ABSTRACT.
Prevalence data on severe dental infections is scarce, particularly for low-income countries. Patients with dental abscess complications who presented from September 2020 until December 2020 in two hospitals in Tonkolili District, Sierra Leone, were included into this case series. We report on a total of 20 patients, median age 28 years, with severe complications of dental abscesses, with a mortality rate of 45%. This case series illustrates the severity of the dire consequences of the absence of access to basic dental and oral healthcare.
INTRODUCTION
Good dental and, in general, oral health is an important contributor to overall health and quality of life. Oral health inequalities are a global problem and affect all age groups. Social determinants of health, most importantly socioeconomic status, relate directly to the prevalence and severity of oral diseases.1–4
Whereas in high- and middle-income countries and those countries, in general, undergoing rapid transition toward western lifestyle and dietary patterns, excess sugar consumption is the main driving force behind caries up from young age.4,5 Beyond nutritional factors, lack of knowledge on and access to oral health; financial hardship and the difficulty to gain access to dental toiletries such as toothbrushes, (fluoridated) toothpaste, and the lack of dental services are assumed to contribute to an overall poor state of oral health in low-income countries.4 In Sierra Leone, over 50% of children experience toothache as a result of severe decay (caries), without access to appropriate care.6 Poor oral hygiene or oral neglect can result in severe tooth decay in the adult population, which may progress to serious odontogenic infections including apical dental abscesses and exacerbations, with peri-mandibular or pterygomandibular abscess formation, or spread into the soft tissues such as cellulitis or phlegmon.1–3 Severe odontogenic infections originate mostly from decayed teeth with non-vital pulps leading to periapical abscess formation (periodontitis apicalis).7,8 Often, the infection spreads lingually rather than buccally, originating from decayed mandibular molars, because the lingual aspect of the tooth socket is thinner. A diffuse cellulitis in the submandibular or peri-mandibular spaces tends to spread fast to the cervical tissues.1 A rapidly spreading bilateral submandibular soft tissue infection is often referred to as Ludwig’s angina (generally without abscess formation), provided that the symptoms include additional symptoms such as stridor and impaired airway function. Severe, spreading odontogenic infections are potentially life-threating due to a compromised airway, tissue necrosis, mediastinitis, and sepsis including multiple organ failure. The infection is usually polymicrobial, involving oral flora, aerobes, and anaerobes.6 Immunocompromised patients or patients with a compromised nutritional status are at higher risk of severe exacerbations of odontogenic infections.9
Identifying and treating these odontogenic infections in an early stage is life-saving.1–4 Case fatality rates regularly exceeded over 50% prior to development of antibiotics due to life-threatening complications.9,10 Nowadays, in high-income settings, the mortality related to exacerbation of severe odontogenic infections has been reduced to less than 8%.1 The key to successful treatment is the early start of IV broad-spectrum antibiotics, surgical intervention with drainage of the infected tissues or abscesses, and, if required, timely intubation. Furthermore, dental extraction is recommended in a second stage when the infection is controlled and the patient is stable.9 In case of necrotizing fasciitis or abscess formation, surgical intervention is the first choice of treatment.11
The Global Burden of Disease Study 2017 estimated that 3.5 billion people worldwide are affected by oral diseases, with over 2.3 billion people suffering from caries of permanent teeth. On the prevalence of severe dental infections, in general, few data is available. Data are even scarcer for low-income countries. McDonnough et al. reported a total amount of 5,855 patients nationwide in the United States between 2006 and 2014, which makes it a rare disease in the United States.12 In Ghana, between 2012 and 2017, there were 243 patients reported with incidence proportions ranging between 8.2 and 27.7 per 1,000 cases of tooth-related infections for the respective years.2
CASE SERIES
The patients of this case series were identified and included at Masanga Hospital (MH) (district hospital with 120 beds, catchment area of 440,000 people) and Lion Heart Medical Center (LHMC) (district hospital with 70 beds, catchment area of 110,000 people) in rural TD from September 1, 2020 until December 31, 2020.
Patients with severe dental abscess complications that presented to our hospitals were eligible for inclusion. Clinical photographs were taken upon admission, during every surgical procedure, and upon discharge. Clinical information on the preadmission history was gathered by the attending clinicians.
