Abstract
Background:
Articular cartilage pathology can result from a spectrum of origins, including trauma, osteochondritis dissecans, avascular necrosis, or degenerative joint disease.
Purpose:
To compare the differences in clinical and patient-reported outcomes after autologous chondrocyte implantation (ACI) versus osteochondral allograft transplantation (OCA) in patients with focal articular cartilage defects without underlying bone loss.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A retrospective review identified patients who underwent ACI or OCA between 2008 and 2016 for isolated grades 3 and 4 articular cartilage defects without underlying bone loss. Outcome measures included the Knee injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS JR), International Knee Documentation Committee (IKDC) evaluation, and 12-Item Short Form Health Survey–Physical Component (SF-12-P) scores. Defect location, size, complications, and rate of subsequent surgery were determined.
Results:
Overall, 148 patients were included: 82 (55%) underwent ACI and 66 (45%) underwent OCA. The mean age at the time of surgery was 31.2 years within the ACI cohort and 37.7 years within the OCA cohort (P < .001); the mean follow-up for both cohorts was 6.7 years (P = .902). Within the ACI group, 28 (34%) patients had multifocal defects, 21 (26%) had defects confined to the femoral condyles, and 33 (40%) had defects in the patellofemoral region. Within the OCA group, 23 (35%) patients had multifocal defects, 30 (46%) had confined femoral condyle lesions, and 13 (20%) had patellofemoral defects. When comparing by lesion location, there were no significant differences in KOOS JR, and IKDC scores between the ACI and OCA cohorts (P < .05). There was, however, a significant difference for SF-12-P scores for FDD trochlear lesions. In both cohorts, traumatic patellofemoral pathology demonstrated lower patient-reported outcomes and higher failure rates than degenerative lesions. The overall rate of failure, defined as graft failure with revision surgery and/or conversion to arthroplasty, was significantly greater in the OCA group (21% vs 4%; P = .002).
Conclusion:
Study results indicated that ACI provides similar outcomes to OCA with or without concomitant procedures for the treatment of symptomatic articular cartilage defects in all lesion locations and may have a lower revision rate for multifocal and condylar lesions.
Keywords: osteochondral allograft transplantation, articular cartilage defect, autologous chondrocyte implantation
In the United States, approximately 200,000 to 300,000 chondral-related surgeries are performed annually. 44 Chondral lesions are commonly encountered during knee arthroscopic surgery, with a reported prevalence of 63% to 66%. 2,12 Patients with symptomatic lesions may experience pain, locking, catching, recurrent effusions, and functional impairment. 13,51 When damaged, articular cartilage has limited capacity to regenerate because of poor vascularity and limited chondrocytes. 1 Prior studies 1,2,14,35,41,57 have demonstrated that unaddressed lesions and excised fragments result in poor knee function and progression to diffuse osteoarthritis.
There are several options available to address focal chondral defects, including palliative, reparative, and restorative treatments. Palliative measures such as chondroplasty and debridement can help reduce irritation and inflammation. 24 Reparative techniques involve fixation of the loose fragment or microfracture. 11,18,50 Restorative techniques attempt to recreate the type 2 hyaline cartilage via autologous chondrocyte implantation (ACI), osteochondral autograft transfer (OAT), or osteochondral allograft transplantation (OCA). 5,23,40 Ultimately, treatment selection depends on a variety of factors including lesion size, location, extension to the underlying subchondral bone, patient age, function, and concomitant pathology. 11 Prior studies 3,6,28,51 have demonstrated that microfracture has the best results in lesions <2 cm 2 ; lesions between 2 and 4 cm2 can be addressed using ACI, OAT, or OCA; and lesions >4 cm2 can be addressed using ACI or OCA. Although unicompartmental and total knee arthroplasty are alternative options, they are generally cost-inefficient because of concerns regarding implant survivorship in younger patients and limitations in activity level. 20
OCA transfers mature viable chondrocytes with subchondral bone into size-matched chondral defects while avoiding donor site morbidity seen with OATs. 32,48,60 The use of OCA has increased in recent years as new studies have demonstrated satisfactory long-term outcomes, with 5- and 10-year survival rates of 95% and 85%, respectively. § There are several limitations to the use of OCA, including the availability of donor tissue, contour matching, limited time from graft harvest to implantation, high cost, and potential for disease transmission. 27,53,54
With ACI, autologous chondrocytes are harvested from a donor site and cultured in vitro. These chondrocytes are then reimplanted into the chondral defect in a second operation. There are various methods of applying the chondrocytes to the cartilage defect, including applying them under a periosteal cover (ACI-P), applying them under a porcine-derived type 1/type 3 collagen cover (ACI-C), or implanting the cells into a membrane using a matrix-induced ACI (MACI) technique. The former 2 methods involve implantation of chondrocytes in suspension leading to concerns with uneven distribution of cells as well as leakage. The MACI technique alleviates these concerns. 4,49 The results of several randomized controlled trials have indicated favorable short-term results for MACI when used to treat focal articular cartilage defects. 31,37
When there is significant subchondral bone damage or loss, OCA may be superior to ACI since it has the advantage of restoring the subchondral bone. In the setting of articular cartilage damage without subchondral bone loss, both OCA and ACI are viable options. Because of the increased popularity of cartilage restoration techniques for treatment of focal cartilage defects, the purpose of this study was to compare differences in clinical and patient-reported outcomes in patients treated with OCA or ACI for grades 3 and 4 chondral defects without underlying bone loss or defects.
Methods
After receiving institutional review board approval, we conducted a database query to identify patients at a single institution who had undergone ACI or OCA between 2008 and 2016. Patients in whom articular cartilage disease occurred in the setting of concomitant bone loss and/or unaddressed malalignment were excluded from the study. In addition, patients with <2 years of postoperative follow-up were excluded.
