Abstract
Background
Dietary fatty acids intake affects the composition of erythrocyte fatty acids, which is strongly correlated with glycolipid metabolism disorders. This study aimed at investigating the different effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acid (n-3 PUFA) on the fatty acids of erythrocytes and glycolipid metabolism in patients with type 2 diabetes mellitus (T2DM).
Methods
The randomized double-blinded trial that was performed on 180 T2DM patients. The participants were randomly assigned to three groups for the six-month intervention. The participants were randomly assigned to three groups for the six-month intervention. The fish oil (FO) group was administered with FO at a dose of 3 g/day containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the perilla oil (PO) group was administered with PO at a dose of 3 g/day containing α-linolenic (ALA), the linseed and fish oil (LFO) group was administered with mixed linseed and fish oil at a dose of 3 g/day containing EPA, DHA and ALA. Demographic information were collected and anthropometric indices, glucose and lipid metabolism indexes, erythrocyte fatty acid composition were measured. Statistical analyses were performed using two-way ANOVA.
Results
A total of 150 patients finished the trial, with 52 of them in the FO group, 50 in the PO group and 48 in the LFO group. There were significant effects of time × treatment interaction on fast blood glucose (FBG), insulin, HOMA-IR and C-peptide, TC and triglyceride (TG) levels (P < 0.001). Glucose and C-peptide in PO and LFO groups decreased significantly and serum TG in FO group significantly decreased (P < 0.001) after the intervention. Erythrocyte C22: 5 n-6, ALA, DPA, n-6/n-3 PUFA, AA/EPA levels in the PO group were significantly higher than FO and LFO groups, while EPA, total n-3 PUFA and Omega-3 index were significantly higher in the FO and LFO groups compared to PO group.
Conclusion
Supplementation with perilla oil decreased FBG while fish oil supplementation decreased the TG level. Marine-based and plant-based n-3 PUFAs exhibit different effects on fatty acid compositions of erythrocytes and regulated glycolipid metabolism.
Trial registration
This trial was recorded under Chinese Clinical Trial Registry Center (NO: ChiCTR-IOR-16008435) on May 28 2016.
Keywords: Type 2 diabetes, Glucose, Polyunsaturated fatty acids, Erythrocyte
Introduction
Type 2 Diabetes mellitus (T2DM) is a major chronic diseases that is characterized by elevated glucose levels and insulin resistance in the body [1]. In 2019, it was postulated that the global number of adults (20–79 years) with diabetes was 463 million. This figure is projected to increase by 51% to reach 700 million by the year 2045 [2]. More than half of the T2DM population were in the high-risk status of dyslipidemia and vascular disease complications [3]. A reasonable diet structure is key for T2DM prevention and treatment. T2DM patients are frequently associated with dyslipidemia, which results in atherosclerosis (AS) and cardiovascular diseases (CVD). Approximately 80% of T2DM patients in the USA suffer from dyslipidemia [4].
Omega-3 polyunsaturated fatty acids (n-3 PUFA) are irreplaceable fatty acids for human from fish oils that contains eicosapentaenoic acids (EPA) and docosahexaenoic acid (DHA). In addition to this marine-based n-3 PUFA, α-Linolenic acid (ALA) is a plant-based n-3 PUFA that is an essential precursor of the n-3 PUFA family. Researches have explored the beneficial effects of major marine-based n-3 PUFA on human health, particularly on lipid profiles and glucose metabolism [5, 6]. Due to the poor palatability of fish oil and heavy metal pollution, the search for alternative plant-based n-3 PUFA sources is important [7].
Dietary n-3 PUFA is the most important determinant for balancing fatty acid status in the human body [8]. Fatty acid composition changes such as increased saturated fatty acids (SFA) and n-6/n-3 PUFA ratios are health risks for diabetes and dyslipidemia [9–12]. Fatty acids are involved in the de novo lipogenesis pathway, which is the key pathway in the pathogenesis of T2DM [13]. Previous studies have established that changes in serum, plasma, and erythrocyte fatty acids are biomarkers of diet exposure. However, erythrocytic changes reflect n-3 PUFA intake from food and metabolic conversion in the last 90 days. The conversion ratios from plasma to erythrocytes are stable [14].
Studies on fatty acid composition and their association with different diseases have focused on serum and plasma fatty acids. A few cross-sectional studies have been performed to investigate the biomarkers for n-3 PUFA in erythrocytes of diabetic and CVD patients. Only two RCT explored the effects of n-3 PUFA from different sources on erythrocyte n-3 fatty acid composition. These studies established that the blood glucose and lipid levels were within normal ranges. They focused on the n-3 PUFA of the erythrocyte but not SFA, monounsaturated fatty acids (MUFA), or n-6 PUFA [15, 16].
Our previous research found that fish oil supplement for 6 months improved serum EPA, DHA and TG levels inT2DM patients. The hypothesis of this trial was that plant n-3 PUFA supplement has the different effects on glucolipid metabolism in T2DM patients. Therefore, a double-blind, randomized trial with three groups was performed. The purpose was to compare the different effects of marine-based and plant-based n-3 PUFA on glucose, lipids profiles and erythrocyte fatty acids compositions in T2DM patients.
Materials and methods
Study participants
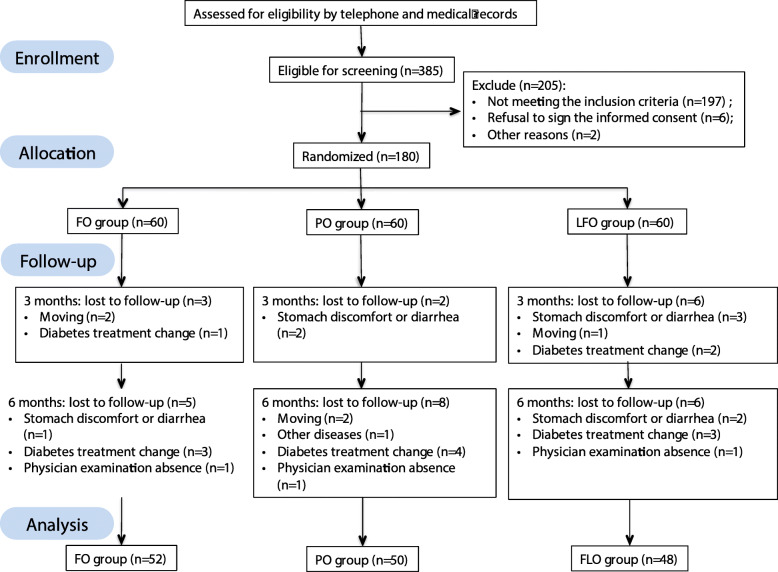
One hundred fifty type 2 diabetic and dyslipidemia people aged between 18 to 70 years were recruited from among 180 patients in Guanlin Public Hospital, Yixing City, China. The inclusion criteria were that the participants had to have been diagnosed with T2DM that fasting blood glucose (FBG) ≥ 7.0 mmol/L and dyslipidemia (TG ≥1.7 mmol/L, or TC ≥5.2 mmol/L, or LDL-C ≥ 3.4 mmol/L, or HDL-C < 1.0 mmol/L, or no HDL-C ≥ 4.1 mmol/L). Patients who had been on omega-3 supplements within 6 months or on lipid-lowering drugs, those with complicated chronic cardiovascular diseases, asthma, alcoholism, hyperthyroidism, tumor patients, pregnancy, lactation period were excluded from the study. Finally, the physical examination of patients for participation in the study was done by a competent physician. Figure 1 shows the process of participant selection and allocation in the trial.
Fig. 1.
Flow chart of the randomized controlled trial
Using the formula N = 2(Zα + Zβ)2σ2/d2 for sample size calculation, 48 individuals were required per group in this trial. According to the previous trial and reported literature, 0.5 mmon/L, 0.3 mmon/L and 0.4 mmon/L was set up as the TG decreasing mean after fish oil, perilla oil and blend oil intervention, respectively. The standard deviations were 0.3 mmon/L, 0.2 mmon/L, 0.3 mmon/L respectively [17, 18]. With α = 0.05 and β = 0.1, at least 48 subjects were needed per group. According to the 20% lost to follow-up, 180 participants were selected for final inclusion.
The trial was approved by the Ethical Committee of Zhongda hospital affiliated to Southeast University, Nanjing, China (NO. 2015ZDSYLL089.0). The purpose and protocol of the trial were exhaustively explained to the study participants. All participants were required to sign the informed consent before enrollment. The trial was recorded under http://www.chictr.org.cn/showprojen.aspx?proj=14291 (ChiCTR-IOR-16008435).