As we retrospectively compiled an observational noninterventional case series, no ethics clearance was required. All participants, however, were consenting, and provided written permission to use clinical picture and data material.
Post-discharge follow-up was not performed routinely. Patients were advised to return to our outpatient departments for medical review, but since most of them came from far and transport cost were an issue; in practice, follow-up was only done if telephone consultation was possible.
A total of 20 patients, seven female and 13 male, were included. Three further eligible patients could not be included due to death shortly after arrival. The median age for the patients included was 28 years. Eighteen patients (90%) said to brush teeth daily; 14 (70%) visited the traditional healer before admission; 12 (60%) needed surgical intervention; and three (15%) required tracheostomy. The median duration of admission was 7 days (range 0–112 days); the overall mortality rate was 45% (9/20 cases); one female and eight males. Main causes of death reported were necrotizing fasciitis (6/20 cases; 30%) and bacterial meningitis (2/20 cases; 10%). One cause of death remained unknown. Table 1 summarizes the key characteristics of the case series, Case Vignettes 1–3 (Figures 1–3) highlight selected cases.
Table 1.
Clinical course summary for all 20 patients
| Age | Sex | TH | TB | ID in cm | Hb oA g/dl | HR oA bpm | AB bA | DB | Sepsis | SI | Tracheostomy | DoA | Mortality | Cause of death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 28 | F | Y | Y | 50 | 5.6 | 119 | Y | N | N | 2 | N | 113 | N | |
| P2 | 21 | M | Y | Y | 30 | 15.3 | 110 | Y | Y | N | 3 | Y | 51 | N | |
| P3 | 30 | M | N | Y | 35 | 10.9 | 116 | N | N | N | 2 | N | 5 | Y | Necr. fasciitis + pneumonia |
| P4 | 70 | M | N | Y | 20 | 11.8 | 111 | N | Y | Y | 0 | N | 1 | Y | Necr. fasciitis-sepsis |
| P5 | 19 | M | Y | Y | 10 | 14.2 | 103 | N | N | N | 0 | N | 28 | N | |
| P6 | 40 | M | N | Y | 60 | 12.0 | 88 | N | Y | N | 2 | Y | 22 | N | |
| P7 | 35 | F | Y | Y | 40 | 10.1 | 116 | N | Y | N | 2 | N | 9 | N | |
| P8 | 24 | M | Y | N | 50 | 7.7 | 116 | Y | N | N | 0 | N | 7 | Y | Unknown |
| P9 | 21 | M | Y | Y | 30 | 11.7 | 146 | Y | Y | Y | 2 | Y | 2 | Y | Compromised airway + bacterial meningitis |
| P10 | 35 | M | Y | Y | 40 | 11.4 | 134 | Y | Y | Y | 1 | Y | 7 | Y | Necr. fasciitis-sepsis |
| P11 | 28 | M | Y | Y | 50 | 6.0 | 123 | Y | N | N | 1 | N | 1 | Y | Necr. fasciitis-sepsis |
| P12 | 20 | F | Y | Y | 50 | 9.0 | 165 | Y | Y | Y | 1 | N | 9 | Y | Encephalitis |
| P13 | 3 | M | Y | Y | 20 | 6.7 | 172 | Y | M | N | 0 | N | 7 | N | |
| P14 | 39 | M | Y | Y | 10 | x | 86 | N | N | N | 5 | N | 31 | N | |
| P15 | 35 | F | N | Y | 10 | x | 102 | N | N | N | N | N | 2 | N | |
| P16 | 46 | M | Y | Y | 10 | 11,5 | 155 | N | N | Y | 4 | N | 10 | Y | Necr. fasciitis - septic shock |
| P17 | 45 | F | Y | Y | 15 | 9,0 | 98 | N | N | N | 1 | N | 12 | N | |
| P18 | 25 | M | Y | Y | 10 | x | 47 | N | Y | Y | N | N | 0 | Y | Septic shock, death on D0 |
| P19 | 9 | F | Y | N | 15 | 8,3 | x | N | N | N | N | N | 10 | N | |
| P20 | 25 | F | Y | Y | 10 | 9,8 | 111 | Y | N | N | Y | N | N | N |
AB = antibiotics; bA = before admission; DB = difficulty breathing; DoA = days of admission; ID = interincisal distance (i.e., mouth opening in cm); F = female; M = male; N = no; oA = on admission; P = patient; SI = surgical interventions; TB = toothbrushing; TH = traditional healer (Was a traditional healer visited prior to presentation? Y/N); x = missing data; Y = yes.
