ABSTRACT.
Visceral leishmaniasis (VL) is a tropical disease endemic to Brazil. The clinical manifestations of the infection range from asymptomatic to severe. In VL, changes in lipid metabolism, such as hypocholesterolemia and hypertriglyceridemia, occur that are believed to be related to its progression and severity. This study investigated the associations between serum levels of cholesterol, triglycerides, and lipoproteins (high-density lipoprotein, low-density lipoprotein, and very low-density lipoprotein) with clinical and hematological parameters that predict severity in a case series of 83 VL patients. Severely ill patients had higher mean serum triglyceride levels than non-severely ill patients. There was a significant positive correlation between disease severity score and serum triglyceride levels, very low-density lipoprotein, international normalized ratio for prothrombin time test, total bilirubin, and age. An inverse correlation was detected between the disease severity score and mean platelet and neutrophil counts. Hypertriglyceridemia can be a prognostic indicator of severity in patients diagnosed with VL.
INTRODUCTION
Visceral leishmaniasis (VL) is a chronic protozoan disease endemic to Brazil that is lethal if untreated. Disorders of lipid metabolism in patients with VL have been reported since the 1980s.1 Studies have described hypocholesterolemia, alteration of lipoproteins, and hypertriglyceridemia in VL patients.2–5 Mohapatra et al.3 and Lal et al.4 suggested using hypertriglyceridemia as a possible diagnostic and prognostic factor for the disease, but the first study included only nine patients and the second study, despite including a good number of patients, based its severity criteria on only the anemia and parasite load determined on a microscopic examination. None of these factors correlated the lipid profile with disease severity score.
Regarding the interaction of Leishmania with the host’s lipid metabolism and evidence of the use of lipids by the parasite in the disease’s infection, maintenance, and progression,5,6 it is important to evaluate the biochemical changes and their association with disease severity in a larger sample of patients. The severity classification scores of LV patients are heterogeneous.7,8 Considering that the definition of severity in an early and practical way using accessible and low-cost laboratory tests influences therapeutic decisions and indicates the need for early hospitalization with a consequential reduction in morbidity and mortality, we aimed to associate the lipid changes in VL patients with more objective parameters of disease severity to improve the prediction of clinical outcomes in these patients.
MATERIALS AND METHODS
Study design/sample.
This cross-sectional analytical study included 83 patients with a confirmed diagnosis of VL who were admitted to the University Hospital of the Federal University of Sergipe between 2012 and 2019. The inclusion criteria were confirmed diagnosis of VL by compatible symptoms/signs and a positive rK39 rapid test and/or direct visualization of amastigote forms of the parasite in bone marrow aspirate and/or bone marrow positive culture (gold standard). The exclusion criteria were the history of any other chronic disease (kidney or liver disease, cancer, HIV/AIDS, immunodeficiencies, hypothyroidism, or hyperthyroidism); alcoholism or use of illicit drugs, hypersensitivity to drugs used to treat VL, immunosuppressive drug treatment, current pregnancy, or previous diagnosis or treatment of dyslipidemia.
Procedures.
The study was approved by the Ethics Committee of the University Hospital of the Universidade Federal de Sergipe. Before admission, written informed consent was obtained from the patients/guardians of patients under 18 years of age. Serum total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and triglycerides were measured after a 12-hour fast using the automated calorimetric enzymatic method but before VL treatment was started. The samples were processed using a Wiener apparatus and are expressed as milligrams per deciliter. Low-density lipoprotein values were estimated using the Friedewald equation: LDL (mg/dL) = TC (mg/dL) − HDL (mg/dL) − triglycerides. Other examinations performed during follow-up, such as blood counts, hepatic enzyme levels, and coagulation test results (international normalized ratio [INR] for prothrombin time test), bilirubin as well as clinical follow-up to ascertain clinical complications and disease evolution were annotated from the patients’ medical records. The classification of severity adopted to evaluate these patients was published by Sampaio et al.9 and included dyspnea (1 point), associated infections (1 point), neutrophil count < 500/mm3 (1 point), jaundice (2 points), bleeding mucous membranes (2 points), and platelet count < 50,000/mm3 (3 points).9 The presence of one criterion indicates greater severity. Each sample was categorized according to severity into one of two groups: severe (score > 1) or nonsevere (score < 1). Scoring ≥ 1 point gives the case greater severity and a score ≥ 3 points is the best predictor of death, with a combination of high sensitivity (88.7%), specificity (78.5%), predictive negative value (78.5%), and area under the receiver operating characteristic curve (89.5%).9 Based on these data, we categorized VL cases into three groups: critically ill (≥ 3 points), moderate severity (1–2 points), and nonsevere (score < 1) according to their association with lipid profile.
