Abstract
Background
In this study we tested the hypothesis that in patients with cystic fibrosis (pwCF) respiratory rate (RR) is associated with antibiotic treatment, exacerbation status, forced expiratory volume in one second (FEV1) and C-reactive protein (CRP).
Methods
Between June 2018 and May 2019, we consecutively enrolled pwCF who were referred to our hospital. We determined RR and heart rate (HR) by using the minimal-impact system VitaLog during the hospital stay. Furthermore, we performed spirometry and evaluated CRP.
Results
We included 47 patients: 20 with pulmonary exacerbation and 27 without. RR decreased in patients with exacerbation (27.5/min (6.0/min) vs. 24.4/min (6.0/min), p = 0.004) and in patients with non-exacerbation (22.5/min (5.0/min) vs. 20.9/min (3.5/min), p = 0.024). Patients with exacerbation showed higher RR than patients with non-exacerbation both at the beginning (p = 0.004) and at the end of their hospital stay (p = 0.023). During the hospital stay, HR did not change in the total cohort (66.8/min (11.0/min) vs. 66.6/min (12.0/min), p = 0.440). Furthermore, we did not find significant differences between patients with exacerbation and patients with non-exacerbation (67.0/min (12.5/min) vs. 66.5/min (10.8/min), p = 0.658). We observed a correlation of ρ = -0.36 between RR and FEV1. Moreover, we found a correlation of ρ = 0.52 between RR and CRP.
Conclusion
In pwCF requiring intravenous therapy, respiratory rate is higher at their hospital admittance and decreased by the time of discharge; it is also associated with C-reactive protein. Monitoring RR could provide important information about the overall clinical conditions of pwCF.
Keywords: Cystic fibrosis, Respiratory rate, Telemedicine, Home monitoring, Exacerbation
Background
Cystic fibrosis (CF) is an autosomal recessive disease leading to shorter life expectancy. More than 80.000 individuals are affected by CF worldwide [1–3]. The highest prevalence occurs in Europe, North America and Australia [4].
Thanks to continuous medical progress, median survival age increased significantly to above 40 years since the first description of CF in 1938 [4, 5]. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene influence the conductance of chloride and bicarbonate ions through the cell membrane leading to dehydration of the epithelial liquid film in affected organs. Thus, CF is a multisystem disease. Morbidity and mortality are mainly determined by chronic inflammation and frequent infections of the lungs [6]. CF related bacterial overgrowth is associated with frequent pulmonary exacerbations that require antibiotic treatment. Typical exacerbation symptoms are more frequent cough, increased purulent secretion, fever, weight loss, reduced resilience, decline of lung function and tachypnoea [7].
Previous studies have shown that respiratory rate (RR) can be used as a marker for clinical outcome of various diseases. For instance, in chronic obstructive pulmonary disease (COPD) a correlation between RR and exacerbation-related hospitalizations has been reported [8–10]. Moreover, RR is a predictor for mortality in patients with community-acquired pneumonia [11]. Consequently, RR determination is integrated in common prognosis tools such as the CRB 65 index (confusion, respiratory rate, blood pressure, age > = 65 years) [12].
In general, there is strong evidence that RR is an important vital sign to predict morbidity and mortality in some lung disorders. Hence, it can be suggested that RR is an important clinical marker for the disease course in patients with cystic fibrosis (pwCF) as well. However, knowledge on the role of RR in CF is scarce. Therefore, in pwCF we tested the hypothesis that RR is associated with antibiotic treatment, exacerbation status, forced expiratory volume in one second (FEV1), and C-reactive protein (CRP).
Methods
Patients
Between June 2018 and May 2019 we consecutivley enrolled pwCF who were referred to the Ruhrlandklinik Essen, Germany in order to receive intravenous antibiotic treatment. Hospitalization was indicated due to pulmonary exacerbation or prophylactic antibiotic treatment. The diagnosis of CF was based on the patient’s history and the detection of two disease causing mutations.
Pulmonary exacerbations were determined according to the criteria defined by Fuchs et al. [7]. An exacerbation is present if four of the following criteria are fulfilled and an antibiotic treatment is required: increased secretion, newly occurred haemoptysis, more frequent cough, increasing dyspnoea, fatigue/indisposition, temperature above 38 °C, weight loss, sinus pain or change in sinus discharge, decline of lung function of about 10% and radiological alterations that indicate an infect [7].
