ABSTRACT.
The management of visceral leishmaniasis (VL) in HIV-infected patients is complex because of high mortality rates, toxic drug-related side effects, and a high risk of treatment failure and relapse. We report a case of active chronic VL in an HIV-1-infected woman presenting multiple secondary VL episodes over 7 years leading to massive splenomegaly and blood transfusion–dependent anemia despite several treatment courses and secondary prophylaxis. The patient was finally successfully treated with rescue treatment based on intravenous pentamidine. Twenty months after discontinuation of pentamidine the patient presented complete clinical and parasitological response. In patients with active chronic VL, treatment with intravenous pentamidine can be effective and should be considered as rescue treatment.
INTRODUCTION
Visceral leishmaniasis (VL) is a vector-born infectious disease transmitted by a bite of infected sand flies and caused by parasites of the Leishmania donovani complex, which include two species, the anthroponotic L. donovani and the zoonotic L. infantum.1 Leishmania infantum is the prevalent species in the Mediterranean basin where the epidemiology and clinical manifestations of human VL are strictly linked with the HIV-1 coinfection. The management of VL in HIV-infected patients is often complex because of high mortality rates, toxic drug-related side effects, and a high risk of treatment failure and relapse.2–4 Some years ago the term “active chronic VL” was proposed to identify the nosological entity observed in HIV-1/leishmania-coinfected patients presenting multiple secondary VL episodes despite appropriate treatment and secondary prophylaxis.2,5 We report a case of active chronic VL in an HIV-1-infected woman resolved with intravenous pentamidine 7 years after the first VL episode.
CASE REPORT
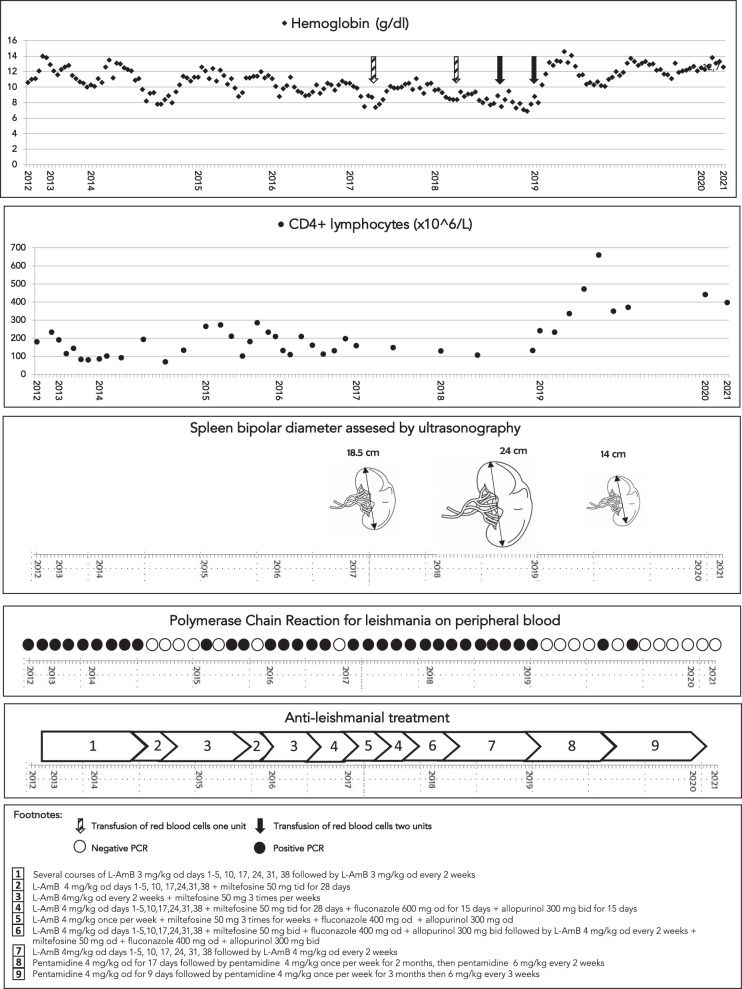
A 52-year-old Italian woman with HIV-1 infection was reevaluated for worsening of her clinical conditions due to active chronic VL. HIV-1 infection was diagnosed in late 80s and for several years the patient was poorly compliant to antiretroviral regimen leading to progressive immunological impairment and uncontrolled viral replication; however, the patient did not developed significant symptoms for years. In 2012, she developed persistent fever and pancytopenia and was diagnosed for the first time with VL based on positive polymerase chain reaction (PCR) on peripheral blood and bone marrow. The patient was treated with a full course of intravenous liposomal amphotericin B (L-AmB) leading to clinical improvement. The patient was then putted in secondary prophylaxis with L-AmB, and antiviral treatment was initially modified with the introduction of abacavir, lamivudine, and darunavir/ritonavir. Since 2014 she was switched to dolutegravir plus darunavir/cobicistat. Since mid-2014 HIV-1 RNA was undetectable, however, PCR