ABSTRACT.
Leptospirosis is endemic in New Caledonia. Clinical diagnosis is often difficult and its evolution can be fatal. Leptospirosis requires specific management before biological confirmation. Modified Faine criteria (Faine Score) have been suggested to diagnose leptospirosis on epidemiological (parts A and B) and biological (part C) criteria. The main objective of our study was to assess the relevance of the epidemiological–clinical modified Faine score, parts A and B (MF A + B), in patients with suspected leptospirosis in New Caledonia. A monocentric case–control study was conducted in suspect patients for whom a Leptospira polymerase chain reaction (PCR) test was done within the first 7 days of signs onset at the tertiary hospital from January 2018 to January 2019. Cases and control subjects were matched 1:2 in the gender and age categories. Bivariate, and then multivariable, analyses studied the association between the MF A + B score and a positive Leptospira PCR test, adjusted on the variables retained. In all, 35 cases and 70 control subjects matched for age and gender were analyzed. Multivariable analysis by logistic regression found a significant association between an MF A + B score taken from the categories “possible leptospirosis” (score, 20–25) and “presumed leptospirosis” (score, > 26), and the case or control subject status (P < 0.0001). Model performance was high, with an area under the curve value of 99.27%, 93.55% sensitivity, and 96.36% specificity, which classified subjects correctly in 95.35% of cases. Our study suggests using the MF A + B score to identify possible cases of leptospirosis and initiate antibiotic therapy before biological confirmation in New Caledonia. This score should be evaluated in areas where more differential diagnoses exist and where PCR is not widely available.
INTRODUCTION
Leptospirosis is a ubiquitous bacterial zoonosis with a yearly incidence estimated at more than 1 million symptomatic cases each year.1 Although most infections remain symptom free, leptospirosis is a potentially severe infection with a case fatality rate among hospitalized cases ranging from less than 5% to more than 30%, depending on the availability and quality of health care.1 Its clinical presentation varies greatly. Most cases present as an undifferentiated acute and self-resolving flu-like illness, making clinical diagnosis difficult in tropical settings. In some cases, this is followed by aggravation with signs including sepsis of rapid onset, myalgia, headaches, eye redness, or Weil’s disease.2,3 Pulmonary signs, polyuria, and meningism may also be present.4,5 Jaundice typically appears in the second/immunological phase. In severe cases, sepsis progresses to multiple organ failure—usually renal, liver, and/or pulmonary—leading to hemorrhaging and coma.6,7 Aside from prompt referral to intensive care if needed, timely probabilistic antibiotic treatment may reduce lethality.8 Increased monitoring is also warranted in leptospirosis. Clinicians in endemic areas therefore must initiate adequate management without, or while waiting for, diagnostic confirmation. Polymerase chain reaction (PCR) testing is a specific and rapid diagnostic tool, but is not accessible in many affected countries. Withholding antibiotics while waiting for serology or microscopy agglutination test (MAT) results would likely jeopardize patient survival. In 1982, the WHO published a clinical, epidemiological, and bacteriological score referred to as “Faine’s criteria.”3 This score was revisited in 2012 as the “modified Faine (MF) criteria,” adding a clinical variable and molecular biological test results.9 Other scores have been developed by teams in Thailand10,11 and Sri Lanka,12 but these scores also integrate biological variables and therefore require access to a laboratory.
New Caledonia is a French island territory in the Pacific. Like many other Pacific Island countries and territories, it is highly endemic for leptospirosis, especially in rural areas with limited access to health structures and diagnostic facilities.2 The lack of syndromic diagnosis for timely pre-test assessment may lead to the overuse of probabilistic antibiotic therapy in patients with a disease other than leptospirosis, as well as an underuse in patients with leptospirosis who may need it the most. We conducted a study to evaluate the usefulness of Faine’s modified epidemiological and clinical criteria in predicting Leptospira PCR test results in patients admitted to the Territorial Hospital for New Caledonia (THNC).
METHODS
Our study was monocentric and conducted at the tertiary care THNC. Data were documented retrospectively in hospital records for patients admitted between January 1, 2018 to July 31, 2018, and prospectively for patients admitted between August 1, 2018 and April 1, 2019. Diagnostic real-time PCR was conducted at THNC on 1,000 μL of blood serum using EasyMag (Laboratoire Biomérieux, Lyon, France) and it targeted the lipL32 gene.13 Leptospirosis real-time PCR has optimal sensitivity and specificity within the first week after signs onset.14 A case of leptospirosis was defined as a clinically symptomatic adult patient (≥ 18 years old) for whom a medical doctor suspected leptospirosis and prescribed a PCR test that returned positive within 7 days after signs onset. A control subject in our study was a clinically symptomatic patient for whom a medical doctor suspected leptospirosis, but in whom PCR return negative within 7 days after signs onset. Because leptospirosis risks are very age dependent, control subjects were frequency-matched to cases 2:1 on gender at birth and age using two categories: 18 to 44 years and 45 to 77 years. To achieve 2:1 matching, the more numerous control subjects were selected randomly using a random number generator. Clinicians documented the sociodemographic (including self-declared ethnicity), anamnestic, epidemiological, and clinical characteristics of cases and control subjects using an anonymized questionnaire. Modified Faine criteria were defined per the literature.9 Epidemiological factors were documented for 30 days before signs onset.
