Abstract
Background:
Exposure to polycyclic aromatic hydrocarbons (PAHs) is a risk factor for esophageal squamous cell carcinoma (ESCC) in high-incidence areas of China, Iran and Brazil, but PAH assessments have not been conducted in East Africa, another ESCC hot spot.
Objective:
To evaluate demographic or lifestyle factors associated with the PAH biomarker concentrations in the study population, and whether PAH metabolite concentrations showed any associations with esophageal precancerous lesions.
Methods:
We recruited a community-based sample of 289 asymptomatic adults from a rural area of Kenya and performed Lugol’s chromoendoscopy to detect esophageal squamous dysplasia (ESD); participants completed a questionnaire and provided a spot urine specimen. We analyzed urine for seven hydroxylated metabolites of naphthalene, fluorene, phenanthrene, and pyrene at the U.S. National Center for Environmental Health, and compared creatinine-corrected PAH metabolite concentrations with questionnaire data and the presence of ESD.
Results:
PAH metabolite concentrations among never tobacco users in these rural Kenya residents were 2.4 – 28.1 times higher than those reported from never tobacco users in Iran, Brazil and the USA. Female sex, cooking indoors, having no post-primary education, and age <50, but not tobacco use, were positively and significantly associated with PAH metabolite concentrations. Almost all participants used wood as cooking fuel. Nine participants had advanced ESD. Adjusted logistic regression showed a significant association between 2-hydroxynaphthalene (OR=4.19, 95%CI:1.01-17.47) and advanced ESD. All other PAH metabolites had positive but non-significant associations with advanced ESD.
Conclusions:
Urinary PAH metabolite concentrations among never tobacco users are markedly higher in this group from Kenya than in other populations and are associated with indoor cooking with wood on open, unvented stoves. These metabolite concentrations were also associated with the presence of advanced esophageal dysplasia. Our findings underline the importance of assessing alternative cooking conditions to reduce PAH exposure in this population.
Keywords: Esophageal Neoplasms, Polycyclic Aromatic Hydrocarbons, Kenya
Introduction
Esophageal cancer is the seventh most common cancer and the sixth most common fatal cancer worldwide, with an estimated 572,000 new cases and 509,000 deaths in 2018 (1). Esophageal cancer rates vary greatly by geography and are especially high in parts of central and east Asia, the “Asian esophageal cancer belt,” and parts of Brazil (2). Another high-risk region has been identified along the east coast of Africa (3). The highest national incidence, mortality and burden of esophageal cancer globally are found in Malawi (4), and esophageal cancer is the leading cause of cancer mortality in Kenyan men (1). In these high-risk regions in East Africa, esophageal squamous cell cancer (ESCC) comprises 90% of esophageal cancer cases (5). Poor oral health (6, 7), use of local or commercial alcohol drinks (8), drinking hot beverages (9), poverty, diet and smoke exposure (10) have been identified as potential risk factors.
The geographic variation of ESCC suggests the important role of environmental risk factors in its etiology, and unlike low-incidence areas, tobacco use does not seem to be a strong risk factor for ESCC in high-risk regions (11). However, consistently high exposure to tobacco-related carcinogens such as polycyclic aromatic hydrocarbons (PAHs) has been reported in several high incidence areas in China, Brazil, and Iran, (12–15). Similar biomarker studies have not yet been conducted in high-risk parts of East Africa. Internal exposure to PAHs can be assessed by measuring PAHs or their metabolites in body fluids and tissues (16, 17). Urinary metabolites are stable measures of total body PAH exposure in the previous 24-48 hours (18).
PAHs are formed during incomplete combustion of organic materials, and, besides tobacco use, people are exposed to PAHs through vehicle exhaust, forest or crop burning, certain foods and beverages, and cooking and heating methods that involve open burning of biomass fuels (wood, coal or dung) (19–21). A recent systematic review and meta-analysis of case-control studies has shown an independent association between the use of biomass fuels and ESCC, which was strongest when wood was used as fuel (OR=3.90, 95%CI: 2.25-6.77) (22). Most families in rural Kenya cook on open stoves fueled by wood, with no chimneys and little room ventilation, leading to high levels of ambient smoke exposure and blackened walls and ceilings in the cooking areas (Figure 1). We hypothesized that this type of exposure to ambient smoke can result in high concentrations of PAH metabolites in the body and contribute to the increased risk of esophageal cancer.
Figure 1.

Typical cooking area in rural Kenya with blackened walls due to cooking over open stove, and view of smoke billowing through small open window on the outside of the cooking room
In the current study, we measured the concentrations of seven PAH metabolites in urine collected from participants in a community-based study to assess the prevalence of the ESCC precursor lesion, esophageal squamous dysplasia (ESD) (23), in asymptomatic adults residing in southwestern Kenya. The PAH assays were conducted at the Centers for Disease Control and Prevention (CDC) National Center for Environmental Health laboratory. These methods have been used previously to analyze samples from the U.S. National Health and Nutrition Examination Survey (NHANES) (24), as well as a number of other studies in ESCC high-risk populations. The availability of these results measured in the same laboratory using the same standards, gave us the opportunity to compare concentrations of these metabolites across different high-risk and low-risk populations. We also evaluated whether there were demographic or lifestyle factors that were associated with the PAH metabolite concentrations in our study population, and whether PAH metabolite concentrations showed any associations with concurrent biopsy-proven ESD.
