ACL grafts in vancomycin was associated with a reduction in the rate of SA in a large series of patients. The secondary objective was to perform an updated systematic review and meta-analysis to determine the efficacy of vancomycin in reducing the rate of SA.
Study Design:
Cohort study and systematic review; Level of evidence, 3.
Methods:
A retrospective analysis of patients who underwent primary ACL reconstruction (ACLR) at our institution was undertaken. Rates of postoperative SA were determined and analyzed according to whether patients had received grafts presoaked in vancomycin. A systematic review of the literature and meta-analysis was performed. Odds ratios (ORs) for the risk of SA were calculated according to the inverse variance approach. Results were presented using forest plots, funnel plots, and the fragility index.
Results:
A total of 5300 patients underwent primary ACLR during the study period. The rate of SA was 0.34% (11/3228) in the control group and 0.05% (1/2072) in the presoaked group. There was a 5-fold greater risk of SA in patients who did not receive grafts presoaked in vancomycin (OR, 5.13 [95% CI, 1.16-48.30]; P = .04). Overall, 11 studies were included in the systematic review (29,659 ACLR procedures). The meta-analysis demonstrated a significantly greater risk of SA in those patients who did not receive grafts presoaked in vancomycin (OR, 14.39 [95% CI, 5.90-35.10]; fragility index = 23). This finding held true for the subpopulation receiving hamstring tendon grafts (fragility index = 16), but only a trend was demonstrated for bone–patellar tendon–bone grafts.
Conclusion:
The meta-analysis demonstrated that presoaking ACL grafts in vancomycin was associated with significant reductions in the rates of SA when all graft types were analyzed together. This finding held true specifically for hamstring tendon autografts. The fragility index of these findings allows for a strong recommendation for the universal use of vancomycin presoaking. However, it should be noted that only a trend toward reduced SA rates was demonstrated with presoaking bone–patellar tendon–bone autografts in vancomycin.
Keywords: knee, ligaments, ACL, anterior cruciate ligament reconstruction, septic arthritis, vancomycin
Septic arthritis (SA) is an infrequent complication of anterior cruciate ligament (ACL) reconstruction (ACLR), with an incidence ranging from 0.14% to 2.6%, despite the use of intravenous antibiotic prophylaxis. 3,13,19,41 The current standard of care for the management of SA after ACLR includes arthroscopic debridement, graft retention (with or without hardware removal), and antimicrobial treatment. Although some authors 16,20 have reported good results with such approaches, particularly with regards to their efficacy in eradicating infections, Presti et al 32 cautioned that both arthrofibrosis and severe chondral defects are observed frequently, even at short-term follow-up. Additional potential complications include the need for multiple reoperations, the need for the removal of grafts and implants; prolonged duration of antimicrobial treatment and potentially devastating sequelae, including rapidly progressive chondrolysis.
In 2012, Vertullo et al 45 reported that presoaking ACL hamstring tendon (HT) grafts in vancomycin (in combination with routine intravenous antibiotic prophylaxis) was successful in reducing the rate of SA. Vancomycin appears to be an ideal drug to presoak the graft because of its low allergenicity, good heat stability, and safety for local use as well as the fact that it has bactericidal action against most of the common pathogens isolated in ACLR infections such as Staphylococcus aureus and coagulase-negative staphylococci. 15,26,27,29,31,37,40,47 Since the landmark publication of Vertullo et al, 45 numerous groups have also reported that vancomycin presoaking could reduce the rate of SA. 2,5,10,21,23,24,26,27,29 However, it is important to note that despite this supporting evidence, important concerns exist, and this has limited the ability to make strong recommendations for practice guidelines.
Although several meta-analyses have confirmed an overall significant reduction in the rate of SA with vancomycin presoaking, they have been met with some skepticism because of concerns about the fragility of the findings. Fragility reflects the fact that a change in outcomes for a small number of cases could significantly change the overall findings of the meta-analysis. This issue arises because of the infrequency with which SA occurs and the resulting requirement for very large study populations to evaluate any intervention intended to reduce the risk. A further reason that a blanket policy advocating for presoaking all grafts in vancomycin has not been recommended to date is because the majority of studies have focused on HT grafts and its efficacy has not been clearly demonstrated for other graft types. It appears that these unanswered questions have resulted in a lack of widespread acceptance of vancomycin presoaking, with infrequent utilization reported in some countries. 9,35,50
The primary objective of this study was to investigate whether presoaking ACL grafts in vancomycin was associated with a significant reduction in the rate of SA. The secondary objectives were to perform an updated systematic review and meta-analysis (including the current study) to determine the efficacy of vancomycin in reducing the rate of SA and to report the fragility index of the findings. The hypothesis was that an updated meta-analysis with a very large study population would demonstrate that vancomycin significantly reduces the risk of SA after ACLR and that the fragility index would give confidence in the findings and allow for firm recommendations for practice guidelines.
Methods
Cohort Study Design
Institutional review board approval was granted for this study, and all participants gave valid consent to participate. A retrospective analysis of prospectively collected data from the SANTI Study Group Database was undertaken. All patients who underwent primary ACLR performed by the senior surgeon (B.S.-C.) between January 2008 and September 2019 and had a minimum follow-up of 12 months were considered for study eligibility. Patients were excluded if they had a history of ipsilateral knee injuries or surgery; had a previous knee infection; sustained a multiligamentous injury that required complex surgical treatment; underwent other concomitant major procedures (eg, osteotomy for coronal alignment correction or posterior tibial slope correction); underwent reconstruction using an allograft; had a known allergy to penicillin, cephalosporin, glycopeptide, or iodine; or had any history of diabetes or immunosuppression.
Preoperative Infection Prophylaxis Protocol
The same preoperative infection prophylaxis measures were undertaken for all patients. The affected limb was chemically epilated 1 week before surgery. The patients took a shower using a povidone-iodine scrub brush 24 hours before surgery. In the operating theater, a prophylactic intravenous cefazolin bolus of 2 g was administered at the time of the induction of anesthesia, approximately 30 minutes before applying the pneumatic tourniquet. Next, the skin was precleaned via a brush application of alcoholic betadine. The surgeon subsequently performed a final preparation, again using alcoholic betadine, before setting up sterile surgical drapes. Lastly, a stockinette and an Ioban drape (3M) were applied. Postoperative antibiotics were not utilized.