Data were entered using EpiData (v. 4.6.0.2; EpiData Association, Odense, Denmark). The database were imported and analyzed using Stata (v. 13; Stata Corp., College Station, TX). After database cleaning and correction, the data were described and recategorized. The score was computed based on part A + part B modified criteria values and the probabilistic leptospirosis status established based on modified score thresholds.15 Clinical categories were termed “improbable” (score, < 20), “possible” (score, 20–25), and “presumed” (score, >25) leptospirosis. Bivariate analysis tested the association between variables and patients’ case or control subject status. Clinical variables were excluded from multivariable analysis because the latter included the modified score values to which they contributed. The association between percentages and status were tested using a χ2 test or a Fisher test, as applicable. The association between quantitative variables and status was tested using a non-parametric Wilcoxon-Mann-Whitney test. A χ2-for-trend test was undertaken to explore the dose–response relationship between patient status as cases or control subjects and their Faine modified criteria score category. After dichotomization or categorization, a multivariable analysis explored the association between patient status and score category after adjustment for other variables using a stepwise ascending procedure. The variables included in the model were those associated statistically at the 0.2 significance threshold in bivariate analysis. Regardless of whether they were associated significantly with case or control subject status, variables composing the modified Faine, parts A and B (MF A + B) score were not included individually in the multivariable analysis, but were aggregated into the score result, and then the statistical significance of composite score values with case and control subject status was assessed.
Admission C-reactive protein (CRP) titers were analyzed by category (< 10 mg/L, 10–39 mg/L, 40–79 mg/L, and ≥ 80 mg/L). These limits were chosen specifically to match results delivered in daily clinical practice when using the semiquantitative BIOSYNEX® CRP (Biosynex, Strasbourg, France) semiquantitative rapid tests, as is the case in most community health centers in New Caledonia.
The variables year and month are linked to seasonality and epidemic years and were forced into the model. Models were tested for interaction between variables. Respective model performance was compared using several criteria: McFadden r2; area under the receiver operating characteristic (AUC) curve, Akaike’s criterion, and number of patients included. The performance of the final model was documented using AUC, sensitivity, specificity, and percentage classified correctly. All associations were tested using a 0.05 statistical threshold. The THNC ethics committee approved the study.
RESULTS
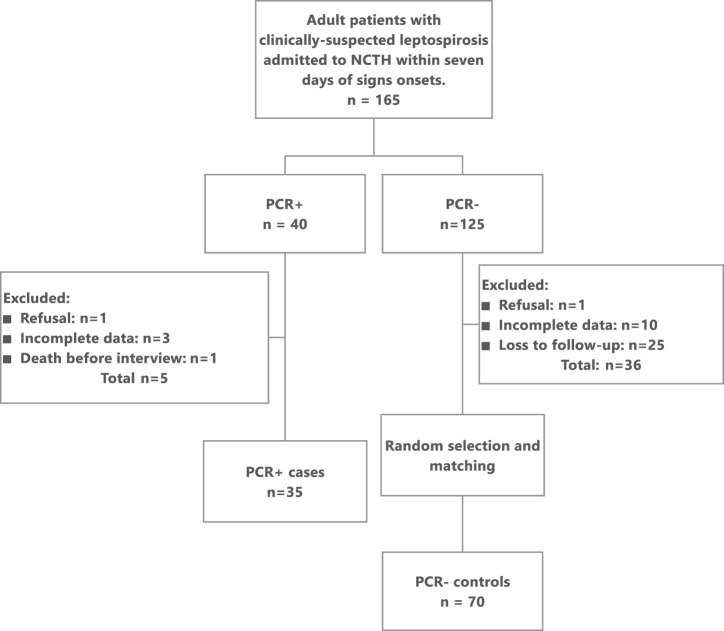
Between January 1, 2018 and April 1, 2019, 165 adult patients were admitted to THNC with clinically suspected leptospirosis and underwent PCR texting within 7 days after symptoms onset (Figure 1). Of these patients, 40 (24.2%) had PCR-confirmed leptospirosis. Five of the 40 patients were excluded from the study: one refused to participate, three were insufficiently documented to compute the MF score, and one died before interview. The remaining 35 patients were all included as cases in the study. PCR test results were negative in 125 patients (75.8%). Thirty-six of these patients (28.8%) were insufficiently documented or could not be contacted to participate in the study. Of the remaining 89 PCR-negative patients, 70 control subjects were selected and matched randomly on gender and age with the 35 cases. Of these control subjects, final diagnosis included dengue in 20 (28.6%), bacterial pneumonia in 11 of 70 (15.7%), and undetermined etiology in eight patients (11.4%). Etiologies are shown in Supplemental Table S1. Patient age ranged from 18 to 77 years (median, 45 years). Twenty-seven of 35 cases (77.1%) were male, as were matched control subjects (Table 1). Self-declared ethnicity was most frequently Melanesian (Kanak, 61 of 105; 58% of all patients). Among the clinical criteria for the MF A + B score, acute headache, eye redness, meningism, muscle tenderness, and jaundice were significantly more frequent among cases compared with control subjects (P < 0.001; Table 2). Albuminuria and oligoanuria were also significantly more frequent among cases (P < 0.001). The presence of fever was not significantly different among the two groups. Among the epidemiological criteria for the MF A + B score, recent rains, high-risk environments, and animal contacts were significantly more frequent among cases compared with control subjects (Table 3). The median delay to THNC admission after signs onsets was 3 days among PCR-positive cases (interquartile range [IQR], 2–5 days) and 2 days among PCR-negative control subjects (IQR, 1–3 days), with delays ranging from 0 to 7 days in both groups. The difference in medians was significant (P = 0.0016). CRP titers differed significantly between groups with a median of 235.5 mg/L (IQR, 177–334 mg/L) among 34 documented cases and 56 mg/L (IQR, 17–187 mg/L) among 63 control subjects (P < 0.0001).