Methods
Study Population
Between 2010 and 2013, 305 asymptomatic adults were recruited from communities in rural Bomet County, in southwestern Kenya, for the STEP study, a community-based endoscopic screening study investigating the prevalence of ESD within this population (25). Inclusion criteria included residing within 50 kilometers of Tenwek Hospital, age between 20 and 79 years, and absence of esophageal disease symptoms. Persons with weight loss or medical diseases that would increase risk of endoscopy were excluded. Potential participants were asked to fast for their appointment at Tenwek Hospital. Upon arrival, study personnel explained the study and those who wished to enroll signed an informed consent. Each participant then completed an in-person interview conducted by trained study personnel. Questionnaire items included socio-demographics, family history of esophageal cancer, typical ESCC risk factors found in western populations, such as regular tobacco use (at least once a day for 6 months) or alcohol use (at least once a week for 6 months), as well as possible sources of PAH exposure such as indoor cooking over an open fire, type of fuel used in cooking, and drinking mursik (a traditional drink of fermented milk which is mixed with charcoal before consumption). The study protocol was approved by the Human Subjects Review Committee of Tenwek Hospital and the Ethics Review Committee of the University of Nairobi (Protocol Number P172/06/2010; Clinicaltrials.gov identifier NCT01981876). Analysis of blinded specimens and anonymized data was exempted from review by the corresponding human subjects research offices at the US National Cancer Institute (NCI) and the Centers for Disease Control and Prevention (CDC).
Urine collection and quantification of polycyclic aromatic hydrocarbon metabolites
Prior to endoscopy, each fasting participant collected a spot urine specimen in a sterile container that was snap frozen and stored at −80 °C. Frozen urine samples were shipped on dry ice to CDC for analysis using solid phase extraction high performance liquid chromatography tandem mass spectrometry (26). Seven urinary PAH metabolites were quantified for each participant, along with laboratory quality controls that were used to evaluate the between-batch coefficients of variation (CV) for each metabolite. The seven metabolites were 1-hydroxynaphthalene (CV=2.4%), 2-hydroxynapthalene (CV=4.7%), 2-hydroxyfluorene (CV=3.3%), 3-hydroxyfluorene (CV=4.4%), 1-hydroxyphenanthrene (CV=5.6%), the sum of 2- and 3-hydroxyphenanthrene (CV=5.0%), and 1-hydroxypyrene (CV=4.0%). Urine creatinine was also measured at the CDC by enzymatic colorimetry. All PAH and creatinine measurements were above the limits of detection.
Detection of esophageal squamous dysplasia by chromoendoscopy
After the interview was completed, 294 participants successfully underwent Lugol’s iodine chromoendoscopy to detect ESD. The sensitivity of this chromoendoscopy to detect either advanced (moderate or severe) dysplasia or malignancy is greater than 90% (27). Pathological findings of endoscopically directed biopsies included normal esophagus, esophagitis and esophageal squamous dysplasia (ESD). ESD was further divided into mild, moderate, or severe ESD, the last two (advanced dysplasia) being very strong predictors of progression to ESCC (28).
Classification of cooking location
Most families in the study area cook in a separate room detached from the main house, both to keep the smoke from the cooking fires away from the main house and to protect the main house if the cooking room catches on fire. In some homes, however, cooking is done in a separate room inside the main house, and in others (for example one-room homes), cooking is done in the main living area. Finally, a few families cook outside and do not have an indoor area for cooking. In nearly all homes, women do all the cooking, and if there is a separate cooking room it is uncommon for men to enter it. In this study, we asked all participants “Where do you [or your family] do your cooking?, and the participants’ answers were recorded as “inside the house main living area”; “in a separate room inside the house”; “in a separate room outside the house”; or “outside”. For analysis, the first two answers were analyzed as “cooking indoors”, and the last two answers were analyzed as “cooking outdoors”, which is how they are understood in Kenya.
Statistical Analysis
The urine volume of five of the 294 endoscoped patients was insufficient for biomarker analysis, so the analytic cohort for this study included 289 individuals (including 233 never tobacco users). All PAH metabolite concentrations were divided by urinary creatinine to adjust for urine dilution. We calculated geometric means (GM) and 95% confidence intervals (95%CI) of these creatinine-corrected concentrations. We compared metabolite concentrations by strata of the questionnaire variables thought to be most relevant for PAH exposure, and by ESD and advanced ESD status. We also calculated GMs and 95%CIs stratified by sex and household cooking location. Due to the skewed PAH metabolite concentration distributions, p-values for these concentration comparisons were calculated using the nonparametric Wilcoxon-Mann-Whitney rank sum test for two-sample comparisons. We also examined independent associations between PAH concentrations and the same variables using multivariate linear regression with long-transformed creatinine-corrected PAH metabolite concentrations as the outcome variables. We investigated the correlation among log-transformed urinary metabolite concentrations by Pearson correlation.