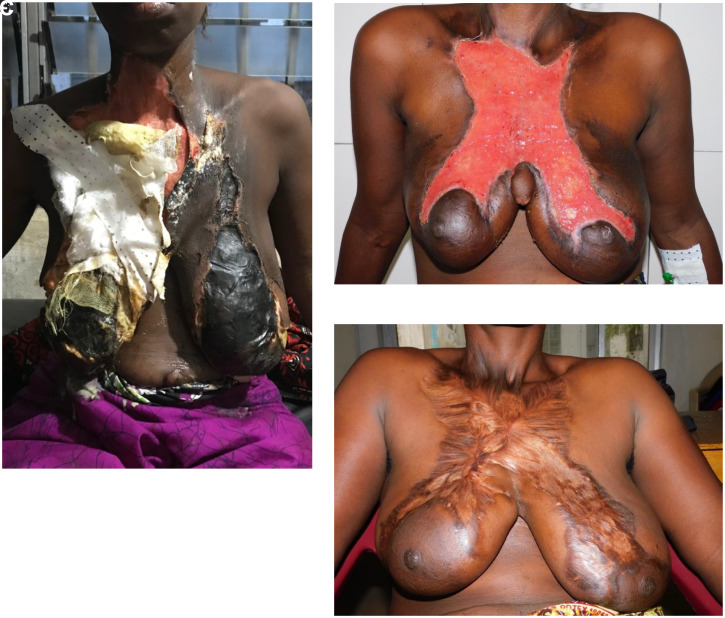
Figure 1.
Case vignette #1. (A) on admission; (B) before skin graft surgery; (C) four months after skin graft surgery. A 28-year-old lactating mother had toothache for 3 weeks. She first sought help from a traditional healer, who applied leaves on the skin and gave her several smoke baths. Because the swelling and pain increased, she went to another hospital first where she received metronidazole, ceftriaxone IV, and surgical debridement. As the situation deteriorated, the patient was referred to our district hospital. On admission, the patient was alert and stable, she had no difficulty breathing. The mouth opening was 50 mm (interincisal distance). Her vital parameters were stable, and she was afebrile. She had a deep and expansive necrotizing lesion on her chest, starting at the jaw reaching up to both upper parts of the areolae mammae (A). There was no drainage of pus into the mouth. Her hemoglobin was 5.6 g/dL, a malaria RDT was positive, and an HIV-test was negative. A provisional diagnosis of necrotizing fasciitis due to a dental abscess secondary to a tooth infection was made. The patient was stabilized with 2 units of fresh blood and empirical intravenous antibiotics were administered (ceftriaxone 2 g OD, and metronidazole 500 mg TID). She was treated for concomitant malaria with co-artem standard-dose BID. The patient underwent debridement under ketamine anesthesia within 3 hours. Postoperatively, she developed septicemia indicated by a HR 162 bpm, BP 98/60 mm of Hg, RR 25 bpm, Sat 90%, Temp 38.9°C. The urine output remained adequate with > 30 cc/h. Gentamicin 240 mg OD and aggressive fluid resuscitation were initiated. The patient stabilized within two days. A skin graft operation was performed on D32 post-admission (B), using both upper legs as donor sites. On D35, the skin graft was infected, and 80% of the graft failed. Small graft “islands” remained, and further growth was established. The patient discharged herself against medical advice. Six months later, she returned to our facility. At that time, she had no complaints, her chest wound was fully granulated and scarred. There was no pain or dysfunction (C). This figure appears in color at www.ajtmh.org.
