Abstract
Background
The purpose was to evaluate the relationship of component size and position to postoperative range of motion following reverse shoulder arthroplasty. The hypothesis was that increased lateralization, larger glenospheres, and a decreased acromiohumeral distance would be associated with excellent postoperative range of motion.
Methods
A retrospective multicenter study was performed at a minimum of one year postoperatively on 160 patients who underwent primary reverse shoulder arthroplasty with a 135° humeral component. Outcomes were stratified based on postoperative forward flexion and external rotation into excellent (n = 42), defined as forward flexion >140° and external rotation > 30°, or poor (n = 36), defined as forward flexion <100° and external rotation < 15°. Radiographic measurements and component features were compared between the two groups.
Results
A larger glenosphere size was associated with an excellent outcome (p = 0.009). A 2-mm posterior offset humeral cup (p = 0.012) and an increased inferior glenosphere overhang (3.1 mm vs 1.4 mm; p = 0.002) were also associated with excellent outcomes. Humeral lateralization and distalization were not associated with an excellent outcome.
Conclusion: Larger glenosphere size and inferior positioning as well as posterior humeral offset are associated with improved postoperative range of motion following reverse shoulder arthroplasty.
Level of Evidence
Level 3, retrospective comparative study.
Keywords: Reverse shoulder arthroplasty, range of motion, implant position, lateralization, distalization, forward flexion, external rotation
Introduction
Reverse shoulder arthroplasty (RSA) has revolutionized the treatment of several shoulder conditions, leading to satisfactory functional outcome based on mean preoperative to postoperative improvement of cohorts.1–3 However, up to 38% of patients remain unsatisfied or have minimal improvement in range of motion (ROM) following RSA.4,5
A variety of implant options are available for RSA, which lead to different postoperative positions of the glenoid and humeral components. Lateralization and distalization have been associated with improvement in ROM in at least one study, 6 while others have evaluated radiographic factors associated with outcomes following a cohort of RSAs and failed to find substantial relationships. 7 However, such analyses have been limited by interpretation of the mean. Another approach is to stratify outcomes and compare the outliers (excellent vs poor) in order to identify factors associated with an unsatisfactory outcome.
The purpose of the current study was to evaluate the relationship of component size or position to postoperative ROM following RSA. The hypothesis was that increased lateralization, larger glenospheres, and a decreased acromiohumeral distance would be associated with excellent postoperative ROM.
Materials and methods
A retrospective comparative study was performed of data collected in a multicenter prospective arthroplasty database. Institutional review board approval was obtained prior to commencing the study. Inclusion criteria included a primary RSA performed between August 2015 and February 2018 and minimum clinical and radiographic follow-up of one year postoperative. Exclusion criteria included revision arthroplasty and a treatment for acute fracture. A total of 200 RSAs meeting the study criteria were performed during the study period, of which 160 (80%) had complete clinical and radiographic follow-up.
Postoperative outcomes were stratified as excellent or poor based on postoperative ROM. Forward flexion (FF) and external rotation (ER) at the side were measured with a goniometer preoperatively and at final follow-up by an independent examiner at each site. An excellent outcome was defined as FF ≥ 140° and ER at the side ≥ 30°. A poor outcome was defined as FF < 100° or ER < 15° based on a previous study. 4 Based on this division, a total of 42 cases (26.3%) were defined as an excellent outcome and 36 cases (22.5%) were defined as a poor outcome.
Surgical technique
The RSAs were performed at eight institutions by nine different surgeons. A deltopectoral approach and the same implant (Univers Revers; Arthrex, Inc., Naples, FL) was used in all cases. On the humeral side, a 135° press-fit component was used with an inlay humeral cup. Polyethylene thickness, the use of metallic spacers, humeral cup offset, and glenoid components were based on surgeon preference. In 51 cases (65.4%), a 3-mm polyethylene component was placed, and in 27 cases (34.6%), a 6-mm polyethylene was placed. In 14 cases (17.9%), a metallic spacer was used in conjunction with the 3-mm polyethylene. The humeral liner (polyethylene) was of standard depth in 75 cases (96.2%) and constrained in three cases (3.8%). A 2-mm posterior offset humeral cup was used in 35 cases (45.5%), a neutral humeral cup was used in 38 cases (49.4%), a 2-mm anterior offset humeral cup was used in four cases (5.2%), and data were missing regarding offset in one case. On the glenoid side, glenosphere options included a 36-mm glenosphere (44 cases; 56.4%), a 39-mm glenosphere (12 cases; 15.4%), or a 42-mm glenosphere (22 cases; 28.2%). In 12 cases (15.4%), the glenospheres had neutral offset; in 65 (83.3%), the glenospheres had 4 mm of lateral offset; and in one case (1.3%), a 2.5-mm inferior eccentric glenosphere was used.