To compare PAH exposure in the current study to PAH exposure in different populations, we used data from three other populations with PAH biomarker measurements performed by the same laboratory. We compared GMs of creatinine-corrected PAH metabolite concentrations (ng/g creatinine) and restricted the comparison to never tobacco users in all four populations, to eliminate confounding by tobacco use prevalence and intensity. These other populations included an ESCC low-risk population, i.e. the NHANES 2011-2012 from the USA, (24) and two high-risk populations, the MATCH study from southern Brazil (29) and the Golestan Cohort Study from northeastern Iran (30). All the assays were performed in the same lab, using similar standards and procedures and creatinine correction method.
Crude and multivariate associations between PAH biomarker concentrations and participant dysplasia status were examined in logistic regression models. Crude models were adjusted only for creatinine level. Fully adjusted models also included potentially confounding variables including age, sex, education, family history of ESCC, smoking status, alcohol drinking, indoor cooking, and drinking mursik. P-values were two-sided and p< 0.05 was considered statistically significant. All analyses were conducted using Stata 15.0 (StataCorp, College Station, Texas).
Results
Most participants were male (55%), under 50 years of age (54%), completed no more than a primary school education (65%), and had no family history of esophageal cancer (94%). Regular tobacco use (cigarette smoking) and alcohol use were reported by 19% and 32%, respectively. Table 1 also shows the prevalence of other possible sources of PAH exposure in this population, including drinking mursik, (87%) and cooking indoors (12%). All participants except one used wood as their fuel for cooking. One hundred and seven participants (37%) had an endoscopically and histologically normal esophagus, 140 (48%) had esophagitis, and 42 (15%) had some degree of ESD. Of the 42 with ESD, 9 (3%) had advanced (moderate or severe) dysplasia (Table 1).
Table 1:
Geometric means (GM) and 95% confidence intervals (95% CI) for creatinine-corrected urine PAH metabolite concentrations (ng/g creatinine) by the characteristics of the STEP study population
| Samples, N (%) | 1-hydroxy naphthalene GM (95% CI) |
2-hydroxy naphthalene GM (95% CI) |
2-hydroxy fluorene GM (95% CI) |
3- hydroxy fluorene GM (95% CI) |
1- hydroxy phenanthrene GM (95% CI) |
2 & 3-hydroxy phenanthrene GM (95% CI) |
1-hydroxy pyrene GM (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 158 (55) | 21466 (18614-24754) |
17603 (15817-19591) |
1993 (1805-2201) |
1384 (1245-1538) |
1151 (1045-1268) |
1495 (1346-1661) |
1488 (1311-1689) |
| Female | 131 (45) | 36206 (31875-41125)** |
36942 (32851-41544)** |
4490 (4030-5002)** |
2927 (2617-3273)** |
2317 (2094-2565)** |
2957 (2637-3315)** |
4509 (3952-5144)** |
|
| ||||||||
| Age | ||||||||
| < 50 | 157 (54) | 27613 (24339-31327) |
26659 (23819-29838) |
3146 (2827-3501) |
2203 (1984-2446) |
1642 (1485-1817) |
2183 (1957-2435) |
2786 (2413-3218) |
| >=50 | 132 (46) | 26729 (22679-31502) |
22423.0 (19436-25869)* |
2593 (2255-2982)* |
1674 (1450-1933)** |
1511 (1327-1720) |
1876 (1634-2153) |
2120 (1785-2518)** |
|
| ||||||||
| Education | ||||||||
| Post-primary | 100 (35) | 24414 (20007-29792) |
20242 (17153-23887) |
2413 (2072-2811) |
1685 (1447-1963) |
1369 (1185-1580) |
1765 (1522-2047) |
1989 (1644-2406) |
| Primary or less | 189 (65) | 28809 (25715-32276)** |
27330 (24649-30301)** |
3163 (2852-3506)** |
2096 (1884-2331)** |
1706 (1550-1878)** |
2197 (1976-2442)** |
2752 (2403-3152)** |
|
| ||||||||
| Family history of esophageal cancer | ||||||||
| Present | 17 (6) | 32791 (17711-60712) |
20819 (13663-31721) |
2519 (1623-3912) |
1628 (1049-2528) |
1327 (950-1855) |
1762 (1163-2668) |
2039 (1208-3444) |
| Absent | 272 (94) | 26330 (23502-29498) |
24911 (22715-27320) |
2909 (2662-3179) |
1970 (1801-2155) |
1601 (1473-1740) |
2058 (1882-2249) |
2492 (2222-2796) |
|
| ||||||||
| Regular tobacco Use | ||||||||
| Yes | 56 (19) | 31172 (24929-38980) |
25108 (20755-30375) |
2714 (2283-3228) |
1941 (1632-2309) |
1504 (1248-1813) |
1967 (1613-2400) |
2068 (1665-2569) |
| No | 233 (81) | 26952 (23734-30607) |