Experimental oil capsule preparation
The fish oil (FO), perilla oil (PO), or mixed linseed and fish oil (LFO) capsules were standardized to 500 mg. Each FO capsule contained 143 mg EPA and 172 mg DHA. Each PO capsule contained 322 mg of ALA. The major fatty acids in each LFO capsule were EPA (105 mg), DHA (60 mg) and ALA (140 mg). These capsules were manufactured by the Zhanwang Company (Shanghai, China). They exhibited the same shape and were packaged in transparent bottles. The bottles were labeled with ‘PUFA A’, ‘PUFA B’ and ‘PUFA C’ for FO, PO and LFO, respectively. Fatty acid contents of intervention capsules were shown in Table 1. Except for the analyzer, participants, nurses and outcomes assessor were blinded during the intervention.
Table 1.
Fatty acid contents of intervention capsules (%)
| Fatty acids (%) | Perilla oil capsules | Fish oil capsules | Linseed and fish oil capsules |
|---|---|---|---|
| <C14 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C14:0 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C16:0 | 5.6 | 5.6 | 5.6 |
| C17:0 | 0.0425 | 0.0425 | 0.0425 |
| C17:1 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C18:0 | 2.29 | 2.29 | 2.29 |
| C18:1 | 13.7 | 13.7 | 13.7 |
| C18:2 | 14 | 14 | 14 |
| C18:3 n3 | 63.4 | ——(< 0.0066) | 28 |
| C20:0 | 0.108 | 0.108 | 0.108 |
| C20:1 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C20:2 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C20:5 n3 | ——(< 0.0066) | 28.6 | 21 |
| C22:0 | ——(< 0.0026) | ——(< 0.0026) | ——(< 0.0026) |
| C22:1 | ——(< 0.0013) | ——(< 0.0013) | ——(< 0.0013) |
| C22:6 n3 | ——(< 0.0066) | 34.4 | 12 |
| C24:0 | ——(< 0.0026) | ——(< 0.0026) | ——(< 0.0026) |
—— not detected
Omega-3 fatty polyunsaturated fatty acid intervention
After the baseline examination and stratified by gender and age, patients were randomly distributed in three groups (at a 1:1:1 ratio) by reference to the computer-generated random number list, with 60 patients in each group. The intervention duration was 6 months. FO group participants were administered with two capsules of fish oil three times a day; PO group participants were administered with perilla oil at the same dose; LFO group participants were administered with mixed linseed oil and fish oil capsules. During the experimental period, subjects were instructed to maintain their habitual dietary behaviors and lifestyles.
Questionnaire survey and physical evaluation
The unified questionnaires were used to obtain information on demographic characteristics, diseases history, diabetes duration, treatment modality, smoking status and alcohol intake. Physical evaluation was performed. Blood pressure was measured twice for each subject (Yuwell medical equipment & supply Co., Ltd., Jiangsu, China).
Biochemical analysis
Overnight fasting venous blood samples were obtained between 7:00 am to 9:00 am into vacationer tubes containing ethylenediaminetetraacetic acid (EDTA). C peptide and insulin were measured by an electrochemiluminescence analyzer (Roche Cobas e 602). FBG levels and TG, TC, HDL-C, LDL-C lipoprotein a (LPa), apolipoprotein A1 (Apo A1), apolipoprotein B (Apo B), free fatty acids (FFA), interleukin 6 (IL-6), markers of hepatic or renal function were determined by an automatic blood biochemical analyzer (Mindray BS-800, Shenzhen, China). Glycated haemoglobin (HbAlc) was determined by chromatography (Audicom, AC6601, Jiangsu, China).
Lipid and erythrocyte fatty acid analysis
Approximately 7 mL fasting blood was obtained from forearm veins. After centrifugation at 3000 rpm/min for 15 min, red blood cells were separated from the serum. Lipids were extracted from the erythrocyte by chloroform/methanol (2:1) solution with 10 mg/L butylated-hydroxytoluene. The total weight of lipids was determined by gravimetric analysis. Fatty acid content and composition in the erythrocyte were analyzed by gas chromatography (GC) after total lipid extraction. Toluene and H2SO4 in methanol (5%, v/v) were used for the transesterification of erythrocyte fatty acid methyl esters in sealed tubes at 70 °C for 2 h. FAME was analyzed using a Shimadzu GC-14C gas chromatography-flame ionization detector (Shimadzu Corporation, Japan). The GC column was a DB-23 fused silica bonded phase column 60 m with 0.25 mm diameter and 0.25 μm film thickness (Agilent Corporation, California, USA). The column temperature was programmed as follows: the initial temperature was 150 °C increased to 180 °C at a rate of 10 °C/min and held for 2 min; then further increased to 215 °C and maintained for 6 min. It was finally raised to 230 °C followed by a 5 min hold time. The retention time and FAME mixture standards (Nu-Chek Prep, MN, USA) were used to identify individual fatty acids. Before extraction, 1 mg internal standard was added to each 500 mg samples. The relative quantities of individual fatty acids were expressed as a percentage of total fatty acids in the erythrocytes based on the peak area.
Statistical analysis
Survey data and testing results were double-entered by two independent entry clerks into the EpiData 3.1. Data were checked for consistency. Statistical calculations were conducted by the SPSS software system (version 22.0). The present report was based on the pre-protocol analysis. If the data met the normal distribution and homogeneous variance, they were expressed as mean ± SD and two-way ANOVA was performed to test statistical significance among three groups, followed by Tukey’s post hoc test. If the data were not homogenous, it was expressed as median (25, 75% quartile) and Kruskal-Wallis test was performed to test statistical significance among three groups comparisons, followed by Dunn’s test. The two-factor repeated ANOVA was performed to determined the treatment-by-time interaction effects on all outcomes, with baseline as covariates. Categorical variables were expressed as counts. The paired sample t-test was performed for the comparison of before and after intervention in the same group. Chi-square test was performed for counting data. P-value ≤0.05 was set as the threshold for statistical significance. Non HDL = TC − HDL. .
Results
Baseline characteristics
As shown in Fig. 1, a total of 150 participants completed the trial, with 52 of them in the fish oil group, 50 in the PO group and 48 in the LFO group. Table 2 shows the anthropometric characteristics of the three groups of participants. The mean age was 62.45 ± 8.58 years old and the mean BMI was 26.58 ± 3.23 kg/m2 with 67.3% of the study participants’ exhibiting excess body weight. At the beginning of the study, age, gender composition, diabetes duration, treatment programme, smoking habits, alcohol consumption, body weight, BMI, waist circumference, hip circumference did not exhibit significant differences among the three groups. The mean BMI was 26.58 ± 3.23 kg/m2 with 67.3% of the study participants’ exhibiting excess body weight. The levels of markers of hepatic or renal function were not different among the FO, PO and LFO groups. The renal and liver functions were all normal. After 6 months of intervention, both SBP and DBP were significantly decreased (Table 3).
Table 2.
