Abstract
Background
To describe persistent symptoms in long COVID-19 non-severe outpatients and report the 6-month clinical recovery (CR) rate.
Methods
Observational study enrolling outpatients (≥ 18 years) with confirmed non-severe COVID-19 (positive nasopharyngeal RT-PCR or presence of SARS-CoV-2 antibodies) who consulted for persistent symptoms after the first pandemic wave (March-May 2020). CR was assessed at the 6-month visit and defined as complete (no symptom), partial (persistent symptoms of lower intensity) or lack of recovery (no improvement).
Results
Sixty-three patients (79% women, mean age: 48 years) enrolled; main symptoms (mean 81 days after acute infection): asthenia/myalgia (77%), dyspnea (51%), headaches (35%), cough (33%). At 6 months (n = 56), 30% had complete, 57% partial, and 13% lack of recovery. The proportion of patients with > 2 persistent symptoms was 26% at 6 months (main symptoms: dyspnea [54%] and asthenia/myalgia [46%]).
Conclusion
We observed a slow but high recovery rate at 6 months among these outpatients.
Keywords: Recovery, Post-infectious disorders, Long COVID-19, Outpatients
1. Introduction
By the end of March 2021 the pandemic of Corona Virus disease (COVID-19) had exceeded 100 million confirmed cases and 3,000,000 deaths [1], [2]. The clinical pattern of COVID-19 encompasses an heterogeneous spectrum of illness, ranging from asymptomatic/mild infections to severe cases (4–16%) [3], [4]. Among patients with non-severe COVID-19, there is now clear evidence that symptoms may persist [5], [6], [7], with 7% to 65% of persistent disorders according to the delay in follow-up. In a review, Nalbandian et al. [8] gathered post-acute COVID-19 entity as, firstly, subacute or ongoing symptomatic COVID-19 when occurring from 4-12 weeks after acute COVID-19 and, secondly, chronic or post-COVID-19 syndrome when persisting beyond 12 weeks after acute COVID-19 onset. Longitudinal data on the evolution from subacute to chronic stage of long COVID-19 and clinical recovery rate still need to be clarified. We aimed to report our clinical experience with confirmed non-severe COVID-19 outpatients complaining of persistent symptoms. We performed a longitudinal study to describe:
-
•
the persistent disorders defined as long COVID-19 syndrome;
-
•
the clinical management;
-
•
and the 6-month clinical recovery rate in this population.
2. Methods
2.1. Study design
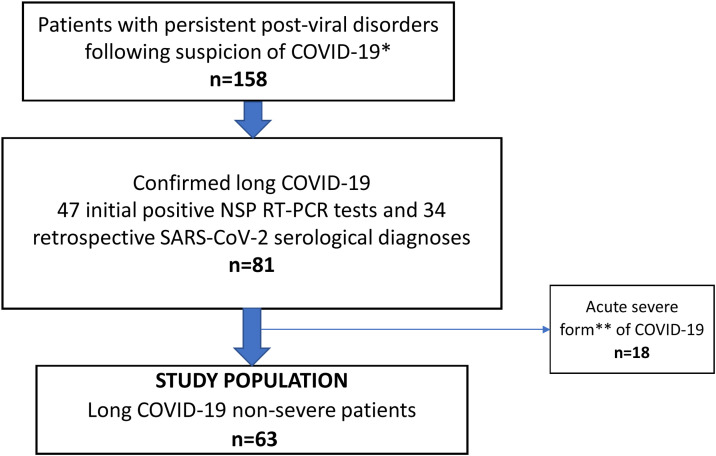
Between May 1 and July 31, 2020 visits were proposed at the Infectious Diseases Department of the Pitié-Salpêtrière Hospital (Paris, France) to adults ≥18 years of age complaining of persistent post viral symptoms following clinical suspicion of COVID-19 (Fig. 1 ). Patients directly contacted the center or were referred by their family physician.
Fig. 1.
Flow chart to identify non-severe long COVID-19 patients. *suspected COVID-19 if a history of the following symptoms was reported: fever (≥ 37.8°), asthenia, myalgia, anosmia, dysgeusia, cough, dyspnea, headaches or diarrhea occurring between March 2020 and the day of the visit ** according to WHO guidelines (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1)
A nasopharyngeal (NSP) RT-PCR test (Roche Cobas® SARS-CoV-2 assay on Cobas 6800 system) was proposed to all patients and SARS-CoV-2 antibody assessment (Abbott SARS-CoV-2 IgG assay [9]) was performed in patients without initial documentation of SARS-CoV-2 (no initial PCR test nor history of immunological SARS-CoV-2 status) to identify confirmed COVID-19 patients.