24520 (22147-27148) |
2921 (2645-3227) |
1944 (1757-2151) |
1600 (1462-1750) |
2054 (1865-2262) |
2564 (2256-2914) |
|
| ||||||||
| Regular alcohol use | ||||||||
| Yes | 92 (32) | 27368 (23171-32325) |
22844 (19819-26331) |
2588 (2258-2967) |
1747 (1525-2002) |
1461 (1273-1677) |
1916 (1658-2214) |
2049 (1730-2428) |
| No | 197 (68) | 26952 (23734-30607) |
25336 (22617-28381) |
3010 (2697-3359) |
2030 (1815-2271) |
1632 (1478-1802) |
2080 (1868-2316) |
2672 (2316-3082)* |
|
| ||||||||
| Drinking mursik | ||||||||
| Yes | 252 (87) | 27865 (24984-31078) |
24710 (22453-27194) |
2858 (2601-3139) |
1935 (1761-2127) |
1568 (1436-1710) |
2035 (1853-2234) |
2416 (2140-2727) |
| No | 37 (13) | 23111 (17598-30352) |
24116 (18528-31389) |
3039 (2427-3805) |
2001 (1562-2563) |
1675 (1353-2073) |
2048 (1617-2593) |
2778 (2088-3695) |
|
| ||||||||
| Cooking indoors † | ||||||||
| Yes | 34 (12) | 39776 (29570-53506) |
39431 (31403-49511) |
4337 (3458-5439) |
3048 (2452-3787) |
2166 (1768-2653) |
2959 (2358-3714) |
3823 (2852-5124) |
| No | 254 (88) | 25862 (23246-28773)* |
23136 (21047-25432)** |
2727 (2488-2989)** |
1830 (1667-2009)** |
1516 (1391-1652)** |
1938 (1768-2124)** |
2319 (2059-2611)** |
|
| ||||||||
| Any Dysplasia | ||||||||
| Present | 42 (15) | 27527 (21143-35840) |
26128 (20188-33815) |
3099 (2462-3901) |
2019 (1567-2600) |
1749 (1418-2158) |
2158 (1695-2749) |
2842 (2127-3797) |
| No | 247 (85) | 27151 (24320-30312) |
24270 (22158-26583) |
2844 (2590-3124) |
1931 (1758-2121) |
1554 (1424-1696) |
2017 (1838-2213) |
2400 (2126-2709) |
|
| ||||||||
| Moderate/Severe Dysplasia | ||||||||
| Present | 9 (3) | 34698 (22225-54172) |
39122 (24371-62800)* |
3849 (2384-6215) |
2735 (1563-4786) |
2259 (1430-3568) |
2663 (1618-4382) |
3579 (1810-7076) |
| Absent | 280 (97) | 26994 (24338-29938) |
24270 (22158-26583) |
2853 (2613-3116) |
1922 (1758-2102) |
1563 (1440-1696) |
2019 (1849-2205) |
2430 (2170-2721) |
p<0.05;
p<0.01 using Wilcoxon-Mann-Whitney nonparametric rank-sum test
One person was missing the information on cooking location.
Abbreviations: 2-3- hydroxyphenanthrene: the sum of 2-and 3- hydroxyphenanthrene.
Table 1 also compares the geometric means and 95% confidence intervals for PAH metabolites across different strata in the study. PAH biomarker concentrations were significantly higher in women, those who cooked indoors, those with no post-primary education, and those who were less than 50 years old. Noteworthy, PAH metabolite concentrations in women were approximately twice those in men, suggesting much higher PAH exposure. This was true across all quartiles of age and seven PAH biomarkers (Supplementary Figure 1). Regular tobacco use or drinking mursik were not associated with PAH metabolite concentrations. Because all participants except one used wood as fuel, we could not make a formal comparison based on fuel type. In multivariate analyses adjusted for all the factors listed in Table 1, cooking indoors, female sex, younger age and lower education were all significant independent predictors of the PAH biomarker concentrations. The concentrations of all seven PAH biomarkers highly correlated with each other (correlation coefficients 0.6-0.9; all p<0.0001) (Supplementary Figure 2).
The urine PAH biomarker geometric means and 95% confidence intervals of all participants, women, and men, stratified by their family’s cooking location, are shown in Supplementary Table 1. For each cooking location, the concentrations in women were much higher than in men, and for both women and men concentrations for those cooking indoors were greater than those for cooking outside. Overall, the highest concentrations were for participants cooking inside the house main living area, followed by those cooking in a separate room inside the house, those cooking in a separate room outside (detached from) the house, and by those cooking outside.
Figure 2 and Supplementary Table 2 compare the geometric means and 95% CI of the seven creatinine-corrected PAH biomarkers measured in never tobacco users in the U.S. NHANES, the MATCH study from southern Brazil, the Golestan Cohort Study from northeast Iran, and the present study. The metabolite concentrations were 2.4 – 28.1 times higher in the STEP Kenyan never tobacco users than in any of the other populations.