The subscapularis was repaired in 80.8% of cases (59 of 73), and not repaired in 19.2% of cases (14 of 73). Postoperative rehabilitation was not standardized.
Radiographic evaluation
Immediate postoperative anteroposterior radiographs were reviewed in DICOM format by an independent examiner who measured multiple parameters to assess superior–inferior (distalization) position and medial–lateral (lateralization) component position (GH). Distances were measured to the nearest millimeter and angles were measured to the nearest 0.1°.
Measurements of superior–inferior position included:
Acromiohumeral distance (AHD); 8
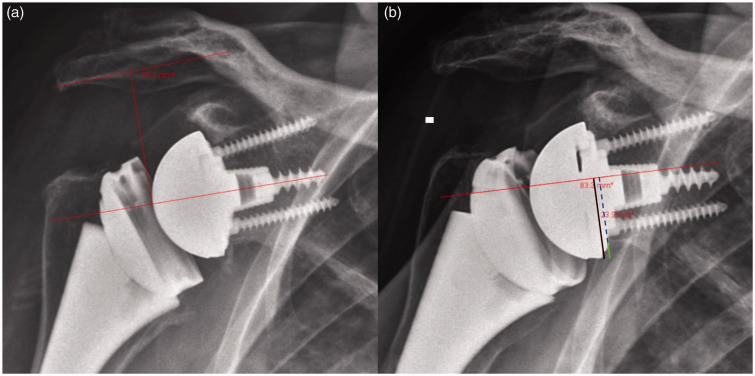
Acromial–center of rotation distance (ACD) or distance from the acromion to the center of the glenosphere (Figure 1(a));
Inferior glenosphere offset (IGO) or overhang of the glenosphere relative to glenoid (Figure 1(b)); and
Distalization angle; 6
Figure 1.
Radiographic examples of measurements obtained for the analysis of superior–inferior positioning differences between the excellent and poor outcome groups. (a) Acromial–center of rotation distance (ACD) or distance from the acromion to the center of the glenosphere. (b) Inferior glenosphere offset (IGO) or overhang of the glenosphere relative to glenoid (solid green line) is the difference between the distance from the center of the glenoid to the inferior border of the glenosphere (solid black line) and the distance from the center of the glenoid the inferior border of the scapular neck (dashed blue line).
Measurement of medial–lateral position included:
Acromion index; 9
Lateral humeral offset (LHO) or perpendicular distance from the lateral acromion to the most lateral post point of the humerus; 8
Glenosphere lateral offset (GLO) or perpendicular distance from the glenoid to the most lateral point of the glenosphere; 10 and
Lateralization angle. 6
Statistical analysis
Mean and standard deviations were used to describe continuous data. To examine the differences in means between groups were compared with a t test or Wilcoxon Rank Sum were according to variable distribution. Fishers exact test were used to compare frequencies between groups. The impact of glenosphere size was examined controlling for sex using a Mantel–Haenszel test. Following univariate analysis, two multivariate analyses were performed. The first included on an evaluation of patient factors of age, sex, and body mass index (BMI) with potentially implant factors (glenosphere size and offset) and radiographic factors (IGO, GLO, and ACD). The second included preoperative FF and ER with implant factors and radiographic factors. Two separate analyses were performed because of the high number of variables and by limiting the variables more precise estimates can be obtained. Two-tailed p values of < 0.05 were considered significant. Statistical analyses were carried out by a trained statistician.