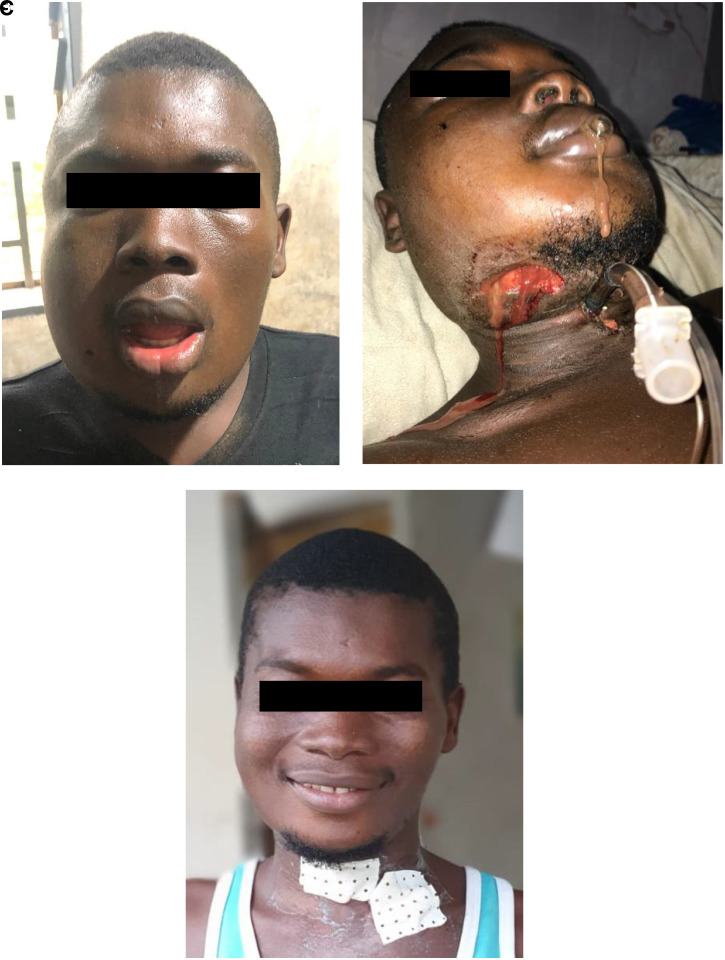
Figure 2.
Case vignette #2. (A) on admission; (B) after initial surgery: tracheostomy, incision and drainage; (C) on discharge. A 21-year-old male with an empty past medical history presented with a large bilateral submandibular swelling (A). He had toothache for 1 week and visited a traditional healer who had prescribed a topical herbal medication. On admission, the patient was alert, circulatory stable, and afebrile but had difficulty breathing; there was an inspiratory stridor, and the maximum mouth opening was 30 mm (inter-incisal distance); with pus draining into the oral cavity. The Hb level was 15.3 g/dL. The diagnosis of peri-mandibular cellulitis with airway compression was made, and intravenous antibiotics were administered (ceftriaxone 2 g OD, metronidazole 500 mg TID, and gentamycin 320 mg OD). Single-dose dexamethasone 10-mg IV was administered considering the stridor, continued with 4-mg IV QID. Also, the patient was nebulized with adrenaline in normal saline solution. The right-sided submandibular abscess was incised and drained. One day after admission, the patient deteriorated, with saturation dropping to 69% and prolonged apnea periods. During an emergency cricothyroidotomy, the previous incision on the right was extended to enhance drainage of pus (B). Three days later, there were crepitations and swelling in the left supraclavicular region, and an incision and drainage as well as a debridement were performed. On D6, the patient was considered stable enough for removal of the tracheostoma. He recovered uneventfully, with the prominent complaint of excess of saliva in the mouth cavity and trismus, both improving over the next 3–4 weeks. Three weeks after admission the mouth opening was sufficient for removal of the infected and decayed molar. Six weeks after admission the patient was discharged, with a mouth opening of 40 mm (C). At that time he received paracetamol for gum pain; his wounds were not fully granulated yet. Six weeks after discharge the patient was consulted by phone, and no complications were reported. This figure appears in color at www.ajtmh.org.