Main Characteristic of subjects completing trial at baseline
| Variables | Total sample | FO (n = 52) | PO (n = 50) | LFO (n = 48) | p-value |
|---|---|---|---|---|---|
| Age (years) | 62.45 ± 8.58 | 62.29 ± 7.55 | 63.84 ± 9.67 | 61.19 ± 8.38 | 0.308 a |
| Sex (male/female, n) | 57/93 | 17/35 | 20/30 | 20/28 | 0.612 b |
| Diabetes duration (years) | 7 (3, 11) | 8 (4, 11) | 7 (3, 13) | 8 (3, 10) | 0.890 c |
| Diabetes treatment | 0.599 b | ||||
| • Oral hypoglycemic agents (n) | 83 | 27 | 32 | 24 | |
| • Insulin injection (n) | 22 | 8 | 4 | 10 | |
| • Two items above (n) | 38 | 15 | 11 | 12 | |
| • Diet controlled (n) | 7 | 2 | 3 | 2 | |
| Smoking habits (male/female, n) | 0.295 b | ||||
| • Never | 25/91 | 8/34 | 7/30 | 10/27 | |
| • Previous | 3/2 | 2/1 | 0/0 | 1/1 | |
| • Current | 29/0 | 7/0 | 13/0 | 9/0 | |
| Alcohol consumption (n) | |||||
| • Never | 113 | 37 | 39 | 37 | 0.280 b |
| • Alcohol less than once per week | 4 | 1 | 3 | 0 | |
| • Alcohol once a week or more | 33 | 14 | 8 | 11 | |
| Weight (cm) | 67.90 ± 10.26 | 66.34 ± 9.59 | 69.27 ± 10.58 | 68.13 ± 10.61 | 0.353 a |
| Height (cm) | 159.72 ± 8.61 | 158.40 ± 8.40 | 160.14 ± 8.33 | 160.69 ± 9.11 | 0.384 a |
| Body mass index (kg/m2) | 26.58 ± 3.23 | 26.38 ± 2.68 | 26.99 ± 3.44 | 26.38 ± 3.56 | 0.560 a |
| Overweight n(%) | 101 (67.3%) | 17 (32.7%) | 14 (28%) | 18 (37.5%) | 0.605 b |
| Waist circumference (n) | 91.63 ± 8.05 | 90.18 ± 7.27 | 91.48 ± 8.50 | 93.26 ± 8.21 | 0.155 a |
| Hip circumference (n) | 100.42 ± 5.90 | 100.18 ± 5.34 | 101.54 ± 6.57 | 99.50 ± 5.67 | 0.218 a |
| Waist-to-hip ratio | 0.91 ± 0.05 | 0.90 ± 0.05 | 0.92 ± 0.06 | 0.92 ± 0.06 | 0.138 a |
| ALT (U/L) | 24.36 ± 11.46 | 24.66 ± 13.26 | 24.23 ± 10.73 | 24.17 ± 10.27 | 0.973 a |
| AST (U/L) | 22.22 ± 8.96 | 22.40 ± 9.87 | 22.13 ± 5.71 | 22.11 ± 10.72 | 0.984 a |
| Creatinine (umol/L) | 61.83 ± 13.08 | 62.51 ± 14.67 | 60.20 ± 12.19 | 62.81 ± 12.23 | 0.559 a |
| Uric acid (umol/L) | 329.64 ± 83.85 | 313.94 ± 85.88 | 342.27 ± 88.09 | 333.50 ± 75.75 | 0.218 a |
| Urea nitrogen (mmol/L) | 6.19 ± 1.54 | 6.13 ± 1.33 | 6.40 ± 1.73 | 6.05 ± 1.54 | 0.482 a |
| Total protein (g/L) | 77.35 ± 3.52 | 77.50 ± 3.35 | 77.56 ± 3.21 | 76.97 ± 4.02 | 0.663 a |
| Albumin (g/L) | 46.91 ± 2.27 | 46.80 ± 2.18 | 47.09 ± 2.37 | 46.83 ± 2.30 | 0.784 a |
| Globulin (g/L) | 30.45 ± 3.46 | 30.70 ± 3.48 | 30.47 ± 3.18 | 30.14 ± 3.75 | 0.782 a |
a Data are mean ± standard deviation, ANOVA test for comparing difference among groups;
b Data are presented as number (%), χ2 test for comparing difference among groups;
c Data are presented as median (P25, P75), Kruskal-Wallis tests for comparing difference among groups
FO Fish oil, PO Perilla oil, LFO Mixed linseed oil and fish oil; ALT glutamic-pyruvic transaminase; AST glutamic-oxalacetic transaminease
Table 3.
Blood pressures at baseline and after 6-months of each treatment
| Blood Pressures | FO | PO | LFO | T Effect | t Effect | T × t Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 month | Baseline | 6 month | Baseline | 6 month | p | p | p | |
| SBP (mm Hg) | 144.78 ± 18.86 | 139.23 ± 18.68 | 148.36 ± 18.19 | 139.04 ± 16.34 | 143.02 ± 18.53 | 139.13 ± 17.10 | 0.998 | < 0.001 | < 0.001 |
| DBP (mm Hg) | 85.76 ± 9.11 | 80.77 ± 8.18 | 87.36 ± 10.41 | 82.36 ± 8.22 | 88.48 ± 8.33 | 83.42 ± 7.73 | 0.256 | < 0.001 | < 0.001 |
T: treatment effect. ANOVA test for comparing difference among groups;
t, time effect. Paired t test for comparing differences before and after intervention;
T × t: treatment×time interaction effect. Two-way repeated ANOVA test for assess the interaction effects adjusted the baseline values of each biochemical variables as covariate
Values are expressed as mean ± SD
FO Fish oil, PO Perilla oil, LFO Mixed linseed oil and fish oil, SBP Systolic blood pressure, DBP Diastolic blood pressure
Blood biochemical indices
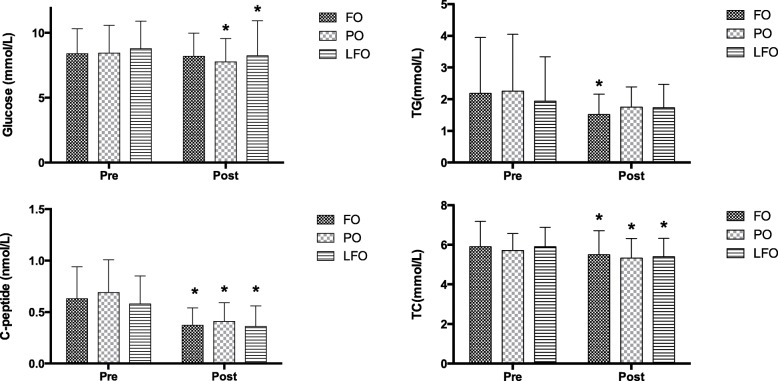
The serum glucose and lipid profiles are presented in Table 4 and Fig. 2. There were no statistical differences in glucose indices and lipid profiles among groups before treatments. Two-way ANOVA showed statistical effects of time × treatment interaction on FBG, insulin, HOMA-IR and C-peptide. Administration of PO and LFO significantly decreased the FBG and glycated hemoglobin (HbA1c) levels. Administration of all n-3 PUFA significantly reduced insulin and C-peptide concentrations in the three groups after treatments. The significant time × treatment interaction effects for lipid profiles, FFA and IL-6 were observed (P < 0.001). Administration of FO significantly decreased serum TG levels and TG/HDL ratio. Serum TC, Apo A1 and IL-6 levels in the all of the treatment groups decreased after the interventions.
Table 4.
Glucose and lipid index at baseline and after 6-month of each treatment
| Blood biochemical index | FO (n = 52) | PO (n = 50) | LFO (n = 48) | T Effect | t Effect |
T × t Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 month | Baseline | 6 month | Baseline | 6 month | p | p | p | |
| Glucose (mmol/L) | 8.38 ± 1.94 | 8.19 ± 1.80 | 8.43 ± 2.15 | 7.76 ± 1.79 * | 8.77 ± 2.13 | 8.24 ± 2.69 * | 0.464 | 0.005 | < 0.001 |
| Insulin (mU/L) | 7.84 (4.64,13.66) | 3.97 (2.44,6.93) * | 9.42 (5.63,14.06) | 4.77 (2.96,8.82) * | 9.45 (5.60,15.21) | 4.28 (2.52,14.84) * | 0.656 | < 0.001 | < 0.001 |
| HOMA-IR a | 2.93 (1.76,5.36) | 1.45 (0.78,2.53) * | 3.39 (1.94, 4.94) | 1.60 (0.87,3.42) * | 3.35 (2.22, 5.66) | 1.41 (0.87,4.87) | 0.454 | < 0.001 | < 0.001 |
| C-peptide (nmol/L) | 0.63 ± 0.31 | 0.37 ± 0.17 * | 0.69 ± 0.32 | 0.41 ± 0.18 * | 0.58 ± 0.27 | 0.36 ± 0.20 * | 0.311 | < 0.001 | < 0.001 |
| HbA1c | 6.79 ± 1.05 | 6.54 ± 1.19 | 6.94 ± 1.09 | 6.62 ± 1.29 * | 6.74 ± 1.09 | 6.27 ± 1.17 * | 0.324 | < 0.001 | < 0.001 |
| TG (mmol/L) | 2.18 ± 1.77 | 1.52 ± 0.64 * | 2.25 ± 1.80 | 1.75 ± 0.64 | 1.94 ± 1.40 | 1.73 ± 0.74 | 0.227 | 0.324 | < 0.001 |
| TC (mmol/L) | 5.91 ± 1.28 | 5.50 ± 1.21 * | 5.72 ± 0.85 | 5.33 ± 0.99 * | 5.91 ± 0.98 | 5.40 ± 0.92 * | 0.697 | < 0.001 | < 0.001 |
| HDL-C (mmol/L) | 1.28 ± 0.32 | 1.36 ± 0.29 | 1.25 ± 0.24 | 1.24 ± 0.23 | 1.30 ± 0.30 | 1.28 ± 0.30 | 0.083 | 0.980 | < 0.001 |
| LDL-C (mmol/L) | 2.83 ± 0.99 | 2.90 ± 0.76 | 2.74 ± 0.60 | 2.57 ± 0.57 * | 2.99 ± 0.71 | 2.91 ± 0.73 | 0.022 | 0.661 | < 0.001 |
| non HDL-C (mmol/L) | 4.63 ± 1.20 | 4.18 ± 1.18 * | 4.46 ± 0.80 | 4.11 ± 1.00 * | 4.61 ± 0.84 | 4.12 ± 0.79 * | 0.926 | < 0.001 | < 0.001 |
| LPa (mg/L) a | 121.23 (54.63, 406.75) | 138.95 (61.66,334.77) | 197.58 (62.57,410.12) | 132.45 (44.77,308.22) * | 175.61 (62.94,326.93) | 134.90 (61.52,277.00) * | 0.997 | < 0.001 | < 0.001 |
| Apo A1 (g/L) | 1.42 ± 0.24 | 1.34 ± 0.20 * | 1.40 ± 0.18 | 1.33 ± 0.17 * | 1.44 ± 0.21 | 1.34 ± 0.19 * | 0.874 | < 0.001 | < 0.001 |