Results
Patient and implant analysis
Patient characteristics of the two groups are summarized in Table 1. An excellent outcome was more common in patients with a larger BMI. Regarding implant choice, the use of a larger glenosphere was associated with an excellent outcome (p = 0.009). When controlling for sex, the difference remained significant (p = 0.012). Among women, an excellent outcome was obtained in 42.9% (15/35) with a 36-mm glenosphere, and 100% (2/2) with a 39-mm glenosphere. Among men, an excellent outcome was obtained in 25% (2/8) with a 36-mm glenosphere, 60% (6/10) with a 39-mm glenosphere, and 77.3% (17/22) with a 42-mm glenosphere. Glenosphere offset did not reach statistical significance (p = 0.279). The use of a 2-mm posterior offset humeral cup (p = 0.012) was also associated with an excellent outcome.
Table 1.
Baseline patient characteristics, patient-reported measures, ROM, and implant characteristics.
| Poor ROM (n = 36) | Excellent ROM (n = 42) | p value | |
|---|---|---|---|
| Age | 71 (±7) | 68 (±8) | 0.066 |
| Sex | 21 females (58%) 15 males (42%) | 17 females (40%) 25 males (60%) | 0.173 |
| BMI | 30 (±6) | 33 (±7) | 0.012 |
| ASES | 38 (±21) | 36 (±17) | 0.523 |
| SSV | 30 (±25) | 39 (±31) | 0.218 |
| VAS | 5.5 (±2.8) | 6.2 (±2.3) | 0.385 |
| Glenosphere size | 27 size 36 mm (75%) 4 size 39 mm (11%) 5 size 42 (14%) | 17 size 36 mm (41.5%) 8 size 39 mm (19%) 5 size 42 (41.5%) | 0.007 |
| Glenosphere offset | 7 neutral (19.4%) 28 lateral 4 mm (77.8%) 1 inferior eccentric (2.8%) | 5 neutral (12%) 37 lateral 4 mm (88%) 0 inferior eccentric (0%) | 0.279 |
| Humeral cup offset | 20 neutral (55.5%) 11 posterior (30.5%) 5 anterior (14%) | 18 neutral (43%) 24 posterior (57%) 0 anterior (0%) | 0.006 |
ASES, American Shoulder and Elbow Surgeons; BMI, body mass index; mm, millimeters; ROM, range of motion, SSV, subjective shoulder value; VAS, visual analog scale. p values based on comparing differences between the two groups.
Radiographic analysis
The relationship between radiographic measurements and outcome is presented in Table 2. The only factor associated with an excellent outcome was the IGO. Patients with an excellent outcome had a mean inferior overhang of the glenosphere of 3.1 mm beyond the inferior glenoid, compared to 1.4 mm for patients with a poor outcome (p = 0.002). Another representation of inferior position, the ACD trended toward, but did not reach statistical significance (42.6 mm vs 39.4 mm; p = 0.072). Lateralization of the glenosphere (GLO) also trended toward but did not reach statistical significance (27.2 mm vs 25.8 mm; p = 0.089).
Table 2.
Radiographic measurements and outcomes.
| Poor ROM (n = 36) | Excellent ROM (n = 42) | p value | |
|---|---|---|---|
| Preoperative AHD (mm) | 8.0 (±4.9) | 10.0 (±6.8) | 0.277 |
| Postoperative AHD (mm) | 32.6 (±8.3) | 34 (±7.6) | 0.417 |
| Δ AHD (mm) | 24.6 (±7.5) | 24.1 (±8.4) | 0.782 |
| ACD (mm) | 39.4 (±7.9) | 42.6 (±7.5) | 0.072 |
| IGO (mm) | 1.4 (±2.5) | 3.1 (±2.1) | 0.002 |
| Distalization angle | 46.5° (±8.9°) | 47.9° (±10.2°) | 0.545 |
| Acromial index | 0.75 (±0.11) | 0.76 (±0.14) | 0.813 |
| LHO (mm) | 14.1 (±7.0) | 13.3 (±9.5) | 0.694 |
| GLO (mm) | 25.8 (±3.8) | 27.2 (±3.5) | 0.089 |
| Lateralization angle | 85.0° (±9.5°) | 81.4° (±6.5°) | 0.165 |
| Forward flexion | 96° (±34°) | 152° (±8°) | <0.001 |
| External rotation | 22° (±20°) | 46° (±11°) | <0.001 |
| ASES | 68 (±22) | 83 (±14) | <0.001 |
| SSV | 63 (±28) | 73 (±22) | 0.154 |
| VAS | 2.2 (±2.7) | 1 (±1.3) | 0.069 |
ACD, acromial–center of rotation distance; AHD, acromiohumeral distance; ASES, American Shoulder and Elbow Surgeons; GLO, glenosphere lateral offset; IGO, inferior glenosphere offset; LHO, lateral humeral offset; mm, millimeters; SSV, subjective shoulder value; VAS, visual analog scale; Δ: the difference between postoperative and preoperative values. p values based on comparing differences between the two groups.