Vancomycin Presoaking of Grafts and Group Allocation
Presoaking grafts in vancomycin was not utilized at our institution before July 2016. Therefore, all patients who underwent surgery before this date received intravenous antibiotic prophylaxis only and were allocated to the no vancomycin group. Patients undergoing surgery after this date received both intravenous antibiotics and grafts presoaked in vancomycin and were allocated to the vancomycin group. ACL grafts used for patients in the vancomycin group were wrapped in gauze soaked in a 2.5-mg/mL vancomycin solution (125 mg/50 mL) immediately after harvest for approximately 10 minutes.
Surgical Techniques
All procedures were performed with the patient under general anesthesia and using a tourniquet. Bone–patellar tendon–bone (BPTB), quadriceps tendon, or HT autografts were utilized, and all tunnels were made using an outside-in technique. ACL grafts were fixed at 30° of knee flexion. Graft choices were based on patient factors and the evolving indications for performing a concomitant lateral extra-articular procedure (either modified Lemaire lateral extra-articular tenodesis [LET] or anterolateral ligament [ALL] reconstruction) over the study period, including younger age (<20 years), participation in pivoting sports, high-demand athletes, high-grade pivot shift, lateral femoral notch sign, and Segond fractures.
ACLR With BPTB Graft
A 2-incision technique was used. 12 BPTB grafts were harvested with a patellar bone plug (10 × 15 mm) and a tibial bone plug (9-11 × 25 mm). Press-fit fixation was performed on the femoral side. Graft fixation on the tibial side was performed using a bioabsorbable screw (Bio-Interference screw; Arthrex).
ACLR With HT Graft
The semitendinosus and gracilis tendons were harvested using an open-ended tendon stripper. The tibial insertion was preserved to improve fixation and vascularity. 42 Tendons were quadrupled and then fixed using an interference screw and a cortical suspensory device (TightRope; Arthrex).
ACLR With Quadriceps Tendon Graft
A 10 mm–wide quadriceps tendon graft was harvested with a patellar bone plug (10 × 15 mm). The graft was routed proximally through the knee, with the bone plug placed on the tibial side. Fixation was performed using bioabsorbable screws on both sides (Arthrex).
Concomitant Modified Lemaire LET
A 1 cm–wide strip of the iliotibial band (ITB) was harvested up to 2 cm proximal to the lateral epicondyle, keeping its tibial attachment to the Gerdy tubercle intact. The ITB graft was passed beneath the fibular collateral ligament from distal to proximal using a right-angled clamp. A 4.5 mm–diameter socket was created at the isometric point, slightly posterior and proximal to the lateral epicondyle. The ITB graft was then fixed within the socket using a bioabsorbable screw (Arthrex), with the knee placed in full extension and neutral rotation.
Combined ACLR + ALL Reconstruction
Semitendinosus and gracilis tendons were harvested as described above. A combined ACL + ALL graft was prepared using a tripled semitendinosus tendon with an additional length of gracilis tendon sutured to it. The ACL portion of the graft (3 parts semitendinosus and 1 part gracilis) was fixed on both sides using bioabsorbable screws (Arthrex). The additional length of gracilis tendon that emerged from the femoral tunnel, at the lateral cortex, formed the ALL portion of the graft. This was passed under the ITB using a suture grasper, then through a tunnel in the proximal tibia, and back to the ALL origin, where it was tensioned and fixed with the knee in extension, completing double-strand anatomic ALL reconstruction. 36
Diagnosis and Management of Postoperative SA
The main outcome of interest was the occurrence of SA within 12 months after ACLR. All patients with clinical symptoms suggestive of SA were admitted to the hospital urgently for physical examinations and laboratory tests (including C-reactive protein, erythrocyte sedimentation rate, and leukocyte count). The diagnosis of SA was clinical, depending on the integration of history, examination, and investigation findings. Clinical suspicion was based on fever, rigor, knee pain, and loss of articular mobility. White cell count, erythrocyte sedimentation rate, and C-reactive protein concentration were usually measured because, when raised, they aid in diagnosis and are useful to monitor responses to treatment. Patients who were suspected of having a deep infection underwent arthroscopic lavage using 9 L of normal saline and careful debridement of inflamed soft tissue. All knee compartments were inspected, and graft integrity was assessed. Samples from intra-articular synovial fluid and debrided tissue were sent for culture and antibiotic sensitivity. Postoperatively, patients received empirical antibiotic therapy (intravenous penicillin and gentamicin). This was subsequently adapted according to bacterial identification and antibiotic sensitivities. Antibiotics were administered intravenously for 3 days and then given orally for 6 weeks.
Systematic Review and Meta-analysis: Search Strategy and Eligibility Criteria
A systematic review of the literature relating to the influence of presoaking ACL grafts on the rate of SA was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The systematic review protocol was registered with the PROSPERO database (registration No. CRD42020223628). A literature search was performed using subject mapping and the following keywords: “anterior cruciate ligament” and “vancomycin” in combination with “septic arthritis” or “infection.” The search strategy was applied to the PubMed and Embase databases by 2 investigators independently on December 30, 2020 (A.C. and A.S.).
Each article was reviewed for relevance, and the references of included articles were reviewed to identify further relevant studies. All comparative studies evaluating the influence of vancomycin on the rates of SA after ACLR were included. Patients from our previously presented clinical series were included in the meta-analysis to increase the sample size because of the rare nature of SA after ACLR. In addition, our population comprised a substantial number of patients who had undergone ACLR using a BPTB graft, which was an underrepresented group in the previously published work included in the meta-analysis. Studies were only excluded if they were not published as a full article in a peer-reviewed journal, were duplicated or overlapped populations with included studies, or were unavailable in the English language. Any disagreements between investigators regarding study eligibility were resolved by the senior author (B.S.-C.).