Figure 2.
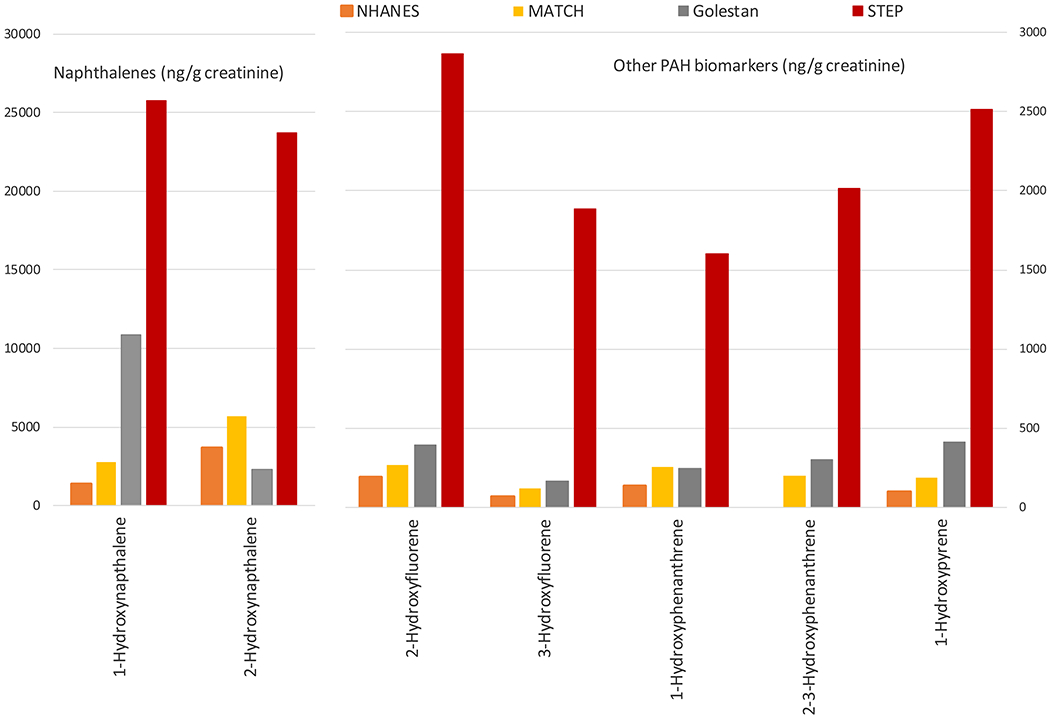
Geometric means of urinary biomarkers of polycyclic aromatic hydrocarbons among never tobacco users in different populations. NHANES 2011-2012 from the United States; MATCH study from southern Brazil; Golestan Cohort Study from northeast Iran; the STEP study from Kenya (the present study)
Individuals with moderate or severe dysplasia had higher geometric means of all PAH metabolites than those without these lesions, but these differences did not reach statistical significance except for 2-hydroxynaphthalene (Table 1). Logistic regression models adjusted for confounders also showed a significant association between 2-hydroxynaphthalene and biopsy-proven advanced dysplasia (adjusted OR 4.19, 95% CI: 1.01-17.47, p<0.05), and all other PAH metabolites had positive but non-significant associations with advanced dysplasia (Table 2).
Table 2:
Associations between continuous creatinine-corrected urinary PAH biomarker concentrations and risk of moderate or severe dysplasia.
| Model | Crude OR (95% CI)* |
p value | Adjusted OR (95% CI)ƚ |
p value |
|---|---|---|---|---|
| 1-hydroxynaphthalene | 1.63 (0.64-4.16) |
0.31 | 1.45 (0.48-4.33) |
0.51 |
| 2-hydroxynaphthalene | 3.14 (1.05-9.40) |
0.04 | 4.19 (1.01-17.47) |
0.05 |
| 2-hydroxyfluorene | 2.10 (0.71-6.19) |
0.18 | 2.21 (0.57-8.62) |
0.25 |
| 3-hydroxyfluorene | 2.59 (0.80-8.35) |
0.11 | 2.81 (0.69-11.35) |
0.15 |
| 1-hydroxyphenanthrene | 3.22 (0.85-12.20) |
0.09 | 4.96 (0.84-29.22) |
0.08 |
| 2-3-hydroxyphenanthrene | 1.77 (0.59-5.27) |
0.31 | 1.58 (0.46-5.44) |
0.19 |
| 1-hydroxypyrene | 1.82 (0.74-4.47) |
0.47 | 2.28 (0.67-7.78) |
0.19 |
OR: odds ratio per 1 natural log (~2.7 times) increase in PAH metabolite concentration
95%CI: 95% confidence interval
Crude models adjusted only for creatinine
Adjusted for age, sex, ≥ primary education, family history of esophageal cancer, reported smoking status, drinking alcohol, indoor cooking, mursik drinking, and creatinine
Discussion
In this community-based study conducted in rural, southwestern Kenya, we found very high concentrations of all of the measured PAH biomarkers; these concentrations were 2.4 – 28.1 times higher among Kenyan non-smokers than any previously reported sample of never tobacco users selected from a general population, including not only a population at low risk of ESCC from the USA, but also populations with high ESCC risk from northeast Iran and southern Brazil, which are also known for their high exposure to PAHs. Female sex, cooking indoors, younger age, and no post-primary education were positively associated with concentrations of these biomarkers, while tobacco use did not seem to play a role.