Multivariate analysis
In the first multivariate analysis, basic patient factors were evaluated with glenosphere size, humeral cup offset, the ACD, GLO, and IGO. Age, sex, and BMI were not significantly associated with outcome (p > 0.05). In this analysis, glenosphere size and IGO remained significant. A glenosphere size of 39 mm was associated with a 9.2 times increased chance of achieving an excellent outcome (p = 0.03). Each millimeter of inferior overhang of the glenosphere was associated with a 1.6 times increased chance of achieving an excellent outcome (p < 0.001). Posterior offset of the humeral cup did not remain significant, although anterior offset was negatively associated with an excellent outcome (Table 3).
Table 3.
Multivariate analysis of radiographic and implant factors.
| Variable | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| 39-mm glenosphere | 9.1 | 1.2–122.5 | 0.030 |
| 42-mm glenosphere | 5.2 | 1.2–27.9 | 0.024 |
| 1 mm of IGO | 1.6 | 1.2–2.2 | <0.001 |
| Posterior offset humeral cup | 1.7 | 0.4–6.7 | 0.512 |
| Anterior offset humeral cup | 0.1 | 0–0.7 | 0.026 |
IGO, inferior glenosphere overhang; mm, millimeters.
A second multivariate analysis was performed in an attempt to control for preoperative ROM. In this model, a higher preoperative FF trended toward slightly increasing the chance of obtaining an excellent outcome (odds ratio = 1.01, p = 0.057), whereas preoperative ER did not reach statistical significance. The IGO remained significant, with every 1-mm increase in inferior overhang being associated with a 1.5 time increase in the chance of achieving an excellent outcome (p = 0.006). On the other hand, glenosphere size did not reach significance (p > 0.05) (Table 4).
Table 4.
Second multivariate analysis of radiographic and implant factors.
| Variable | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Preoperative FF | 1.01 | 1–1.03 | 0.057 |
| Preoperative ER | 1.02 | 0.99–1.05 | 0.218 |
| ACD | 0.99 | 0.91–1.07 | 0.795 |
| GLO | 1.04 | 0.86–1.26 | 0.674 |
| 39-mm glenosphere | 4.05 | 0.6–26.4 | 0.144 |
| 42-mm glenosphere | 2.75 | 0.55–13.74 | 0.217 |
| 1 mm of IGO | 1.51 | 1.13–2.02 | 0.006 |
| Posterior offset humeral cup | 1.22 | 0.36–4.20 | 0.748 |
| Anterior offset humeral cup | 0.03 | <0.01–1.23 | 0.065 |
ACD, acromial–center of rotation distance; ER, external rotation; FF, forward flexion; GLO, glenosphere lateral offset; IGO, inferior glenosphere overhang; mm, millimeters.
Discussion
The aim of this study was to evaluate the relationship between postoperative ROM following RSA and component selection as well as position, using different radiographic markers. The major findings were that larger glenospheres, a posterior offset humeral cup, and increased IGO were associated with excellent postoperative outcomes. These findings may help better understand what factors lead to different postoperative results as they can have implications on patient counseling, in addition to surgical technique and implant choices.