| Apo B (g/L) | 1.05 ± 0.27 | 1.07 ± 0.30 | 1.05 ± 0.20 | 0.95 ± 0.24 * | 1.11 ± 0.21 | 1.06 ± 0.20 * | 0.029 | 0.037 | < 0.001 |
| LDL/HDL ratio | 2.24 ± 0.80 | 2.23 ± 0.60 | 2.23 ± 0.56 | 2.17 ± 0.52 | 2.34 ± 0.57 | 2.33 ± 0.59 | 0.385 | 0.982 | < 0.001 |
| TG/HDL ratio a | 1.33 (0.88,2.27) | 1.04 (0.71,1.42) * | 1.41 (0.89,2.27) | 1.26 (0.95,1.90) | 1.34 (0.72,2.20) | 1.26 (0.77,1.99) | 0.733 | 0.686 | < 0.001 |
| Apo A1/Apo B a | 1.40 (1.22,1.56) | 1.28 (1.12,1.54) | 1.35 (1.14,1.54) | 1.36 (1.18,1.60) | 1.27 (1.12,1.44) | 1.31 (1.10,1.44) | 0.313 | 0.488 | 0.475 |
| FFA (mmol/L) | 0.50 ± 0.24 | 0.45 ± 0.19 | 0.52 ± 0.23 | 0.54 ± 0.23 | 0.50 ± 0.25 | 0.43 ± 0.16 | 0.024 | 0.167 | < 0.001 |
| IL6 (mg/L) | 10.20 ± 2.90 | 2.95 ± 1.44 * | 10.05 ± 2.95 | 3.68 ± 2.47 * | 9.76 ± 4.45 | 3.18 ± 2.15 * | 0.188 | < 0.001 | 0.003 |
a Data presented as median (P25, P75), Kruskal-Wallis tests for comparing difference among three groups
T: treatment effect. ANOVA test for comparing difference among groups;
t, time effect. Paired t test for comparing differences before and after intervention;
T × t: treatment×time interaction effect. Two-way repeated ANOVA test for assess the interaction effects adjusted the baseline values of each biochemical variables as covariate
* Significantly different as compared to baseline (p < 0.05). Values are expressed as mean ± SD
FO Fish oil, PO Perilla oil, LFO mixed linseed oil and fish oil, HbA1c Glycated hemoglobin, HOMA-IR Homeostasis model assessment of insulin resistance, TG Triglyceride, TC Total cholesterol, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, LPa Lipoprotein a, Apo A1 Apolipoprotein A1, Apo B Apolipoprotein B, FFA Free fatty acids, IL-6 Interleukin
Fig. 2.
Main glucose and lipid index at baseline and after 6-month of each treatment. * Significantly different as compared to baseline (p < 0.05). Values are expressed as mean ± SD. FO fish oil; PO perilla oil; LFO mixed linseed oil and fish oil; TG Triglyceride; TC total cholesterol
Fatty acids composition of erythrocyte
The fatty acids composition of erythrocyte was shown in Table 5 and Fig. 3. There were significant differences in C20:0, C22:5 n-6, ALA, EPA, C22:5 n-3 (docosapentaenoic acid, DPA), DHA, total n-6 PUFAs, total n-3 PUFAs, n-6 PUFAs/n-3 PUFAs ratio, arachidonic acid (AA)/EPA ratio and Omega-3 index among the three groups after adjusting the age, sex and free fatty acids levels. C22: 5 n-6, ALA, DPA, n-6/n-3 PUFAs, AA/EPA in the PO group were significantly higher, while EPA, total n-3 PUFAs and Omega-3 index were significantly lower in the PO group compared with FO and LFO groups. In addition, DHA was significantly higher in the FO than that in the PO group. In contrast, total n-6 PUFAs in FO was significantly lower than that in the PO. After intervention, similar trends were observed for total n-3 PUFAs and Omega-3 index in the three treatment groups; the FO was highest, followed by LFO and PO, while the n-6 PUFAs/n-3 PUFAs ratio had the opposite trend.
Table 5.
Fatty acid compositions of erythrocyte after 6-months of each treatment
| Fatty Acid (%) | PO | LFO | FO | p-value | Adjusted p-value a |
|---|---|---|---|---|---|
| SFAs | |||||
| C14:0 (myristic acid) b | 0.21 (0.16, 0.28) | 0.20 (0.15, 0.25) | 0.19 (0.13, 0.27) | 0.341 | 0.253 |
| C15:0 (pentadecanoic acid) | 0.29 ± 0.06 | 0.31 ± 0.06 | 0.30 ± 0.08 | 0.347 | 0.349 |
| C16:0 (palmitic acid) | 13.86 ± 1.57 | 14.22 ± 0.97 | 13.93 ± 1.17 | 0.322 | 0.149 |
| C17:0 (margaric acid) | 0.81 ± 0.18 | 0.87 ± 0.17 | 0.91 ± 0.17 * | 0.019 | 0.091 |
| C18:0 (stearic acid) b | 16.74 (15.72, 17.91) | 17.09 (16.43, 17.71) | 16.76 (15.62, 17.86) | 0.387 | 0.575 |
| C20:0 (arachidic acid) | 0.24 ± 0.05 | 0.26 ± 0.07 | 0.27 ± 0.08 * | 0.030 | 0.034 |
| C21:0 b | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.250 | 0.293 |
| C22:0 (behenic acid) | 0.50 ± 0.18 | 0.55 ± 0.17 | 0.50 ± 0.16 | 0.238 | 0.303 |
| C23:0 (tricosanoic acid) b | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.075 | 0.413 |
| C24:0 (lignoceric acid) | 1.22 ± 0.49 | 1.36 ± 0.49 | 1.20 ± 0.46 | 0.164 | 0.222 |
| MUFAs | |||||
| C14:1 | 0.27 ± 0.38 | 0.20 ± 0.12 | 0.23 ± 0.12 | 0.321 | 0.147 |
| C15:1 b | 3.23 (2.68, 3.65) | 3.30 (2.87, 3.62) | 3.09 (2.53, 3.52) | 0.371 | 0.109 |
| C16:1 b | 0.60 (0.41, 0.84) | 0.50 (0.35, 0.79) | 0.61 (0.41, 0.92) | 0.321 | 0.506 |
| C17:1 | 4.12 ± 1.37 | 4.25 ± 1.41 | 4.03 ± 1.38 | 0.721 | 0.578 |
| C18:1 trans-9 | 0.27 ± 0.07 | 0.26 ± 0.04 | 0.25 ± 0.07 | 0.231 | 0.289 |
| C18:1 cis-9 (oleic acid) b | 12.46 (11.38, 13.55) | 12.04 (11.35, 17.70) | 11.96 (11.33, 12. 90) | 0.308 | 0.505 |
| C20:1 n-9 (eicosenoic acid) | 0.55 ± 0.26 | 0.55 ± 0.18 | 0.58 ± 0.33 | 0.750 | 0.713 |
| C22:1 b | 0.15 (0.00, 0.35) | 0.23 (0.00, 0.46) | 0.18 (0.00, 0.44) | 0.314 | 0.190 |
| C24:1 n-9 (nervonic acid) | 2.52 ± 1.16 | 3.20 ± 1.36 * | 2.97 ± 1.54 | 0.043 | 0.080 |
| n-6 PUFAs | |||||
| C18:2 trans-6 | 1.69 ± 0.64 | 1.59 ± 0.74 | 1.69 ± 0.66 | 0.696 | 0.609 |
| C18:2 cis-6 (linoleic acid) | 18.58 ± 2.66 | 17.81 ± 2.23 | 17.49 ± 2.69 | 0.084 | 0.167 |
| C18:3 n-6 (γ-linolenic acid) | 0.14 ± 0.07 | 0.14 ± 0.07 | 0.16 ± 0.07 | 0.544 | 0.408 |
| C20:3 n-6 | 1.82 ± 0.76 | 1.65 ± 0.26 | 1.72 ± 0.34 | 0.203 | 0.337 |
| C20:4 n-6 (Arachidonic acid) | 12.03 ± 2.31 | 12.46 ± 1.71 | 11.73 ± 1.59 | 0.147 | 0.173 |
| C22:2 n-6 b | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | – | 0.540 |
| C22:5 n-6 b | 0.71 (0.58, 0.85) | 0.63 (0.51, 0.78) * | 0.53 (0.46, 0.64) * | < 0.001 | < 0.001 |
| n-3 PUFAs | |||||
| C18:3 n-3 (α-linolenic acid) | 0.58 ± 0.23 | 0.46 ± 0.18 * | 0.46 ± 0.18 * | 0.012 | 0.037 |
| C20:3 n-3 b | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.618 | 0.681 |
| C20:5 n-3 (eicosapentaenoic acid) b | 0.52 (0.45, 0.67) | 1.13 (0.93, 1.34) * | 1.23 (0.99, 1.62) * | < 0.001 | < 0.001 |
| C22:5 n-3 (docosapentaenoic acid) b | 0.71 (0.58, 0.85) | 0.63 (0.51, 0.78) * | 0.53 (0.46, 0.64) * | < 0.001 | < 0.001 |
| C22:6 n-3 (docosahexaenoic acid) | 2.66 (2.10, 3.14) | 3.80 (3.37, 4.10) | 4.58 (3.93, 5.27) * | < 0.001 | < 0.001 |
| Others C20:2 (eicosadienoic acid) | 0.60 ± 0.10 | 0.59 ± 0.07 | 0.60 ± 0.09 | 0.724 | 0.034 |
| Fatty acids composition of erythrocytes | |||||
| Total SFAs | 33.86 ± 3.00 | 34.90 ± 2.09 | 34.23 ± 2.75 | 0.138 | 0.129 |
| Total MUFAs | 26.27 ± 5.54 | 24.49 ± 2.30 | 25.10 ± 4.44 | 0.122 | 0.185 |
| Total PUFAs | 37.92 ± 3.36 | 38.76 ± 2.31 | 38.74 ± 3.49 | 0.298 | 0.460 |
| Total n-6 PUFAs b | 33.35 (31.67, 34.69) | 32.20 (30.74, 33.94) | 30.95 (29.23, 34.04) * | 0.019 | 0.050 |
| Total n-3 PUFAs b | 4.56 (3.97, 5.03) | 5.99 (5.57, 6.50) *# | 6.71 (6.12, 7.82) * | < 0.001 | < 0.001 |
| n-6/n-3 PUFA b | 7.17 (6.69, 8.42) | 5.31 (4.88, 5.88) *# | 4.59 (3.88, 5.20) * | < 0.001 | < 0.001 |
| AA/EPA | 22.68 ± 7.93 | 13.18 ± 7.86 * | 10.94 ± 6.13 * | < 0.001 | < 0.001 |