Statistical Analysis
Calculations were made using SAS for Windows (Version 9.4; SAS Institute), with the level of statistical significance set at P < .05. Descriptive data analysis was conducted depending on the nature of the considered criteria. For quantitative data, this included the number of observed (and missing, if any) values and the mean, SD, median, range and lower and upper quartiles. For qualitative data, this included the number of observed (and missing, if any) values and the number and percentage of patients per group. The characteristics of the studied population were described according to group allocation and the rate of SA reported. Because of the small number of infections, 95% CIs were calculated using the exact binomial method.
For the meta-analysis, data were extracted from included studies to determine the number of cases of SA and the total number of patients in each group. Missing SDs were determined according to the sample size and means from reported P values. 11 When the required data were not available for extraction from published articles, they were requested directly from the corresponding author. Odds ratios (ORs) for the risk of SA in each group were calculated according to the inverse variance approach. Heterogeneity across publications was assessed using I 2 values, with moderate heterogeneity defined as I 2 between 30% and 60%. A random-effects Mantel-Haenszel model was used to account for between-study variation. Results were presented using forest plots, and 95% CIs were calculated using the exact binomial method. Funnel plots were generated to assess bias due to small study effects. 28 The fragility index, which describes the extent to which the attribution of statistical significance is subject to random influences and indicates the number of patients whose results would have to change to alter the statistical interpretation of the study, was determined using the R software package.
Results
Results of Cohort Study
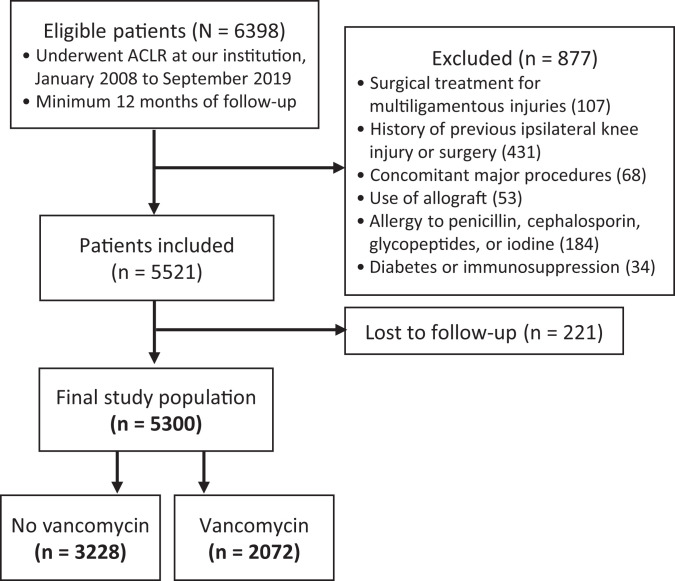
The study population comprised 5300 patients who underwent primary ACLR during the study period: 3228 patients (60.9%) in the no vancomycin group and 2072 patients (39.1%) in the vancomycin group (Figure 1). Patients in the vancomycin group were significantly younger (age, 28.0 ± 10.0 vs 29.3 ± 10.4 years, respectively; P = .001); otherwise, there were no differences in preoperative patient characteristics between the groups. There were no differences between the no vancomycin and vancomycin groups regarding the operating time (31.7 ± 16.4 vs 31.1 ± 15.9 minutes, respectively; P = .788), but there was a significant difference in the overall proportion that underwent a lateral extra-articular procedure (either modified Lemaire LET or ALL reconstruction) (35.5% vs 69.2%, respectively; P < .001). The population characteristics and details of graft choice (as well as extra-articular procedures) are reported in Tables 1 and 2, respectively.
Figure 1.
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) flow diagram of the cohort study arm. ACLR, anterior cruciate ligament reconstruction.
TABLE 1.
Patient Characteristics a
| Total (n = 5300) | No Vancomycin (n = 3228) | Vancomycin (n = 2072) | P Value | |
|---|---|---|---|---|
| Age, y | 28.8 ± 10.3 | 29.3 ± 10.4 | 28.0 ± 10.0 | .001 |
| Sex | .319 | |||
| Female | 1454 (27.5) | 904 (28.0) | 559 (26.98) | |
| Male | 3830 (72.5) | 2324 (72.0) | 1513 (73.02) | |
| Body mass index | 23.9 ± 3.3 | 23.9 ± 3.3 | 24.0 ± 3.5 | .341 |
| Side | .864 | |||
| Right | 2810 (53.2) | 1722 (53.3) | 1089 (52.99) | |
| Left | 2475 (46.8) | 1506 (46.7) | 974 (47.01) |
a Data are reported as mean ± SD or n (%). Bolded P value indicates a statistically significant difference between groups (P < .05).
TABLE 2.
Surgical Procedure Characteristics a
| Total (n = 5300) | No Vancomycin (n = 3228) | Vancomycin (n = 2072) | |
|---|---|---|---|
| Graft type | |||
| BPTB | 729 (13.8) | 487 (15.1) | 242 (11.7) |
| HT | 4510 (85.1) | 2699 (83.6) | 1811 (87.4) |
| Quadriceps tendon | 61 (1.2) | 42 (1.3) | 19 (0.9) |
| Isolated ACLR | 2720 (51.3) | 2082 (64.5) | 638 (30.8) |
| ACLR + modified Lemaire LET | 203 (3.8) | 150 (4.6) | 53 (2.6) |
| ACLR + ALL reconstruction | 2376 (44.8) | 996 (30.9) | 1380 (66.6) |
a Data are reported as n (%). ACLR, anterior cruciate ligament reconstruction; ALL, anterolateral ligament; BPTB, bone–patellar tendon–bone; HT, hamstring tendon; LET, lateral extra-articular tenodesis.
Occurrence of SA
Overall, 12 patients were diagnosed with SA during the study period (0.23% [95% CI, 0.12%-0.40%]). There were 11 infections that occurred in the no vancomycin group (0.34% [95% CI, 0.17%-0.61%]) and 1 in the vancomycin group (0.05% [95% CI, 0.01%-0.27%]) (Table 4). The infections were sustained by bacteria belonging to the Staphylococcus genus in 10 of 12 patients (83.3%). Patients who did not receive grafts presoaked in vancomycin were at >5-fold greater risk of SA compared with those who did (OR, 5.13 [95% CI, 1.16-48.30]; P = .04). The characteristics of the infected patients are reported in Table 3.
TABLE 4.