Figure 1.
Patient flow, CaledoFaine study, New Caledonia, 2018 to 2019. NCTH = New Caledonia Territorial Hospital; PCR = polymerase chain reaction.
Table 1.
Sociodemographic characteristics of study participants and association with patient status as cases or control subjects, CaledoFaine study, New Caledonia, 2018 to 2019
| Factors | Cases (n = 35) | Control subjects (n = 70) | Total (N = 105) | P value* |
|---|---|---|---|---|
| Age, y; median (IQR) | 43 (33–56) | 45 (30–61) | 45 (31–59) | 0.539 |
| Age category, y | ||||
| 18–24 | 3 (8.6%) | 11 (15.7%) | 14 (13.3%) | |
| 25–44 | 15 (42.9%) | 22 (31.4%) | 37 (35.2%) | |
| 45–54 | 8 (22.8%) | 9 (12.9%) | 17 (16.2%) | |
| 55–64 | 7 (20.0%) | 14 (20.0%) | 21 (20.0%) | |
| 65+ | 2 (5.7%) | 14 (20.0%) | 16 (15.23%) | |
| Age matching category | ||||
| 18–44 | 18 (51.4%) | 33 (47.1%) | 51 (48.6%) | 0.685 |
| 45+ | 17 (48.6%) | 37 (52.9%) | 54 (51.4%) | |
| Gender | ||||
| Male | 27 (77.1%) | 54 (77.1%) | 81 (77.1%) | 1.0 |
| Female | 8 (22.9%) | 16 (22.9%) | 24 (22.9%) | |
| Self-declared ethnicity | ||||
| Kanak (Melanesian) | 25 (71.4%) | 36 (51.4%) | 61 (58.1%) | |
| European | 2 (5.7%) | 23 (32.9%) | 25 (23.8%) | |
| Ni-Vanuatu | 0 (0%) | 0 (0%) | 0 (0%) | |
| Tahitian | 1 (2.8%) | 2 (2.9%) | 3 (2.9%) | |
| Vietnamese | 0 (0%) | 0 (0%) | 0 (0%) | |
| Wallisian/Futunian | 1 (2.8%) | 6 (8.6%) | 7 (6.6%) | |
| Other Asian | 1 (2.8%) | 0 (0%) | 1 (0.9%) | |
| Caledonian | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other | 1 (2.8%) | 0 (0%) | 1 (0.9%) | |
| Mixed | 3 (8.6%) | 0 (0%) | 3 (2.9%) | |
| ND | 1 (2.8%) | 3 (4.3%) | 4 (3.8%) | |
| ONENA | 27 (77.1%) | 44 (62.9%) | 71 (67.6%) | 0.185 |
| Province (ND = 4) | ||||
| Southern province | 19 (54.3%) | 57 (81.4%) | 76 (72.4%) | 0.01 |
| Northern province | 13 (37.1%) | 6 (8.6%) | 19 (18.1%) | |
| Island province | 0 (0%) | 6 (8.6%) | 6 (5.7%) | |
| Substance abuse | ||||
| Alcohol | 17 (48.6%) | 18 (52.9%) (ND = 36) | 35 (50.7%) | 1.000 |
| Tobacco | 25 (71.4%) | 22 (59.5%) (ND = 33) | 47 (65.3%) | 0.329 |
| Kava | 2 (5.7%) (ND = 2) | 0 (0%) (ND = 40) | 2 (3.2%) | 0.493 |
| Long-term treatment | 8 (22.8%) | 25 (35.7%) | 33 (31.4%) | 0.265 |
IQR = interquartile range; ND = Nondocumented; ONENA = Oceanians of non-European or non-Asian ancestry.