Determining outcomes after shoulder arthroplasty is not always straightforward. A study by Roy et al. evaluating the relationship between different patient-reported outcome measures following RSA showed that even though 93% of patients were very satisfied with their care, not all of them were able to achieve comparable scores on the other outcome tests studied. Their results also showed that, depending on the test used, ROM between the good outcome groups can vary by up to 20° for FF and 3° for ER. 11 Moreover, there is substantial evidence that not all patients achieve satisfactory improvements in ROM following RSA.4,5,11 For instance, Simovitch et al. showed that when ROM was stratified based on substantial clinical benefit (SCB) (i.e. a pre- to postoperative improvement of 35° for FF and 12° for ER), 38% of patients did not achieve the SCB for FF and 31% were not able to achieve the SCB for ER. 5 Moreover, Jeon and Rhee allocated patients who underwent RSA using a Grammont implant into satisfactory and unsatisfactory groups based on their postoperative FF (FF < 100° considered as unsatisfactory) and found that 22.1% of patients did not achieve a satisfactory result. Based on the definition used in our study, we found that 22.5% of patients had a poor outcome. 4
Previous evidence has pointed toward larger glenosphere diameters having a positive effect on ROM.12–14 Berhouet et al. showed that a larger glenosphere (42 mm) is associated with better rotation ROM compared to a smaller one (36 mm) (p < 0.05). 12 Moreover, a study comparing functional scores and ROM differences between two groups of patients, one receiving a 36-mm glenosphere and the other receiving a 44-mm glenosphere, found that patients with the larger glenosphere had a 12° increase in ER in adduction compared to those with the smaller glenosphere (p < 0.001), at 12 months and 60 months follow-up intervals. 14 Similarly, Mollon et al. showed that a 42-mm glenosphere size generated a 15° improvement in FF and a 6° improvement in ER compared to the 38 mm size, with an overall improvement in pain scores. They also suggested that these results could be gender specific, with males experiencing less improvement in FF. 13 Although we found similar associations with respect to glenosphere size and ROM, we found no evidence of gender being a significant factor in achieving and excellent outcome. Nevertheless, in the absence of patient-specific criteria to guide glenosphere size choices, this process is still surgeon dependent and is strongly influenced by gender and stature. 15 Moreover, it is still unclear how big is too big with respect to diameter size, with evidence pointing toward certain tradeoffs that come with larger glenospheres, such as limitation in internal rotation 16 and higher polyethylene volumetric wear rates. 17 This highlights the need for more variation in size and design, which could help surgeons manage patient-to-patient variability and lead to more predictable outcomes.
Previous research has suggested that humeral component offset can influence ROM. A posterior position would be better expected to mimic normal anatomy given that the humeral head is typically offset posterior and medial relative to the humeral canal. 18 Biomechanical studies evaluating humeral tray positioning showed that posterior and postero-lateral offset maximized impingement-free ROM.19,20 On the other hand, in a biomechanical study, Dedy et al. reported a decrease in FF with a posterior offset humeral cup. 21 However, their biomechanical model utilized a medialized glenosphere with a 155° humeral stem. In the current study, we used a 135° humeral stem. We theorize that the higher percentage of excellent results in the posterior offset group was due to better reduction of anatomy and thus less bony impingement.
In a computer modeling study examining the effect of different RSA designs on both deltoid and rotator cuff muscles, Roche et al. found that, regardless of implant design, the center of rotation (COR) shifted medially and inferiorly compared to the normal shoulder, but to different degrees between designs. When they controlled for stem design (Grammont stem) and component parameters such as size and position, lateralized glenosphere constructs resulted in similar distalization but an 8-mm increase in lateralization. 22 Clinical studies have shown that glenospheres with a lateralized COR can improve ER. 23 Greiner et al. observed a trend toward improved ER in lateralized glenosphere constructs compared to non-lateralized ones, with a statistically significant improvement in ER in patients with an intact teres minor. 24 In the current study, lateralization of the glenoid components trended toward being higher in the excellent group, but this difference did not reach statistical significance (GLO 27.2 mm vs 25.8 mm; p = 0.089). However, computer modeling studies have demonstrated that 5 mm or more of lateralization (with a 135° humerus) is ideal for decreasing bony impingement.25,26 For instance, Keener et. al performed 3D RSA preplanning on 10 shoulders with severe glenoid retroversion deformities. They demonstrated that in the setting of advanced glenoid osteoarthritic deformities, optimal ROM was achieved with 10-mm baseplate lateralization, and neutral to 5° of retroversion coupled with a varus stem having a 135° neck shaft angle. Failure to find a significant association between increased lateralization and having an excellent outcome could be attributed to the fact that with the glenosphere used in this study, only 4 mm of lateralization was available. It is thus possible that differences could be detected with greater amounts of lateralization (i.e. 6–8 mm of lateralization).Yet, with the glenosphere evaluated in the current study, only 4 mm of lateralization was available. Lateralization can also be achieved on the humeral side. Merolla et al. compared clinical outcomes of a lateralized stem construct to a non-lateralized one and found that although there was a higher delta improvement of ER in the onlay group, 27 we did not find a relationship between the different measurements of humeral lateralization (LHO and AI) and outcomes. However, this interpretation is limited by the fact that all patients received the same stem design.