SA Incidence and Antibiotic Prophylaxis Protocols a
| No. of Patients With SA | Total No. of Patients | SA Incidence (95% CI), % | Vancomycin Presoaking Protocol | |
|---|---|---|---|---|
| Current study | Vancomycin wrapping: 2.5 mg/mL (10-15 min). IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 12 | 5300 | 0.23 (0.12-0.40) | |
| No vancomycin | 11 | 3228 | 0.34 (0.17-0.61) | |
| Vancomycin | 1 | 2072 | 0.05 (0.00-0.27) | |
| Phegan 29 (2016) | Vancomycin wrapping: 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 4 | 1585 | 0.25 (0.07-0.64) | |
| No vancomycin | 4 | 285 | 1.40 (0.38-3.55) | |
| Vancomycin | 0 | 1300 | 0.00 (0.00-0.28) | |
| Wan 46 (2020) | Vancomycin dipping (1 min) + wrapping (15-20 min): 5 mg/mL. IV antibiotic preoperative prophylaxis: 1 g of cefazolin. | |||
| Overall | 3 | 305 | 0.98 (0.20-2.85) | |
| No vancomycin | 3 | 185 | 1.62 (0.34-4.67) | |
| Vancomycin | 0 | 120 | 0.00 (0.00-3.03) | |
| Pérez-Prieto 27 (2016) | Vancomycin dipping + wrapping (10-15 min): 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 15 | 1544 | 0.97 (0.54-1.60) | |
| No vancomycin | 15 | 810 | 1.85 (1.04-3.04) | |
| Vancomycin | 0 | 734 | 0.00 (0.00-0.50) | |
| Offerhaus 23 (2019) | Vancomycin dipping + wrapping: 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 22 | 1779 | 1.24 (0.78-1.87) | |
| No vancomycin | 22 | 926 | 2.38 (1.49-3.58) | |
| Vancomycin | 0 | 853 | 0.00 (0.00-0.43) | |
| Figueroa 10 (2019) | Vancomycin wrapping (15-20 min): 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 4 | 490 | 0.82 (0.22-2.08) | |
| No vancomycin | 4 | 230 | 1.74 (0.48-4.39) | |
| Vancomycin | 0 | 260 | 0.00 (0.00-1.41) | |
| Banios 2 (2021) | Vancomycin wrapping: 5 mg/mL. IV antibiotic preoperative and postoperative prophylaxis. | |||
| Overall | 7 | 1835 | 0.38 (0.15-0.78) | |
| No vancomycin | 7 | 1242 | 0.56 (0.23-1.16) | |
| Vancomycin | 0 | 593 | 0.00 (0.00-0.62) | |
| Schuster 39 (2020) | Vancomycin wrapping: 5 mg/mL. IV antibiotic preoperative prophylaxis. | |||
| Overall | 35 | 10,516 | 0.33 (0.23-0.46) | |
| No vancomycin | 35 | 8222 | 0.43 (0.30-0.59) | |
| Vancomycin | 0 | 2294 | 0.00 (0.00-0.16) | |
| Bohu 5 (2020) | Vancomycin dipping (10 min): 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 7 | 1674 | 0.42 (0.17-0.86) | |
| No vancomycin | 7 | 1184 | 0.59 (0.24-1.21) | |
| Vancomycin | 0 | 490 | 0.00 (0.00-0.75) | |
| Vertullo 45 (2012) | Vancomycin wrapping: 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 4 | 1155 | 0.35 (0.10-0.90) | |
| No vancomycin | 4 | 285 | 1.40 (0.38-3.55) | |
| Vancomycin | 0 | 870 | 0.00 (0.00-0.42) | |
| Chaturvedi 7 (2020) | Vancomycin wrapping: 5 mg/mL. IV antibiotic preoperative prophylaxis: 2 g of cefazolin. | |||
| Overall | 18 | 1836 | 0.98 (0.58-1.55) | |
| No vancomycin | 18 | 963 | 1.87 (1.11-2.94) | |
| Vancomycin | 0 | 873 | 0.00 (0.00-0.42) | |
| Baron 4 (2019) | Vancomycin dipping (10 min) + wrapping: 1 mg/mL. IV antibiotic preoperative prophylaxis: cefazolin. | |||
| Overall | 11 | 1640 | 0.67 (0.34-1.20) | |
| No vancomycin | 10 | 842 | 1.19 (0.57-2.17) | |
| Vancomycin | 1 | 798 | 0.13 (0.00-0.70) |
a IV, intravenous; SA, septic arthritis.
TABLE 3.
Characteristics of Patients Diagnosed With Septic Arthritis (n = 12) a
| Patient | Age, y | Sex | Graft | Lateral Extra-articular Procedure | Additional Procedures | Bacterial Organism | Time From Reconstruction to Infection, d |
|---|---|---|---|---|---|---|---|
| 1 | 25 | M | BPTB | No | No | SC | 14 |
| 2 | 44 | M | HT | Yes | No | SA | 20 |
| 3 | 27 | M | BPTB | Yes | MM + LM repair | PBA | 149 |
| 4 | 32 | M | HT | Yes | No | SC | 12 |
| 5 | 24 | F | HT | Yes | No | SA | 15 |
| 6 | 33 | M | HT | Yes | No | SE | 34 |
| 7 | 33 | M | HT | Yes | MM + LM repair | SM | 10 |
| 8 | 37 | M | HT | No | MM repair + LM meniscectomy | SL, SC, SCA | 15 |
| 9 | 24 | M | HT | No | No | SA | 60 |
| 10 | 28 | F | HT | No | No | SA | 20 |
| 11 | 18 | M | HT | Yes | MM repair | SA | 40 |
| 12 b | 27 | M | HT | Yes | No | SC | 13 |
a BPTB, bone–patellar tendon–bone; F, female; HT, hamstring tendon; LM, lateral meniscus; M, male; MM, medial meniscus; PBA, Propionibacterium acnes; SA, Staphylococcus aureus; SC, Staphylococcus caprae; SCA, Staphylococcus capitis; SE, Staphylococcus epidermidis; SL, Staphylococcus lugdunensis; SM, Serratia marcescens.
b Patient 12 was in the vancomycin group.