Fisher or Wilcoxon tests.
Table 2.
Clinical and paraclinical variables and associations with patient status as cases or control subjects, CaledoFaine study, New Caledonia, 2018 to 2019
| Variable | Control subjects, n (%) | Cases, n (%) | Total, N | P value* | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| Headache with brutal onset | 32 (45.7) | 38 (54.3) | 4 (10.4) | 31 (88.6) | 105 | < 0.0001 |
| Fever | 5 (7.2) | 65 (92.8) | 2 (5.7) | 33 (94.3) | 105 | 1 |
| Fever ≥ 39°C | 54 (73.2) | 16 (22.8) | 14 (40) | 21 (60) | 105 | 0.299 |
| Clinical signs of inflammation | ||||||
| Eye redness | 21 (80.0) | 14 (20.0) | 16 (53.3) | 14 (46.6) | 105 | < 0.0001 |
| Meningism | 68 (97.2) | 2 (2.8) | 15 (42.9) | 20 (57.1) | 105 | < 0.0001 |
| Myalgia | 30 (42.9) | 40 (57.1) | 3 (8.6) | 32 (91.4) | 105 | < 0.0001 |
| All three signs | 68 (97.2) | 2 (2.8) | 20 (57.1) | 15 (42.9) | 105 | < 0.0001 |
| Jaundice | 68 (97.2) | 2 (2.8) | 15 (42.9) | 20 (57.1) | 105 | < 0.0001 |
| Proteinuria or oligo-anuria | 34 (48.6) | 36 (51.4) | 3 (8.6) | 32 (91.4) | 105 | < 0.0001 |
| Hemoptysis/dyspnea | 55 (79.6) | 15 (21.4) | 12 (34.3) | 23 (65.7) | 105 | < 0.0001 |
| Rainfall or flooding | 31 (44.3) | 39 (55.7) | 2 (5.7) | 33 (94.3) | 105 | < 0.0001 |
| Contact with contaminated environment (water and/or soil) | 35 (50) | 35 (50) | 1 (2.9) | 34 (97.1) | 105 | < 0.0001 |
| Presence of rats (home or area) | 41 (59.6) | 29 (41.4) | 2 (5.7) | 33 (94.3) | 105 | < 0.0001 |
Fisher text.
Table 3.
Modified Faine criteria part A and part B score categories among cases and control subjects, bivariate analysis and χ2 for trend, CaledoFaine study, New Caledonia, 2018 to 2019
| Score category | Cases, n | Control subjects, n | Total, N | Unadjusted odds ratio | Mantel Haenszel (compared with reference) | P value |
|---|---|---|---|---|---|---|
| Leptospirosis unlikely | 3 | 61 | 64 | 1 | 1 | – |
| Possible leptospirosis | 10 | 8 | 18 | 25.42 | 25.42 | < 0.00001 |
| Presumed leptospirosis | 22 | 1 | 23 | 447.33 | 447.33 | < 0.00001 |
| Total | 35 | 70 | 105 | – | – | – |
For Faine’s modified A + B criteria: leptospirosis presumed if A score ≥ 26 or A + B score ≥ 25, leptospirosis possible if score between 20 and 25.
Bivariate analysis found a statistically significant association between the MF A + B score and the “possible” (A + B score, ≥ 20) and “presumed” (A + B score, > 25) leptospirosis status of cases and control subjects (P < 0.001; Table 4). As leptospirosis progressed from the systemic phase to the immune phase, the Faine criteria performance score was assessed, taking into account the time elapsed since signs onset. In our study, the difference in the MF A + B score between cases and control subjects remained statistically significant after stratification for time elapsed, but not after adjustment for other variables. Other significantly associated variables in bivariate analysis were age category (18–44 years and 45–77 years), province of residence, year (2018 and 2019) and month (March and April) of diagnosis, time to referral, and ethnicity (Oceanians of non-European or non-Asian ancestry). Compared with the “unlikely leptospirosis” group used as reference, bivariate regression estimated the unadjusted odds ratio between “possible” (A + B score, ≥ 20) and “presumed” (A + B score, > 25) leptospirosis status of cases at 25.41 points (95% CI, 5.75–112.31) and 447.5 points (95% CI, 44.18–4,529.51), respectively (P < 0.0001).
Table 4.