For all patients inflammation marker CRP and leucocytes, body temperature and body-mass-index (BMI) were determined on the day of hospitalization. Forced vital capacity (FVC) and FEV1 were determined with a JAEGER MasterScreen Body (CareFusion, Hoechberg, Germany) according to ATS guidelines [11]. We used the Quanjer GLI-2012 regression equations for spirometric indices [13]. Spirometry was performed at the first day.
Referring to the findings of Hart et al. [14] for the correlation between RR and FEV1 we presume ρ = -0.41. Thus, we need a sample size of n = 44 using an alpha of 0.05 and a power of 0.8.
The ethics committee of the University Duisburg-Essen (17-7891-BO) approved the study and all patients provided a written informed consent.
Biomotion sensor
We continuously determined RR and heart rate (HR) during night sleep by using the VitaLog (SWG Sportwerk, Dortmund, Germany) system. This device is composed of a sensor and an analysis unit (Fig. 1). The sensor is layed under the bed sheet close to the thorax in order to deviate body movements. Technology is based on the piezoelectric effect leading to a composed movement signal. Due to this minimal-impact setting VitaLog does not interfere with patients’ comfort. The analysis unit is positioned next to the bed. In recent studies our research group evaluated the VitaLog sensor for various vital signs including HR with a very good correlation compared to ECG [15] and RR with a very good correlation compared to nasal flow [16].
Fig. 1.

The VitaLog system
Statistics
Data are presented as mean (standard deviation). Mann–Whitney-U-test was applied to test differences between subgroups and Wilcoxon signed rank test was performed to test differences during treatment. Uncertainty was reported using 95% confidence interval (CI). Fisher’s exact test was applied for categorical variables. Correlation analysis was performed using Spearman correlation coefficients (ρ). We used box plots in order to present the variability of RR and HR. All statistics were performed with Matlab2020b (MathWorks®, Natick, Massachusetts, USA). A P-value < 0.05 was considered statistically significant.
Results
We included 47 patients. In general, our study group was young, mainly male and had several comorbidities (Table 1). Our study cohort consisted of patients who received prophylactic antibiotic treatment in hospital 57% (27/47) and patients suffering from an acute exacerbation 43% (20/47). Age ranged from 18 to 59 y, BMI from 11.0 to 32.3 kg/m2, FEV1 from 17.9 to 81.0%, FVC from 18.0 to 88.7%, Leukocyte from 3.7*10^9/l to 15.3*10^9/l, and CRP from 0.1 to 27.4 mg/dl. Length of hospital stay ranged from 1 to 18 days.
Table 1.
Patient characteristics at baseline
| All (n = 47) | Exacerbated (n = 20) | Non-Exacerbated (n = 27) | |
|---|---|---|---|
| Age, y (SD) | 30.7 (10.4) | 27.9 (8.9) | 32.7 (11.0) |
| BMI, kg/m2 (SD) | 20.4 (3.8) | 18.5 (3.5)* | 21.8 (3.5) |
| Sex | |||
| Female, n (%) | 20 (43) | 9 (45) | 11 (41) |
| Male, n (%) | 27 (57) | 11 (55) | 16 (59) |
| Genotype | |||