Inferior glenoid positioning, which leads to COR inferiorization, has been shown to decrease scapular notching and improve adduction and abduction angles,28,29 but its effect on axial and sagittal ROM is not fully understood. Li et al. evaluated the effect of different glenosphere positions and found that inferior translation resulted in improved rotation at different degrees of abduction, whereas superior translation limited ROM. 30 Another study evaluating radiographic and outcome differences between a concentric and 4-mm eccentric glenosphere showed that inferior offset measured was 1 mm higher in the eccentric group, and that this group witnessed an increase of 4° in ER and 15° in FF compared to the concentric group. 31 Our results showed that inferior offset was 1.7 mm higher in the excellent group compared to the poor group (3.1 mm vs 1.4 mm p = 0.002). Moreover, ACD, another measurement of inferiorization of the COR, trended toward but did not reach statistical significance (42.6 mm vs 39.4 mm; p = 0.072).
Choi et al., 32 however, found that a 4-mm increase in inferior offset in the eccentric group (5.8 mm vs 2.0 mm), was not associated with a statistically significant difference in postoperative ROM between the eccentric and concentric groups, which could indicate that there might be a certain degree of inferiorization beyond which ROM benefits could diminish.
Humeral lengthening, represented by an increase in AHD, has been linked to improvements in FF and can be affected by stem design as well as surgical technique, which includes the amount of humeral head resection and how inset or onset a stem is placed with respect to the level of the resection.22,33 In excess, however, arm lengthening can have deleterious effects such as nerve injury34,35 and increased risk of scapular spine fracture. 36 Lengthening has been shown to be a key factor in obtaining adequate deltoid tension, where postoperative FF was found to be significantly greater in patients with arm lengthening compared to shortening (145° vs 122° p < 0.001). 37 Jobin et al. showed that deltoid lengthening resulting in >38-mm increase in AHD had a 90% positive predictive value of obtaining 135° of active FF. 38 Another study showed that an increase in subacromial space distance of 33–50% was associated with FF ≥ 120° (p = 0.001). 34 Sabesan et al., however, suggested that deltoid lengthening does not correlate with improvements in FF or ER. They showed a trend toward a negative correlation between increased AHD and FF, which could signify a negative effect from deltoid over tensioning. 39 Moreover, a study evaluating the association between radiographic markers and outcomes, found no association between AHD and ROM, 7 which was similar to our findings that showed that there was no difference in AHD between the two groups (32.6 mm vs 34.1 mm; p = 0.417). Distalization angle (DSA) is another measurement of distalization that can affect FF, with the highest improvement reported to be between 40° and 65°. 6 Our study showed that there was no association between DSA and ROM, which could be due to the fact that different configurations of inlay and onlay stems were used in the original study that described this measurement, as compared to the inlay stem used in this study.
There are several limitations to the current study. First, the design of the study was retrospective and component position was not controlled but rather left to surgeon choice. Since this was a multi-surgeon study, differences in surgical experience, technique, as well as postoperative rehabilitation protocols are factors that were not controlled for and might have influenced the association between the evaluated parameters and ROM outcomes. Second, we did not examine patient factors that could impact ROM such as patient height and muscle quality. Third, our measurements are based on radiographic analysis in the coronal plane only; computed tomography would provide a better assessment of three-dimensional position. Finally, and most importantly, we are unable to provide patient-specific guidelines based on the current study. It is likely that there is an ideal component size and position for each patient based on patient size and remaining muscle quality. Future studies should be directed toward defining patient-specific recommendations.