Eligible patients completed functional outcome surveys including the Knee injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS JR), International Knee Documentation Committee (IKDC), and 12-Item Short Form Health Survey–Physical Component (SF-12-P) scores. A retrospective chart review was performed to obtain patient information, including age at time of surgery, sex, and body mass index (BMI). We also collected information related to ACI and OCA, including technique, laterality, injury origin, chondral lesion grade, defect size, defect location, number of defects, concomitant procedures, previous and subsequent procedures on the ipsilateral knee, and complications. Failure after ACI and OCA was defined as graft failure with revision surgery and/or conversion to arthroplasty.
The decision for ACI or OCA was previously determined based on surgeon experience, preference, patient age, and incorporated lesion characteristics (location, size, containment, and presence of subchondral cysts/sclerosis). ACI was performed using a porcine collagen patch with no subchondral bone grafting. Postoperative rehabilitation included nonweightbearing for 6 weeks with continuous passive motion use. There were slight variabilities with regard to range of motion restrictions initially based on lesion location, size, and concomitant procedures. Within each group, patients with lesions in both the femoral condyle and patellofemoral (patella, trochlea) regions were categorized as multifocal, while those with isolated lesions were grouped as femoral condyle (medial or lateral), patellar, or trochlear.
Descriptive statistics including the mean, standard deviation, and range were calculated. The Fisher exact test or chi-square test was used to compare categorical data. The Student t test was used to compare means of parametric data between 2 groups, while analysis of variance was used to compare means of parametric data among 3 groups. Linear regression was used to determine the association between age at surgery and patient-reported outcome scores. Statistical significance was set at P < .05. All statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) Statistics software 26 (IBM Corp., Armonk, NY, USA).
Results
A total of 202 patients were identified as being eligible to participate in the study. Of these, 54 (27%) patients were lost to follow-up. The remaining 148 (73%) patients were included in the final analysis. The patient cohort comprised 82 (55%) patients who had underwent ACI and 66 (45%) patients who underwent OCA (Table 1). In both cohorts, cartilage injuries were a result of acute trauma, or they were atraumatic and characterized as focal degenerative defect (FDD). Within the ACI group, acute trauma was further categorized as athletic (11 patients, 34%), fall (5 patients, 16%), work-related (5 patients, 16%), motor vehicle accident (2 patients, 6%), acute patellar dislocation (2 patients, 6%), and unspecified (7 patients, 22%). Within the OCA group, acute trauma was further categorized as athletic (11 patients, 32%), fall (12 patients, 35%), work-related (4 patients, 12%), motor vehicle accident (2 patients, 6%), gunshot (1 patient, 3%), and unspecified (4 patients, 12%).
Table 1.
Comparison of Characteristics Between the ACI and OCA Groups a
| ACI (n = 82) | OCA (n = 66) | P Value | |
|---|---|---|---|
| Age at surgery, y | 31.2 ± 9.5 | 37.7 ± 10.7 | <.001 |
| Follow-up, y | 6.7 ± 1.7 | 6.7 ± 1.6 | .902 |
| Sex | .284 | ||
| Male | 35 (42.7) | 34 (51.5) | |
| Female | 47 (57.3) | 32 (48.5) | |
| BMI | 27.4 ± 5.2 | 29.1 ± 5.3 | .056 |
| Laterality | .123 | ||
| Right | 44 (53.7) | 27 (40.9) | |
| Left | 38 (46.3) | 39 (59.1) | |
| Origin | .040 | ||
| Acute trauma | 32 (39.0) | 34 (51.5) | |
| FDD | 50 (61.0) | 32 (48.5) | |
| Lesion grade | .001 | ||
| Grade 3 | 1 (1.2) | 12 (18.2) | |
| Grade 4 | 81 (98.8) | 54 (81.8) |
a Data are presented as mean ± SD or n (%). Bolded P values indicate a statistically significant difference between groups (P < .05). ACI, autologous chondrocyte implantation; BMI, body mass index; FDD, focal degenerative defect; OCA, osteochondral allograft transplantation.
Articular cartilage defects were grouped into the following locations: multifocal (lesions involving >1 area), condylar (isolated medial or lateral femoral condyle), patellar, and trochlear regions. The only statistically significant difference was seen in degenerative patellar lesions, with 18 in the ACI cohort and 5 in the OCA cohort, as demonstrated in Table 2 (P = .016).
Table 2.
Comparison of Lesion Location Between the ACI and OCA Groups Based on Origin a
| Lesion Location | ACI (n = 82) | OCA (n = 66) | P Value |
|---|---|---|---|
| Multifocal | |||
| FDD | 19 (23) | 11 (17) | .328 |
| Trauma | 9 (11) | 12 (18) | .212 |
| P value | .358 | .938 | |
| Combined | 28 (34) | 23 (35) | .928 |
| Condylar | |||
| FDD | 10 (12) | 15 (23) | .089 |
| Trauma | 11 (13) | 15 (23) | .139 |
| P value | .146 | .822 | |
| Combined | 21 (26) | 30 (45) | .011 |
| Patellar | |||
| FDD | 18 (22) | 5 (8) | .016 |
| Trauma | 12 (15) | 5 (8) | .181 |
| P value | .891 | .917 | |
| Combined | 30 (37) | 10 (16) | .004 |
| Trochlear | |||
| FDD | 3 (4) | 1 (2) | .424 |
| Trauma | 0 (0) | 2 (3) | .437 |
| P value | .555 | .591 | |
| Combined | 3 (4) | 3 (5) | .786 |
a Data are presented as n (%). Bolded P values indicate a statistically significant difference between groups (P < .05). ACI, autologous chondrocyte implantation; FDD, focal degenerative defect; OCA, osteochondral allograft transplantation.