Statistical analysis.
R Core Team 2019 and Graph Pad Prisma v9 software were used. Associations were tested using Fisher’s exact test and Pearson’s χ2 test. Differences in measures of central tendency were tested using Student’s t-test or the Mann–Whitney U test for independent samples. Analysis of variance or the Kruskal–Wallis test was used to analyze the three groups. Multiple comparisons were performed using Dunn’s test. Cohen’s D test was used to standardize the magnitude of the effect, which is considered small at 0.2–0.3, medium at 0.4–0.7, and large at ≥ 0.8. The correlations between the severity score based on clinical and laboratory parameters with other relevant clinical data were done by the Spearman correlation test. The significance level (α) was set at 5% (P < 0.05).
RESULTS
Of the 83 patients, 54 (65.1%) were male; the patient age range was 4 months to 58 years (mean, 18.8 ± 16.8 years). The mean serum levels of TC, HDL, and LDL (110.8 ± 31.2 mg/dL, 10.0 ± 5.8 mg/dL, and 54.8 ± 30.2 mg/dL, respectively) were lower than the reference values for each age group, while the mean serum levels of triglyceride and VLDL (243.2 ± 114.8 mg/dL and 49.3 ± 27.4 mg/dL, respectively) were higher than the reference values for each age group (Table 1).
Table 1.
Clinical and laboratory characteristics of VL patients by severity score
| Severity score* | |||||
|---|---|---|---|---|---|
| Demographic and clinical data | Severe (≥ 1), N = 47 | Nonsevere (< 1), N = 36 | P value | D | |
| Age (years, mean ± SD) | 24.0 ± 17.9 | 12.1 ± 12.4 | 0.004‡ | 0.749 | |
| Female sex n (%) | 31 (66.0) | 23 (63.9) | 0.845§ | 0.326 | |
| Male sex n (%) | 16 (34.0) | 13 (36.1) | 0.119‡ | 0.055 | |
| Spleen size (cm) | 8.8 ± 5.7 | 7.1 ± 3.8 | 0.913‡ | −0.088 | |
| Liver size (cm) | 4.5 ± 3.6 | 4.3 ± 2.6 | 0.317‡ | ||
| Hemoglobin (g/dL, mean) | 8.0 ± 1.9 | 8.1 ± 1.1 | |||
| Platelet count (cells/mm3) (mean ± SD) | 90,918.1 ± 49,421.4 | 122,947.2 ± 42,695.7 | 0.005‡ | −0.687 | |
| Neutrophil count (cells/mm3) (mean ± SD) | 834.7 ± 579.3 | 1114.8 ± 608.4 | 0.020‡ | −0.473 | |
| INR (mean ± SD) | 1.48 ± 0.80 | 1.24 ± 0.15 | 0.275‡ | 0.384 | |
| Total bilirubin | 2.37 ± 4.84 | 0.61 ± 0.34 | < 0.001‡ | 0.474 | |
| Mucous bleeds, n (%) | 14 (29.8) | 0 (0) | < 0.001† | ||
| Jaundice, n (%) | 12 (25.5) | 0 (0) | 0.001† | ||
| Dyspnea, n (%) | 10 (21.3) | 0 (0) | 0.003† | ||
| Associated infections, n (%) | 26 (55.3) | 0 (0) | < 0.001† | ||
| Edema, n (%) | 12 (26.1) | 1 (2.8) | 0.004† | ||
| Total cholesterol (mg/dL, mean ± SD) | 114.0 ± 30.1 | 106.6 ± 31.6 | 0.295 | ||
| HDL (mg/dL, mean ± SD) | 9.8 ± 6.3 | 10.0 ± 7.5 | 0.383 | ||
| LDL (mg/dL, mean ± SD) | 54.4 ± 31.8 | 55.2 ± 28.4 | 0.794 | ||
| VLDL (mg/dL, mean ± SD) | 54.8 ± 31.5 | 40.0 ± 15.0 | 0.096 | ||
| Triglycerides (mg/dL, mean ± SD) | 270.8 ± 132.6 | 207.2 ± 71.1 | 0.011 | 0.577 | |
n = absolute frequency; % = percentage relative frequency; HDL = high-density lipoprotein; LDL = low-density lipoprotein; VL = visceral leishmaniasis; VLDL = very low-density lipoprotein.