Results of Literature Search and Meta-analysis
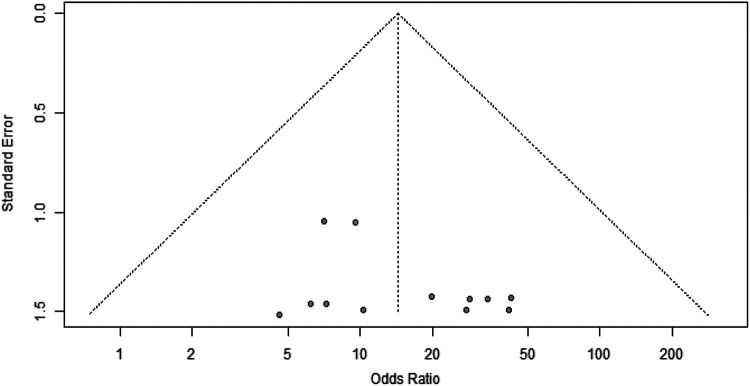
The search strategy yielded 11 eligible studies, †† from which data were extracted and pooled alongside the current study (Figure 2). The final population for the pooled data analysis comprised 29,659 patients. Table 4 shows the incidence of SA and the associated 95% CIs for all studies, including the current study, and Table 5 shows the incidence of SA according to graft type. The meta-analysis demonstrated a significantly greater risk of SA in those patients who did not receive grafts presoaked in vancomycin (OR, 14.39 [95% CI, 5.90-35.10]) (Figure 3). The funnel plot analysis did not demonstrate asymmetry (Figure 4). Analyses of the relative risk of SA after ACLR using HT and BPTB autografts in each included study are reported in Figures 5 and 6, respectively.
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the systematic review and meta-analysis arm.
TABLE 5.
SA Incidence According to Graft Type From Current Study and Included Studies a
| Graft Type | Without Vancomycin (n = 18,402) | With Vancomycin (n = 11,257) | Overall (n = 29,659) |
|---|---|---|---|
| BPTB | 7/1019 (0.687) | 0/599 (0.000) | 7/1618 (0.433) |
| HT | 84/13,711 (0.613) | 1/7926 (0.013) | 85/21,637 (0.393) |
| Other/not specified | 49/3672 (1.334) | 1/2736 (0.036) | 50/6404 (0.781) |
| Any | 140/18,402 (0.761) | 2/11,261 (0.018) | 142/29,659 (0.479) |
a Data are reported as the ratio of SA per population. BPTB, bone–patellar tendon–bone; HT, hamstring tendon; SA, septic arthritis.
Figure 3.
Forest plot demonstrating the relative risk of septic arthritis (SA) after anterior cruciate ligament reconstruction using any type of graft in each included study and the pooled summary estimate. Sizes of data markers are proportional to the weight of each study. Horizontal bars represent the 95% CI of individual studies. Pooled analysis demonstrated that the failure to presoak grafts in vancomycin was associated with a significantly greater risk of SA (odds ratio [OR], 14.39 [95% CI, 5.90-35.10]). The fragility index was 23.
Figure 4.
Funnel plot demonstrating that the effect estimates derived from each of the included studies are symmetrical and scatter widely at the bottom. No publication bias was detected. Odds ratios >1 are in favor of the vancomycin group.
Figure 5.
Forest plot demonstrating the relative risk of septic arthritis (SA) after anterior cruciate ligament reconstruction using hamstring tendon autografts in each included study and the pooled summary estimate. Sizes of data markers are proportional to the weight of each study. Horizontal bars represent the 95% CI of individual studies. Pooled analysis demonstrated that the failure to presoak grafts in vancomycin was associated with a significantly greater risk of SA (odds ratio [OR], 13.50 [95% CI, 4.03-45.21]). The fragility index was 16.
Figure 6.
Forest plot demonstrating the relative risk of septic arthritis (SA) after anterior cruciate ligament reconstruction using bone–patellar tendon–bone (BPTB) autografts in each included study and the pooled summary estimate. Sizes of data markers are proportional to the weight of each study. Horizontal bars represent the 95% CI of individual studies. Pooled analysis demonstrated that the failure to presoak grafts in vancomycin was associated with a nonsignificant trend toward a higher risk of SA (odds ratio [OR], 3.42 [95% CI, 0.60-19.58]). Three studies reporting the use of BPTB autografts were excluded because there were no events in either group or because the authors did not provide the necessary data. 2,4,23
Discussion
The main finding of this study was that not presoaking ACL grafts in vancomycin was associated with a significantly greater risk of SA after ACLR. This finding was demonstrated in the cohort study and subsequently confirmed in the overall meta-analysis (OR, 14.39 [95% CI, 5.90-35.10]). Although these findings are consistent with those of the previous literature, the very large study population allows for greater confidence in the findings than that conferred by previous smaller meta-analyses. This was best expressed via the fragility index. In the current meta-analysis, a change in status of 23 patients (representing 16.2% of the total number of SA events) was required for the study to lose statistical significance. Although there is no specific fragility index that is considered acceptable, interpretation is aided by considering that among statistically significant meta-analyses from the Cochrane database, the median fragility index was found to be 12, and in 29% of meta-analyses, the overall fragility index was <5. 1 Furthermore, 9% of meta-analyses would have become nonsignificant if the event status was modified for <1% of the total number of events. On that basis, it is our opinion that the overall findings of this meta-analysis are robust.
In the meta-analysis, we further sought to determine the efficacy of vancomycin presoaking specifically for the 2 most popular graft choices in clinical practice. For HT autografts, which according to some authors are at a higher risk of SA, 17 a very large population was available for study, and the meta-analysis demonstrated that the failure to utilize vancomycin presoaking resulted in a significantly greater risk of SA (OR, 13.50 [95% CI, 4.03-45.21]). The fragility index for this finding was 16, and this allows for a strong recommendation in favor of vancomycin presoaking for all HT autografts. The same evaluation for BPTB grafts showed no significant increase in the risk of SA when vancomycin presoaking was omitted (OR, 3.42 [95% CI, 0.60-19.58]). However, this finding should be interpreted cautiously because the population available for study was relatively small (n = 1618), and therefore, this part of the analysis was likely to have been underpowered. It is noteworthy that no cases of SA occurred in those patients receiving a BPTB graft treated with vancomycin presoaking (0/599) but that the rate of SA was 0.99% (7/1019) in the control group. Although this trend was nonsignificant, it lends some support to a recommendation for presoaking of all ACL grafts in vancomycin, particularly given the significant overall reduction in the rate of SA when all graft types were grouped together.