Two visits were organized: at entry (visit 1,V1) and 6 months after first COVID-19 symptoms onset (visit 2, V2). History and clinical data related to the acute infection and current clinical state were assessed at V1 using questionnaires (Table 1 ). V2 consisted in a phone call to assess persistent manifestations. Patients with acute severe COVID-19 infection according to the World Health Organization (WHO) guidelines [10] were excluded from our analysis.
Table 1.
Questionnaires at visit 1 (at entry) and visit 2 (6-month follow-up) to assess long COVID-19 symptoms among non-severe COVID-19 outpatients.
|
QUESTIONNAIRE VISIT 1 DATE: |
| Previous history of medical disease: Smoking □ yes □ no Current medications: History of acute COVID-19 disease: Date of first COVID-19 symptoms: Fever □ Asthenia/myalgia □ Cough □ Dyspnea □ Chest pain □ Anosmia, dysgeusia □ Headaches □ Sensitive disorders □ Motor disorders □ Digestive disorders □ Cutaneous manifestations □ Arthralgia □ Other: Initial form of acute COVID-19 infection: Severe (including hospitalization)a □ Non severe □ Positive SARS-CoV-2 RT-PCR □ yes (date:) □ no □ not performed If unconfirmed COVID-19 (RT-PCR negative or not performed), specify: clinical suspicion only □ clinical and radiological □ (date of CT chest and type of lesion: minimal □ moderate □ severe □) Treatment during acute COVID-19 infection: none □; antibiotic no □ yes □ (macrolide □ cephalosporin/penicillin □ fluoroquinolone □ other); steroid yes □ no □; specific experimental COVID-19 therapy no □ yes □ (hydroxychloroquine □, lopinavir/r □, tocilizumab □ remdesivir □) Clinical evolution of COVID-19 symptoms from onset of first symptoms: Complete regression: yes □ (specify date) No □ Persistent symptoms: intermittently □ continuously □ Type of persistent disorders: Fever □ Asthenia/myalgia □ Cough □ Dyspnea □ Chest pain □Anosmia, dysgeusia □ Headaches □ Sensitive disorders □ Motor disorders □ Digestive disorders □ Cutaneous manifestations □ Arthralgia □ Any new symptoms? if yes, specify type and date of onset: Controlled CT chest (date and result): normal □ type of lesion minimal □ moderate □ severe □ other: Clinical examination (time since onset of first COVID-19 symptoms) Temperature/blood pressure/pulse/respiratory rate/SpO2 on room air Fever □ Asthenia □ Myalgia □ Cough □ Dyspnea □ Chest pain □ Anosmia, dysgeusia □ Headaches □ Sensitives disorders □ Motor disorders □ Digestive disorders □ Cutaneous manifestations □ Arthralgia □ Physical exam: |
|
QUESTIONNAIRE VISIT 2 (phone call at 6-month follow-up) DATE: |
|
Clinical evolution of COVID-19 symptoms since V1: Hospitalization needed: yes □ no □ Complete regression □ persistent symptoms of lower intensity □ absence of improvement □ Persistent symptoms: intermittently □ continuously □ Type of persistent symptoms: Fever □ Asthenia/myalgia □ Cough □ Dyspnea □ Chest pain □ Anosmia, dysgeusia □ Headaches □ Sensitive disorders □ Motor disorders □ Digestive disorders □ Cutaneous manifestations □ Arthralgia □ |
Severe according to WHO guidelines (respiratory rate > 30 breaths/min or sign of severe respiratory distress or SpO2 < 90% on room air).
2.2. Clinical and biological outcomes
Clinical and biological data were prospectively collected during the visit using an electronic medical data system (NADIS). A blood test was performed including whole blood cell count, dosage of C-reactive protein (CRP), serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), creatininemia, D-Dimer, and interleukin-6. Chest CT scan and cardiac echography were also performed according to clinical symptoms.
Confirmed COVID-19 was defined by a positive SARS-CoV-2 NSP RT-PCR test and/or SARS-CoV-2 serology at any time after initial symptom onset. At V2, complete clinical recovery and partial recovery were respectively defined as complete regression of symptoms and persistent symptoms of lower intensity. Lack of recovery was defined by the absence of clinical improvement.
2.3. Ethical considerations
All patients provided oral informed consent and did not object to their data analysis for research purposes. The research was conducted according to the recommendations outlined in the Helsinki declaration and was approved by an institutional review board (CPP Île-de-France X, Paris, France, No 47-2020).
2.4. Statistical method
Continuous variables (medians and interquartile ranges) were compared using Student's t-test when their distribution was normal and Mann-Whitney test otherwise. Nominal variables (absolute values and percentages) were compared using the Chi-2 test. All statistical analyses were performed using STATVIEW.