Conclusion
Approximately 20% of patients may not achieve satisfactory ROM following a primary RSA. Larger glenosphere size and inferior positioning as well as posterior humeral offset are associated with improved postoperative ROM following RSA.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PJD is a paid consultant for and receives royalties from Arthrex, Inc. PJD and GH, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article. AL is a paid consultant for Arthrex, Wright, and Medacta and receives royalties from Wright. RUH is a paid consultant for Arthrex, Inc. BOP, ESL, and JMT are paid consultants for and receive royalties from Arthrex, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Arthrex Inc., Naples, FL.
Ethical Review and Patient Consent: Obtained prior to the study. SO IRB#: 16-014. Consent not applicable.
Guarantor: PD.
ORCID iD: Patrick J Denard https://orcid.org/0000-0002-2641-5920
References
- 1.Boileau P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15(5): 527–540. [DOI] [PubMed] [Google Scholar]
- 2.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004; 86(3): 388–395. [DOI] [PubMed] [Google Scholar]
- 3.Werner CM, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005; 87(7): 1476–1486. [DOI] [PubMed] [Google Scholar]
- 4.Jeon YS, Rhee YG. Factors associated with poor active anterior elevation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27(5): 786–793. [DOI] [PubMed] [Google Scholar]
- 5.Simovitch R, Flurin PH, Wright T, et al. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg 2018; 27(2): 298–305. [DOI] [PubMed] [Google Scholar]
- 6.Boutsiadis A, Lenoir H, Denard PJ, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27(7): 1226–1234. [DOI] [PubMed] [Google Scholar]
- 7.Roberson TA, Shanley E, Abildgaard JT, et al. The influence of radiographic markers of biomechanical variables on outcomes in reverse shoulder arthroplasty. JSES Open Access 2019; 3(1): 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lädermann A, Denard PJ, Boileau P, et al. What is the best glenoid configuration in onlay reverse shoulder arthroplasty?. Int Orthop 2018; 42(6): 1339–1346. [DOI] [PubMed] [Google Scholar]
- 9.Nyffeler RW, Werner CM, Sukthankar A, et al. Association of a large lateral extension of the acromion with rotator cuff tears. J Bone Joint Surg Am 2006; 88(4): 800–805. [DOI] [PubMed] [Google Scholar]
- 10.Ferle M, Pastor MF, Hagenah J, et al. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg 2019; 28(5): 966–973. [DOI] [PubMed] [Google Scholar]
- 11.Roy JS, MacDermid JC, Goel D, et al. What is a successful outcome following reverse total shoulder arthroplasty?. Open Orthop J 2010; 4: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berhouet J, Kontaxis A, Gulotta LV, et al. Effects of the humeral tray component positioning for onlay reverse shoulder arthroplasty design: a biomechanical analysis. J Shoulder Elbow Surg 2015; 24(4): 569–577. [DOI] [PubMed] [Google Scholar]
- 13.Mollon B, Mahure SA, Roche CP, et al. Impact of glenosphere size on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 297 shoulders. J Shoulder Elbow Surg 2016; 25(5): 763–771. [DOI] [PubMed] [Google Scholar]
- 14.Müller AM, Born M, Jung C, et al. Glenosphere size in reverse shoulder arthroplasty: is larger better for external rotation and abduction strength?. J Shoulder Elbow Surg 2018; 27(1): 44–52. [DOI] [PubMed] [Google Scholar]
- 15.Schoch BS, Vasilopoulos T, LaChaud G, et al. Optimal glenosphere size cannot be determined by patient height. J Shoulder Elbow Surg 2020; 29(2): 258–265. [DOI] [PubMed] [Google Scholar]
- 16.Langohr GD, Giles JW, Athwal GS, et al. The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg 2015; 24(6): 972–979. [DOI] [PubMed] [Google Scholar]
- 17.Haggart J, Newton MD, Hartner S, et al. Neer Award 2017: wear rates of 32-mm and 40-mm glenospheres in a reverse total shoulder arthroplasty wear simulation model. J Shoulder Elbow Surg 2017; 26(11): 2029–2037. [DOI] [PubMed] [Google Scholar]