It is our opinion that there have been several previous barriers to the widespread adoption of presoaking all ACL grafts in vancomycin. One of these is perhaps a misconception that early arthroscopic washout and graft retention result in a relatively benign course. This message has perhaps been reinforced by a previous systematic review in which Makhni et al 18 concluded that outcomes in patients after SA are broadly comparable to those in patients in whom an infection does not develop (including range of motion, residual instability, Lysholm score, and return to preinjury levels of activity). However, pooling studies with inconsistent reporting, a short-term follow-up, and conflicting findings may have resulted in a lack of clarity regarding the severity and spectrum of morbidity. It is therefore important to highlight that several authors have reported inferior outcomes after SA compared with those in patients in whom an infection did not develop and furthermore that resultant limitations can be severe. 16,20,37,40,43 Waterman et al 48 reported on 31 (of a series of 9511 ACLR procedures) cases of SA from the military health care system. At a mean follow-up of 26.9 months, only 33.3% of those who underwent graft resection and 54.5% of those who underwent graft retention were able to return to military function. Significant risk factors for the inability to return to duty included symptomatic postinfection arthritis and arthrofibrosis. These risk factors appear to be surprisingly common after SA, with Presti et al 32 reporting high rates of arthrofibrosis (81%) and severe chondral defects (63%) at a mean follow-up of 56 months. Although very long-term studies are sparse, Schub et al 38 reported the outcomes of 4 patients with a mean follow-up of 17.9 years. The authors reported that all patients had a Kellgren-Lawrence arthritis severity grade of at least 3 (in at least 1 compartment). They also reported diminished long-term subjective and functional outcomes compared with historical reports of uncomplicated cases and attributed these inferior outcomes to advanced arthritis. These findings serve to highlight that the sequelae of SA are considerable and that the condition is associated with significant morbidity.
Further barriers to the widespread use of vancomycin presoaking were highlighted by a 2021 survey of ACL study group members. These included concerns regarding its cost-effectiveness, the effect on mechanical properties of the graft, and antibiotic resistance. 50 Ruelos et al 34 recently demonstrated that vancomycin presoaking was highly cost-effective (assuming the following costs in US Dollars: vancomycin, $44; arthroscopic debridement and ACL graft retention, $6424; ACL graft revision, $24,178), although limitations included questionable external validity, given the widely varying costs within the United States and internationally. However, these findings are supported by Offerhaus et al, 23 who demonstrated cost-effectiveness in the German health care system. Concerns about the effect of vancomycin on graft integrity are not supported by Jacquet et al, 14 who demonstrated no difference in the biomechanical properties of HT autografts treated with and without vancomycin presoaking, or by Pérez-Prieto et al, 25 who reported no difference in graft rupture rates in a small comparative series. Still, Xiao et al 49 examined the in vitro toxicity of various doses of vancomycin on the patellar tendon, showing that even high concentrations (12.8 mg/mL) of vancomycin, up to 6 hours of exposure, do not lead to cell death and toxicity to tenocytes. To our knowledge, there were no cases of vancomycin resistance in this SA population. The vancomycin presoaking protocol of our institution, employing vancomycin at a 2.5-mg/mL concentration, was established before there was a proliferation of literature regarding this prophylactic wrap. This corresponds to a lower concentration compared with that used in other studies but did not lead to a higher incidence of SA than that in studies that used vancomycin at higher concentrations. Although concerns about antibiotic resistance are more difficult to address, it seems that widespread global issues surrounding the use of antibiotics (including misuse, ease of availability, excessive use in the food chain, and lack of surveillance of resistance development 8 ) are of greater concern rather than specifically focused and appropriately indicated prophylactic antibiotics for orthopaedic surgery.
In a recent editorial commentary, Vertullo 44 addressed whether vancomycin presoaking should become a universal recommendation for all ACL grafts. It was highlighted that infection prophylaxis guidelines are typically based on level 1 evidence and that the risks of unrecognized bias (potentially including performance bias, sequence bias, and selection bias) are important concerns in study designs using historical cohorts as a comparator (per the majority of studies in this meta-analysis). Although we agree with Vertullo 44 in this regard and with the suggestion that further study in the form of a randomized trial nested in a registry is needed, it is clear that this is a huge undertaking and that a definitive answer will not be obtained in the near future. It is for that reason that we consider the fragility index of this study to be an important strength, providing confidence that the findings are robust and can be used to form guidelines in the absence of level 1 evidence. A final and compelling reason to adopt a recommendation for presoaking all grafts in vancomycin is that SA is one of the most common complications leading to litigation after ACLR in the United States 6 and, in those cases that end in settlement, results in one of the highest rates of settlement ($499,800 ± $770,471 US Dollars). Similar findings have also been reported internationally, with an infection documented to be one of the most common causes for compensation after ACLR in Norway, 33 Finland, 22 and France, where Pioger et al 30 recently demonstrated that a verdict for the plaintiff was significantly associated with the occurrence of a postoperative infection.
Limitations
The limitations of the cohort study included its retrospective design and the use of a historical cohort for comparison. Although retrospective studies are frequently criticized, they also offer important advantages. The main advantage of this design was the ability to collect a very large study population, which is essential for the study of infrequent events and in which the only change about the protocol for the prevention of SA was to add topical vancomycin to the current practice of intravenous antibiotic prophylaxis. The main limitation of the meta-analysis was the lack of randomized controlled studies. However, the very large study population and the associated fragility index of the findings give confidence in the main results. Another limitation of the included studies was the heterogeneity in the presoaking protocol; however, all seem to be effective. Further important limitations included the inability to study (because of the unavailability of data) patient, surgical, and socioeconomic factors that could have influenced the rates of infections, as well as a lack of correlation between vancomycin presoaking and functional outcomes and ACL graft rupture rates.