Within the ACI group, the defect locations from most to least frequent were the patella (46%), followed by the lateral femoral condyle (19%), medial femoral condyle (18%), medial trochlea (14%), and lateral trochlea (3%). Within the OCA group, the defect locations from most to least frequent were the medial femoral condyle (44%), patella (17%), lateral femoral condyle (17%), medial trochlea (13%), and lateral trochlea (8%). When comparing the mean defect area per patient, the OCA group was found to have a significantly greater mean area at the medial femoral condyle (486 ± 264 vs 326 ± 137 mm2; P = .003) and lateral femoral condyle (458 ± 250 vs 284 ± 130 mm2; P = .019). The mean area of the patellofemoral region was not statistically different between the 2 groups (OCA, 453 ± 296 vs ACI, 509 ± 320 mm2; P = .409).
Surgical history was also collected and compared. A significantly greater proportion of patients within the OCA group had a previous procedure on their affected knee compared with the ACI group (76% vs 18%; P < .001). In contrast, patients were significantly more likely to have undergone a concomitant procedure at the time of ACI compared with OCA (93% vs 53%; P < .001). Detailed surgical history is shown in Table 3. Although the rate of subsequent surgery was greater in the OCA group, the difference was not significant (46% vs 31%; P = .061).
Table 3.
Comparison of Concomitant and Subsequent Procedures of Patients Who Underwent ACI or OCA a
| Concomitant Surgeries | Subsequent Surgeries | |||
|---|---|---|---|---|
| Additional Treatment | ACI (n = 76; 92.7%) |
OCA (n = 35; 53.0%) |
ACI (n = 25; 30.5%) |
OCA (n = 30; 45.5%) |
| ACI | 0 | 0 | 0 | 1 |
| ACLR | 2 | 3 | 0 | 3 |
| ACLR + meniscal repair | 1 | 1 | 0 | 0 |
| ACLR + meniscectomy | 2 | 0 | 0 | 1 |
| ACLR + meniscal transplant | 0 | 1 | 0 | 0 |
| Partial knee arthroplasty | 0 | 0 | 1 | 8 |
| Curettage and bone grafting | 0 | 0 | 0 | 0 |
| Debridement | 44 | 8 | 23 | 15 |
| Fulkerson TTO | 26 | 6 | 1 | 0 |
| Fulkerson TTO + MPFLR | 10 | 3 | 0 | 0 |
| Lateral retinacular lengthening | 22 | 7 | 3 | 0 |
| Meniscal repair | 1 | 0 | 0 | 0 |
| Meniscectomy | 8 | 6 | 7 | 8 |
| Meniscal transplant | 4 | 1 | 0 | 2 |
| Microfracture | 0 | 1 | 1 | 3 |
| MPFLR | 1 | 5 | 0 | 0 |
| OCA | 0 | 0 | 2 | 0 |
| Synovectomy | 8 | 1 | 4 | 2 |
a Data are presented as n. Debridement includes lysis of adhesions, chondroplasty, removal of hard, and excision of loose bodies. Arthroplasty includes total and unicompartmental. ACI, autologous chondrocyte implantation; ACLR, anterior cruciate ligament reconstruction; MPFLR, medial patellofemoral ligament reconstruction; OCA, osteochondral allograft transplantation; TTO, tibial tubercle osteotomy.
When comparing the ACI and OCA groups, patient-reported outcomes including KOOS JR (72.4 ± 16.6 vs 69.7 ± 20.3, P = .385), IKDC (61.6 ± 20.8 vs 59.1 ± 23.1, P = .491), and SF-12 (48.9 ±7.6 vs 45.9 ±10.4, P = .060) did not significantly differ. The patient-reported outcome scores were also compared based on lesion location and between FDD versus traumatic lesions (Table 4). When comparing KOOS JR, IKDC, and SF-12-P scores in FDD lesions between ACI and OCA, the only statistically significant difference was seen in trochlear lesions, where ACI demonstrated higher SF-12-P scores compared with OCA. There were no differences between groups in the scores for traumatic lesions.
Table 4A.
Comparison of Patient-Reported Outcomes in Focal Degenerative Defects for ACI and OCA Based on Location a
| KOOS JR | IKDC | SF-12-P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lesion Location | ACI | OCA | P | ACI | OCA | P | ACI | OCA | P |
| Multifocal | 75.3 ± 20.2 | 67.2 ± 14.0 | .245 | 65.2 ± 23.4 | 63.7 ± 15.6 | .854 | 48.3 ± 8.9 | 46.6 ± 10.0 | .628 |
| Femoral Condyle | 68.0 ± 16.2 | 71.7 ± 17.8 | .602 | 52.4 ± 20.0 | 57.5 ± 22.3 | .569 | 46.7 ± 9.4 | 46.9 ± 10.8 | .955 |
| Patellar | 78.8 ± 15.8 | 70.4 ± 11.0 | .282 | 68.2 ± 21.1 | 63.0 ± 15.1 | .614 | 50.6 ± 6.3 | 47.9 ± 5.5 | .400 |
| Trochlear | 66.5 ± 9.6 | 100.0 ± 0.0 | .094 | 54.6 ± 2.4 | 73.6 ± 0.0 | .099 | 50.9 ± 0.8 | 36.0 ± 0.0 | .042 |
a Values presented as mean ± SD. Bolded P value indicates a statistically significant difference between groups (P < 0.05). ACI, autologous chondrocyte implantation; IKDC, International Knee Documentation Committee; KOOS JR, Knee injury and Osteoarthritis Outcome Score for Joint Replacement; OCA, osteochondral allograft transplantation; SF-12-P, Short Form Health Survey Physical Component.
