ABSTRACT.
Several neurological manifestations are recognized in dengue infection, but stroke is a rare complication. We report a case of ischemic stroke in a patient with dengue hemorrhagic fever. A 52-year-old previously healthy male presented with a history of fever for 2 days, and left-sided weakness and numbness of sudden onset. MRI scanning showed a right-sided thalamic lacunar infarct. Diagnosis of dengue fever was made based on leuco-thrombocytopenia, positive dengue nonstructural protein-1 (NS-1) antigen, and positive dengue IgM antibodies. Severity of limb weakness correlated with the critical phase of dengue hemorrhagic fever (DHF). He was discharged home with good recovery from neurological symptoms and disability. Strokes are rare in dengue, and are mainly hemorrhagic strokes related to thrombocytopenia. Ischemic stroke is even rarer. More evidence is needed for confirmation of dengue as a pathogenic mechanism of ischemic stroke.
CASE PRESENTATION
A 52-year-old previously healthy man presented to a Sri Lankan tertiary care hospital with a history of fever, headache, and arthralgia of 2 days. There was associated nausea and loss of appetite but no respiratory, urinary, gastrointestinal, or musculoskeletal symptoms were reported. He had a dull global headache without photophobia, phonophobia, or symptoms suggestive of increased intracranial pressure. On the second day of his illness, he had developed left sided face-arm-leg weakness and numbness of sudden onset. There was no altered sensorium, dysarthria, or dysphagia, and he was able to mobilize independently despite the weakness. He was a nonsmoker and a social drinker.
On admission, he was febrile with a temperature of 38.6°C and was dehydrated. There was no plethora or lymphadenopathy. He had a blood pressure of 120/80 mm of Hg and a pulse rate of 96 bpm, and examination of the precordium, lungs and abdomen were normal. The Glasgow Coma Scale was 15/15 and he was well oriented. A left face-arm-leg hemiparesis was noted (power grade 4/5 on the Medical Research Council [MRC] scale). Cranial nerve examination was normal, except for the left upper motor neuron type facial palsy. Deep tendon reflexes were normal and the plantar reflexes were flexor bilaterally. There was no objective sensory impairment or cerebellar involvement. He was able to walk independently. Stroke severity as measured by the National Institutes of Health Stroke Scale (NIHSS) score was 3 (on a scale of 0–42, higher scores indicating more severe strokes) on admission.
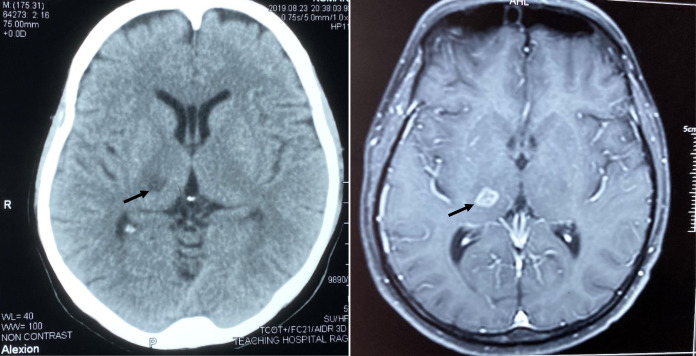
A diagnosis of dengue fever was made based on leucopenia, thrombocytopenia, and positive non-structural protein-1 (NS-1) antigen test for dengue, and subsequently by positive dengue IgM antibody test. His initial random blood glucose, renal functions, coagulation profile, C-reactive protein, and erythrocyte sedimentation rate (ESR) values were within normal limits. Urine analysis did not reveal hematuria. Liver transaminases were elevated compatible with dengue fever. A noncontrast CT scan of the brain showed a right-sided thalamic lacunar infarct, which was later confirmed by a MRI scan that showed a T2 and FLAIR high intensity area with mild diffusion restriction and peripheral enhancement in the right thalamus (Figure 1). Lumbar puncture was not performed because of severe thrombocytopenia. He was managed according to the current Sri Lankan dengue practice guidelines. Summary of important investigations available from the day of admission (third day of the illness) is shown (Supplemental Table 1).
Figure 1.
Non contrast CT scan (left) and Contrast-enhanced MRI brain (right) showing right thalamic infarct (arrow).
