ABSTRACT.
It is important for malaria-endemic countries to address malaria control across international borders, and in particular to prioritize appropriate rapid diagnosis, treatment and surveillance. Bhutan and India aim to achieve malaria elimination by 2023 and 2030 respectively. Malaria elimination along the Indo–Bhutan border is of common concern. We delineated malaria epidemiology along the border to provide a blueprint for focusing malaria control efforts in key foci within this region. Epidemiological data from 2015 to 2019 were analyzed, as the most drastic reductions in malaria burden across most parts of India were witnessed in this time frame. Several areas of concern include low surveillance in most border districts, favorable climatic conditions for perennial malaria transmission, and movement of potential parasite carriers because of the porous borders. India and Bhutan need to control the importation/exportation of malaria cases. We highlight the foci of concern for which implementing tailor-made malaria control strategies may benefit both countries.
INTRODUCTION
In 2019, an estimated 229 million malaria cases were reported in 87 malaria-endemic countries worldwide.1 Nine of the World Health Organization’s (WHO) south-east Asia region member countries account for ∼3% of the global malaria burden, whereas the remaining two countries—Maldives and Sri Lanka—were certified malaria-free by WHO in 2015 and 2016, respectively.2 In the south-east Asia region India is the major contributor of malaria cases (∼88%), and Bhutan—India’s neighbor—is accelerating its malaria elimination program and aims to achieve elimination by 2023.
Bhutan is divided into 20 administrative districts, of which 7 districts namely Chukha, Dagana, Jongkhar, Pemagatshel, Samdrup, Sarpang Samtse, and Zhemgang share borders with India and show perennial malaria transmission. Another eight districts have seasonal or periodic transmission.3 The seven India-lining districts mentioned above are located in the foothills of southern Himalayan, bordering the Indian states of Assam and West Bengal.4 Between 2000 and 2009, continuous decline in malaria morbidity (from 5,935 to 972 cases) and mortality (from 15 to 4) was reported in Bhutan.3 Since 2009, Bhutan has decreased overall malaria incidence with cases below 1 per 1,000 population and thus the country is moving towards malaria elimination.5 In 2019, Bhutan reported 42 malaria cases (2 indigenous cases) as compared with 104 in 2015 (34 indigenous cases).6 Bhutan’s malaria control has been credited to the implementation of a multi-pronged approach that includes early diagnosis, prompt treatment, use of indoor residual spraying, long-lasting insecticidal nets, and integrated vector management.4 These measures have resulted in an interruption of indigenous transmission in 18 out of 20 districts.4,5
The national framework for malaria elimination of India aims to eliminate malaria by 2030.7 As of 2019, ∼7.5% (∼25K/∼338K) of the total malaria cases and ∼14.5% (∼23K/∼157K) of Plasmodium falciparum cases have been reported from the north-eastern states of India, namely Assam, Arunachal Pradesh, Manipur, Meghalaya, Mizoram, Nagaland, Sikkim and Tripura.8 In the last two decades (2000–2019), the percentage of total malaria cases reported in north-eastern states ranged from ∼4.3% (2017) to ∼14.9% (2009) whereas the percentage of P. falciparum ranged from ∼5.7% (2017) to ∼21.6% (2009).8 These north-eastern states of India share ∼4,600 kilometers of international borders with Bangladesh, Bhutan, China, and Myanmar.9 In Assam, both P. falciparum and P. vivax occur but the former accounts for > 60% of the cases.10,11 The Sikkim state is almost malaria free, and only few reported cases are due to migrants from malaria endemic areas within India.9
Bhutan and India share a ∼700 kilometers international border, which spans 10 districts of 4 Indian states i.e., Assam (Baksa, Chirang, Kokrajhar, and Udalguri districts), Arunachal Pradesh (Tawang and West Kameng districts), Sikkim (East District), and West Bengal (Alipurduar, Jalpaiguri, and Kalimpong) as shown in Figure 1. Assam and West Bengal, as well as seven districts in Bhutan, have observed significant cross-border activities,12 and the majority of malaria cases in Bhutan have been reported from areas bordering Assam in India.13 Curbing cross-border malaria transmission is a major challenge for both countries. The aim of this analysis is to delineate malaria epidemiology along the India–Bhutan border districts so as to provide a blueprint for focusing malaria control efforts in key foci within this region. We have analyzed epidemiological data in the 2015–2019 window as India witnessed the most significant gains in malaria control during this period.
Figure 1.
Location of Indian districts that border Bhutan. This figure appears in color at www.ajtmh.org.
MATERIALS AND METHODS
Study site and data collection.