| MUFAs/SFAs ratio b | 0.73 (0.68, 0.78) | 0.69 (0.65, 0.76) | 0.72 (0.63, 0.81) | 0.253 | 0.124 |
| PUFAs/SFAs ratio | 1.12 ± 0.10 | 1.12 ± 0.11 | 1.14 ± 0.13 | 0.636 | 0.542 |
| Omega-3 index(%) b | 3.24 (2.66, 3.70) | 4.86 (4.50, 5.38) *# | 5.70 (5.06, 6.91) * | < 0.001 | < 0.001 |
Data are mean ± standard deviation, ANOVA test for comparing difference among groups, followed by Tukey’s pos hoc test
a ANOVA test for comparing difference among groups after adjusting the age, sex and free fatty acids levels
b Data presented as median (P25, P75), Kruskal-Wallis tests for comparing difference among groups, followed by Dunn’s test
* significantly different as compared to PO (p < 0.05)
# significantly different as compared to FO (p < 0.05)
FO Fish oil, PO Perilla oil, LFO Mixed linseed oil and fish oil, SFA Saturated fatty acid, MUFA Molyunsaturated fatty acid, PUFA Polyunsaturated fatty acid, AA Arachidonic acid, EPA Eicosapentaenoic acid
Fig. 3.
Main n-3 PUFA of erythrocyte after 6-months of each treatment. * significantly different as compared to PO (p < 0.05). FO fish oil; PO perilla oil; LFO mixed linseed oil and fish oil; ALA α-linolenic acid; EPA eicosapentaenoic acid; DPA docosapentaenoic acid; DHA docosahexaenoic acid
Discussion
For diabetic patients, it remains unknown whether different sources of n-3 PUFA have the same effects on glucose and lipid metabolism. The present trial compared the different effects of marine-based n-3 PUFA and plant-based n-3 PUFA on the glucose and lipid profiles. It was found that administration of marine-based n-3 PUFA significantly increased erythrocyte EPA, DHA, total n-3 PUFA levels and omega-3 index compared to plant-derived n-3 PUFA intake. However, administration of plant-derived n-3 PUFA significantly increased C22:5n-6, ALA and DPA levels of erythrocyte significantly. Moreover, both marine and plant-derived n-3 PUFA administration reduced insulin, C-peptide and serum TC levels in T2DM patients. There was a decrease in serum TG after administration of marine-based n-3 PUFAs. There was the significant decrease in FBG after the administration of plant-derived n-3 PUFA.
Most studies have indicated that n-3 PUFA especially marine-based n-3 PUFA intake results in favorable lipid profiles. Many meta-analyses showed that EPA and DHA consumption can significantly reduce the levels of TG, LDL in T2DM patients [5, 19]. And n-3 PUFA intake for both ≤8 and > 8 weeks could significantly increase the HDL level [20]. It is well established that T2DM is associated with dyslipidemia and that EPA and DHA supplementation has long been indicated in the treatment of hyperlipidemia in T2DM and pre-diabetes patients. The beneficial actions of n-3 PUFA on inflammation have been reported in patients with high inflammatory states [21, 22]. Fish oil and ALA have also been shown to exhibit anti-inflammatory effects [23–25]. Although the meta-analysis indicated that the reduction in the c-reactive protein were not statistically significant [20, 26]. The reduction of IL-6 after EPA and DHA supplementation was significant in T2DM patients [27]. One recent cross-sectional study showed that DHA levels in erythrocytes was negatively related to the IL-6 and EPA levels in erythrocytes was negatively associated with TNF-α [28]. The aetiology of T2DM was associated with metabolic abnormality and inflammation. Therefore, the benefits of marine and plant n-3 PUFA may be not consistent in T2DM patients. EPA and DHA have the positive effects on lipid profile markers and may be more likely to improve dyslipidemia, which is one of the main risk factors and complications of T2DM. On the other hand, n-3 PUFA inhibited the pro-inflammatory cytokines and promoted resolution of inflammation, which will improve insulin resistance.
Supplementation with n-3 PUFA has protective effects on diabetes factors, while the effects of different sources n-3 PUFA on glycose were controversial. The present results showed that the influences of EPA and DHA vs ALA on FBG are different. Administration with EPA and DHA did not affect fasting glucose. These results are in tandem with two recent meta-analyses which showed no significant relationship between EPA and DHA supplementation and blood glucose level [5, 27]. Even EPA and DHA are associated statistically significantly with increased levels of FBG among T2DM patients in Natto et al’s meta-analysis. This analysis excluded studies that were administered less than 1000 mg/day of n-3 PUFA [26]. However, Delpino et al’s meta-analysis presented the opposite results, which was probably due to the inclusion of gestational diabetes studies [29]. Besides FBG, O′Mahoney’s study revealed that a small reduction in HbA1c following EPA and DHA supplementation. But sensitivity analysis showed that removal of two trials changed the statistical results from significant to non-significant [27]. And two meta-analyses also showed that the level change of HbA1c was not statistically significant [26, 29]. But in Khalili’s study, n-3 PUFA consumption including DHA, EPA and ALA can significantly reduce HbA1c (− 0.74 (− 1.13 to − 0.35)). The possible reason for these inconsistent results is that the n-3 PUFA sources were different in the inclusion trials. Khalili’s meta-analysis included the trials using ALA as the intervention [20]. Another reason is the intervention time. HbA1c reflects the integrated FBG level for the past 3 months, so HbA1c is less affected by short-term n-3 PUFA interventions. The results of this trial showed that FBG and HbA1c decreased significantly after PO intervention compared with FO intervention, which suggested the potential ability of ALA to improve glycemic factors. In accordance with the present results, a 12-week treatment of rapeseed oil (with ALA 9.1%) resulted in a greater decrease in serum HbA1c in the intervention group compared to control group [30]. Another study showed that flaxseed intake could reduce blood glucose and insulin levels and increase insulin sensitivity in overweight and obese people with pre-diabetes [31]. Besides, Khalili et al’s meta-analysis found that there was the significant decrease of glucose after n-3 PUFA intake and the suggested dose and duration for T2DM patients was 1–2 g/d for more than 8 weeks [20]. This meta-analysis included studies administrating not only marine-based n-3 PUFA but also plant-based n-3 PUFA. Perilla oil is a common plant source of n-3 PUFA with rich ALA. ALA promotes glycolipid metabolism [32, 33]. Erythrocytic ALA levels were negatively correlated with T2DM risk in participants with low genetic risk [34]. The large-scale findings also suggested the inverse association of ALA but no convincing association of EPA and DHA with glucose in T2DM [35].