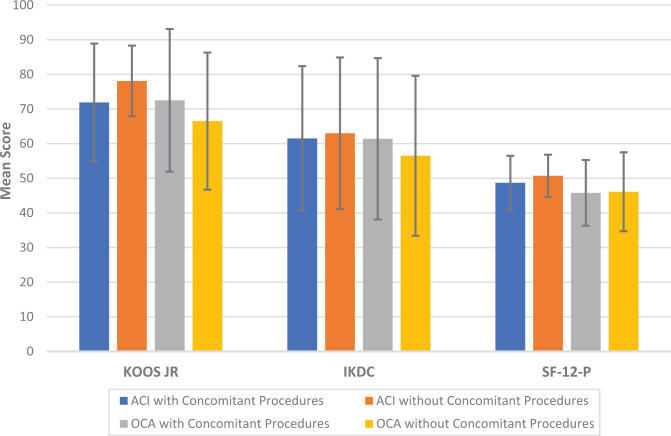
Within the ACI group, we noted only 1 statistically significant difference with degenerative patellar lesions (n=18) having superior KOOS Jr scores compared with traumatic patellar lesions (n=12). Comparing degenerative lesions to traumatic lesions in the OCA cohort, we also found 1 statistically significant difference, with degenerative trochlear lesions (n = 1) having superior IKDC scores to those of traumatic injuries (n = 2). Patient-reported outcome scores were also compared within and between the ACI and OCA groups based on the presence or absence of concomitant procedures at the time of surgery (Figure 1). No significant differences were found in any of the 3 patient-reported outcome scores among the 4 subgroups (P > .05). Within the ACI group, age was associated with significantly worse KOOS JR (P = .003), IKDC (P < .001), and SF-12-P (P = .007) scores. However, age was not significantly associated with KOOS JR (P = .281), IKDC (P = .110), or SF-12-P (P = .196) scores after OCA.
Figure 1.
Comparison of mean patient-reported outcome scores by surgical procedure with and without concomitant procedures. Error bars indicate SDs. No outcome scores were significantly different among the 4 groups. ACI, autologous chondrocyte implantation; IKDC, International Knee Documentation Committee; KOOS JR, Knee injury and Osteoarthritis Outcome Score for Joint Replacement; OCA, osteochondral allograft transplantation; SF-12-P, 12-Item Short Form Health Survey–Physical Component.
The rate of failure based on lesion location and origin is depicted in Table 5. Multifocal degenerative lesions within the OCA cohort demonstrated statistically significantly higher failure rates (n = 3) compared with the ACI group (n = 0) (P = .041). Furthermore, the overall condylar lesions (degenerative plus traumatic) in the OCA group demonstrated statistically significantly higher failure rates than did the ACI group, with 7 total compared with 0, respectively (P = .017). There were no statistically significant differences comparing the rate of failure between degenerative and traumatic lesions within the patella; however, there was a trend toward greater failure rates in traumatic lesions in both groups than in degenerative lesions. Within the ACI group, 3 patients with isolated patellar lesions required an additional surgery because of failure. Two of these patients underwent revision using OCA, and 1 patient underwent conversion to patellofemoral arthroplasty. Within the OCA group, 14 patients required an additional surgery because of OCA failure. Three patients underwent OCA revision because of implant failure, 1 underwent microfracture for a delaminated plug, 2 required removal of a loose plug, and 8 patients required conversion to total or unicompartmental knee arthroplasty. The overall rate of failure was significantly greater in the OCA group (21% vs 4%; P = .002). Complications after ACI included complex regional pain syndrome in 1 (1.2%) patient and an incisional stitch abscess that was successfully managed nonoperatively in 1 (1.2%) patient. No other complications were reported in the OCA group.
Table 4B.
Comparison of Patient-Reported Outcomes in Traumatic lesions for ACI and OCA Based on Location a
| KOOS JR | IKDC | SF-12-P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lesion Location | ACI | OCA | P | ACI | OCA | P | ACI | OCA | P |
| Multifocal | 68.3 ± 19.8 | 62.9 ± 29.7 | .644 | 57.2 ± 26.0 | 51.0 ± 33.8 | .650 | 51.5 ± 7.2 | 43.5 ± 13.7 | .179 |
| Femoral Condyle | 72.1 ± 12.4 | 61.8 ± 12.9 | .588 | 61.8 ± 12.9 | 65.8 ± 20.0 | .534 | 48.6 ± 6.6 | 48.2 ± 9.4 | .890 |
| Patellar | 66.6 ± 12.9 | 65.5 ± 20.0 | .900 | 58.2 ± 19.7 | 52.0 ± 30.3 | .615 | 47.4 ± 8.2 | 41.9 ± 12.0 | .294 |
| Trochlear | - | 57.1 ± 0.0 | - | - | 45.4 ± 0.8 | - | - | 42.8 ± 3.5 | - |
a Values presented as mean ± SD. Dashes indicate no patients were in the category or comparison could not be made. ACI, autologous chondrocyte implantation; IKDC, International Knee Documentation Committee; KOOS JR, Knee injury and Osteoarthritis Outcome Score for Joint Replacement; OCA, osteochondral allograft transplantation; SF-12-P, Short Form Health Survey Physical Component.
Table 5.