| F508del homozygous, n (%) | 23 (49) | 11 (55) | 12 (45) |
| F508del heterozygous, n (%) | 16 (34) | 7 (35) | 9 (33) |
| Other, n (%) | 8 (17) | 2 (10) | 6 (22) |
| FEV1, L (SD) | 1.48 (0.69) | 1.11 (0.39)* | 1.74 (0.74) |
| FEV1, % predicted (SD) | 38.0 (15.5) | 28.4 (7.9)* | 45.2 (15.9) |
| FVC, L (SD) | 2.32 (1.06) | 1.76 (0.59)* | 2.72 (1.15) |
| FVC, % predicted (SD) | 50.0 (17.1) | 38.7 (9.0)* | 57.9 (17.1) |
| Leucocyte, 10^9/l (SD) | 8.9 (3.1) | 10.9 (3.0)* | 7.5 (2.2) |
| CRP, mg/dl (SD) | 3.5 (5.3) | 6.9 (6.7)* | 1.0 (0.8) |
| Pancreatic insufficiency, n (%) | 43 (91) | 18 (90) | 25 (93) |
| P. aeruginosa positive, n (%) | 33 (70) | 13 (65) | 20 (74) |
| Cystic fibrosis-related diabetes, n (%) | 16 (34) | 7 (35) | 9 (33) |
| O2-Supplementation, n (%) | 21 (45) | 14 (70)* | 7 (26) |
| NIV, n (%) | 7 (15) | 6 (30)* | 1 (4) |
| tVitaLog (days) (SD) | 6.4 (3.7) | 7.6 (4.1) | 5.5 (3.2) |
NIV: non invasive ventilation
*p < 0.05 between subgroups
RR ranged from 14.3/min to 36.2/min (n = 43). At the end of the hospital stay we observed a decrease of RR in the total cohort (24.6/min (5.9/min) vs. 22.4/min (5.0/min), p < 0.001) meaning a drop of − 2.2/min (95% CI: − 3.3\min to − 1.0/min). Moreover, RR decreased in patients with exacerbation (27.5/min (6.0/min) vs. 24.4/min (6.0/min), p = 0.004) meaning a drop of − 3.1/min (95% CI: − 5.2/min to − 0.9/min) and in patients with non-exacerbation (22.5/min (5.0/min) vs. 20.9/min (3.5/min), p = 0.024) meaning a drop of − 1.5 (95% CI: − 2.8/min to − 0.2/min) (Fig. 2). Patients with exacerbation (n = 18) showed higher RR than patients with non-exacerbation (n = 25) both at the beginning (p = 0.004) and at the end of their hospital stay (p = 0.023).
Fig. 2.
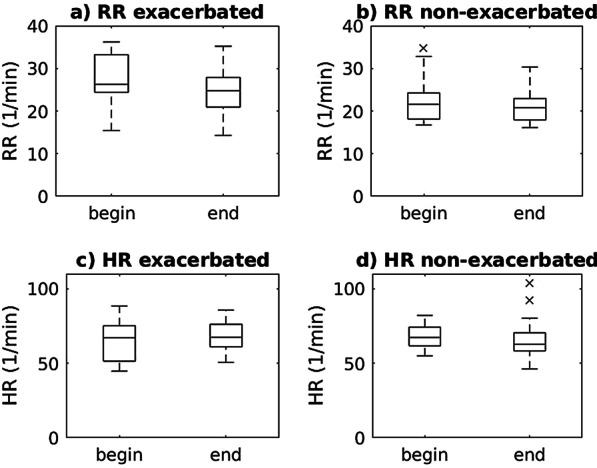
RR (a and b) and HR (c and d) changes during hospital stay for exacerbated (a and c) and non-exacerbated (b and d) pwCF. The lowest point on the box-plot describes the minimum value and the highest point the maximum value of the analyzed data. The box is drawn from the lower quartile (Q25) to the upper quartile (Q75) with a horizontal line in the middle to label the median
HR ranged from 44.8/min to 103.9/min (n = 43). We did not observe any HR changes during the hospitalization (66.8/min (11.0/min) vs. 66.6/min (12.0/min), p = 0.440). For patients with exacerbation (n = 18) we did not observe statistically significant HR changes (65.2/min (14.2/min) vs. 68.8/min (10.5/min), p = 0.184). Of note, patients with non-exacerbation (n = 25) showed a statistically significant decrease in HR (68.0/min (8.2/min) vs. 65.0/min (12.9/min), p = 0.031). However, this decrease is not of clinical relevance. Furthermore, we did not find any significant differences between exacerbated and patients with non-exacerbation (67.0/min (12.5/min) vs. 66.5/min (10.8/min), p = 0.658).
We found a weak negative correlation ρ = − 0.36 (p = 0.015) between RR and FEV1 measurements (n = 45) as well as a moderate positive correlation ρ = 0.52 (p < 0.001) between RR and CRP measurements (n = 72) (Fig. 3).
Fig. 3.