ALA can be converted into EPA, DPA and DHA in specific tissues through the elongation and desaturation enzymatic machinery [36, 37]. However, the conversion efficacy is limited [38, 39]. ALA is the essential precursor of n-3 PUFA that is mainly oxidized to acetyl coenzyme A in the mitochondria, with only a tiny part being transformed into DHA [40]. Through the endoplasmic reticulum and peroxisome processing, ALA does not lead to the accumulation of total omega-3 index [38]. The exact mechanisms underlying the regulation of FBG by ALA remains elusive, but there is evidence suggesting that the receptors involved probably differ from ALA, EPA and DHA. The in vitro study indicated that ALA may induce insulin secretion from the β-cells via direct actions on the pancreas as well as via a GLP-1-mediated indirect mechanism.
The erythrocytic fatty acids compositions were detected. As biomarkers for disease risk and reliable biological indicators of dietary intake, erythrocytic fatty acids are reliable indicators for increased n-3 PUFA intake [41, 42]. After n-3 PUFAs intake, erythrocytic n-3 PUFAs levels were shown to be significantly elevated after the washout period [43]. The omega-3 index is an objective marker for dietary n-3 PUFA intake. Erythrocytic PUFA levels and omega-3 indices reflect the relative n-3 PUFA composition in the main organs [44]. Due to the 90-day turnover rate for erythrocyte, erythrocyte fatty acids composition is likely to be more representative of long-term dietary intake, compared with the fatty acids composition of plasma and serum, which response quickly after n-3 PUFA intake [43]. Previous studies found that fish oil consumption sufficiently increased erythrocytic DHA, DPA, EPA and total n-3 PUFAs levels [45, 46]. Flaxseed oil supplementation (2.4 or 3.6 g/d) has also been shown to significantly increase ALA, EPA and DPA levels [33]. Because of the comparatively low ALA dose (2.0 g/d) in this study, erythrocytic EPA contents in the PO group were lower compared to FO and LFO groups. It has been shown that erythrocytic EPA and omega-3 indices remained unaltered after administering ALA (4 g/d) for 6 weeks [47]. ALA is not a sufficient source for increasing total EPA and DHA, especially among people with high-fat diet habits [48]. However, consumption of marine products exhibited a positive correlation between erythrocytic EPA and DHA levels. Food or supplements that are rich in EPA and DHA can explain half change of n-3 PUFA in the body [49].
Erythrocytic membrane fatty acid patterns could predict the development of T2DM [50, 51]. Levels of specific fatty acids that denaturize enzyme activities in the erythrocytes have been associated with T2DM onset. These fatty acids are considered to be significant biomarkers for poor T2DM outcomes [9, 52, 53]. Long-chain n-3PUFA levels are low in diabetic patients [54]. A significant relationship between EPA or DHA with T2DM risks was not established in eight European countries. However, an inverse association was obtained between ALA and T2DM incidents [35]. Among Asian countries, a Chinese study documented an inverse association between EPA/DPA contents and risks for T2DM. The study did not find any associations between DHA and risks for T2DM [11]. The detailed mechanisms, causal pathways for EPA, DHA and T2DM risks warrant further studies. A possible mechanism is that a high EPA status leads to improving glucagon like peptide 1 release that promotes insulin secretion by the β-cells [55]. In this study, erythrocytic DPA concentrations after PO administration were significantly elevated compared to the other two groups. This result is in tandem with a previous finding [56]. By the fatty acid elongase 2 enzyme, the increased amounts of EPA resulted in DPA changes, which may act as long chain n-3 PUFA reservoirs in humans, incorporated into lipids components [43, 57, 58].
The association between n-6 PUFA and T2DM is heavily influenced by the race, ethnicity, gender or basal levels of n-6 PUFA in the body. There has been a cross-sectional study conducted in China which indicated that higher n-6 PUFAs status was associated with lower risk of T2DM in male [59]. In one Multi-Ethnic Study, biomarker levels of LA were inversely and AA were positively associated with incident T2DM patients. Analyzing results including twenty prospective cohort studies from 10 regions indicated that high LA concentrations related to a 43% lower risk of T2DM, whereas AA levels were not associated with T2DM [60]. In the EPIC-InterAct study, AA also is not associated with T2DM, while intermediate metabolites 18:3 n6, 20:3 n6 are positively associated [35]. Particularly, a combination of fatty acids, especially characterized by high levels of LA, may be potentially more important in T2DM prevention than individual fatty acid or fatty acid subclasses [61]. Besides long-chain PUFA, some SFAs have been shown to exhibit positive associations with T2DM incidents. Higher proportions of C15:0 have been correlated with low risks for T2DM [50]. Other studies have documented that higher C16:0, C17:0 [13], C18:0 and C20:0 are associated with increased risks for T2DM while there were no association between C14:0 and C24:0 and risks for T2DM [62]. Two prospective studies found inverse associations between plasma long chain SFA with T2DM [63, 64]. A previous study did not find any relationship between erythrocytic long chain SFA with T2DM [52]. Therefore, more evidence is needed for evaluating the associations between SFAs status and T2DM. In this trial, FO did not reduce the fast glucose levels while C17:0 and C20:0 were significantly elevated in FO group versus PO and LFO groups.
Strengths and limitations
This study provided the first direct comparison of EPA, DHA and ALA effects on erythrocytic membrane fatty acid and glucose metabolism in T2DM patients. Besides, the sample size of this trial provided sufficient power. Several sources of potential bias were present. First, rates of loss to follow-up were 16.7% for 3 months, and pre-protocol analyses were carried out in all of outcomes, which may cause overestimation. Second, trials implement blinding of patients and outcomes assessor, the assessors who gathered the information and analysts were not fully blinded. The limitations include; first, most participants were overweight and with higher blood pressure. Therefore, the finding of present study may not apply to diabetic patients with normal weight and blood pressures. Second, due to the end-point measurement point, fatty acid composition changes during the intervention could not be determined. Third, common edible oils such as corn oil were not used as the placebo control in the present trials. The beneficial effects of FO on glucose and lipid profiles have been proved in our previous studies. The focus of this study lies in the comparison of the influences of perilla oil with that of fish oil. Fourth, most of participants took oral hypoglycemic agents or injected insulin, which probably was a potential source of bias, although the diabetes treatments had no significant difference among three groups and were not likely to affect the overall results. Finally, because of the small numbers of participants in all groups, a subgroup analysis would have produced unreliable results.
Conclusion
Perilla oil supplementation decreased glucose levels while fish oil supplementation reduced serum TG levels. Erythrocytic ALA, DPA and total n-6 PUFAs were elevated after perilla oil administration. Erythrocytic EPA, DHA, total n-3 PUFAs and omega-3 index were highly elevated in the FO group, followed by the LFO group. Marine-based and plant-based n-3 PUFAs exhibit different effect on erythrocytic fatty acid composition and regulate glycolipid metabolism. Careful attention should be paid to evaluated T2DM patient’s complications, FBG, TG levels and eating habits when translated into clinical applications of fish oil and perilla oil.
Acknowledgements
We are indebted to all the people who kindly participated in this study.
Abbreviations
- ALA
Alpha-linolenic acid
- Apo A1
Apolipoprotein A1
- Apo B
Apolipoprotein B
- AS
Atherosclerosis
- BMI
Body mass index
- CVD
Cardiovascular diseases
- DBP
Diastolic blood pressure
- DHA
Docosahexenoic acid
- DPA
Docosapentaenoic acid
- EPA
Eicosapentaenoic acid
- RBCm
Erythrocyte membrane
- FO
Fish oil
- FFA
Free fatty acids
- GC
Gas chromatography
- ALT
Glutamic pyruvic transaminase
- AST
Glutamic oxalacetic transaminase
- HbAlc
Glycated haemoglobin
- IL-6
Interleukin 6
- LFO
Linseed and fish oil
- LPa
Lipoprotein a
- MUFA
Monounsaturated fatty acids
- n-3 PUFA
-
Omega-3 polyunsaturated
fatty acid
- PO
Perilla oil
- SFA
Saturated fatty acids
- SBP
Systolic blood pressure
- TG
Triglyceride
- TC
Total cholesterol
- T2DM
Type 2 diabetes
- TG
Triglycerides
Authors’ contributions
HL and FW designed research; HL, FW, XL, YX conducted research; HX analyzed data; SW provided essential reagents or provided essential materials; HL wrote the paper; GS had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was supported by the two National Natural Science Foundations of China (No 81872618 and No 81573144).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Ethical Committee of Zhongda hospital affiliated to Southeast University, Nanjing, China (NO. 2015ZDSYLL089.0). The purpose and protocol of the trial were exhaustively explained to the study participants. All participants were required to sign the informed consent before enrollment. The trial was registered under http://www.chictr.org.cn (identifier no. ChiCTR-IOR-16008435).
Consent for publication
All participants signed the consent form and agreed to use data for publish.