Bivariate and multivariable regression testing of association between patient case or control status and the results of the Faine’s criteria part A and part B score before and after adjustment for other variables, CaledoFaine study, New Caledonia, 2018 to 2019
| Variable category | Control subjects, n | Cases, n | P value* | Unadjusted odds ratio | Adjusted odds ratio in final model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P value | Estimate | 95% CI | P value | |||||
| MF A + B† | ||||||||||
| Leptospirosis unlikely | 61 | 3 | < 0.001 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| Leptospirosis possible | 8 | 10 | – | 25.41 | 5.75–112.31 | < 0.001 | 67.24 | 2.04 – 2,214.47 | 0.02 | |
| Leptospirosis presumed | 1 | 22 | – | 447.33 | 44.18–4,529.51 | < 0.001 | 148,426.9 | 1.54–1.4 × 1010 | 0.04 | |
| Leptospirosis possible or presumed | 9 | 32 | < 0.001 | 72.30 | 18.28–285.91 | < 0.001 | 379.18 | 15.29–9,405.54 | < 0.001 | |
| Continuous | 45.3 (30–61.2) | 43.5 (33.6–56) | 0.66 | 0.99 | 0.96–1.01 | 0.55 | – | – | – | |
| Province of residence | ||||||||||
| South | 57 | 19 | < 0.001 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| North | 6 | 13 | – | 6.50 | 2.17–19.48 | 0.001 | 7.01 | 0.42–115.86 | 0.17 | |
| Islands | 6 | 0 | – | – | – | – | – | – | – | |
| Year | ||||||||||
| 2018 | 31 | 25 | 0.013 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| 2019 | 38 | 10 | – | 0.33 | 0.14–0.78 | 0.012 | 0.19 | 0.014–2.46 | 0.22 | |
| Month | 0.38 | 0.13–1.10 | – | |||||||
| January | 13 | 4 | 0.001 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| February | 8 | 3 | – | 1.22 | 0.21–6.92 | 0.823 | – | – | – | |
| March | 17 | 14 | – | 2.68 | 0.71–10.07 | 0.145 | 8.26 | 0.01–2.46 | 0.97 | |
| April | 1 | 3 | – | 9.75 | 0.78–121.83 | 0.077 | 0.84 | 0.05–14.14 | 0.05 | |
| May | 0 | 0 | – | – | – | – | – | – | – | |
| June | 0 | 2 | – | Collinearity | – | – | – | – | – | |
| July | 0 | 3 | – | Collinearity | – | – | – | – | – | |
| August | 5 | 4 | – | 2.6 | 0.46–14.63 | 0.278 | – | – | – | |
| September | 3 | 2 | – | 2.17 | 0.26–17.89 | 0.473 | – | – | – | |
| October | 3 | 0 | – | Collinearity | – | – | – | – | – | |
| November | 10 | 0 | – | Collinearity | – | – | – | – | – | |
| December | 8 | 0 | – | Collinearity | – | – | – | – | – | |
| Oceanians of non-European or non-Asian ancestry | ||||||||||
| No | 38 | 26 | 0.058 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| Yes | 32 | 9 | – | 2.43 | 1.0–5.94 | 0.051 | 1.42 | 0.10–20.79 | 0.80 | |
| Time lapse since signs onset, Faine | ||||||||||
| Continuous | 2 (1–3) | 3 (2–5) | 0.002 | 1.42 | 1.12–1.80 | 3 | 2.25 | 0.99–5.10 | 0.17 | |
| 0–3 d | 52 | 18 | – | 1.0 (Ref.) | – | – | – | – | – | |
| 4–7 d | 12 | 15 | – | 3.37 | 1.38–8.17 | 0.007 | – | – | – | |
| CRP (mg/L) | ||||||||||
| Continuous | 56 (17–187) | 235 (177–334) | < 0.001 | 1.01 | 1.0–1.01 | < 0.001 | 1.23 | 0.93–1.63 | 0.15 | |
| < 10 | 7 | 0 | 0 | 1.0 (Ref.) | – | – | – | – | – | |
| 10–39 | 21 | 1 | – | 0.041 | 0.05–0.331 | – | – | – | – | |
| 40–79 | 6 | 0 | – | Collinearity | – | – | – | – | – | |
| ≥ 80 | 29 | 33 | – | Collinearity | – | – | – | – | – | |
| CRP > 30 and leptospirosis presumed or possible | ||||||||||
| No | 64 | 3 | < 0.001 | 1.0 (Ref.) | – | – | 1.0 (Ref.) | – | – | |
| Yes | 6 | 32 | – | 113.78 | 26.70–484.76 | < 0.001 | 104.81 | 13.31–825.10 | < 0.001 | |
A = part A; B = part B; CRP = C-reactive protein; MF = modified Faine.
Fisher test for qualitative variables; Wilcoxon test for continuous variables.
For Faine’s modified A + B criteria only: leptospirosis presumed if A score ≥ 26 or A + B score ≥ 25, leptospirosis possible if score between 20 and 25, and leptospirosis unlikely if score < 20.
Logistic regression multivariable analysis found a statistically significant association between patient status and MF A + B score, with odds ratios of 67.24 (95% CI, 2.04–2,214.47) for “possible leptospirosis” (A + B score, ≥ 20) and of 148,426.9 (95% CI, 1.54–1.4 × 1010) for “presumed leptospirosis” after adjustments for other variables (P < 0.0001). The final model was based on 84 patients documented for all variables included in the model. The AUC curve was 99.27%, sensitivity was 93.55%, specificity was 96.36%, and 95.35% of patients were categorized correctly as cases or control subjects (Figure 2). Including CRP titers led to lower model performance (AUC curve, 96.24%).