- 18.Boileau P, Walch G. The three-dimensional geometry of the proximal humerus: implications for surgical technique and prosthetic design. J Bone Joint Surg Br 1997; 79(5): 857–865. [DOI] [PubMed] [Google Scholar]
- 19.Berhouet J, Garaud P, Favard L. Influence of glenoid component design and humeral component retroversion on internal and external rotation in reverse shoulder arthroplasty: a cadaver study. Orthop Traumatol Surg Res 2013; 99(8): 887–894. [DOI] [PubMed] [Google Scholar]
- 20.Glenday J, Kontaxis A, Roche S, et al. Effect of humeral tray placement on impingement-free range of motion and muscle moment arms in reverse shoulder arthroplasty. Clin Biomech 2019; 62: 136–143. [DOI] [PubMed] [Google Scholar]
- 21.Dedy NJ, Stangenberg M, Liem D, et al. Effect of posterior offset humeral components on range of motion in reverse shoulder arthroplasty. Int Orthop 2011; 35(4): 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis 2013; 71(4): 284–293. [PubMed] [Google Scholar]
- 23.Helmkamp JK, Bullock GS, Amilo NR, et al. The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2018; 27(11): 2099–2107. [DOI] [PubMed] [Google Scholar]
- 24.Greiner S, Schmidt C, Herrmann S, et al. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg 2015; 24(9): 1397–1404. [DOI] [PubMed] [Google Scholar]
- 25.Keener JD, Patterson BM, Orvets N, et al. Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: a computer-enhanced range of motion analysis. J Shoulder Elbow Surg 2018; 27(2): 339–349. [DOI] [PubMed] [Google Scholar]
- 26.Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26(10): 1726–1731. [DOI] [PubMed] [Google Scholar]
- 27.Merolla G, Walch G, Ascione F, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg 2018; 27(4): 701–710. [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez S, Levy JC, Frankle MA, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg 2008; 17(4): 608–615. [DOI] [PubMed] [Google Scholar]
- 29.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005; 14(5): 524–528. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Dines JS, Warren RF, et al. Inferior glenosphere placement reduces scapular notching in reverse total shoulder arthroplasty. Orthopedics 2015; 38(2): e88–e93. [DOI] [PubMed] [Google Scholar]
- 31.De Biase CF, Ziveri G, Delcogliano M, et al. The use of an eccentric glenosphere compared with a concentric glenosphere in reverse total shoulder arthroplasty: two-year minimum follow-up results. Int Orthop 2013; 37(10): 1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi CH, Kim SG, Lee JJ, Kwack BH. Comparison of Clinical and Radiological Results According to Glenosphere Position in Reverse Total Shoulder Arthroplasty: A Short-term Follow-up Study. Clin Orthop Surg 2017; 9: 83–90. DOI: 10.4055/cios.2017.9.1.83. [DOI] [PMC free article] [PubMed]
- 33.Lädermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop 2015; 39(11): 2205–2213. [DOI] [PubMed] [Google Scholar]
- 34.Lädermann A, Edwards TB, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop 2014; 38(5): 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lädermann A, Lübbeke A, Mélis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am 2011; 93(14): 1288–1293. [DOI] [PubMed] [Google Scholar]
- 36.Werthel JD, Schoch BS, van Veen SC, et al. Acromial fractures in reverse shoulder arthroplasty: a clinical and radiographic analysis. J Shoulder Elbow Arthroplasty 2018; 2: 2471549218777628. [Google Scholar]
- 37.Lädermann A, Walch G, Lubbeke A, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21(3): 336–341. [DOI] [PubMed] [Google Scholar]
- 38.Jobin CM, Brown GD, Bahu MJ, et al. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and center of rotation medialization. J Shoulder Elbow Surg 2012; 21(10): 1269–1277. [DOI] [PubMed] [Google Scholar]
- 39.Sabesan VJ, Lombardo D, Josserand D, et al. The effect of deltoid lengthening on functional outcome for reverse shoulder arthroplasty. Musculoskelet Surg 2016; 100(2): 127–132. [DOI] [PubMed] [Google Scholar]