Competing interests
There are no conflicts to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . IDF Diabetes Atlas. 10. Brussels: International Diabetes Federation; 2021. [PubMed] [Google Scholar]
- 3.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 4.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 5.Gao C, Liu Y, Gan Y, Bao W, Peng X, Xing Q, Gao H, Lai J, Liu L, Wang Z, Yang Y. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a Meta-analysis of randomized controlled trials. Lipids Health Dis. 2020;19(1):87. doi: 10.1186/s12944-020-01214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark LF, Thivierge MC, Kidd CA, McGeoch SC, Abraham P, Pearson DWM, Horgan GW, Holtrop G, Thies F, Lobley GE. Fish oil supplemented for 9 months does not improve glycaemic control or insulin sensitivity in subjects with impaired glucose regulation: a parallel randomised controlled trial. Br J Nutr. 2015;115(1):75–86. doi: 10.1017/S0007114515004274. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo G, Baroni L. Health and ecological implications of fish consumption: a deeper insight. Mediterr J Nutr Metab. 2016;9(1):7–22. doi: 10.3233/MNM-160054. [DOI] [Google Scholar]
- 8.Abbott SK, Else PL, Atkins TA, Hulbert AJ. Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim Biophys Acta Biomembr. 2012;1818(5):1309–1317. doi: 10.1016/j.bbamem.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Lankinen MA, Stančáková A, Uusitupa M, Ågren J, Pihlajamäki J, Kuusisto J, Schwab U, Laakso M. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58(11):2533–2544. doi: 10.1007/s00125-015-3730-5. [DOI] [PubMed] [Google Scholar]
- 10.Harris K, Oshima M, Sattar N, Würtz P, Jun M, Welsh P, Hamet P, Harrap S, Poulter N, Chalmers J, Woodward M. Plasma fatty acids and the risk of vascular disease and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE study. Diabetologia. 2020;63(8):1637–1647. doi: 10.1007/s00125-020-05162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng J-S, Lin J-S, Dong H-L, Zeng F-F, Li D, Song Y, Chen Y-M. Association of erythrocyte n-3 polyunsaturated fatty acids with incident type 2 diabetes in a Chinese population. Clin Nutr. 2019;38(5):2195–2201. doi: 10.1016/j.clnu.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Zong G, Li H, Lin X. Fatty acids and cardiometabolic health: a review of studies in Chinese populations. Eur J Clin Nutr. 2020;75(2):253–266. doi: 10.1038/s41430-020-00709-0. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi W, Santaren ID, Hanley AJ, Watkins SM, Lorenzo C, Wagenknecht LE. Risk of diabetes associated with fatty acids in the de novo lipogenesis pathway is independent of insulin sensitivity and response: the insulin resistance atherosclerosis study (IRAS) BMJ Open Diabetes Res Care. 2019;7(1):e000691. doi: 10.1136/bmjdrc-2019-000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu XF, Sandhu SK, Harris WS, Chan HM. Conversion ratios ofn-3 fatty acids between plasma and erythrocytes: a systematic review and meta-regression. Br J Nutr. 2017;117(8):1162–1173. doi: 10.1017/S0007114517001052. [DOI] [PubMed] [Google Scholar]
- 15.Manninen S, Lankinen M, de Mello V, Ågren J, Laaksonen D, Schwab U, Erkkilä A. The effect of camelina sativa oil and fish intakes on fatty acid compositions of blood lipid fractions. Nutr Metab Cardiovasc Dis. 2019;29(1):51–61. doi: 10.1016/j.numecd.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88(3):801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Wang Y, Zhu Y, Liu X, Xia H, Yang X, Sun G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2017;56(7):2415–2422. doi: 10.1007/s00394-016-1352-4. [DOI] [PubMed] [Google Scholar]
- 18.Zheng JS, Lin M, Fang L, Yu Y, Yuan L, Jin Y, Feng J, Wang L, Yang H, Chen W, Li D, Tang J, Cai W, Shi M, Li Z, Wang F, Li D. Effects of n-3 fatty acid supplements on glycemic traits in Chinese type 2 diabetic patients: a double-blind randomized controlled trial. Mol Nutr Food Res. 2016;60(10):2176–2184. doi: 10.1002/mnfr.201600230. [DOI] [PubMed] [Google Scholar]
- 19.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, dela Cruz L, Frazier-wood AC, et al. ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease. JAMA Intern Med. 2016;176(8):1155–1166. doi: 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalili L, Valdes-Ramos R, Harbige LS. Effect of n-3 (Omega-3) polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers and body weight in patients with type 2 diabetes mellitus: a systematic review and Meta-analysis of RCTs. Metabolites. 2021;11(11). 10.3390/metabo11110742. [DOI] [PMC free article] [PubMed]
- 21.Johnson KM, Weinhold KR, Andridge R, Arnold K, Chu PP, Orchard TS. Associations of erythrocyte polyunsaturated fatty acids with inflammation and quality of life in post-menopausal women with obesity completing a pilot dietary intervention. Nutrients. 2019;11(7):1589. doi: 10.3390/nu11071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enzenbach C, Kröger J, Zietemann V, Jansen EHJM, Fritsche A, Döring F, Boeing H, Schulze MB. Erythrocyte membrane phospholipid polyunsaturated fatty acids are related to plasma C-reactive protein and adiponectin in middle-aged German women and men. Eur J Nutr. 2011;50(8):625–636. doi: 10.1007/s00394-011-0169-4. [DOI] [PubMed] [Google Scholar]
- 23.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Hashemzadeh AA, Nasoohi N, Raygan F, Aghadavod E, Akbari E, Taghizadeh M, Memarzadeh MR, Asemi Z. Flaxseed oil supplementation improve gene expression levels of PPAR-γ, LP(a), IL-1 and TNF-α in type 2 diabetic patients with coronary heart disease. Lipids. 2017;52(11):907–915. doi: 10.1007/s11745-017-4295-5. [DOI] [PubMed] [Google Scholar]
- 25.Raygan F, Taghizadeh M, Mirhosseini N, Akbari E, Bahmani F, Memarzadeh MR, Sharifi N, Jafarnejad S, Banikazemi Z, Asemi Z. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: a randomized, double-blinded, placebo-controlled trial. Phytother Res. 2019;33(7):1943–1951. doi: 10.1002/ptr.6393. [DOI] [PubMed] [Google Scholar]
- 26.Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and Meta-analysis. Sci Rep. 2019;9(1):18867. doi: 10.1038/s41598-019-54535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Mahoney LL, Matu J, Price OJ, Birch KM, Ajjan RA, Farrar D, Tapp R, West DJ, Deighton K, Campbell MD. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol. 2018;17(1):98. doi: 10.1186/s12933-018-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer BB, Hopkins SE, Wiener HW, Purnell JQ, O'Brien DM, Zhang CX, et al. Habitual intake of marine-derived n-3 polyunsaturated fatty acids is inversely associated with a Cardiometabolic inflammatory profile in Yup’ik Alaska native people. J Nutr. 2021. 10.1093/jn/nxab412. [DOI] [PMC free article] [PubMed]
- 29.Delpino FM, Figueiredo LM, da Silva BGC, da Silva TG, Mintem GC, Bielemann RM, et al. Omega-3 supplementation and diabetes: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021:1–14. 10.1080/10408398.2021.1875977. [DOI] [PubMed]
- 30.Jenkins DJA, Kendall CWC, Vuksan V, Faulkner D, Augustin LSA, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L, Blanco Mejia S, Nishi S, Sahye-Pudaruth S, Patel D, Bashyam B, Vidgen E, de Souza RJ, Sievenpiper JL, Coveney J, Josse RG, Leiter LA. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care. 2014;37(7):1806–1814. doi: 10.2337/dc13-2990. [DOI] [PubMed] [Google Scholar]