for leishmania on peripheral blood was persistently positive and associated with a persistently low CD4 count (usually < 200/mL) and pancytopenia. She was treated sub continuously with several course of intravenous L-AmB (dose 3 mg/kg od days 1–5, 10, 17, 24, 31, 38) followed by secondary L-AmB secondary prophylaxis (3 mg/kg every 2 weeks) (Figure 1). In October 2014 and April 2015, the patient was treated with combination of intravenous L-AmB (4 mg/kg od days 1–5, 10, 17, 24, 31, 38) plus oral miltefosine (50 mg tid for 28 days) followed by secondary prophylaxis with the same two drugs (intravenous L-AmB 4 mg/kg od every 2 weeks + miltefosine 50 mg 3 times per weeks). Transitory resolution of pancytopenia and negativization of PCR for Leishmania was observed, followed by recurrence of pancytopenia and positivity of leishmania PCR on peripheral blood. In November 2016, a new treatment attempt was carried out with intravenous L-AmB (4 mg/kg od days 1–5, 10, 17, 24, 31, 38), miltefosine (50 mg tid for 28 days), fluconazole (600 mg od for 15 days), and allopurinol (300 mg bid for 15 days) followed by a maintenance treatment with the same four drugs at lower dosage (L-AmB 4 mg/kg once per week + miltefosine 50 mg 3 times per week + fluconazole 400 mg od + allopurinol 300 mg bid) for 5 months. The treatment leaded to a transient negativization of leishmania PCR on peripheral blood and improvement of pancytopenia. However, because of new parasitological and clinical relapses, the combination treatment was repeated twice in 2017 with unsatisfactory results. In the period 2018–2019, the patient was maintained on L-AmB at secondary prophylaxis dose but she developed progressive loss of weight, pancytopenia with transfusion-dependent anemia, hypergammaglobulinemia, and massive splenomegaly (bipolar diameter 24 cm). In January 2019, the treatment with intravenous pentamidine was started (4 mg/kg od for 17 days followed by 4 mg/kg once per week for 2 months, then 6 mg/kg every 2 weeks for 5 months). The treatment was administered in the outpatient department. The drug was diluted in 500 cc of normal saline infused over 2–3 hours in supine position and twice a week monitoring of blood count, electrolytes, urine analysis, renal and liver function, as well as electrocardiogram was carried out. On day 33^ after pentamidine treatment was started the patient was hospitalized as a result of the sudden onset of left arm hypostenia, visual disturbance, and cognitive impairment. A brain magnetic resonance imaging (MRI) revealed presence of multiple focal lesions. Cerebral spinal fluid (CSF) was clear with normal protein concentration (0.30 g/L), serum/CSF glucose ratio (101%), and cell count (4/mL). Extensive microbiological investigations, including PCR for leishmania on CSF were all negative.
Figure 1.
Laboratory results, ultrasonography findings and antileishmanial treatment.