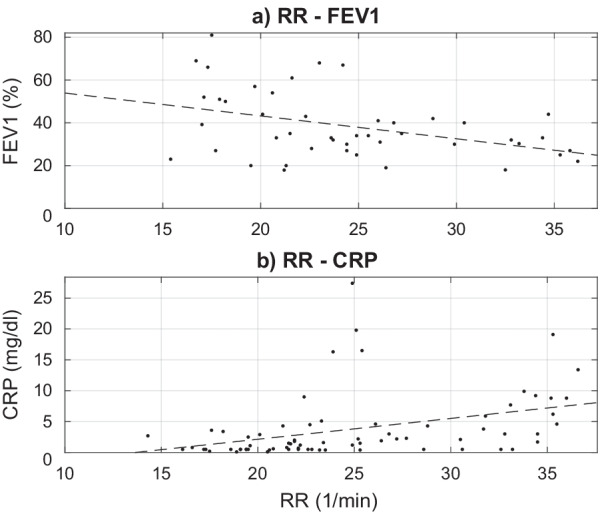
Relationship of RR with FEV1 (a) and CRP (b), respectively
Discussion
With the presented study we provided some novel insights and confirmed observations of previous studies. First, during hospital stay RR is increased in pwCF compared to known RR of the general population. Second, we observed that RR decreased in the course of antibiotic treatment. Third, RR was higher in patients with exacerbation compared to patients with non-exacerbation. Fourth, we found that RR is associated with FEV1. Fifth, HR did not change relevantly during antibiotic treatment.
For healthy adults RR ranges between 12/min and 20/min [17]. In our cohort we observed that only a minority has an RR in the normal range whereas most of our CF patients have increased RR compared to healthy subjects. Previously, it was reported that mean RR was 34/min for adult pwCF with mean FEV1 of 22.5% prior to lung transplantation [18]. However, knowledge on RR in adult pwCF is limited. Thus, evidence does not exist on common RR for this cohort. Therefore, further investigations are required in order to elucidate this research topic including possible clinical implications. Though, a few studies showed that infants and young adults have increased RR compared to healthy peers [14, 19, 20]. Korten et al. observed for pwCF during the first year of life a consistently higher RR of 4/min independent of respiratory infections [19]. Paranjape et al. found significantly increased RR (19.5/min vs. 16.5/min) for pwCF with median age of 9.6 years and median FEV1 of 87% [20]. For clinically stable CF patients with a mean age of 14.2 years and a mean FEV1 of 28.7% Hart et al. observed a mean RR of 22.6/min which is similar to our observations [14]. Hence, our findings of increased RR in adult pwCF are in line with reported elevated RR values in adolescent pwCF.
We could confirm the results reported earlier that RR decreases in the course of antibiotic treatment. Bell et al. found in a smaller cohort of 22 adult pwCF that RR went down from 24/min (on day 1) to 19/min (on day 15) [21]. However, this cohort from 1999 differs to our patient group since treatment progress over the last 20 years had a positive impact on clinical outcome. For instance, the cohort from Bell et al. had a mean age of 23.7 years and a mean FEV1 of 28.5% as opposed to our patient group with a mean age of 30.7 years and a mean FEV1 of 38.0%. Moreover, in patients with non-exacerbation during prophylactic antibiotic treatment we also observed decreasing RR caused by lowering of bacterial load, reduced secretion and therby improved respiration. Thus, RR may be an additional marker for response to antibiotic treatment. This finding provides some new procedures for the monitoring of treatment success beyond the laboratory diagnosis of common clinical infection parameters. Monitoring of RR provides the opportunity to develop a telemedicine-based approach of surveillance in the home environment in order to decrease hospitalization rate.
Evidence suggests that RR is an established risk parameter for hospital mortality for patients with community acquired pneumonia. McFadden et al. noticed that increased RR (mean 29.7/min) indicated pulmonary infections prior to the clinical diagnosis [22]. Furthermore, RR is known to increase during exacerbations of different underlieing pulmonary diseases. For instance, Yanez et al. observed that in COPD patients RR rises significantly days before hospitalization [8]. The authors concluded that continuous RR monitoring may offer a window of opportunity for early intervention.
To our knowledge, this is the first study observing that RR and FEV1 are negatively associated in adult patients. This complements the findings of a previous study. Hart et al. reported that in clinically stable, adolescent pwCF a reduction in FEV1 was accompanied by an increase in RR [14].