High PAH exposure is a consistent finding in high-risk areas for ESCC (11), and we expected similar findings in this understudied high-risk population from East Africa. But the urinary PAH metabolite concentrations in the current study were dramatically higher than those reported in previous urine metabolite studies of other populations. As with many other high-risk populations (15), the exposure to PAHs in this population seems to come mainly from non-tobacco sources: only 19% of the current study participants reported regular tobacco use, and tobacco use was not positively associated with metabolite concentrations. Identifying the non-tobacco source(s) that result in high PAH biomarker concentrations are important, since this knowledge can translate into public health interventions to reduce exposure. For example, in southern Brazil, the yerba maté brands commonly used in maté drinks contain high PAH concentrations (31), which can be reduced if the leaves are not smoked during commercial processing (32).
The present study suggests two important sources for the exceptionally high PAH exposures: cooking in an enclosed room on open, unvented stoves, without chimneys or adequate room ventilation; and the use of wood as cooking fuel. As noted previously, most families in rural Kenya cook indoors on open unvented stoves, leading to high levels of ambient smoke exposure and blackened walls and ceilings in the cooking area (Figure 1). Usually, this cooking area is a separate room, detached from the main house, and only the women and small children go into that room. Indeed, some think it is taboo for men to enter or even step anywhere near these cooking rooms, and those who do usually come away coughing because they are not used to the dense smoke inside. This is probably the main reason that the urine PAH biomarkers in women in our study were twice those of men. Stratifying the PAH biomarker concentrations by cooking location also suggests that cooking smoke is an important source of PAH exposure in this population, since the highest PAH biomarkers concentrations, in all participants and in men, were found when cooking was done in the main house living area (where everyone is exposed to cooking smoke, which lingers in the house all day and night), followed by cooking in a separate room within the main house (where some but not all cooking smoke filters out and contaminates other rooms), followed by cooking in a separate room outside the house (where women are exposed while cooking but not in the main house), and by cooking outside. If cooking smoke is indeed a prominent source of PAH exposure in this population, this exposure could probably be significantly mitigated by installing closed cookstoves with chimneys and/or improving the ventilation in the cooking rooms, as has been shown in Xuanwei, China, an area with very high lung cancer rates where residents use predominantly “smoky” (bituminous) coal for cooking and heating. Over a period of 15 years, 81% of the people in Xuanwei changed permanently from unvented indoor firepits to stoves with chimneys, and this was accompanied by a 65% reduction in indoor air pollution and a 40-45% reduction in lung cancer incidence (33).
Previous field studies have shown quite high levels of particulate and PAH emissions from residential wood combustion (similar to the levels from residential coal combustion), including especially high levels of high molecular weight PAHs such as benzo[a]pyrene, which are often carcinogenic (34). Another study showed that the mutagenicity emission factor (which is highly correlated with PAH emissions) for wood-burning stoves can be even higher than that of a diesel generator (35). The investigators concluded that even the most efficient wood-burning stove would expose individuals in the area to highly mutagenic emissions if the stove was indoors without adequate ventilation. Munyeza et al. studied the effects of fuel type on gas-phase PAH emissions in rural and urban households in Kenya, and found that wood, charcoal, kerosene, and gas stoves emitted 454, 88, 43, and 0 benzo[a]pyrene equivalents, respectively (36). We could not formally analyze the effects of fuel type on PAH biomarker concentrations, because all but one person in this study used wood as their cooking fuel. Nevertheless, it seems clear from these earlier studies that burning wood for cooking fuel exposes individuals to much higher levels of PAHs than other fuels (charcoal, kerosene or gas) that are available in Kenya. The difficulty in mitigating this component of the observed high PAH exposure is the higher expense of these other fuels compared to wood.
Presence of moderate or severe squamous dysplasia detected by endoscopy increases the risk of ESCC development 9.8 and 28 times, respectively (28), so these two lesions (collectively referred to as advanced dysplasia) are recognized as precursor lesions for this cancer. Exposure to indoor air pollution from solid fuel use with poor ventilation has been shown to be associated with the risk of both ESCC (22, 37, 38), and ESD (39). However, to the best of our knowledge, this is the first study to examine a direct link between biomarkers of a specific environmental exposure in indoor pollution and ESD. In our study, we found elevated mean concentrations of all 7 PAH biomarkers in the people with advanced dysplasia, and this finding was confirmed in regression models adjusted for other ESCC risk factors, but only the association with 2-hydroxynaphthalene reached statistical significance, probably because we had only nine cases of advanced dysplasia in our study. Other studies with larger sample sizes and more cases of ESD and advanced ESD are needed to evaluate this association more completely.