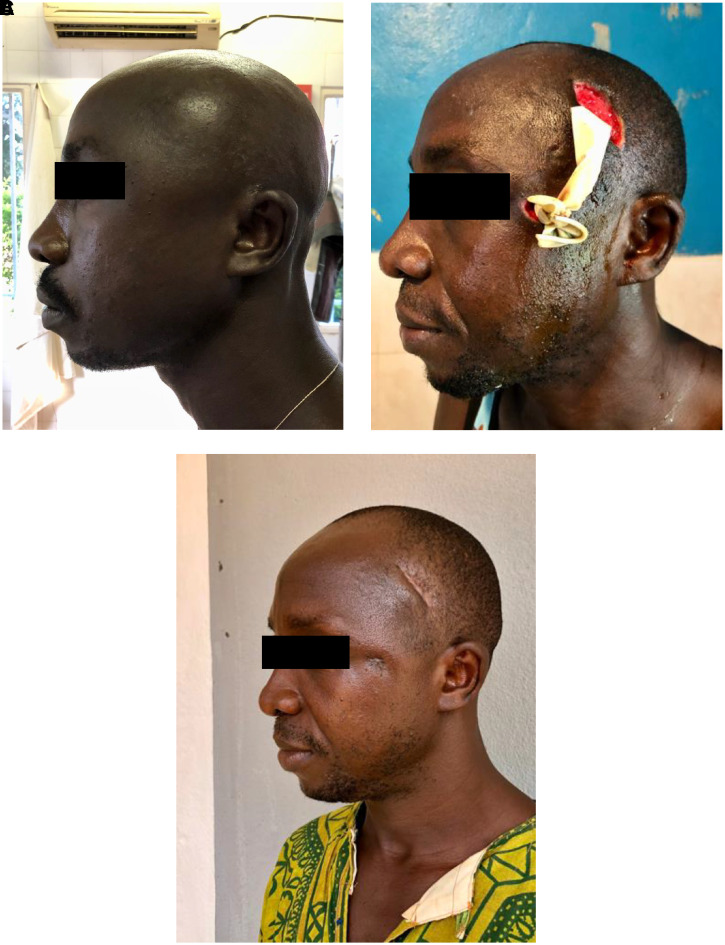
Figure 3.
Case vignette #3. (A) on admission; (B) post-second debridement; (C) eight weeks post-admission. A 39-year-old male with no relevant past medical history presented at LHMC with swelling of the left temporo-parietal region of the head (A). Other complaints were toothache, ear pain, and pain at the left mandibular notch for 2.5 weeks. He had visited a traditional healer who applied herbs with boiled water inside his mouth around the affected tooth before presentation at our facility. On admission the patient was alert with stable vitals and a clear trismus with a mouth opening of 10 mm (inter-incisal distance). The BP was 149/74 mm of Hg, HR 86 bpm, saturation of 97% without O2 support, RR 22/min, and temperature 37.1°C. There was a fluctuant swelling left temporo-parietal, but no pus draining in the mouth, and the lungs were clear. The patient was diagnosed with an odontogenic infection with abscess formation at the temporo-parietal region of the scalp and an osteomyelitis of the left mandibula. The abscess on the scalp was incised and drained and cloxacillin was started. Three days later, the patient underwent a new debridement because of increasing drainage of pus from the wound and inside the mouth (B). There was a necrotizing fasciitis of the scalp extending to the left cheek. The abscess pocket extended until the lateral corner of the left eye and into the left maxillary sinus and the left mandibular notch. Counter-incisions were made and the wound was flushed. During surgery (under ketamine anesthesia), the patient vomited. Antibiotic therapy was switched to ampicillin 1 g QID, gentamycin 160 mg OD, and metronidazole 500 mg TID intravenously because of the necrotizing fasciitis and the possible risk of aspiration pneumonia. On days 6 and 10, additional debridements were performed in theatre. The patient fully recovered, 5 weeks after admission the scalp wound was surgically closed and the patient was discharged. Eight weeks after the admission, several teeth were extracted (C). This figure appears in color at www.ajtmh.org.
DISCUSSION
In a relatively small time frame (4 months), in two rural hospitals in Sierra Leone, a large number of patients with severe odontogenic infection and abscess complications was seen. The mortality rate of 45% in this case series is unacceptably high, and highlights a public health problem that demands urgent attention. The affected population is young and otherwise healthy. This case series illustrates patients’ present late, and that (in our series 70%) visited a traditional healer before attending a health care facility. In our series, the most common cause of death was a severe necrotizing fasciitis.
Our case series shows that most patients know about oral health and practice daily tooth-brushing. Nevertheless, we reported a 100% patient delay after onset of swelling and/or pain, with 70% of the patients first seeking help at the traditional healer. Neither patients nor traditional healers seemed to be aware of the high incidence of catastrophic outcomes in case of a severe odontogenic infection, or did not expect rapid progression of their odontogenic infection. WHO also suggests, for settings as the one described here, to include oral health in the Universal Health Coverage, which should help reorient oral health policy away from a conventional model of dentistry toward a preventive model of care that promotes oral health to be integrated into health systems at all levels.13 For Sierra Leone, this would be clearly beneficial, and particularly so if the traditional healers were to be included into the discourse.