Figure 2.
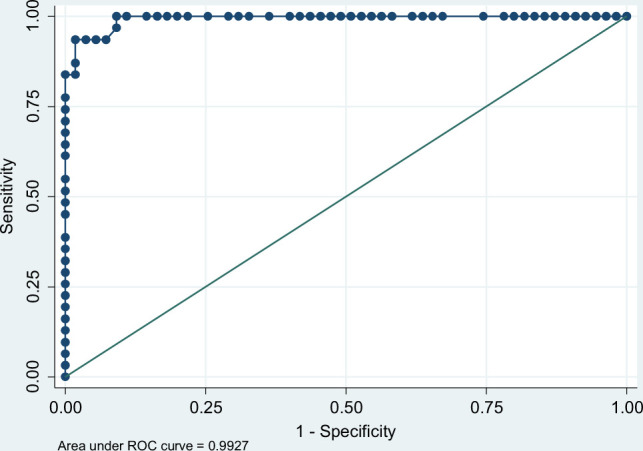
Area under the receiver operating characteristic (ROC) curve and performance of the multivariable model, CaledoFaine study, New Caledonia, 2018 to 2019.
DISCUSSION
Since 1982, the WHO has recommended using a score to establish a diagnostic probability of leptospirosis after examining clinical (part A), epidemiological (part B), and biological (part C) criteria.3 This score was evaluated and modified by a team in India in 2012 to improve its performance, resulting in a sensitivity and specificity of 39.39% and 79.41%, respectively, when only A + B criteria were considered.3 Score performance rose in that study only when biological diagnosis results (part C, Leptospira PCR or MAT) were included. Based on clinical and epidemiological criteria alone, the MF score had excellent predictive probability of Leptospira PCR test results in patients with clinically suspected leptospirosis within 7 days of signs onset in New Caledonia in 2018 to 2019. After adjusting for other factors, score sensitivity and specificity were estimated at 93.55% and 96.36%, respectively, resulting in 95.35% patients being categorized correctly as cases or control subjects in our study. All epidemiological and clinical modified criteria were more often present among cases and control subjects, except for fever. A study was conducted in Thailand to identify predictive factors of leptospirosis and develop a prediction score.1 The Thai Lepto study, a recently published prospective multicenter study from Thailand, examined the performance of a prediction score among hospital inpatients.10 The Thai Lepto score was based on three clinical criteria (hypotension, jaundice, and myalgia) as well as four biological criteria (acute kidney failure, anemia, neutrophilia, and hypokalemia with concurrent hyponatremia). It had moderate statistical performance, with an AUC curve of 0.78. Jaundice, myalgia, or meningism were especially discriminant in our study, the latter being present in 57.1% of cases but in only 2.8% of control subjects. Hypotension was not observed frequently in our study, which is in line with findings published in 2003.14 The presence of rats in the house or area of residence was also highly discriminant and was found in 54.3% of cases and 10.1% of control subjects (P < 0.001).
Another score was tested in outpatients in Thailand.11 The outpatient department Lepto score was tested in primary care patients with suspected leptospirosis. The study included 262 patients with suspected leptospirosis, including 82 confirmed cases. Many criteria were evaluated and five of them showed high discrimination: exposure to wet soil at work (mainly paddy fields), contact with a water source used by animals, presence of protein and/or blood in urine, and a predominance of neutrophils > 80% of white blood cells. These findings were published after we implemented our study. We did not investigate biological markers because our study focused on syndromic screening. We did, however, take into account urine dipstick and CRP results, because these are routinely available to clinicians in community centers in New Caledonia.
A study conducted in French Guyana compared CRP titers among leptospirosis cases and control subjects with confirmed Dengue virus. That study showed that a CRP value of > 50 mg/L was strongly in favor of leptospirosis and was associated with an odds ratio of 168 (95% CI, 23.3–1,212).16 Integrating CRP titers in our syndromic screening score in New Caledonia, however, reduced model performance, with the percentage of correctly classified subjects decreasing from 95.35% to 92.55%. This is likely the result of our study comparing leptospirosis not only to dengue, but also to a host of conditions (including dengue, but also those of bacterial origin) that are real-world differential diagnoses in tropical or subtropical areas such as New Caledonia.
Our study may suffer from several biases and limitations. First, it focused solely on patients admitted to the THNC. This may have introduced a selection bias because most patients resided in the southern province, which has a more urban population and a higher socioeconomic status than New Caledonia’s two other provinces.17 The risk of leptospirosis is closely linked with the environment and socioeconomic activities, and therefore varies across provinces. The number of control subjects residing in the northern province, which is known for a greater leptospirosis risk, was low (6 of 70). The number of cases residing in that province, however, was high (13 of 35). Furthermore, the diseases considered as differential diagnoses are ubiquitous in New Caledonia. The validity of the MF criteria is therefore likely maintained across populations referring to health centers in all three provinces.