The neurological findings were finally interpreted as vasculitic nature and brain lesions and symptoms completely resolved with treatment based on high-dose methylprednisolone 1 g per day for 5 days followed by slow decalage within a period of 5 months. Secondary prophylaxis with intravenous pentamidine (6 mg/kg every 2 weeks) was continued. The clinical and immunological conditions of the patient gradually improved as revealed by the normalization of the full blood count, increase of CD4 count, negativization of leishmania PCR on peripheral blood, and reduction of spleen diameter (14.5 cm). In September and October 2019, leishmania PCR on peripheral blood was again weakly positive in two determination and treatment with pentamidine (4 mg/day per 9 days) was repeated, followed by reintroduction of secondary prophylaxis 4 mg/kg once per week for 3 months, then 6 mg/kg every 3 weeks. In April 2020, due to persistently negative PCR for leishmania, normal full blood count and given the CD4 count persistently above 350/mL, the secondary prophylaxis was discontinued. The patient remained negative to leishmania PCR in the following twenty months achieving a complete and stable clinical and parasitological response without taking any antiparasitic drug.
DISCUSSION
HIV-1-infected individuals have a 100–2,300 fold increased risk of developing VL compared with HIV-negative subjects.6 Active chronic VL is reported in up to 37% of HIV infected patients with VL and is associated with lack of immune reconstitution and long-lasting morbidity.2 Current guidelines recommend L-AmB monotherapy for treatment of VL in immunocompromised subjects and combination treatment with L-AmB and miltefosine in refractory cases.7 Secondary prophylaxis is recommended in immunocompromised HIV-infected subjects. Some investigators suggest that secondary prophylaxis can be discontinued after a sustained (> 3–6 months) increase in CD4 count to > 200–350 cells/mm3 in response to antiretroviral therapy, but others suggest that prophylaxis should be continued indefinitely.8
Nevertheless HIV patients may develop severe chronic uncontrolled VL with massive splenomegaly and blood transfusion dependent anemia despite appropriate treatment and secondary prophylaxis. In these cases, even splenectomy has been considered to partially ameliorate the patient conditions.9 Our patient presented multiple clinical and parasitological relapses despite several course of L-AmB and combination treatment.
Moreover, 1 month after the onset on treatment with pentamidine the patient developed neurological symptoms and brain lesions, which were interpreted as cerebral vasculitis and successfully treated with steroids. Since the development of the neurological disturbance was concurrent with a significant rise of the values of red blood cells, platelets as well as leukocytes including CD4 + lymphocytes, an immunoreconstitution syndrome may be hypothesized as trigger for vasculitis. Neurological involvement is seldom reported in human with leishmaniasis, with peripheral neuropathy being the most frequent complication. Meningoencephalic involvement is exceptionally reported.10,11 In the present case, we cannot exclude an asymptomatic leishmania central nervous system invasion unmasked by an immunoreconstitution induced by treatment with pentamidine, similar to those reported in some cases of cryptococcal meningitis.12
The complicated case was finally resolved with a long course of intravenous pentamidine. Pentamidine is a broad-spectrum anti-infective agent active against several parasitic and fungal agents. It is an aromatic diamidine drug consisting of pentane-1,5-diol, which interferes with polyamine synthesis, RNA polymerase activity, enters the protozoal cell binding to transfer RNA, and prevents the synthesis of protein, nucleic acids, phospholipids, and folate.13 More precisely, evidence suggests that the site of action for pentamidine is the mitochondria of leishmania parasites. An initial study showed that when L. amazonensis parasites were treated with pentamidine, the morphology of mitochondria was enormously expanded, followed by condensation and disruption of kinetoplast DNA. Later, other groups showed that exposure of L. donovani promastigotes to pentamidine rapidly collapsed the parasites’ mitochondrial membrane potential, further supporting the hypothesis that the mitochondrion is the target organ. Furthermore the activities of topoisomerase IB and topoisomerase II, two DNA replication–associated enzymes, were also inhibited by pentamidine in vitro, implying that this compound have the potential to disrupt the replication processes of Leishmania pathogens.14 The adverse effects are various and require close monitoring because they could be fatal. Among the most important drug related adverse events there are QT prolongation and arrhythmias including torsades de pointes, hypotension, hypoglycemia, nephrotoxicity, electrolyte imbalances, hepatotoxicity (transaminase increase), pancreatitis, peripheral neuropathies, leukopenia, and thrombocytopenia.13 According to a recent systematic review of literature the cure of rate pentamidine in VL was above 84% and, concerning the safety profile, among almost 2,000 treated subjects, only few severe adverse events were reported, namely six cases of arrhythmia (including four cases of fatal ventricular fibrillation), 20 cases of irreversible diabetes, and 26 cases of muscular aseptic abscess following intramuscular administration.15
In our patient the treatment was effective and, excluding the transitory and unclear neurological complication, well tolerated by the patient, in the absence of drug-specific adverse reactions.