Comparison of Treatment Failure Based on Lesion Location Between Patients Who Underwent ACI or OCA a
| Lesion Location | ACI | OCA | P value |
|---|---|---|---|
| Multifocal | |||
| FDD | 0 (0.0) | 3 (27.3) | .041 |
| Trauma | 0 (0.0) | 0 (0.0) | .999 |
| P value | .999 | .093 | |
| Condylar | |||
| FDD | 0 (0.0) | 3 (20.0) | .250 |
| Trauma | 0 (0.0) | 4 (26.7) | .113 |
| P value | .999 | .999 | |
| Patellar | |||
| FDD | 0 (0.0) | 0 (0.0) | .999 |
| Trauma | 3 (25.0) | 3 (60.0) | .280 |
| P value | .054 | .167 | |
| Trochlear | |||
| FDD | 0 (0.0) | 0 (0.0) | .999 |
| Trauma | – | 1 (50.0) | – |
| P value | – | .999 | |
a Data are presented as n (%). Bolded P value indicates a statistically significant difference between groups (P < .05). Dashes indicate no patients matched the criteria. ACI, autologous chondrocyte implantation; FDD, focal degenerative defect; OCA, osteochondral allograft transplantation.
Discussion
Prior studies have evaluated long-term outcomes after ACI and OCA, but direct comparisons between these two techniques for isolated chondral defects have not been reviewed. The present study evaluated clinical and patient-reported outcomes of patients who underwent either ACI or OCA for focal grades 3 and 4 articular cartilage defects. Both patient cohorts were found to have similar patient-reported outcome scores at a mean follow-up of 6.7 years. The OCA group had a significantly higher rate of failure compared with the ACI group (21% vs 4%; P = .002). Traumatic patellofemoral pathology in both cohorts demonstrated lower patient-reported outcomes and higher failure rates than degenerative lesions. Although both interventions produced similar patient-reported outcomes, the findings of this study suggest that ACI may have a lower failure rate than OCA in select patients and lesion locations.
Patient data between the ACI and OCA cohorts were similar with respect to sex, BMI, and laterality. However, the OCA group was significantly older than the ACI group at the time of surgery. While Frank et al 17 reported that age was not predictive of functional outcomes after OCA, previous studies 43,64 have found age to be associated with worse outcomes after ACI. Similar findings were seen in the present study, with advanced age demonstrating a significant association with worse functional outcome scores after ACI but not OCA. This may be because of the inherent quality of the chondrocytes harvested in the ACI group, with older patients having less viable and active cells, while OCA directly replaces the lost chondrocytes with donor tissue. The younger population within the ACI group may have contributed to the similar patient-reported outcome scores seen at final follow-up. In addition to being an older cohort, patients within the OCA group were more likely to have undergone a previous procedure compared with patients in the ACI group. With no baseline subjective data because of the retrospective design, it is not possible to determine the degree of symptoms at the time of surgery. Frank et al 19 found that past surgical history was associated with worse functional outcomes after OCA. The higher rate of prior operations may have resulted in lower-than-expected patient-reported outcomes within the OCA group in comparison with published literature. In contrast, patients within the ACI group were more likely to have undergone a concomitant procedure at the time of surgery. Harris et al 29 examined patient-reported outcomes after cartilage repair (ACI, OCA, microfracture) with or without meniscal transplantation and osteotomy. Aside from the KOOS quality of life subscale, the authors found no significant differences in IKDC, KOOS subscale (pain, symptoms, activities of daily living, sport), Lysholm, and SF-12 scores between the groups after a minimum follow-up of 2 years. 29 In the present study, no significant difference was found in regard to patient-reported outcome scores between the ACI and OCA groups when comparing patients based on the presence or absence of concomitant surgery (Figure 1).
Previous literature has provided mixed conclusions regarding the effect of defect size on long-term outcomes after ACI and OCA. Some studies 38,41,42,47 have found correlations between larger lesions with worse outcomes at long-term follow-up, while others 56,59 have not found an association between these 2 variables. The results of the present study indicate that lesion size was not associated with patient-reported outcome scores at the final follow-up, as the OCA group was found to have a significantly greater mean defect area at the medial and lateral femoral condyle but similar patient-reported outcome scores in comparison with the ACI group. Although the OCA group had a significantly greater proportion of patients with grade 3 injuries, previous literature 56 has found lesion grade to be a poor predictor of functional outcomes. In contrast, lesion location, specifically the patellofemoral joint, has been established as a risk factor for worse outcomes after both ACI and OCA. 8,21,25,43 In our study, when comparing isolated patellofemoral lesions in the OCA and ACI cohorts, patient-reported outcomes in both cohorts were similar to those of patients with multifocal or confined condylar lesions.
The patient-reported outcomes of the present study aligned with those of previous literature. With regard to the ACI group, the mean KOOS JR score (72.4) was slightly lower than the range of 74.7 to 84.6 as reported by Saris et al 52 and Hoburg et al. 30 In addition, the mean IKDC score (61.6) aligned with the scores of Viste et al, 58 who reported a mean score of 60.2 at an average follow-up of 6 years, and Martincic et al, 36 who reported a mean score of 67 at an average follow-up of 5 years. The mean SF-12-P score (48.9) aligned with those of Gillogly and Arnold, 22 who reported a mean score of 47.6 at an average follow-up of 7.6 years, and Ogura et al, 45 who reported a mean score of 49.2 at a mean follow-up of 2 years.