The patient entered the critical phase of dengue hemorrhagic fever (DHF) when the platelet count was 12,000/µL, and a focused ultrasound scan confirmed fluid leakage with pericholecystic fluid and free fluid in the hepatorenal pouch. The severity of limb weakness correlated with the disease progression of dengue fever. The left upper and lower limb power was grade 4/5 (MRC scale) at the beginning of the ascending phase of DHF, and weakness progressed to grade 3/5 requiring support for mobilization towards the peak of the ascending phase and during the first half of the descending phase. However, he did not develop other neurological complications and remained hemodynamically stable. He recovered from the critical phase of DHF with only supportive therapy including careful monitoring and fluid management. The timeline of important clinical events is shown below (Supplemental Table 2).
He was transferred to the hospital stroke unit after recovering from dengue for further management. With the recovery of thrombocytopenia, he was started on anti-platelet therapy. Stroke risk factor evaluation revealed elevated fasting blood glucose (198 mg/dL) and HbA1C (6.7%), and he was started on oral hypoglycemic medications. His 2D-echocardiogram and carotid Doppler scan were normal. He made a good neurological recovery with multi-disciplinary rehabilitation and was discharged home. He was followed up at the stroke clinic, and at 6 months was found to have complete recovery with no residual weakness and independence in all daily activities.
DISCUSSION
Dengue fever is an arboviral disease transmitted by mosquito vectors Aedes aegypti and Aedes albopictus.1 It is the most common mosquito-borne viral illness in Sri Lanka,2 with yearly epidemics following monsoon rains when conditions are optimal for mosquito breeding. The highest number of globally notified dengue cases was reported in 2019, with more than four million cases reported from all regions of the world.3 It is now recognized to be a disease with multi-organ involvement.
Neurological complications are well described in dengue but thought to be uncommon. The spectrum of neurological manifestations in dengue is diverse and can be related to neurotropic effects of the virus (e.g., meningitis, encephalitis, myositis, myelitis), systemic complications (e.g., encephalopathy), or post-infectious immune mediated phenomena (e.g., acute disseminated encephalomyelitis, Guillain-Barré syndrome).4
Stroke is a rarely reported neurological manifestation in dengue infection.5 Stroke was not observed among 45 patients with neurological involvement in a study of 486 patients with dengue in India.6 In a Sri Lankan study of 295 patients with dengue fever, neurological complications were reported in 12 patients, but strokes were not seen.7 Similarly, stroke has not been reported in several other case series detailing neurological involvement in dengue.8–11 The majority of strokes reported in dengue are hemorrhagic in nature,12–15 and increased capillary permeability, plasma leakage, and vasculitis are thought to be the underlying pathogenic mechanisms for hemorrhagic stroke.5
Thrombotic phenomena involving both arterial and venous circulations are described in dengue infection. These include myocardial infarction,16,17 cerebral venous thrombosis,18 deep vein thrombosis,19 and pulmonary embolism.20,21 The underlying pathophysiological basis for thrombotic events in dengue is poorly understood. Several hematological disturbances including cytokine storm secondary to antibody-dependent enhancement, increased immune complex formation, and complement activation are suggested as possible mechanisms.22 Loss of endothelial nonthrombogenic protective factors and expression of thrombomodulin by the infected endothelial cells facilitating a procoagulant hemotasis are described even in very early stages of severe dengue infection.23,24 However, none of these mechanisms have been described related to ischemic strokes in dengue.
Published literature on ischemic stroke in dengue fever is limited to a few case reports.5,25–30 The pathogenesis postulated includes meningo-vasculitis and a transient hypercoagulable state following plasma leakage.6,31,32 In our patient, the observed correlation of neurological disability with the severity of thrombocytopenia and plasma leakage lends support to a transient hypercoagulable state being a possible pathogenic mechanism. A similar observation was made in a case report by Liou in 2008.25 Intracranial vasculitis has been reported in a child with ischemic stroke and positive dengue serology.33 However, MRI angiography did not reveal vascular abnormalities in our patient. Cardiac dysfunction is described in dengue34 and can be a potential cause of ischemic stroke in these patients, but there is no evidence of possible cardiogenic embolism in the reported cases. In our patient, cardiogenic embolism was unlikely to be the possible stroke mechanism, as the clinical subtype was a lacunar syndrome, neuroimaging confirmed a lacunar infarct, and ECG and 2D echocardiogram were normal (although Holter monitoring was not performed). A summary of the key findings from previous publications of ischemic stroke in dengue is given in Supplemental Table 3.
Currently, management of dengue fever is limited to supportive care with carefully tailored fluid resuscitation. Immune-mediated mechanisms may play a role in thrombocytopenia in dengue infection,35 and correction of thrombocytopenia with steroids or intravenous immunoglobulin (IVIG) may help improve stroke outcomes.25 However, no benefit of steroids or IVIG has been shown in clinical trials on dengue management,36 and their place in clinical practice is uncertain.