CONCLUSION
Pentamidine is currently considered as a second choice antileishmanial drug; however, a recent systematic review of literature showed that this drug has an efficacy rate above 84% in VL, including in refractory cases, and an acceptable safety profile.15 The case suggests that intravenous pentamidine can be used as rescue treatment in difficult to treat active chronic VL cases in HIV-positive patients in the Mediterranean area.
REFERENCES
- 1.WHO Expert Committee on the Control of the Leishmaniases & World Health Organization, 2010. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22--26 March 2010. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/44412. [Google Scholar]
- 2. Bourgeois N et al. 2010. ‘Active chronic visceral leishmaniasis’ in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med 11: 670–673. [DOI] [PubMed] [Google Scholar]
- 3. Trudel N et al. 2008. Intracellular survival of Leishmania species that cause visceral Leishmaniasis is significantly reduced by HIV-1 protease inhibitors. J Infect Dis 198: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 4. Abongomera C et al. 2017. The risk and predictors of visceral leishmaniasis relapse in human immunodeficiency virus-coinfected patients in Ethiopia: a retrospective cohort study. Clin Infect Dis 65: 1703–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diro E et al. 2015. Use of pentamidine as secondary prophylaxis to prevent visceral leishmaniasis relapse in HIV infected patients, the first twelve months of a prospective cohort study. PLoS Negl Trop Dis 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagliano P, Esposito S, 2017. Visceral leishmaniosis in immunocompromised host: an update and literature review. J Chemother 29: 261--266. [DOI] [PubMed] [Google Scholar]
- 7. Aronson N et al. 2016. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 63: E202–E264. [DOI] [PubMed] [Google Scholar]
- 8.Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf. Accessed October 27, 2021. [Google Scholar]
- 9. Troya J Casquero A Muñiz G Fernández-Guerrero ML Górgolas M , 2007. The role of splenectomy in HIV-infected patients with relapsing visceral leishmaniasis. Parasitology 134: 621–624. [DOI] [PubMed] [Google Scholar]
- 10. Stauffer WM Magill A Kain KC , 2006. Parasitic central nervous system infections in immunocompromised hosts: clarification of malaria diagnosis. Clin Infect Dis 43: 114–115. [DOI] [PubMed] [Google Scholar]
- 11.Llanos-Cuentas A, Valencia BM, Petersen CA, 2013. Neurological manifestations of human leishmaniasis. Handb Clin Neurol 114: 193--198. [DOI] [PubMed] [Google Scholar]
- 12. Kiggundu R Rhein J Meya DB Boulware DR Bahr NC , 2014. Unmasking cryptococcal meningitis immune reconstitution inflammatory syndrome in pregnancy induced by HIV antiretroviral therapy with postpartum paradoxical exacerbation. Med Mycol Case Rep 5: 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafiz S, Kyriakopoulos C. Pentamidine, 2021. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. PMID: 32491518. [PubMed]
- 14. Yang G Choi G No JH , 2016. Antileishmanial mechanism of diamidines involves targeting kinetoplasts. Antimicrob Agents Chemother 60: 6828–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccica M, Lagi F, Bartoloni A, Zammarchi L, 2021. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: a systematic review. J Travel Med 28: taab065. [DOI] [PubMed] [Google Scholar]