Within the OCA group, the mean KOOS JR score (69.7) was slightly less than the mean scores found in previous literature, including the studies by Mirzayan et al, 39 who reported a mean score of 83.1, and Tírico et al, 55 who reported a mean score of 85.2. The superior outcomes reported by Mirzayan et al 39 and Tírico et al 55 are not surprising, as the studies had an average follow-up of 2 years compared with 6 years in the present study. The mean IKDC score (59.1) was within the range of 54.8 to 81.2 established by a number of previous studies. 10,17,19,34,55,59,61 The mean SF-12-P score (45.9) also aligned with the reported range of 43.4 to 48.7. 17,34,46
Comparison of traumatic to degenerative lesions between OCA and ACI also did not demonstrate any major differences in patient-reported outcomes. The only statistically significant difference was noted in trochlear lesions, in which degenerative lesions treated with ACI demonstrated superior SF-12-P compared with those treated with OCA; however, the patient sample was small, with only 3 patients with ACI and 1 with OCA. Subgroup analysis of each cohort demonstrated that degenerative lesions within the patellofemoral joint fared superior to traumatic lesions. Degenerative patellar lesions treated with ACI not only demonstrated superior KOOS JR scores compared with traumatic lesions addressed with ACI but also had zero failures as opposed to the 25% (n = 3) failure rate seen in traumatic lesions (Table 5). A similar trend was seen in the OCA cohort, with degenerative trochlear pathology treated with OCA having better IKDC scores and no failures as opposed to traumatic trochlear lesions. With higher failure rates in traumatic patellofemoral lesions, it was not surprising to see lower patient-reported outcomes. The higher failure rates in traumatic lesions could be a result of underlying subchondral bone damage that may occur at the time of injury, which could affect the integration of both ACI and OCA grafts. Further research exploring traumatic lesions is needed.
Overall, the failure rate of 3.7% within the ACI group was slightly lower than the rates reported in the literature (5%-7%). 31,36,58 While some of these studies focused solely on lesions in the femoral condyle, current literature suggests that lesion location is not associated with rate of failure after ACI. 59 However, our study results suggest the cause of the lesion may affect graft survivorship, with traumatic patellofemoral pathology having inferior outcomes and higher failure rates compared with degenerative lesions. With regard to OCA, the failure rate of 21% was slightly higher than the range of 13% to 18% 16,19,61 reported in the literature. The OCA group had a higher failure rate compared with the ACI cohort in terms of multifocal degenerative lesions and isolated condylar lesions. This finding is likely multifactorial and attributable to the older patient population in the OCA group, larger defect size in the femoral condyles, greater prevalence of prior operations, and differences in etiopathogenesis.
The findings of the present study suggest that both ACI and OCA are effective techniques for the management of grades 3 and 4 articular cartilage defects, as evidenced by the similarity in outcome scores at final follow-up. Furthermore, concomitant procedures during both ACI and OCA can be performed with similar patient-reported outcomes for either procedure in isolation. Advanced age was an independent risk factor for worst outcomes after ACI but not OCA. Overall, ACI had a lower failure rate in regard to degenerative multifocal and isolated condylar lesions compared with OCA; however, the age at the time of surgery, lesion size, and incidence of previous operations were greater in the latter group, which may have contributed to a higher rate of failure. Further research on the efficacy of ACI and OCA for the treatment of articular cartilage defects and on the implications of third-generation ACI techniques (MACI) is required.
The present study had several limitations. The retrospective study design did not allow for the formation of groups with identical patient and lesion characteristics, including defect size. Although similar, the OCA group had a significantly higher average age at the time of surgery, larger condylar lesions, and higher prevalence of previous operations when compared with the ACI group. Because of the retrospective study design, there were no baseline subjective data, and therefore it was not possible to determine the degree of symptomology at the time of surgery within each cohort. Despite collecting data on >70% of patients who underwent ACI or OCA between 2008 and 2016, a power analysis demonstrated that the results were underpowered and at risk of a type 2 error. The mean follow-up of 6.7 years for both patient groups underscored the effectiveness of these surgical techniques in the short and intermediate periods. Extended follow-up would provide more definitive conclusions on the longevity and long-term outcomes after ACI and OCA. The extent of improved patient-reported outcomes purely due to cartilage restoration procedures is also difficult to determine, as concomitant procedures were also performed. There were also slight variations with postoperative protocols among surgeons, which may have contributed to functional recovery. Although clinical outcomes were tracked, long-term postoperative radiographs were not routinely obtained, limiting the ability to grade the progression of osteoarthritis. Furthermore, there was potential for selection bias among surgeons, with preference toward 1 technique to address subchondral cysts or sclerosis. Lastly, this study only included patients treated by several surgeons at 1 institution who perform a high volume of OCAs and ACIs, which may have potentially introduced performance bias.
Conclusion
The results of the current study indicated that ACI provides similar patient-reported outcomes to OCA with or without concomitant procedures for the treatment of symptomatic articular cartilage defects in all lesion locations and may have a lower revision rate for multifocal and condylar lesions.
ACKNOWLEDGMENT
The authors would like to acknowledge Emma E. Johnson for her contribution to revision of the manuscript.
Footnotes
Final revision submitted July 20, 2021; accepted August 24, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: B.S.T. has received education payments from Liberty Surgical and consulting fees from Medical Device Business Services. F.P.T. has received consulting fees from Medical Device Business Services and nonconsulting fees from Medtronic and Smith & Nephew. K.B.F. has received education payments from Liberty Surgical, consulting fees from Medical Device Business Services, and nonconsulting fees and honoraria from Vericel. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Thomas Jefferson University.