This score uses the following parameters: dyspnea (1 point), associated infections (1 point), neutrophil count < 500/mm3 (1 point), jaundice (2 points), bleeding mucous membranes (2 points), and platelet count < 50,000/mm3 (3 points) (Sampaio et al.9).
Fisher’s exact test.
Mann–Whitney U test.
Pearson’s χ2 test; D, Cohen effect size.
Among the patients, 47 (56.6%) were categorized as having severe disease and 36 (43.4%) were categorized as having nonsevere disease. The severe group had a higher mean age (24.0 versus 12.1 years; P = 0.004), lower median platelet count (90,918.1 versus 122,9472.2 cells/mm3; P = 0.005), lower median neutrophil count (834.7 versus 1,114.8 cells/mm3; P = 0.02), and higher median bilirubin level (2.37 versus 0.61 mg/dL; P < 0.001) than the nonsevere group.
The frequency of clinical complications, such as mucosal bleeding, associated infections, jaundice, dyspnea, and edema, was significantly higher in patients with severe disease. The edema variable, which was not included in the severity criteria used to classify these patients, occurred at a higher frequency among the most severe patients in our sample (P = 0.004). The median triglyceride level of the severe group was higher than that of the nonsevere group (270.8 versus 207.2 mg/dL; P = 0.011).
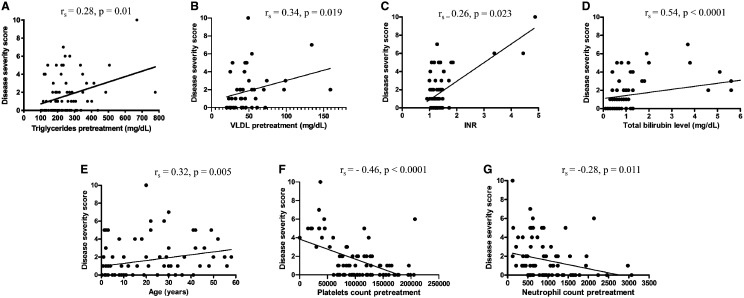
When we categorized the sample into three groups by disease severity, the mean serum triglyceride levels increased as disease severity increased (≥ 3 points: 281.6 ± 141.0 mg/dL) compared with the moderate severity group (1–2 points: 264.9 ± 131.4 mg/dL) and the mild group (< 1 point: 207.2 ± 71.1 mg/dL) (P = 0.045). To rule out the effect of age differences between the severe and nonsevere groups in the lipid profile, we divided the sample by age into ≤ 19 years and > 19 years since this is the division of reference values for serum lipid levels in the general population. We found significantly higher mean values of triglycerides in patients ≤ 19 years old (264.3 ± 120.4 mg/dL) than in patients aged > 19 years old (215.7 ± 100.3 mg/dL); P = 0.026; D = 0.433). Additionally, hypertriglyceridemia, regardless of group categorization, was directly correlated with disease severity score (Figure 1A). There were also direct correlations between disease severity score and VLDL level (Figure 1B), INR (Figure 1C), total bilirubin level (Figure 1D), and age (Figure 1E). There were inverse correlations between disease severity score and platelet count (Figure 1F) and neutrophil count (Figure 1G). There were no correlations between disease severity and serum levels of TC, HDL, and LDL, spleen size, liver size, hemoglobin values, or sex.
Figure 1.
Spearman correlation showing the analysis of severity score based on clinical and laboratory parameters. (A) Pretreatment triglyceride level (mg/dL), (B) very low-density lipoprotein (VLDL) level (mg/dL), (C) international normalized ratio (INR), (D) total bilirubin level, (E) age, (F) platelets count, and (G) neutrophil count.
DISCUSSION
We observed that VL patients had low TC, HDL, and LDL levels and increased VLDL and triglyceride levels compared with the reference values for the general population according to age groups. Using an objective criterion that classifies patients by severity score,9 this study clearly showed a correlation between high triglyceride levels and VL severity as hypothesized by Lal et al.4 Although the mean age of the patients classified as having severe disease was higher than that of those with nonsevere disease, triglyceride levels were higher in patients younger than 19 years, indicating that age is not a confounding variable for this association. Furthermore, the mean ± SD of the lipid profile from normal individuals, in a previous study performed in Sergipe State in children and adolescent (12 ± 3.9 y) shown a mean ±SD of total cholesterol 158 ± 30.4 mg/dL, HDL 47.5 ± 10.5 mg/dL, LDL 91.4 ± 25.4 mg/dL and triglycerides 92.1 ± 48.8 mg/dL.10 Another study in adults (42.02 ± 17.38y) show a mean ± SD of total cholesterol 193.39 ± 43.62 mg/dL, HDL 48.80 ± 11.24 mg/dL, LDL 118.35 ± 36.75 mg/dL, and triglycerides 131.28 ± 82.21 mg/dl.11 These data clearly show that the lipid profile of the patients from the present study is not a trend from the normal population from the same state of Brazil, independently of the age. Importantly, the present findings confirm that these patients classified as severe present a higher frequency of disease complications such as mucosal bleeding, associated infections, lower platelet and neutrophil counts, and edema.