- 31.Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res. 2013;33(5):367–375. doi: 10.1016/j.nutres.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Saleh-Ghadimi S, Kheirouri S, Golmohammadi A, Moludi J, Jafari-Vayghan H, Alizadeh M. Effect of flaxseed oil supplementation on anthropometric and metabolic indices in patients with coronary artery disease: a double-blinded randomized controlled trial. J Cardiovasc Thorac Res. 2019;11(2):152–160. doi: 10.15171/jcvtr.2019.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh-Ghadimi S, Alizadeh M, Jafari-Vayghan H, Darabi M, Golmohammadi A, Kheirouri S. Effect of flaxseed oil supplementation on the erythrocyte membrane fatty acid composition and endocannabinoid system modulation in patients with coronary artery disease: a double-blind randomized controlled trial. Genes Nutr. 2020;15(1):9. doi: 10.1186/s12263-020-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng J-S, Li K, Huang T, Chen Y, Xie H, Xu D, Sun J, Li D. Genetic risk score of nine type 2 diabetes risk variants that Interact with erythrocyte phospholipid alpha-linolenic acid for type 2 diabetes in Chinese Hans: a case-control study. Nutrients. 2017;9(4):376. doi: 10.3390/nu9040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma RCW, Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, et al. Association of Plasma Phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2016;13:e1002094. doi: 10.1371/journal.pmed.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K-B, Nam YA, Kim HS, Hayes AW, Lee B-M. α-linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Barceló-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n−3 fatty acids: benefits for human health and a role in maintaining tissue n−3 fatty acid levels. Prog Lipid Res. 2009;48(6):355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467s–1476s. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 39.de Groot RHM, Emmett R, Meyer BJ. Non-dietary factors associated with n-3 long-chain PUFA levels in humans – a systematic literature review. Br J Nutr. 2019;121(7):793–808. doi: 10.1017/S0007114519000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alessandri J-M, Extier A, Astorg P, Lavialle M, Simon N, Guesnet P. Métabolisme des acides gras oméga-3 : différences entre hommes et femmes. Nutr Clin Métab. 2009;23(2):55–66. doi: 10.1016/j.nupar.2009.03.003. [DOI] [Google Scholar]
- 41.Takayama M, Arai Y, Sasaki S, Hashimoto M, Shimizu K, Abe Y, Hirose N. Association of marine-origin n-3 polyunsaturated fatty acids consumption and functional mobility in the community-dwelling oldest old. J Nutr Health Aging. 2013;17(1):82–89. doi: 10.1007/s12603-012-0389-1. [DOI] [PubMed] [Google Scholar]
- 42.Patterson AC, Chalil A, Aristizabal Henao JJ, Streit IT, Stark KD. Omega-3 polyunsaturated fatty acid blood biomarkers increase linearly in men and women after tightly controlled intakes of 0.25, 0.5, and 1 g/d of EPA + DHA. Nutr Res. 2015;35(12):1040–1051. doi: 10.1016/j.nutres.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Roke K, Mutch D. The role of FADS1/2 polymorphisms on Cardiometabolic markers and fatty acid profiles in young adults consuming fish oil supplements. Nutrients. 2014;6(6):2290–2304. doi: 10.3390/nu6062290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fenton JI, Gurzell EA, Davidson EA, Harris WS. Red blood cell PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins Leukot Essent Fat Acids. 2016;112:12–23. doi: 10.1016/j.plefa.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Escolano-Margarit MV, Campoy C, Ramírez-Tortosa MC, Demmelmair H, Miranda MT, Gil A, Decsi T, Koletzko BV. Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: a randomised controlled trial. Br J Nutr. 2012;109(9):1647–1656. doi: 10.1017/S0007114512003716. [DOI] [PubMed] [Google Scholar]
- 46.Hodson L, Crowe FL, McLachlan KJ, Skeaff CM. Effect of supplementation with flaxseed oil and different doses of fish oil for 2 weeks on plasma phosphatidylcholine fatty acids in young women. Eur J Clin Nutr. 2018;72(6):832–840. doi: 10.1038/s41430-018-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egert S, Lindenmeier M, Harnack K, Krome K, Erbersdobler HF, Wahrburg U, Somoza V. Margarines fortified with α-linolenic acid, Eicosapentaenoic acid, or docosahexaenoic acid Alter the fatty acid composition of erythrocytes but do not affect the antioxidant status of healthy adults. J Nutr. 2012;142(9):1638–1644. doi: 10.3945/jn.112.161802. [DOI] [PubMed] [Google Scholar]
- 48.Greupner T, Kutzner L, Nolte F, Strangmann A, Kohrs H, Hahn A, Schebb NH, Schuchardt JP. Effects of a 12-week high-α-linolenic acid intervention on EPA and DHA concentrations in red blood cells and plasma oxylipin pattern in subjects with a low EPA and DHA status. Food Funct. 2018;9(3):1587–1600. doi: 10.1039/C7FO01809F. [DOI] [PubMed] [Google Scholar]
- 49.Block RC, Harris WS, Pottala JV. Determinants of blood cell Omega-3 fatty acid content. Open Biomark J. 2008;1(1):1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–510. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Mahendran Y, Ågren J, Uusitupa M, Cederberg H, Vangipurapu J, Stančáková A, Schwab U, Kuusisto J, Laakso M. Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am J Clin Nutr. 2014;99(1):79–85. doi: 10.3945/ajcn.113.069740. [DOI] [PubMed] [Google Scholar]
- 52.Herder C, Harris WS, Luo J, Pottala JV, Margolis KL, Espeland MA, Robinson JG. Red blood cell fatty acids and incident diabetes mellitus in the Women’s health initiative memory study. PLoS One. 2016;11(2):e0147894. doi: 10.1371/journal.pone.0147894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EHJM, Döring F, Joost H-G, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European prospective investigation into Cancer and nutrition (EPIC)–Potsdam study. Am J Clin Nutr. 2011;93(1):127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 54.Decsi T, Szabó É, Burus I, Marosvölgyi T, Kozári A, Erhardt É, Soltész G. Low contribution of n-3 polyunsaturated fatty acids to plasma and erythrocyte membrane lipids in diabetic young adults. Prostaglandins Leukot Essent Fat Acids. 2007;76(3):159–164. doi: 10.1016/j.plefa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26(6):571–584. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Abbott KA, Burrows TL, Thota RN, Alex A, Acharya S, Attia J, et al. Association between plasma phospholipid omega-3 polyunsaturated fatty acids and type 2 diabetes is sex dependent: the Hunter Community study. Clin Nutr. 2020;39(4):1059–66. 10.1016/j.clnu.2019.04.007. [DOI] [PubMed]
- 57.Guo X-f, W-f T, Ruan Y, Sinclair AJ, Li D. Different metabolism of EPA, DPA and DHA in humans: A double-blind cross-over study. Prostaglandins Leukot Essent Fat Acids. 2020;158:102033. doi: 10.1016/j.plefa.2019.102033. [DOI] [PubMed] [Google Scholar]
- 58.Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, Turchini GM, Cameron-Smith D, Sinclair AJ. A short-term n-3 DPA supplementation study in humans. Eur J Nutr. 2012;52(3):895–904. doi: 10.1007/s00394-012-0396-3. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Shen H, Li Y, Bi M, Bi Y, Che X, et al. Sex-specific differences in the associations between Omega-6 polyunsaturated fatty acids and type 2 diabetes in Chinese people. Front Nutr. 2021;8. 10.3389/fnut.2021.739850. [DOI] [PMC free article] [PubMed]
- 60.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang W-S, de Oliveira Otto MC, Kröger J, Qureshi W, Virtanen JK, Bassett JK, Frazier-Wood AC, Lankinen M, Murphy RA, Rajaobelina K, del Gobbo L, Forouhi NG, Luben R, Khaw KT, Wareham N, Kalsbeek A, Veenstra J, Luo J, Hu FB, Lin HJ, Siscovick DS, Boeing H, Chen TA, Steffen B, Steffen LM, Hodge A, Eriksdottir G, Smith AV, Gudnason V, Harris TB, Brouwer IA, Berr C, Helmer C, Samieri C, Laakso M, Tsai MY, Giles GG, Nurmi T, Wagenknecht L, Schulze MB, Lemaitre RN, Chien KL, Soedamah-Muthu SS, Geleijnse JM, Sun Q, Harris WS, Lind L, Ärnlöv J, Riserus U, Micha R, Mozaffarian D, Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–974. doi: 10.1016/S2213-8587(17)30307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma RCW, Imamura F, Sharp SJ, Koulman A, Schulze MB, Kröger J, et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2017;14(10). 10.1371/journal.pmed.1002409. [DOI] [PMC free article] [PubMed]
- 62.Lin J-s, Dong H-l, G-d C, Z-y C, Dong X-w, Zheng J-s, Chen Y-M. Erythrocyte Saturated Fatty Acids and Incident Type 2 Diabetes in Chinese Men and Women: A Prospective Cohort Study. Nutrients. 2018;10(10):1393. doi: 10.3390/nu10101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JWJ, van Woudenbergh GJ, Wang L, Summerhill K, Griffin JL, Feskens EJM, Amiano P, Boeing H, Clavel-Chapelon F, Dartois L, Fagherazzi G, Franks PW, Gonzalez C, Jakobsen MU, Kaaks R, Key TJ, Khaw KT, Kühn T, Mattiello A, Nilsson PM, Overvad K, Pala V, Palli D, Quirós JR, Rolandsson O, Roswall N, Sacerdote C, Sánchez MJ, Slimani N, Spijkerman AMW, Tjonneland A, Tormo MJ, Tumino R, van der A DL, van der Schouw YT, Langenberg C, Riboli E, Wareham NJ. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–818. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, Song X, Djoussé L, Siscovick DS, McKnight B, Sotoodehnia N, Kizer JR, Mozaffarian D. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the cardiovascular health study. Am J Clin Nutr. 2015;101(5):1047–1054. doi: 10.3945/ajcn.114.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.