References
- 1. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi:10.1177/0363546504273510 [DOI] [PubMed] [Google Scholar]
- 2. Årøen A, Løken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215. doi:10.1177/0363546503259345 [DOI] [PubMed] [Google Scholar]
- 3. Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214–1220. doi:10.1016/j.arthro.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 4. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–645. doi:10.1302/0301-620X.87B5.15905 [DOI] [PubMed] [Google Scholar]
- 5. Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009. doi:10.2106/JBJS.I.00895 [DOI] [PubMed] [Google Scholar]
- 6. Bekkers JEJ, Inklaar M, Saris DBF. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(1 suppl):148–155. doi:10.1177/0363546509351143 [DOI] [PubMed] [Google Scholar]
- 7. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6(4):203–207. doi:10.1177/1947603515595072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi:10.1056/NEJM199410063311401 [DOI] [PubMed] [Google Scholar]
- 9. Bugbee W, Cavallo M, Giannini S. Osteochondral allograft transplantation in the knee. J Knee Surg. 2012;25(2):109–116. doi:10.1055/s-0032-1313743 [DOI] [PubMed] [Google Scholar]
- 10. Cameron JI, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation of the femoral trochlea. Am J Sports Med. 2016;44(3):633–638. doi:10.1177/0363546515620193 [DOI] [PubMed] [Google Scholar]
- 11. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. Instr Course Lect. 2010;59:181–204. [PubMed] [Google Scholar]
- 12. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi:10.1016/s0749-8063(97)90124-9 [DOI] [PubMed] [Google Scholar]
- 13. Davey A, Frank RM, Wang KC, Southworth TM, Cole BJ. Clinical outcomes of revision osteochondral allograft transplantation. Arthroscopy. 2019;35(9):2636–2645. doi:10.1016/j.arthro.2019.03.055 [DOI] [PubMed] [Google Scholar]
- 14. Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337–342. doi:10.1016/j.joca.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 15. Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2017;24(3):508–517. doi:10.1016/j.knee.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 16. Familiari F, Cinque ME, Chahla J, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2018;46(14):3541–3549. doi:10.1177/0363546517732531 [DOI] [PubMed] [Google Scholar]
- 17. Frank RM, Cotter EJ, Lee S, Poland S, Cole BJ. Do outcomes of osteochondral allograft transplantation differ based on age and sex? A comparative matched group analysis. Am J Sports Med. 2018;46(1):181–191. doi:10.1177/0363546517739625 [DOI] [PubMed] [Google Scholar]
- 18. Frank RM, Cotter EJ, Strauss EJ, Gomoll AH, Cole BJ. The utility of biologics, osteotomy, and cartilage restoration in the knee. J Am Acad Orthop Surg. 2018;26(1):e11–e25. doi:10.5435/JAAOS-D-17-00087 [DOI] [PubMed] [Google Scholar]
- 19. Frank RM, Lee S, Levy D, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864–874. doi:10.1177/0363546516676072 [DOI] [PubMed] [Google Scholar]
- 20. Ghomrawi HM, Eggman AA, Pearle AD. Effect of age on cost-effectiveness of unicompartmental knee arthroplasty compared with total knee arthroplasty in the U.S. J Bone Joint Surg Am. 2015;97(5):396–402. doi:10.2106/JBJS.N.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gigante A, Enea D, Greco F, et al. Distal realignment and patellar autologous chondrocyte implantation: mid-term results in a selected population. Knee Surg Sports Traumatol Arthrosc. 2009;17(1):2–10. doi:10.1007/s00167-008-0635-6 [DOI] [PubMed] [Google Scholar]
- 22. Gillogly SD, Arnold RM. Autologous chondrocyte implantation and anteromedialization for isolated patellar articular cartilage lesions: 5- to 11-year follow-up. Am J Sports Med. 2014;42(4):912–920. doi:10.1177/0363546513519077 [DOI] [PubMed] [Google Scholar]
- 23. Gomoll AH, Farr J, Gillogly SD, Kercher JS, Minas T. Surgical management of articular cartilage defects of the knee. Instr Course Lect. 2011;60:461–483. [PubMed] [Google Scholar]
- 24. Gowd AK, Cvetanovich GL, Liu JN, et al. Management of chondral lesions of the knee: analysis of trends and short-term complications using the National Surgical Quality Improvement Program database. Arthroscopy. 2019;35(1):138–146. doi:10.1016/j.arthro.2018.07.049 [DOI] [PubMed] [Google Scholar]
- 25. Gracitelli GC, Meric G, Pulido PA, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879–884. doi:10.1177/0363546514564144 [DOI] [PubMed] [Google Scholar]
- 26. Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage. 2015;6(2):98–105. doi:10.1177/1947603514566298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gross AE, Aubin P, Cheah HK, Davis AM, Ghazavi MT. A fresh osteochondral allograft alternative. J Arthroplasty. 2002;17(4)(suppl 1):50–53. doi:10.1054/arth.2002.32447 [DOI] [PubMed] [Google Scholar]
- 28. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25–32. doi:10.2106/00004623-200300002-00004 [DOI] [PubMed] [Google Scholar]
- 29. Harris JD, Hussey K, Saltzman BM, et al. Cartilage repair with or without meniscal transplantation and osteotomy for lateral compartment chondral defects of the knee: case series with minimum 2-year follow-up. Orthop J Sports Med. 2014;2(10):2325967114551528. doi:10.1177/2325967114551528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoburg A, Löer I, Körsmeier K, et al. Matrix-associated autologous chondrocyte implantation is an effective treatment at midterm follow-up in adolescents and young adults. Orthop J Sports Med. 2019;7(4):2325967119841077. doi:10.1177/2325967119841077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86(3):455–464. doi:10.2106/00004623-200403000-00001 [DOI] [PubMed] [Google Scholar]
- 32. Krych AJ, Robertson CM, Williams RJ III; Cartilage Study Group. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053–1059. doi:10.1177/0363546511435780 [DOI] [PubMed] [Google Scholar]
- 33. Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sports Med. 2018;6(3):2325967118761871. doi:10.1177/2325967118761871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee S, Frank RM, Christian DR, Cole BJ. Analysis of defect size and ratio to condylar size with respect to outcomes after isolated osteochondral allograft transplantation. Am J Sports Med. 2019;47(7):1601–1612. doi:10.1177/0363546519841378 [DOI] [PubMed] [Google Scholar]