One limitation of this study is that PAH metabolites in urine are only indicative of recent PAH exposure (18), but these biomarkers are probably a good estimate of exposures that are relatively constant from day to day, such as ambient indoor smoke in cooking areas. A previous study of repeated samples taken over 5 years in the same individuals showed that these urinary biomarkers can be useful in assessing long-term exposure patterns, particularly in the presence of a durable source of exposure (30). Another limitation of the study was the small numbers of participants with esophageal dysplasia, especially moderate or severe dysplasia, as outlined above.
To our knowledge, this is the first study to examine a panel of urinary PAH metabolites in a high-risk region for ESCC in East Africa. In this population, we found much higher than expected urinary concentrations of these metabolites, especially in women who cooked indoors with wood on open stoves without chimneys or adequate room ventilation. We also found a suggestive association between PAH exposure biomarkers and advanced esophageal dysplasia, the precursor lesion of ESCC. These findings may justify community-based efforts, such as installing efficient cookstoves with chimneys, changing to less harmful fuels, and increasing ventilation in the cooking area, to decrease exposure to PAHs, combined with studies to evaluate the impact of such interventions.
Supplementary Material
Acknowledgements
The authors would like to express their appreciation to the residents near Bomet, Kenya who participated in the study and thank the research staff of Tenwek Hospital for their dedication and contributions to the study. The authors would also like to acknowledge Yuesong Wang, Lei Meng, Debra Trinidad, Erin Pittman, Kendra Hubbard, and the late Xiaoyun Ye (CDC) for the PAH biomarkers measurements.
Funding
The African Organization for Research and Training in Cancer’s (AORTIC) Beginning Investigator Grant for Catalytic Research (BIG Cat) Program, in collaboration with the U.S. National Institutes of Health’s (NIH) National Cancer Institute (NCI) Office of International Affairs, and The Intramural Research Program of the NCI, NIH.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- ESCC
esophageal squamous cell carcinoma
- ESD
esophageal squamous dysplasia
- NCI
National Cancer Institute
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
- PAH
polycyclic aromatic hydrocarbon
Footnotes
Publisher's Disclaimer: Disclaimer: The views and opinions expressed in this article are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Declaration of interests: None
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G, McCormack V, Abedi-Ardekani B, Arnold M, Camargo MC, Dar NA, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28(9):2086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng ML, Zhang L, Borok M, Chokunonga E, Dzamamala C, Korir A, et al. The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cancer Epidemiology. 2015;39(2):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators GBDOC. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in western Kenya. Dis Esophagus. 2010;23(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menya D, Maina SK, Kibosia C, Kigen N, Oduor M, Some F, et al. Dental fluorosis and oral health in the African Esophageal Cancer Corridor: Findings from the Kenya ESCCAPE case-control study and a pan-African perspective. Int J Cancer. 2019;145(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mmbaga BT, Mwasamwaja A, Mushi G, Mremi A, Nyakunga G, Kiwelu I, et al. Missing and decayed teeth, oral hygiene and dental staining in relation to esophageal cancer risk: ESCCAPE case-control study in Kilimanjaro, Tanzania. Int J Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menya D, Kigen N, Oduor M, Maina SK, Some F, Chumba D, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer. 2019;144(3):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton DR, Menya D, Kigen N, Oduor M, Maina SK, Some F, et al. Hot beverages and oesophageal cancer risk in western Kenya: Findings from the ESCCAPE case-control study. Int J Cancer. 2019;144(11):2669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mmbaga EJ, Mushi BP, Deardorff K, Mgisha W, Akoko LO, Paciorek A, et al. A Case-Control Study to Evaluate Environmental and Lifestyle Risk Factors for Esophageal Cancer in Tanzania. Cancer Epidemiol Biomarkers Prev. 2021;30(2):305–16. [DOI] [PubMed] [Google Scholar]
- 11.Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154(2):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth MJ, Qiao YL, Rothman N, Tangrea JA, Dawsey SM, Wang GQ, et al. High urine 1-hydroxypyrene glucuronide concentrations in Linxian, China, an area of high risk for squamous oesophageal cancer. Biomarkers. 2001;6(5):381–6. [DOI] [PubMed] [Google Scholar]
- 13.Fagundes RB, Abnet CC, Strickland PT, Kamangar F, Roth MJ, Taylor PR, et al. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking mate in healthy subjects from Rio Grande do Sul, Brazil. BMC Cancer. 2006;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamangar F, Strickland PT, Pourshams A, Malekzadeh R, Boffetta P, Roth MJ, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25(1B):425–8. [PubMed] [Google Scholar]