Second, the only intensive care department in New Caledonia is located at the THNC, the reference hospital. Patients admitted to peripheral health structures with a deteriorating condition are transferred to the THNC, thus introducing a recruiting bias and overrepresenting severe leptospirosis even in its early phases. However, we stratified the analysis of the MF A + B score by time elapsed since disease onset. The score remained discriminant between cases and control subjects, whether within the first 4 days or the last 3 days. We therefore believe this score remains relevant, especially as the most severe cases may benefit the most from early probabilistic antibiotic treatment and management of complications.
Third, there is a statistically significant difference in the time elapsed between disease onset and admission among cases and control subjects. As explained earlier, this is a result of frequent initial admissions in a peripheral health center and secondary transfer to the THNC. New Caledonia, however, is a territory of limited size with good road and air connections. Although IQRs differ, the difference in median time elapsed between cases and control subjects is only 1 day. The performance and relevance of the MF A + B score should therefore not be affected unduly.
Fourth, Leptospira PCR-positive patients began to be documented comprehensively at the THNC on January 1, 2018. Control subjects only began to be documented after August 1, 2018. Cases were therefore included over a longer period than control subjects, which included the rainy season months of March and April in 2018 and 2019, during which peak leptospirosis transmission is observed.18 The likelihood is therefore greater that cases have more evident epidemiological risk factors of leptospirosis than control subjects. New Caledonia is malaria free. Our results therefore may not be applicable to areas where malaria, yellow fever, or other diseases that can also cause jaundice and acute kidney failure circulate intensely.19 The principle differential diagnosis during peak leptospirosis transmission in New Caledonia is Dengue virus, which also peaks during the rainy season. Based on our study and clinical experience, the MF A + B score seems sufficiently able to distinguish between leptospirosis and its habitual differential diagnoses in New Caledonia despite the 7-month difference in case and control documentation.
Fifth, our evaluation of this first-line score focused in part on patients who had been referred to the THNC by peripheral health centers. Cases and control subjects not associated with the same degree of baseline clinical suspicion may have generated some information bias. Cases and control subjects, however, were classified on the basis of laboratory confirmation, not clinical suspicion, and our study assessed retrospectively the performance of epidemiological and clinical criteria. Furthermore, even referral centers in leptospirosis-endemic areas widely lack access to biological confirmation methods for leptospirosis, and referred patients are admitted with the same information limited to epidemiological and clinical findings as they would be in first-line centers.
Sixth, patients were categorized as either cases or control subjects based on Leptospira PCR results obtained within 7 days after disease onset. Beyond that optimal period, other tests such as MAT are needed to confirm leptospirosis. The latter are difficult to perform and interpret, and require sequential blood samples that are often hard to obtain from patients after they have been discharged. We therefore restricted our study to PCR confirmation, which may have limited the sample size and power of our study, and introduced classification bias in control subjects with false-negative PCR test results or in those who would have had positive MAT results. The reference MAT confirmation tests, however, are now performed rarely in routine clinical practice in New Caledonia. Furthermore, we show that patients with symptomatic leptospirosis are referred to the hospital early in New Caledonia, and cases and control subjects were included in the study if they were admitted within the first week of symptoms, during which PCR tests have the greatest likelihood of being positive.
Seventh, patients with leptospirosis had a presentation that likely was more highly evocative of leptospirosis than control subjects. This may have led to a greater degree of PCR prescription than among patients with the same presentation as control subjects and artificially dichotomized patients. Table 2, however, shows that although there was a difference in prevalence of various signs, these signs were present to some degree among control subjects. Our study shows that the MF A + B criteria are highly predictive of a positive Leptospira PCR test result when comparing cases to control subjects that referred with a clinical presentation ambiguous enough that a Leptospira PCR test was prescribed.
Eighth, control subjects were defined as not having a positive Leptospira PCR test result. Several control subjects, however, were discharged without a diagnosis. It cannot be excluded that some of these patients did suffer from leptospirosis despite negative PCR test results. The fact that febrile patients may be discharged without a diagnosis is a daily reality in the real-world health-care setting. A possible classification bias would have reduced the performance of the MF A + B score. Our results show, however, that the score remained highly performant in New Caledonia. Our findings may therefore not be applicable to all cases of leptospirosis infection, but are of great importance for patients with clinical leptospirosis who likely most benefit from early, probabilistic antibiotic treatment and early management of complications of severe forms of the disease in real-world conditions.
Ninth, our sample size is comparatively modest and matching age categories, quite large. This may over- or underestimate the association between the MF A + B score and patient case or control subject status, but is no likely cause for systematic bias.
Last, there is a risk of recall bias when using case–control studies that include patient interviews. Patients found positive for leptospirosis may more often recall having been environmentally exposed than control subjects, regardless of whether this is truly the case. Furthermore, investigators may be more insistent when documenting exposure among confirmed leptospirosis cases and may introduce a documentation bias. The PCR results, however, were not yet available to the investigators when interviewing most of the patients, and environmental exposure was assessed only within the previous 30 days.