Conclusion
The meta-analysis demonstrated that presoaking ACL grafts in vancomycin was associated with significant reductions in the rates of SA when all graft types were analyzed together. This finding held true specifically for HT autografts, but only a trend toward reduced SA rates was demonstrated for presoaking BPTB autografts in vancomycin.
Footnotes
Final revision submitted November 9, 2021; accepted November 12, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.C. has received education support from Arthrex. E.M. and B.S.-C. have received consulting fees and royalties from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Scientific Committee of the GCS Ramsay Santé for Education and Research (reference No. COS-RGDS-2021-06-007-SONNERYCOTTET-B).
References
- 1. Atal I, Porcher R, Boutron I, Ravaud P. The statistical significance of meta-analyses is frequently fragile: definition of a fragility index for meta-analyses. J Clin Epidemiol. 2019;111:32–40. [DOI] [PubMed] [Google Scholar]
- 2. Banios K, Komnos GA, Raoulis V, Bareka M, Chalatsis G, Hantes ME. Soaking of autografts with vancomycin is highly effective on preventing postoperative septic arthritis in patients undergoing ACL reconstruction with hamstrings autografts. Knee Surg Sports Traumatol Arthrosc. 2021;29(3):876–880. [DOI] [PubMed] [Google Scholar]
- 3. Bansal A, Lamplot JD, VandenBerg J, Brophy RH. Meta-analysis of the risk of infections after anterior cruciate ligament reconstruction by graft type. Am J Sports Med. 2018;46(6):1500–1508. [DOI] [PubMed] [Google Scholar]
- 4. Baron JE, Shamrock AG, Cates WT, et al. Graft preparation with intraoperative vancomycin decreases infection after ACL reconstruction: a review of 1,640 cases. J Bone Joint Surg Am. 2019;101(24):2187–2193. [DOI] [PubMed] [Google Scholar]
- 5. Bohu Y, Klouche S, Sezer HB, et al. Vancomycin-soaked autografts during ACL reconstruction reduce the risk of post-operative infection without affecting return to sport or knee function. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bokshan SL, Ruttiman R, Eltorai AEM, DePasse JM, Daniels AH, Owens BD. Factors associated with physician loss in anterior cruciate ligament reconstruction malpractice lawsuits. Orthop J Sports Med. 2017;5(11):2325967117738957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaturvedi DBN. Does presoaking of autografts in vancomycin reduce the risk of infection after ACL reconstruction? A retrospective study. J Med Sci Clin Res. 2020;8(9):145–150. [Google Scholar]
- 8. Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Glob Infect Dis. 2019;11(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ekdahl V, Stålman A, Forssblad M, Samuelsson K, Edman G, Kraus Schmitz J. There is no general use of thromboprophylaxis and prolonged antibiotic prophylaxis in anterior cruciate ligament reconstruction: a nation-wide survey of ACL surgeons in Sweden. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2535–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Figueroa D, Figueroa F, Calvo R, Lopez M, Goñi I. Presoaking of hamstring autografts in vancomycin decreases the occurrence of infection following primary anterior cruciate ligament reconstruction. Orthop J Sports Med. 2019;7(9):2325967119871038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. [DOI] [PubMed] [Google Scholar]
- 12. Garofalo R, Mouhsine E, Chambat P, Siegrist O. Anatomic anterior cruciate ligament reconstruction: the two-incision technique. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):510–516. [DOI] [PubMed] [Google Scholar]
- 13. Indelli PF, Dillingham M, Fanton G, Schurman DJ. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002;398:182–188. [DOI] [PubMed] [Google Scholar]
- 14. Jacquet C, Jaubert M, Pioger C, et al. Presoaking of semitendinosus graft with vancomycin does not alter its biomechanical properties: a biomechanical in vitro–controlled study using graft from living donors. Arthroscopy. 2020;36(8):2231–2236. [DOI] [PubMed] [Google Scholar]
- 15. Jefferies JG, Aithie JMS, Spencer SJ. Vancomycin-soaked wrapping of harvested hamstring tendons during anterior cruciate ligament reconstruction: a review of the ‘vancomycin wrap.’ Knee. 2019;26(3):524–529. [DOI] [PubMed] [Google Scholar]
- 16. Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006;22(4):375–384. [DOI] [PubMed] [Google Scholar]
- 17. Kuršumović K, Charalambous CP. Relationship of graft type and vancomycin presoaking to rate of infection in anterior cruciate ligament reconstruction: a meta-analysis of 198 studies with 68,453 grafts. JBJS Rev. 2020;8(7):e1900156. [DOI] [PubMed] [Google Scholar]
- 18. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392–1401. [DOI] [PubMed] [Google Scholar]
- 19. Maletis GB, Inacio MC, Reynolds S, Desmond JL, Maletis MM, Funahashi TT. Incidence of postoperative anterior cruciate ligament reconstruction infections: graft choice makes a difference. Am J Sports Med. 2013;41(8):1780–1785. [DOI] [PubMed] [Google Scholar]
- 20. Monaco E, Maestri B, Labianca L, et al. Clinical and radiological outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. J Orthop Sci. 2010;15(2):198–203. [DOI] [PubMed] [Google Scholar]
- 21. Naendrup JH, Marche B, de Sa D, et al. Vancomycin-soaking of the graft reduces the incidence of septic arthritis following ACL reconstruction: results of a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1005–1013. [DOI] [PubMed] [Google Scholar]
- 22. Nyrhinen KM, Bister V, Helkamaa T, et al. Anterior cruciate ligament reconstruction-related patient injuries: a nationwide registry study in Finland. Acta Orthop. 2019;90(6):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Offerhaus C, Balke M, Hente J, Gehling M, Blendl S, Höher J. Vancomycin pre-soaking of the graft reduces postoperative infection rate without increasing risk of graft failure and arthrofibrosis in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):3014–3021. [DOI] [PubMed] [Google Scholar]
- 24. Offerhaus C, Höher J, Balke M. Pre-soaking of hamstring grafts reduces risk of infection after ACL-reconstruction: a prospective study including 1000 patients. Orthop J Sports Med. 2018;6(4 suppl 2):2325967118S00036. [Google Scholar]