3. Results
3.1. Visit 1
Between May and July 2020, 63 long COVID-19 non-severe patients (79% of women, median age 48 years [39–51]) were enrolled in the study. Previous medical history included: 9.5% of pulmonary disease cases (n = 6), 9.5% of hypertension cases (n = 6), and 3% of patients with a history of cancer; 14% (n = 9) were smokers. At acute infection, the main reported COVID-19 symptoms were asthenia/myalgia (83%), anosmia/dysgeusia (76%), cough (62%), dyspnea (52%), and headaches (52%) while persistent long COVID-19 symptoms were mostly asthenia/myalgia (77%) and dyspnea (51%) (Table 2 ). Time between onset of first COVID-19 symptoms and V1 was 81 days (66-106) and 54% (34/63) of patients reported more than two persistent symptoms (number of symptoms 3 [2,3]).
Table 2.
Clinical disorders among long COVID-19 outpatients over 6 months.
| Acute infection n = 63 |
Visit 1 Median time from onset if the first symptoms were 81 days before (66-106) n = 63 |
Visit 2 Median time from onset if the first symptoms were 181 days before (177-195) n = 39 patients with persistent symptoms |
|
|---|---|---|---|
| Symptoms (n, %): | |||
| - Fever | 42 (67) | 9 (14) | 1 (2.6) |
| - Asthenia and/or myalgia | 52 (83) | 47 (77) | 18 (46) |
| - Cough | 39 (62) | 21 (33) | 4 (10) |
| - Dyspnea | 33 (52) | 32 (51) | 21 (54) |
| - Chest pain | 27 (43 | 20 (32) | 15 (38) |
| - Anosmia, dysgeusia | 48 (76) | 10 (16) | 6 (15) |
| - Headaches | 33 (52) | 22 (35) | 6 (15) |
| - Sensitive disorders | 8 (13) | 16 (25) | 4 (10) |
| - Motor disorders | 1 (2) | 4 (6) | 1 (2.6) |
| - Cutaneous manifestations | 6 (9.5) | 8 (13) | 0 (0) |
| - Digestives disorders | 26 (41) | 14 (22) | 1 (2.6) |
| - Arthralgia | 8 (13) | 6 (9.5) | 4 (10) |
All patients had normal physical examination and biological values were within normal ranges (data not shown). Thirty-nine patients (62%) underwent a control SARS-CoV-2 NSP PCR test, and none were positive. Between the acute infection and V1, discontinuous evolution with periods of relief alternating with relapses was reported in 55% of patients.
3.2. Clinical management
Following V1, all patients were systematically referred to their family physician without any specific treatment and if needed to a specialist including: 40% (25/63) to pulmonologists, 38% (24/63) to psychologists, 33% (21/63) to cardiologists, 22% (14/63) to neurologists, 11% (7/63) to psychiatrists, 6% (4/63) to gastroenterologists, and 5% (3/63) to ENT specialists. Additionally, 21% (13/63) of patients were referred to rehabilitation units and 11% (7/63) to speech therapists or physiotherapists, respectively.
Complementary explorations mainly consisted in chest CT scan (63% of patients, n = 40/63), pulmonary function test (16% of patients, n = 10/63), echocardiography (22% of patients, n = 14/63), brain MRI (17% of patients, n = 11/63); upper gastrointestinal endoscopy (8% of patients, n = 5/63); heart MRI (6% of patients, n = 4/63), and electromyography (6% of patients, n = 4/63). The following abnormalities were observed: 25% of patients (n = 10/40) with abnormal CT chest mainly with mild lesion of COVID-19 (n = 8/10); 20% of patients (n = 2/10) with abnormal pulmonary function test; and 21% of patients (n = 3/14) with cardiac abnormalities (pericarditis, n = 2; dilatation of inferior vena cava, n = 1).
3.3. Visit 2: 6-month follow-up
Overall, 56/63 (7 patients lost to follow-up) long COVID-19 patients were contacted (181 days [177–195] from symptom onset): 30% (n = 17) had complete clinical recovery, 57% (n = 32) partial recovery, and 13% (n = 7) did not achieve recovery. Among the 39 patients with persistent symptoms, 26% (n = 10/39) had more than two symptoms (number of symptoms 2 [1–3]) (Table 2). All patients reported intermittent evolution of symptoms since V1. No patient required hospitalization since the initial visit. Dyspnea at acute infection was associated with symptom persistence (p = 0.027).
4. Discussion
In our cohort of long COVID-19 non-severe outpatients, almost 90% presented signs of recovery but only 30% fully recovered at 6 months. The proportion of patients with more than two persistent symptoms decreased over time reaching 27% at 6 months. The main persistent symptoms were asthenia/myalgia and dyspnea.