Ghosh et al.5 showed the ability of Leishmania to alter cholesterol metabolism through the cleavage of the enzyme DICER1 mediated by the metalloprotease GP63 and leading to an increase in parasitemia in the liver and reduced serum cholesterol levels. Therefore, in patients with VL, the reduction in HDL and LDL lipoproteins may be because of sequestration and degradation of these lipoproteins in the spleen and liver, which are organs that have high concentrations of Leishmania.6,12 Other hypotheses to explain the reduction of HDL in serum include cytokine release during the acute phase response of the disease, which would have an inhibitory effect on lecithin cholesterol acyltransferase.13 Furthermore, hypergammaglobulinemia and elevated serum β2-microglobulin levels described in VL patients6,14 can affect cholesterol metabolism via the formation of immune complexes between HDL and antibodies, which would accelerate lipoprotein degradation, or the impairment of lipoprotein lipase activity, which is responsible for breaking down lipoproteins in the plasma so that the tissue can absorb its components, mainly cholesterol.12
Importantly, hypertriglyceridemia was present in these VL patients and a good marker of disease severity. Hypertriglyceridemia is important for the growth of the parasite in vitro and has a strong correlation with greater parasitic burden in VL patients.4 The increase in triglycerides can be explained by the increase in VLDL production and reduction in its conversion to LDL by affecting the activities of lipoprotein lipase and liver lipase in VL patients.6,15,16 Additionally, reducing the conversion of VLDL to LDL significantly decreases its serum levels.14 The hepatic synthesis of fatty acids can also be increased by the release of inflammatory cytokines demonstrated to occur in VL, thus increasing VLDL and triglyceride levels.17 Therefore, it is possible that these disturbances in lipid metabolism are a consequence of the infection. Disturbances in lipid metabolism have been described in coronavirus disease 2019, which presents a similar cytokine storm.18 Although, due to the cross-sectional nature of this study design, it is not possible to distinguish whether metabolic dysregulation predisposed to, or was caused by, or was exacerbated by VL. However, there is an association between VL disease severity and lipid dysregulation. This information can improve the prediction of clinical outcomes in these patients.
Conversely, metabolic diseases have been considered to increase one’s susceptibility to infections, especially diabetes and obesity.19,20 Diabetes mellitus has also been associated with a more severe clinical presentation and worse response to therapy of cutaneous leishmaniasis (CL),21 and a case report showed an association between diabetes and VL.22 Sernaglia et al.23 demonstrated that diet-induced obesity promotes systemic inflammation and increases susceptibility to murine visceral leishmaniasis. However, obesity is an inflammatory disease, and studies are needed to verify whether disturbances in lipid metabolism alone increase the risk or affect the clinical outcome of these infectious diseases.