Patients may present with symptoms related to stroke prior to the onset of symptoms of dengue.5 Using antiplatelet therapy or thrombolysis oblivious to the underlying dengue infection with thrombocytopenia in such patients may lead to fatal bleeding complications.37
In conclusion, ischemic stroke is a rare complication of dengue fever. The pathogenic mechanisms and treatment options of ischemic stroke in dengue fever need further study. It is important to exclude the possibility of dengue fever in the management of ischemic stroke in endemic countries, as thrombolysis, endovascular treatment and anti-platelet treatment could have fatal consequences in patients with unrecognized thrombocytopenia.
Supplemental Material
ACKNOWLEDGMENTS
We would like to thank all the staff members of the stroke unit, Colombo North Teaching Hospital, Ragama, Sri Lanka. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Kraemer MU et al. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weekly Epidemiological Report—Sri Lanka , 2013. Vector-borne Viral Infections. Available at: http://www.epid.gov.lk/web/images/pdf/wer/2013/vol_40_no_21_english.pdf. Accessed February 16, 2020.
- 3. Cogan JE , 2020. . Dengue and Severe Dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed May 30, 2020.
- 4. Murthy J , 2010. Neurological complications of dengue infection. Neurol India 58: 581. [DOI] [PubMed] [Google Scholar]
- 5. Mathew S Pandian JD , 2010. Stroke in patients with dengue. J Stroke Cerebrovasc Dis 19: 253–256. [DOI] [PubMed] [Google Scholar]
- 6. Sahu R Verma R Jain A Garg RK Singh MK Malhotra HS Sharma PK Parihar A , 2014. Neurologic complications in dengue virus infection: a prospective cohort study. Neurology 83: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 7. Tun MMN Muthugala R Nabeshima T Rajamanthri L Jayawardana D Attanayake S Soe AM Dumre SP Ando T Hayasaka D , 2020. Unusual, neurological and severe dengue manifestations during the outbreak in Sri Lanka, 2017. J Clin Virol 125: 104304. [DOI] [PubMed] [Google Scholar]
- 8. Verma R Sharma P Garg RK Atam V Singh MK Mehrotra HS , 2011. Neurological complications of dengue fever: experience from a tertiary center of north India. Ann Indian Acad Neurol 14: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Misra UK Kalita J Syam UK Dhole TN , 2006. Neurological manifestations of dengue virus infection. J Neurol Sci 244: 117–122. [DOI] [PubMed] [Google Scholar]
- 10. Araújo FM Araújo MS Nogueira RM Brilhante RS Oliveira DN Rocha MF Cordeiro RA Araújo RM Sidrim JJ , 2012. Central nervous system involvement in dengue: a study in fatal cases from a dengue endemic area. Neurology 78: 736–742. [DOI] [PubMed] [Google Scholar]
- 11. Misra UK Kalita J Mani VE Chauhan PS Kumar P , 2015. Central nervous system and muscle involvement in dengue patients: a study from a tertiary care center. J Clin Virol 72: 146–151. [DOI] [PubMed] [Google Scholar]
- 12. Carod-Artal FJ , 2019. Neurological complications associated with dengue virus infection. Rev Neurol 69: 113–122. [DOI] [PubMed] [Google Scholar]
- 13. Verma R Sahu R Singh AS Atam V , 2013. Dengue infection presenting as ischemic stroke: an uncommon neurological manifestation. Neurol India 61: 317–318. [DOI] [PubMed]
- 14. Vargas-Sánchez A Chiquete E Gutiérrez-Plascencia P Castañeda-Moreno V Alfaro-Castellanos D Paredes-Casillas P Ruiz-Sandovala JL , 2014. Cerebellar hemorrhage in a patient during the convalescent phase of dengue fever. J Stroke 16: 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar J Kumar A Gupta S Jain D , 2007. Neurological picture. Dengue hemorrhagic fever: an unusual cause of intracranial haemorrhage. J Neurol Neurosurg Psychiatry 78: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Umakanth M , 2017. Dengue fever complicated with Non-STEMI. Saudi J Med Pharm Sci 3: 704–706. [Google Scholar]
- 17. Lin S-C, Huang C-H, Lin C-Y, Lee W-H, Su H-M, Lin T-H, Lai W-T, Sheu S-H, Hsu P-C, 2016. Dengue virus infection complicated with simultaneous multivessel ST elevation myocardial infarction. J Microb Immun Inf 49: 619. [DOI] [PubMed] [Google Scholar]