The singularity of this French descriptive cohort relies on the longitudinal follow-up of exclusively non-severe long COVID-19 patients with an initial complete physical examination and assessment of specific needs for further referral to a specialist while other studies only used survey questionnaires [5], [11]. Except for rehabilitation, no specific therapy was provided. At 6 months 70% of patients were still experiencing long COVID-19 symptoms. This rate is similar to that reported in a recently published Italian Cohort [7] in which 69.5% of patients who experienced mild acute presentation of COVID-19 presented with post-COVID-19 syndrome (median follow-up of 91 days [172–204]). In a Faroes Island cohort [12], 33% of patients had one or two persistent symptoms which is also in line with our findings. While the rate of long COVID-19 symptoms at 6 months seems to be high, it is important to underline that most of our patients (∼90%) reported signs of improvement over time and that the proportion of patients with > 2 symptoms decreased between V1 and V2. This trend is in line with the Danish cohort of approximately 9,000 patients which showed a low risk of severe post-acute COVID-19 complications among outpatients [6].
We found that the most common ongoing symptoms were fatigue/myalgia and dyspnea which is not surprising since COVID-19 is a respiratory disease [3], with pulmonary manifestations as the main reported symptoms during the acute infection. However, other studies [7], [13] reported lower rates of fatigue, ranging from 11% to 30% in non-hospitalized patients. This can be explained by our gathering of symptoms of asthenia and myalgia to assess fatigue, hence overestimating this symptom. Moreover, fatigue symptom often goes hand-in-hand with anxiety which is likely to be present in the COVID-19 pandemic context and can delay recovery (38% of patients were referred to a psychologist). In the Irish cohort, preexisting depression/anxiety was over-represented among those with post-viral fatigue [14]. Our study did not use any specific scale, such as the Chalder fatigue scale, to characterize fatigue symptoms [15], [16].
Finally, our study illustrated the difficulties of long COVID-19 management at the early stage of the pandemic. The restricted access to testing during the lockdown period in France explains that only 30% of the cohort had initial documentation of COVID-19 with positive NSP PCR tests. Consequently, clinical management in these settings requires a whole-patient perspective as illustrated by our multidisciplinary approach [16] with challenging and time-consuming consultations.
5. Limits
This observational single-center cohort was not designed to assess any intervention or treatment. Additionally, as very few patients had performed RT-PCR testing, the impact of SARS-CoV-2 viral load on symptom persistence could not be addressed. Last, the limited number of patients did not enable multivariate analysis to assess factors associated with recovery.
6. Conclusion
There is clear evidence that long COVID-19 symptoms may persist following non-severe forms of acute COVID-19, with intermittent evolution and up to 6 months. At the end of our study, the proportion of patients with unresolved symptoms was small. Non-specific therapy was required in these cases.
Disclosure of interest
The authors declare that they have no competing interest.
Ethical statement
Consent to participate: all patients provided oral informed consent and did not object to their data analysis for research issues (non-opposition regime).
Consent to publish: participants consented to their data publication.
Ethics approval: the research was conducted according to the recommendations outlined in the Helsinki declaration. The study was approved by an institutional review board (CPP Île-de-France X, Paris, France, No 47-2020).
Authors’ contributions
SS, OI, RT, M-AV, and CK contributed to the conception and design of the study.
SS, OI, RT, M-AV, RP, GM, and AF collected clinical and biological data.
BA and A-GM performed the virology analysis. SS, OI, and RT performed the statistical analyses.
SS, OI, RT, GM, VM, BA, A-GM, MAV, and CK wrote the manuscript.
All authors read and approved the final version of the manuscript.
Funding
This study was carried out as part of our routine work.
Availability of data and materials
All authors certified that data and materials support the published claims and comply with field standards.
Acknowledgements
We would like to thank R. Agher for the biological data analysis.
Preliminary data were presented at the French National Infectious Disease Congress, 2020, France (Poster 33).
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int.
- 2.COVID-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus/#countries.
- 3.Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien J.R., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevinsky J.R., et al. Late conditions diagnosed 1-4 months following an initial COVID-19 encounter: a matched cohort study using inpatient and outpatient administrative data - United States March 1-June 30 2020. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund L.C., et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21:1373–1382. doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peghin M., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EUA Authorized Serology Test Performance. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance.
- 10.https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 11.Cousyn L., et al. Olfactory and gustatory dysfunctions in COVID-19 outpatients: a prospective cohort study. Infect Dis Now. 2021 doi: 10.1016/j.idnow.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen M.S., et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelen M., et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6:e005427. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend L., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler C.X., et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. 2021;8:ofab440. doi: 10.1093/ofid/ofab440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HAS. Rapid responses in the context of COVID-19 -Management of COVID+ patients in Physical Medicine and Rehabilitation (MPR), and on return home. https://www.has-sante.fr/upload/docs/application/pdf/2020-05/388_reponse_rapide_covid19__mpr_srr_03-05-20_anglaise_2020-05-05_14-50-42_202.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors certified that data and materials support the published claims and comply with field standards.