- 35. Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769–776. [PubMed] [Google Scholar]
- 36. Martincic D, Radosavljevic D, Drobnic M. Ten-year clinical and radiographic outcomes after autologous chondrocyte implantation of femoral condyles. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1277–1283. doi:10.1007/s00167-013-2778-3 [DOI] [PubMed] [Google Scholar]
- 37. Micheli LJ, Browne JE, Erggelet C, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11(4):223–228. doi:10.1097/00042752-200110000-00003 [DOI] [PubMed] [Google Scholar]
- 38. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472(1):41–51. doi:10.1007/s11999-013-3146-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mirzayan R, Charles MD, Batech M, Suh BD, DeWitt D. Bipolar osteochondral allograft transplantation of the patella and trochlea. Cartilage. 2020;11(4):431–440. doi:10.1177/1947603518796124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mistry H, Metcalfe A, Smith N, et al. The cost-effectiveness of osteochondral allograft transplantation in the knee. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1739–1753. doi:10.1007/s00167-019-05392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mithoefer K, Hambly K, Della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(suppl 1):167S–176S. doi:10.1177/0363546509351650 [DOI] [PubMed] [Google Scholar]
- 42. Mithoefer K, Williams RJ, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34(9):1413–1418. doi:10.1177/0363546506288240 [DOI] [PubMed] [Google Scholar]
- 43. Nawaz SZ, Bentley G, Briggs TWR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96(10):824–830. doi:10.2106/JBJS.L.01695 [DOI] [PubMed] [Google Scholar]
- 44. Niethammer TR, Holzgruber M, Gülecyüz MF, Weber P, Pietschmann MF, Müller PE. Matrix based autologous chondrocyte implantation in children and adolescents: a match paired analysis in a follow-up over three years post-operation. Int Orthop. 2017;41(2):343–350. doi:10.1007/s00264-016-3321-1 [DOI] [PubMed] [Google Scholar]
- 45. Ogura T, Ackermann J, Barbieri Mestriner A, Merkely G, Gomoll AH. Minimal clinically important differences and substantial clinical benefit in patient-reported outcome measures after autologous chondrocyte implantation. Cartilage. 2020;11(4):412–422. doi:10.1177/1947603518799839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ogura T, Ackermann J, Mestriner AB, Merkely G, Gomoll AH. The minimal clinically important difference and substantial clinical benefit in the patient-reported outcome measures of patients undergoing osteochondral allograft transplantation in the knee. Cartilage. 2021;12(1):42–50. doi:10.1177/1947603518812552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7(4):298–308. doi:10.1177/1947603516630786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32(1):125–131. doi:10.1177/0095399703258614 [DOI] [PubMed] [Google Scholar]
- 49. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi:10.1097/00003086-200005000-00020 [DOI] [PubMed] [Google Scholar]
- 50. Redondo ML, Beer AJ, Yanke AB. Cartilage restoration: microfracture and osteochondral autograft transplantation. J Knee Surg. 2018;31(3):231–238. doi:10.1055/s-0037-1618592 [DOI] [PubMed] [Google Scholar]
- 51. Richter DL, Schenck RC, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health. 2016;8(2):153–160. doi:10.1177/1941738115611350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saris DBF, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–246. doi:10.1177/0363546507311095 [DOI] [PubMed] [Google Scholar]
- 53. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121–133. doi:10.5435/JAAOS-22-02-121 [DOI] [PubMed] [Google Scholar]
- 54. Stoker AM, Stannard JP, Cook JL. Chondrocyte viability at time of transplantation for osteochondral allografts preserved by the Missouri Osteochondral Preservation System versus standard tissue bank protocol. J Knee Surg. 2018;31(8):772–780. doi:10.1055/s-0037-1608947 [DOI] [PubMed] [Google Scholar]
- 55. Tírico LEP, Demange MK, Santos LAU, et al. Development of a fresh osteochondral allograft program outside North America. Cartilage. 2016;7(3):222–228. doi:10.1177/1947603515618484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tírico LEP, McCauley JC, Pulido PA, Bugbee WD. Lesion size does not predict outcomes in fresh osteochondral allograft transplantation. Am J Sports Med. 2018;46(4):900–907. doi:10.1177/0363546517746106 [DOI] [PubMed] [Google Scholar]
- 57. Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee: a long-term study. J Bone Joint Surg Br. 1991;73(3):461–464. doi:10.1302/0301-620X.73B3.1670450 [DOI] [PubMed] [Google Scholar]
- 58. Viste A, Piperno M, Desmarchelier R, Grosclaude S, Moyen B, Fessy MH. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop Traumatol Surg Res. 2012;98(7):737–743. doi:10.1016/j.otsr.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 59. Wang D, Chang B, Coxe FR, et al. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med. 2019;47(1):71–81. doi:10.1177/0363546518808030 [DOI] [PubMed] [Google Scholar]
- 60. Wang D, Eliasberg CD, Wang T, et al. Similar outcomes after osteochondral allograft transplantation in anterior cruciate ligament-intact and -reconstructed knees: a comparative matched-group analysis with minimum 2-year follow-up. Arthroscopy. 2017;33(12):2198–2207. doi:10.1016/j.arthro.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 61. Wang D, Rebolledo BJ, Dare DM, et al. Osteochondral allograft transplantation of the knee in patients with an elevated body mass index. Cartilage. 2019;10(2):214–221. doi:10.1177/1947603518754630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang T, Wang DX, Burge AJ, et al. Clinical and MRI outcomes of fresh osteochondral allograft transplantation after failed cartilage repair surgery in the knee. J Bone Joint Surg Am. 2018;100(22):1949–1959. doi:10.2106/JBJS.17.01418 [DOI] [PubMed] [Google Scholar]
- 63. Williams RJ, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718–726. doi:10.2106/JBJS.F.00625 [DOI] [PubMed] [Google Scholar]
- 64. Windt TS, Bekkers JEJ, Creemers LB, Dhert WJA, Saris DBF. Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am J Sports Med. 2009;37(suppl 1):58S–62S. doi:10.1177/0363546509349765 [DOI] [PubMed] [Google Scholar]