- 15.Etemadi A, Islami F, Phillips DH, Godschalk R, Golozar A, Kamangar F, et al. Variation in PAH-related DNA adduct levels among non-smokers: the role of multiple genetic polymorphisms and nucleotide excision repair phenotype. Int J Cancer. 2013;132(12):2738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strickland P, Kang D, Sithisarankul P. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ Health Persp. 1996;104:927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abedi-Ardekani B, Kamangar F, Hewitt SM, Hainaut P, Sotoudeh M, Abnet CC, et al. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut. 2010;59(9):1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, et al. Excretion Profiles and Half-Lives of Ten Urinary Polycyclic Aromatic Hydrocarbon Metabolites after Dietary Exposure. Chemical Research in Toxicology. 2012;25(7):1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzetti S Polycyclic Aromatic Hydrocarbons in the Environment: Environmental Fate and Transformation. Polycycl Aromat Comp. 2013;33(4):311–30. [Google Scholar]
- 20.Jacob J The Significance of Polycyclic Aromatic Hydrocarbons as Environmental Carcinogens. 35 Years Research on Paha Retrospective. Polycycl Aromat Comp. 2008;28(4-5):242–72. [Google Scholar]
- 21.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res-Gen Tox En. 1999;443(1-2):139–47. [DOI] [PubMed] [Google Scholar]
- 22.Okello S, Akello SJ, Dwomoh E, Byaruhanga E, Opio CK, Zhang R, et al. Biomass fuel as a risk factor for esophageal squamous cell carcinoma: a systematic review and meta-analysis. Environ Health. 2019;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor PR, Abnet CC, Dawsey SM. Squamous Dysplasia-The Precursor Lesion for Esophageal Squamous Cell Carcinoma. Cancer Epidemiology Biomarkers & Prevention. 2013;22(4):540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. (Centers for Disease Control and Prevention). 2019. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019)-volume 2. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019 [cited 2017. 2019:[Available from: https://www.cdc.gov/exposurereport/. [Google Scholar]
- 25.Mwachiro MM, Burgert SL, Lando J, Chepkwony R, Bett C, Bosire C, et al. Esophageal Squamous Dysplasia is Common in Asymptomatic Kenyans: A Prospective, Community-Based, Cross-Sectional Study. American Journal of Gastroenterology. 2016;111(4):500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YS, Meng L, Pittman EN, Etheredge A, Hubbard K, Trinidad DA, et al. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2017;409(4):931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83(2):220–31. [PubMed] [Google Scholar]
- 28.Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes AB, Metzdorf M, Metzdorf L, Sousa MPR, Kavalco C, Etemadi A, et al. Urinary Concentrations of Polycyclic Aromatic Hydrocarbon Metabolites in Mate Drinkers in Rio Grande do Sul, Brazil. Cancer Epidemiology, Biomarkers & Prevention. 2018;27(3):331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etemadi A, Poustchi H, Chang CM, Blount BC, Calafat AM, Wang L, et al. Urinary Biomarkers of Carcinogenic Exposure among Cigarette, Waterpipe, and Smokeless Tobacco Users and Never Users of Tobacco in the Golestan Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019;28(2):337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamangar F, Schantz MM, Abnet CC, Fagundes RB, Dawsey SM. High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1262–8. [DOI] [PubMed] [Google Scholar]
- 32.Golozar A, Fagundes RB, Etemadi A, Schantz MM, Kamangar F, Abnet CC, et al. Significant variation in the concentration of carcinogenic polycyclic aromatic hydrocarbons in yerba mate samples by brand, batch, and processing method. Environ Sci Technol. 2012;46(24):13488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94(11):826–35. [DOI] [PubMed] [Google Scholar]
- 34.Shen G, Tao S, Wei S, Chen Y, Zhang Y, Shen H, et al. Field measurement of emission factors of PM, EC, OC, parent, nitro-, and oxy- polycyclic aromatic hydrocarbons for residential briquette, coal cake, and wood in rural Shanxi, China. Environ Sci Technol. 2013;47(6):2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutlu E, Warren SH, Ebersviller SM, Kooter IM, Schmid JE, Dye JA, et al. Mutagenicity and Pollutant Emission Factors of Solid-Fuel Cookstoves: Comparison with Other Combustion Sources. Environ Health Perspect. 2016;124(7):974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munyeza CF, Osano AM, Maghanga JK, Forbes PBC. Polycyclic Aromatic Hydrocarbon Gaseous Emissions from Household Cooking Devices: A Kenyan Case Study. Environ Toxicol Chem. 2020;39(3):538–47. [DOI] [PubMed] [Google Scholar]
- 37.Kayamba V, Heimburger DC, Morgan DR, Atadzhanov M, Kelly P. Exposure to biomass smoke as a risk factor for oesophageal and gastric cancer in low-income populations: A systematic review. Malawi Med J. 2017;29(2):212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikh M, Poustchi H, Pourshams A, Khoshnia M, Gharavi A, Zahedi M, et al. Household Fuel Use and the Risk of Gastrointestinal Cancers: The Golestan Cohort Study. Environ Health Perspect. 2020;128(6):67002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei WQ, Abnet CC, Lu N, Roth MJ, Wang GQ, Dye BA, et al. Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut. 2005;54(6):759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