Notwithstanding these caveats, our study was carried out in real-world conditions. Our study conclusions on score performance can therefore be extrapolated easily to the daily clinical setting. Ours is the first effort to explore the usefulness of epidemiological and clinical MF criteria as a probabilistic score for leptospirosis in New Caledonia and, to our knowledge, in the Pacific. Our results show a very strong association between score results and patient Leptospira PCR test status. This score is quick and can be used by medical and non-medical staff alike. It can be computed using the THNC digital patient files, and pops up automatically when a Leptospira PCR test is prescribed. This computational module can also be developed as a mobile phone app and made accessible to health centers, doctor clinics, and pharmacies across the territory. Time will tell whether this scoring system will be used by clinicians and whether it will translate into improved management and survival for leptospirosis patients in New Caledonia. The performance of epidemiological and clinical MF criteria should be tested in other leptospirosis-endemic areas where PCR is not readily available to clinicians, especially in highly leptospirosis-endemic Pacific Island countries and territories.
Supplemental Material
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1. Costa F et al. 2015. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis 9: e0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goarant C et al. 2009. Outbreak of leptospirosis in New Caledonia: diagnosis issues and burden of disease. Trop Med Int Health 14: 926–929. [DOI] [PubMed] [Google Scholar]
- 3. Faine S , World Health Organization , 1982. Guidelines for the Control of Leptospirosis. Available at: https://apps.who.int/iris/handle/10665/37219 . Accessed October 11, 2021.
- 4. Katz AR Ansdell VE Effler PV Middleton CR Sasaki DM , 2001. Assessment of the clinical presentation and treatment of 353 cases of laboratory‐confirmed leptospirosis in Hawaii, 1974–1998. Clin Infect Dis 33: 1834–1841. [DOI] [PubMed] [Google Scholar]
- 5. van Samkar A van de Beek D Stijnis C Goris M Brouwer MC , 2015. Suspected leptospiral meningitis in adults: report of four cases and review of the literature. Neth J Med 73: 464–470. [PubMed] [Google Scholar]
- 6. Dolhnikoff M Mauad T Bethlem EP Carvalho CRR , 2007. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz J Infect Dis 11: 142–148. [DOI] [PubMed] [Google Scholar]
- 7. Daher EDF de Abreu KLS da Silva Jr. GB , 2010. Leptospirosis-associated acute kidney injury. Braz. J. Nephrol. 32: 408–415. [PubMed] [Google Scholar]
- 8. Musso D La Scola B , 2013. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect 46: 245–252. [DOI] [PubMed] [Google Scholar]
- 9.Shivakurma S, 2013. Indian Guidelines for the Diagnosis of Human Leptospirosis. Medicine Update. Mumbai, India: The Association of Physicians of India, 23–29.
- 10. Sukmark T et al. 2018. Thai-Lepto-on-admission probability (THAI-LEPTO) score as an early tool for initial diagnosis of leptospirosis: result from Thai-Lepto AKI study group. PLoS Negl Trop Dis 12: e0006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Temeiam N Jareinpituk S Phinyo P Patumanond J Srisawat N , 2020. Development and validation of a simple score for diagnosis of leptospirosis at outpatient departments. PLoS Negl Trop Dis 14: e0007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajapakse S et al. 2016. A diagnostic scoring model for leptospirosis in resource limited settings. PLoS Negl Trop Dis 10: e0004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoddard RA Gee JE Wilkins PP McCaustland K Hoffmaster AR , 2009. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the lipL32 gene. Diagn Microbiol Infect Dis 64: 247–255. [DOI] [PubMed] [Google Scholar]
- 14. Bharti AR et al. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757–771. [DOI] [PubMed] [Google Scholar]
- 15.Bandara K, Weerasekera MM, Gunasekara C, Ranasinghe N, Marasinghe C, Fernando N, 2016. Utility of modified Faine’s criteria in diagnosis of leptospirosis. BMC Infect Dis 16: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Turnier P, Bonifay T, Mosnier E, Blanchet D, Jolivet A, Demar M, Picardeau M, Djossou F, Epelboin L, 2018. Leptospirose versus dengue: et si la CRP suffisait pour guider l’antibiothérapie devant une fièvre au retour ou en zone d’endémie? Une étude cas-témoin appariée, Médecine et Maladies Infectieuses 48 (Suppl): S18. [Google Scholar]
- 17. Institut de la statistique et des études économiques Nouvelle-Calédonie , n.d. Structure de la Population et Évolutions. Available at: http://www.isee.nc/population/recensement/structure-de-la-population-et-evolutions. Accessed October 11, 2021.
- 18. Weinberger D Baroux N Grangeon J-P Ko AI Goarant C , 2014. El Niño southern oscillation and leptospirosis outbreaks in New Caledonia. PLoS Negl Trop Dis 8: e2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO, 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.