In conclusion, this study demonstrated a significant association between hypertriglyceridemia and disease severity in a large sample of VL patients using an objective score that includes clinical and laboratory aspects.9 Serum triglyceride levels were significantly higher in patients with severe VL than in those with nonsevere disease. Additionally, the subdivision of critically ill patients considered at higher risk of mortality revealed that this group had higher triglyceride levels. Furthermore, it has been demonstrated that VL severity correlates positively with serum triglyceride, VLDL, and bilirubin levels as well as INR and correlates negatively with platelet and neutrophil counts. Therefore, the present findings suggest that triglyceridemia may assist in the initial evaluation of patients with a clinical condition compatible with VL who live in endemic disease areas. Hypertriglyceridemia higher than 250 mg/dL can be a predictor of severity in patients with a confirmed diagnosis of VL or be included in the severity score.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. Bertoli A Greco AV Caputo S Caradonna P Grieco A Laghi V , 1982. Visceral leishmaniasis presenting with hypertrigliceridaemia. Lancet 320: 504–505. [DOI] [PubMed] [Google Scholar]
- 2. Bekaert ED Dole E Dubois DY Bouma M-E Lontie J-F Kallel R Malmendier CL Ayrault‐Jarrier M , 1992. Alterations in lipoprotein density classes in infantile visceral leishmaniasis: presence of apolipoprotein SAA. Eur J Clin Invest 3: 190–199. [DOI] [PubMed] [Google Scholar]
- 3. Mohapatra S Ramakrishan L Samantaray J Dash S , 2014. Lipid derangement as diagnostic and prognostic indicator for visceral leishmaniasis patients. Trop Parasitol 4: 134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lal CS et al. 2016. Hypertriglyceridemia: a possible diagnostic marker of disease severity in visceral leishmaniasis. Infection 44: 39–45. [DOI] [PubMed] [Google Scholar]
- 5. Ghosh J Das S Guha R Ghosh D Naskar K Das A Roy S , 2012. Hyperlipidemia offers protection against Leishmania donovani infection: role of membrane cholesterol. J Lipid Res 53: 2560–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liberopoulos E Alexandridis G Bairaktari E Elisaf M , 2002. Severe hypocholesterolemia with reduced serum lipoprotein(a) in a patient with visceral leishmaniasis. Ann Clin Lab Sci 32: 305–308. [PubMed] [Google Scholar]
- 7. Coura-Vital W de Araújo VEM Reis IA Amancio FF Reis AB Carneiro M , 2014. Prognostic factors and scoring system for death from visceral leishmaniasis: an historical cohort study in Brazil. PLoS Negl Trop Dis 8: e3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kämink SS Collin SM Harrison T Gatluak F Mullahzada AW Ritmeijer K , 2017. A clinical severity scoring system for visceral leishmaniasis in immunocompetent patients in south Sudan. PLoS Negl Trop Dis 11: e0005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Queiroz Sampaio MJA Cavalcanti NV Alves JGB Filho MJCF Correia JB , 2010. Risk factors for death in children with visceral leishmaniasis. PLoS Negl Trop Dis 11: e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Araki MVR Barros C Santos EG , 2011. Análise do perfil lipídico de crianças e adolescentes do estado de Sergipe. Sci Plena 6: 12b. [Google Scholar]
- 11. Leao SC, Carvalho TS, Galvão MP, Silva RV, Rocha MF, Queiroz AAF, Almeida RO, Araujo RR, Souto MJS, de Andrade Rodrigues TM, 2016. A decade of lipid profiles: a gender focus. Hear Res - Open J 3: 9–15. [Google Scholar]
- 12. Malmendier CL Lontie JF Dubois DY , 1991. Mechanisms of hypocholesterolemia. Adv Exp Med Biol 285: 173–182. [DOI] [PubMed] [Google Scholar]
- 13. Auerbach BJ Parks JS , 1989. Lipoprotein abnormalities associated with lipopolysaccharide-induced lecithin: cholesterol acyltransferase and lipase deficiency. J Biol Chem 264: 10264–10270. [PubMed] [Google Scholar]
- 14. Lal CS Kumar A Kumar S Pandey K Kumar N Bimal S Sinha PK Das P , 2007. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin Chim Acta 382: 151–153. [DOI] [PubMed] [Google Scholar]
- 15. Feingold KR Memon RA Moser AH Shigenaga JK Grunfeld C , 1999. Endotoxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis 142: 379–387. [DOI] [PubMed] [Google Scholar]
- 16. Patton JS Shepard HM Wilking H Lewis G Aggarwal BB Eessalu TE Gavin LA Grunfeld C , 1986. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci USA 83: 8313–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liberopoulos EN Apostolou F Gazi IF Kostara C Bairaktari ET Tselepis AD Elisaf M , 2014. Visceral leishmaniasis is associated with marked changes in serum lipid profile. Eur J Clin Invest 44: 719–727. [DOI] [PubMed] [Google Scholar]
- 18. Wang G et al. 2020. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis 19: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y Chi J Lv W Wang Y , 2021. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab Res Rev 37: e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luzi L Radaelli MG , 2020. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol 57: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lago AS. et al. , 2020. Diabetes modifies the clinic presentation of cutaneous leishmaniasis. Open Forum Infect Dis 7: ofaa491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwetz V et al. 2018. Visceral leishmaniasis in a patient with diabetes mellitus type 2 and discrete bicytopenia. Clin Case Rep 6: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarnáglia GD Covre LP Pereira FEL De Matos Guedes HL Faria AMC Dietze R Rodrigues RR Maioli TU Gomes DCO , 2016. Diet-induced obesity promotes systemic inflammation and increased susceptibility to murine visceral leishmaniasis. Parasitology 143: 1647–1655. [DOI] [PubMed] [Google Scholar]