In general, data on HR in pwCF is scarce. In contrast to our findings Bell et al. observed that HR decreases were clinically relevant between day 1 and day 8 of exacerbation treatment [21]. Furthermore, mean HR at hospitalization was much higher compared to our cohort (100/min vs. 67/min). As opposed to our study Bell et al. used a short-time single measurement of HR. Thus, it cannot be excluded that at the beginning of the hospital stay the so called white-collar effect may have occurred explaining the different findings. However, as evidence on HR progression during hospital stay is mixed, further studies are necessary in order to gain more profound knowledge on this topic.
Furthermore, we found that RR is associated with CRP. As CRP is a common biomarker for the diagnosis of pulmonary exacerbation [23], but does not always reflect the requirement of antibiotic therapy, it could be suggested that a combination of RR and CRP might be a helpful tool for treatment decision-making. Further studies are needed to elucidate the clinical impact of this approach.
This study has several strengths. First, compared to previous studies we provide a considerably higher sample size. Second, our set-up of long-term monitoring during the hospital stay allows valuable insights in vital parameter trends with clinical implications. Third, we were the first group investigating in long-term continuous RR and HR monitoring of adult pwCF. Fourth, as VitaLog is a simple, reliable and minimal-contact device, RR and HR can be determined with low failure rates.
However, we also acknowledge some limitations. First, in terms of providing common RR and HR values in pwCF we have to admit the bias that only hospitalized patients during treatment were included into our analysis. Second, as we did not adjust for CF-specific features the underlying nature of RR changes is undetermined. Third, we were also only able to determine vital signs during the hospital stay and not until the end of outpatient antibiotic treatment. Therefore, it can be assumed that RR is lower in common non-hospitalized pwCF. Thus, further investigations are required in order to test this hypothesis.
Conclusion
In pwCF requiring intravenous therapy, respiratory rate is higher at their hospital admittance and decreased by the time of discharge; it is also associated with C-reactive protein. Monitoring RR could provide important information about the overall clinical conditions of pwCF.
Acknowledgements
Not applicable.
Abbreviations
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- HR
Heart rate
- pwCF
Patients with cystic fibrosis
- RR
Respiratory rate
Authors' contributions
SS: Conceptualization, Methodology, Writing—original draft. CL: Software, Formal analysis, Writing—review & editing. SB: Resources, Software. CS: Software. CT: Investigation. JG: Resources, Software. FS: Writing—review & editing. SSu: Investigation. MW: Investigation, Writing—review & editing. GW: Conceptualization, Methodology, Writing- review & editing, Supervision. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The ethics committee of the University Duisburg-Essen (17-7891-BO) approved the study and all patients provided a written informed consent. All methods were carried out in accordance with relevant guidelines and regulations such as the Helsinki declaration.
Consent for publication
Not applicable.
Competing interests
SB and JG report to be co-founder of SWG Sportwerk GmbH & Co. KG, which developed the VitaLog system. The other authors have indicated no financial conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Svenja Straßburg, Email: svenja.strassburg@rlk.uk-essen.de.
Carolin-Maria Linker, Email: carolin.linker@tu-dortmund.de.
Sebastian Brato, Email: sebastian.brato@swg-sportwerk.de.
Christoph Schöbel, Email: christoph.schoebel@rlk.uk-essen.de.
Christian Taube, Email: christian.taube@rlk.uk-essen.de.
Jürgen Götze, Email: juergen.goetze@tu-dortmund.de.
Florian Stehling, Email: florian.stehling@uk-essen.de.
Sivagurunathan Sutharsan, Email: sivagurunathan.sutharsan@rlk.uk-essen.de.
Matthias Welsner, Email: matthias.welsner@rlk.uk-essen.de.
Gerhard Weinreich, Email: gerhard.weinreich@rlk.uk-essen.de.
References
- 1.2019 AnnualDataReport Canada.
- 2.Cystic Fibrosis Foundation. 2015 CFF Patient Registry Annual Data Report.
- 3.European Cystic Fibrosis Society. ECFS Patient Registry.