- 18. Vasanthi N Vairamon PM Gowtham T Das AK , 2015. Unusual presentation of dengue fever-cerebral venous thrombosis. J Clin Diagn Res 9: Od09–Od10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ranasinghe KMIU, Dissanayaka D, Thirumavalavan K, Seneviratne M, 2020. An unusual case of dengue shock syndrome complicated by ilio-femoral deep vein thrombosis; a case report. BMC Infect Dis 20: 335. [DOI] [PMC free article] [PubMed]
- 20. Dissanayake N Pathirana A Amarasekara H Siriwardane C Marasinghe I Jayasekara R , 2019. Life threatening thrombo embolic event following dengue hemorrhagic fever. Sri Lankan J Cardiol 2: 69–72. [Google Scholar]
- 21. da Costa PS Ribeiro GM Junior CS da Costa Campos L , 2012. Severe thrombotic events associated with dengue fever, Brazil. Am J Trop Med Hyg 87: 741–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mairuhu AT Mac Gillavry MR Setiati TE Soemantri A ten Cate H Brandjes DP van Gorp EC , 2003. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis 3: 33–41. [DOI] [PubMed] [Google Scholar]
- 23. Cabello-Gutiérrez C Manjarrez-Zavala ME Huerta-Zepeda A Cime-Castillo J Monroy-Martínez V Correa BB Ruiz-Ordaz BH , 2009. Modification of the cytoprotective protein C pathway during dengue virus infection of human endothelial vascular cells. Thromb Haemost 101: 916–928. [PubMed] [Google Scholar]
- 24. Wills BA et al. 2002. Coagulation abnormalities in dengue hemorrhagic fever: serial investigations in 167 Vietnamese children with dengue shock syndrome. Clin Infect Dis 35: 277–285. [DOI] [PubMed] [Google Scholar]
- 25. Liou L-M Lan S-H Lai C-L , 2008. Dengue fever with ischemic stroke: a case report. Neurologist 14: 40–42. [DOI] [PubMed] [Google Scholar]
- 26. Manappallil RG , 2016. Ischemic stroke following dengue fever: a case report. Asian J Med Sci 7: 107–108. [Google Scholar]
- 27. Seet RC Lim EC , 2007. Dysarthria-clumsy hand syndrome associated with dengue type-2 infection. J Neurol 254: 1129–1130. [DOI] [PubMed] [Google Scholar]
- 28. Yoganathan S Sudhakar SV Priyambada L Thomas M , 2017. Stroke in a child with dengue encephalopathy. Ann Indian Acad Neurol 20: 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herath HMM Hewavithana JS De Silva CM Kularathna OAR Weerasinghe NP , 2018. Cerebral vasculitis and lateral rectus palsy—two rare central nervous system complications of dengue fever: two case reports and review of the literature. J Med Case Reports 12: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Estofolete CF Milhim BHGA Zini N Scamardi SN Selvante JD Vasilakis N Nogueira ML , 2020. Flavivirus infection associated with cerebrovascular events. Viruses 12: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li GH Ning ZJ Liu YM Li XH , 2017. Neurological manifestations of dengue infection. Front Cell Infect Microbiol 7: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li HM Huang YK Su YC Kao CH , 2018. Risk of stroke in patients with dengue fever: a population-based cohort study. CMAJ 190: E285–E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nanda SK Jayalakshmi S Mohandas S , 2014. Pediatric ischemic stroke due to dengue vasculitis. Pediatr Neurol 51: 570–572. [DOI] [PubMed] [Google Scholar]
- 34. Estofolete CF de Oliveira Mota MT Bernardes Terzian AC de Aguiar Milhim BHG Ribeiro MR Nunes DV Mourão MP Rossi SL Nogueira ML Vasilakis N , 2019. Unusual clinical manifestations of dengue disease—real or imagined? Acta Trop 199: 105134. [DOI] [PubMed] [Google Scholar]
- 35. Funahara Y Ogawa K Fujita N Okuno Y , 1987. Three possible triggers to induce thrombocytopenia in dengue virus infection. Southeast Asian J Trop Med Public Health 18: 351–355. [PubMed] [Google Scholar]
- 36. Rajapakse S , 2011. Dengue shock. J Emerg Trauma Shock 4: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zulkepli NS , 2018. PP051 Antiplatelet in dengue patient with acute coronary syndrome; a treatment turn misfortune. Malaysian J Emergency Med 3: 73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.