Limitations of this series are the random snapshots in time and the small observation period, the overall limited number of patients, the nonavailability of microbiological services (a bacteriological laboratory is currently established at Masanga Hospital) as well as the impossibility for structured follow-up in a routine setting. The strength is the comprehensive documentation of the courses of disease from admission to discharge.
The cases presented probably represent only a fraction of a by-far larger problem, considerably impacting on morbidity as well as mortality in young and otherwise healthy patients. This case series underlines the importance of oral health, especially for underprivileged communities in a low-income country. This mortality rate and patient load exposes the challenges the country faces in several social determinants of health, for example, on socioeconomical, educational, and political level. Therefore, like Ghotane et al.14 and Hugo et al.7 suggest, any approach toward improvement should be broad and multifactorial. Ghotane and others, looking at Sierra Leone specifically, identified the need for change in political willingness, resources (human and practical), regulation and collaboration of the few dentists the country holds, and lastly patient education.7,14 The WHO positions oral health as part of their strategy toward noncommunicable diseases. In their oral health program, it is stated that greater emphasis is to be put on developing global policies in oral health promotion and oral disease prevention.8 For Sierra Leone, particularly, this seems appropriate, since so far no policy on oral health is rolled out nationally. On a practical level, the goal should be to improve and intensify oral and dental health education, and to optimize early diagnosis and treatment by enhancing basic dental health facilities particularly in rural areas.
ACKNOWLEDGMENTS
We thank all health care workers and patients involved.
REFERENCES
- 1. Pak S Cha D Meyer C Dee C Fershko A , 2017. Ludwig’s angina. Cureus 9: e1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blankson PK Parkins G Boamah MO Abdulai AE Ahmed AM Bondorin S Nuamah I , 2019. Severe odontogenic infections: a 5-year review of a major referral hospital in Ghana. Pan Afr Med J 32: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saifeldeen K Evans R , 2004. Ludwig’s angina. Emerg Med J 21: 242–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peres MA et al. 2019. Oral health 1-oral diseases: a global public health challenge. Lancet 394: 249–260. [DOI] [PubMed] [Google Scholar]
- 5. Kirthiga M Murugan M Saikia A Kirubakaran R , 2019. Risk factors for early childhood caries: a systematic review and meta-analysis of case control and cohort studies. Pediatr Dent 41: 95–112. [PMC free article] [PubMed] [Google Scholar]
- 6. Brook I , 2007. Microbiology and principles of antimicrobial therapy for head and neck infections. Infect Dis Clin North Am 21: 355–391. [DOI] [PubMed] [Google Scholar]
- 7. Hugo FN Kassebaum NJ Marcenes W Bernabé E , 2021. Role of dentistry in global health: challenges and research priorities. J Dent Res 100: 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization WHO , 2016. Oral Health. Geneva, Switzerland: WHO. Available at: https://www.who.int/health-topics/oral-health/#tab=tab_1 Accessed July 17, 2021.
- 9. An J Madeo J Singhal M , 2021. Ludwig Angina. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482354/ Accessed July 23, 2021.
- 10. Bansal A Miskoff J Lis RJ , 2003. Otolaryngologic critical care. Crit Care Clin 19: 55–72. [DOI] [PubMed] [Google Scholar]
- 11. Edetanlen BE Saheeb BD , 2018. Comparison of outcomes in conservative versus surgical treatments for Ludwig’s angina. Med Princ Pract 27: 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonnough JA Ladzekpo DA Yi I Bond WR Jr. Ortega G Kalejaiye AO , 2019. Epidemiology and resource utilization of Ludwig’s angina ED visits in the United States 2006–2014. Laryngoscope 129: 2041–2044. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization , 2021. Universal Health Coverage. Geneva, Switzerland: WHO. Available at: https://www.who.int/health-topics/universal-health-coverage#tab=tab_1. Accessed June 12, 2021.
- 14. Ghotane SG Challacombe SJ Gallagher JE , 2019. Fortitude and resilience in service of the population: a case study of dental professionals striving for health in Sierra Leone. BDJ Open 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]