- 4.Elborn JS. Cystic fibrosis. The Lancet. 2016;388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 5.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344–399. doi: 10.1001/archpedi.1938.01980140114013. [DOI] [Google Scholar]
- 6.Müller F-M, Bend J, Huttegger I, Möller A, Schwarz C, Abele-Horn M, et al. S3-Leitlinie „Lungenerkrankung bei Mukoviszidose“. Monatsschr Kinderheilkd. 2015;163:590–599. doi: 10.1007/s00112-015-3354-3. [DOI] [Google Scholar]
- 7.Fuchs Henry J, Borowitz Drucy S, Christiansen David H, Morris Edward M, Nash Martha L, Ramsey Bonnie W, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. [DOI] [PubMed]
- 8.Yañez AM, Guerrero D, Pérez de Alejo R, Garcia-Rio F, Alvarez-Sala JL, Calle-Rubio M, et al. Monitoring breathing rate at home allows early identification of COPD exacerbations. Chest. 2012;142:1524–9. 10.1378/chest.11-2728. [DOI] [PubMed]
- 9.Perera WR, Hurst JR, Wilkinson TMA, Sapsford RJ, Müllerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29:527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 10.Franciosi LG, Page CP, Celli BR, Cazzola M, Walker MJ, Danhof M, et al. Markers of exacerbation severity in chronic obstructive pulmonary disease. Respir Res. 2006;7:74. doi: 10.1186/1465-9921-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauß R, Ewig S, Richter K, König T, Heller G, Bauer TT. The prognostic significance of respiratory rate in patients with pneumonia: a retrospective analysis of data from 705,928 hospitalized patients in Germany from 2010–2012. Dtsch Arztebl Int. 2014;111(503–8):i–v. doi: 10.3238/arztebl.2014.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischl MA, Pitchenik A, Gardner LB. An index predicting relapse and need for hospitalization in patients with acute bronchial asthma. N Engl J Med. 1981;305:783–789. doi: 10.1056/NEJM198110013051402. [DOI] [PubMed] [Google Scholar]
- 13.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart N, Polkey MI, Clément A, Boulé M, Moxham J, Lofaso F, Fauroux B. Changes in pulmonary mechanics with increasing disease severity in children and young adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:61–66. doi: 10.1164/rccm.2112059. [DOI] [PubMed] [Google Scholar]
- 15.Terjung S, Geldmacher J, Brato S, Werther S, Teschler H, Taube C, et al. Classification of sleep and wake using a novel minimal-contact single-channel device. Somnologie. 2018;22:144–151. doi: 10.1007/s11818-017-0139-z. [DOI] [Google Scholar]
- 16.Dietz-Terjung S, Geldmacher J, Brato S, Linker C-M, Welsner M, Schöbel C, et al. A novel minimal-contact biomotion method for long-term respiratory rate monitoring. Sleep Breath. 2020 doi: 10.1007/s11325-020-02067-4. [DOI] [PubMed] [Google Scholar]
- 17.Lindh WQ, Pooler M, Tamparo CD, et al. Delmar's comprehensive medical assisting: administrative and clinical competencies. New York: Cengage Learning; 2006. [Google Scholar]
- 18.Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, Aliverti A. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010;65:808–814. doi: 10.1136/thx.2009.131409. [DOI] [PubMed] [Google Scholar]
- 19.Korten I, Kieninger E, Yammine S, Cangiano G, Nyilas S, Anagnostopoulou P, et al. Respiratory rate in infants with cystic fibrosis throughout the first year of life and association with lung clearance index measured shortly after birth. J Cyst Fibros. 2019;18:118–126. doi: 10.1016/j.jcf.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Paranjape SM, McGinley BM, Braun AT, Schneider H. Polysomnographic markers in children with cystic fibrosis lung disease. Pediatrics. 2015;136:920–926. doi: 10.1542/peds.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell SC, Bowerman AM, Nixon LE, Macdonald IA, Elborn JS, Shale DJ. Metabolic and inflammatory responses to pulmonary exacerbation in adults with cystic fibrosis. Eur J Clin Invest. 2000;30:553–559. doi: 10.1046/j.1365-2362.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 22.McFadden JP, Price RC, Eastwood HD, Briggs RS. Raised respiratory rate in elderly patients: a valuable physical sign. Br Med J (Clin Res Ed) 1982;284:626–627. doi: 10.1136/bmj.284.6316.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung D, Dong K, Jang J, Lam GY, Wilcox PG, Quon BS. Circulating CRP and calprotectin to diagnose CF pulmonary exacerbations. J Cyst Fibros. 2021;20:46–49. doi: 10.1016/j.jcf.2020.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are available from the corresponding author upon reasonable request.