- 25. Pérez-Prieto D, Perelli S, Corcoll F, Rojas G, Montiel V, Monllau JC. The vancomycin soaking technique: no differences in autograft re-rupture rate. A comparative study. Int Orthop. 2021;45(6):1407–1411. [DOI] [PubMed] [Google Scholar]
- 26. Pérez-Prieto D, Portillo ME, Torres-Claramunt R, Pelfort X, Hinarejos P, Monllau JC. Contamination occurs during ACL graft harvesting and manipulation, but it can be easily eradicated. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):558–562. [DOI] [PubMed] [Google Scholar]
- 27. Pérez-Prieto D, Torres-Claramunt R, Gelber PE, Shehata TMA, Pelfort X, Monllau JC. Autograft soaking in vancomycin reduces the risk of infection after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2724–2728. [DOI] [PubMed] [Google Scholar]
- 28. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. [DOI] [PubMed] [Google Scholar]
- 29. Phegan M, Grayson JE, Vertullo CJ. No infections in 1300 anterior cruciate ligament reconstructions with vancomycin pre-soaking of hamstring grafts. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2729–2735. [DOI] [PubMed] [Google Scholar]
- 30. Pioger C, Jacquet C, Abitan A, et al. Litigation in arthroscopic surgery: a 20-year analysis of legal actions in France. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1651–1658. [DOI] [PubMed] [Google Scholar]
- 31. Plante MJ, Li X, Scully G, Brown MA, Busconi BD, DeAngelis NA. Evaluation of sterilization methods following contamination of hamstring autograft during anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):696–701. [DOI] [PubMed] [Google Scholar]
- 32. Presti ML, Costa GG, Grassi A, et al. Graft-preserving arthroscopic debridement with hardware removal is effective for septic arthritis after anterior cruciate ligament reconstruction: a clinical, arthrometric, and magnetic resonance imaging evaluation. Am J Sports Med. 2020;48(8):1907–1915. [DOI] [PubMed] [Google Scholar]
- 33. Randsborg PH, Bukholm IRK, Jakobsen RB. Compensation after treatment for anterior cruciate ligament injuries: a review of compensation claims in Norway from 2005 to 2015. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruelos VCB, Puzzitiello RN, Menendez ME, et al. Vancomycin presoaking of anterior cruciate ligament tendon grafts is highly cost-effective for preventing infection. Arthroscopy. 2021;37(10):3152–3156. [DOI] [PubMed] [Google Scholar]
- 35. Sailer M, Skråmm I, Sivertsen E, Lygre S, Årøen A. Nationwide survey on the use of local antibiotics during anterior cruciate ligament reconstruction in Norway. Res Square. Published online July 28, 2020. doi.10.21203/rs.2.24413/v1 [Google Scholar]
- 36. Saithna A, Thaunat M, Delaloye JR, Ouanezar H, Fayard JM, Sonnery-Cottet B. Combined ACL and anterolateral ligament reconstruction. JBJS Essent Surg Tech. 2018;8(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schollin-Borg M, Michaëlsson K, Rahme H. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy. 2003;19(9):941–947. [DOI] [PubMed] [Google Scholar]
- 38. Schub DL, Schmitz LM, Sakamoto FA, Winalski CS, Parker RD. Long-term outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(12):2764–2770. [DOI] [PubMed] [Google Scholar]
- 39. Schuster P, Schlumberger M, Mayer P, et al. Soaking of the graft in vancomycin dramatically reduces the incidence of postoperative septic arthritis after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2587–2591. [DOI] [PubMed] [Google Scholar]
- 40. Schuster P, Schulz M, Immendoerfer M, Mayer P, Schlumberger M, Richter J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: evaluation of an arthroscopic graft-retaining treatment protocol. Am J Sports Med. 2015;43(12):3005–3012. [DOI] [PubMed] [Google Scholar]
- 41. Sonnery-Cottet B, Archbold P, Zayni R, et al. Prevalence of septic arthritis after anterior cruciate ligament reconstruction among professional athletes. Am J Sports Med. 2011;39(11):2371–2376. [DOI] [PubMed] [Google Scholar]
- 42. Sonnery-Cottet B, Freychet B, Murphy CG, Pupim BH, Thaunat M. Anterior cruciate ligament reconstruction and preservation: the single-anteromedial bundle biological augmentation (SAMBBA) technique. Arthrosc Tech. 2014;3(6):e689–e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Tongel A, Stuyck J, Bellemans J, Vandenneucker H. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, management and outcome. Am J Sports Med. 2007;35(7):1059–1063. [DOI] [PubMed] [Google Scholar]
- 44. Vertullo CJ. Editorial commentary: is it time for the vancomycin wrap to become a universal recommendation for the prevention of septic arthritis following anterior cruciate ligament reconstruction? Arthroscopy. 2021;37(5):1691–1693. [DOI] [PubMed] [Google Scholar]
- 45. Vertullo CJ, Quick M, Jones A, Grayson JE. A surgical technique using presoaked vancomycin hamstring grafts to decrease the risk of infection after anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(3):337–342. [DOI] [PubMed] [Google Scholar]
- 46. Wan KH, Tang SP, Lee RH, Wong KK, Wong KK. The use of vancomycin-soaked wrapping of hamstring grafts to reduce the risk of infection after anterior cruciate ligament reconstruction: an early experience in a district general hospital. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2020;22:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang C, Lee YH, Siebold R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2136–2144. [DOI] [PubMed] [Google Scholar]
- 48. Waterman BR, Arroyo W, Cotter EJ, Zacchilli MA, Garcia EJ, Owens BD. Septic arthritis after anterior cruciate ligament reconstruction: clinical and functional outcomes based on graft retention or removal. Orthop J Sports Med. 2018;6(3):2325967118758626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiao M, Leonardi EA, Sharpe O, et al. Soaking of autologous tendon grafts in vancomycin before implantation does not lead to tenocyte cytotoxicity. Am J Sports Med. 2020;48(12):3081–3086. [DOI] [PubMed] [Google Scholar]
- 50. Xiao M, Sherman SL, Safran MR, Abrams GD. Surgeon practice patterns for pre-soaking ACL tendon grafts in vancomycin: a survey of the ACL study group. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1920–1926. [DOI] [PubMed] [Google Scholar]