A total of 10 Indian districts that border Bhutan were included in the study (Figure 1). Retrospective district-level malaria data of these districts from 2015 to 2019 was acquired from the National Vector Borne Diseases Control Program (NVBDCP). These data contain statistics on district-level total population, blood slides collected and examined, malaria positive cases that were categorized by P. vivax, P. falciparum, or mixed infections and deaths as a result of malaria. Malaria data of Tawang district of Arunachal Pradesh were not available for 2015 and 2016. Although the West Bengal district of Alipurduar was formed from Jalpaiguri in 2014, malaria data for individual districts were only available from 2018 onwards. As a result, malaria data of both Jalpaiguri and Alipurduar districts are the same from 2015 to 2017. Malaria data of Kalimpong (earlier part of Darjeeling district) were available for 2019 only with no case; therefore, no analysis could be performed for it. Major malaria indices, such as annual blood smears examination rate (ABER), annual falciparum incidence (AFI), annual parasite incidence (API), percentage of P. falciparum, slide falciparum rate (SFR), and slide positivity rate (SPR), were part of data obtained from NVBDCP where:
ABER = Number of slides examined × 100 / Total population
API = Confirmed cases during a year × 1,000 / Population under surveillance
AFI = No. of positive blood smears for P. falciparum malaria parasite in a year × 1,000 / Total population.
SPR = No. of blood smear found positive for malaria parasite × 100 / No. of blood smear examined
SFR = No. of positive blood smears for P. falciparum malaria parasite × 100 / No. of blood smear examined
Plasmodium falciparum percentage = No. of positive blood smears for P. falciparum malaria parasite × 100 / No. of blood smear found positive for malaria parasite
Furthermore, Geographic Information System–based maps were prepared using QGIS 3.14 software,14 a project of the Open-Source Geospatial Foundation, using state and district maps of India.
Statistical methods.
The normality of ABER, API, and SPR data was tested using the Kolmogorov–Smirnov (K–S) test online.15 The relationship between ABER and API was tested using Pearson correlation test in MS-Excel software. API is an extensively used metric for determining the actual intensity of malaria transmission and for comparing malaria transmission risk across different regions. The ABER signifies operational efficiency of surveillance and may indicate adequacy in case detection. To get reasonable assessment of API, the ABER should be typically 10% or above.16 We tested the relationship between API and ABER using quadrant analysis. SPR is an alternative indicator for estimating temporal changes in malaria incidence and may be a better indicator of malaria transmission for low ABER districts. Hence, comparison between API and SPR was also done to analyze the malaria situation in India–Bhutan border districts.
RESULTS
Identification of key districts.
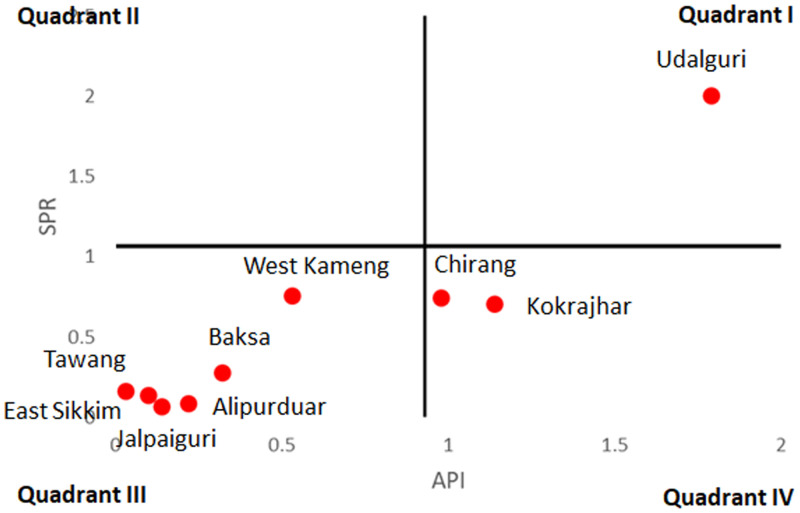
The null hypothesis H0 that the data follows normal distribution was accepted for all three variables, i.e., ABER (D = 0.19, P = 0.84, p(α) > 0.05), API (D = 0.23, P = 0.67, p(α) > 0.05), and SPR (D = 0.26, P = 0.51, p(α) > 0.05) using K–S test. As the data followed normal distribution, the relationship between ABER and API was tested using Pearson correlation test. No significant relationship was found between the ABER and API (R = −0.08, P = 0.84, not significant at P < 0.05) and therefore quadrant analysis was done to analyze the situation. The ABER was plotted on the x axis and the API on the y axis with ABER and API threshold limits of 10 (as per program’s threshold) and 0.9 (mid value of min-max), respectively. This divided the graph into four quadrants (Figure 2). Quadrant I, which includes two districts Kokrajhar and Chirang were thus identified as areas of concern for malaria control as these districts showed both high surveillance (ABER) and high malaria (API). Quadrant II was also of concern as the surveillance was less than the 10% and yet the API was high (Figure 2). This quadrant includes the district of Udalguri, which had an API even higher than the districts falling in Quadrant I. Quadrant III includes three districts, i.e., West Kameng, Tawang, and East Sikkim, and had surveillance below threshold. Thus, its API may not be a true measure of actual malaria cases. Quadrant IV with Baksa, Jalpaiguri, and Alipurduar had surveillance above threshold and low malaria (Figure 2).
Figure 2.
Quadrant analysis between average ABER and API from 2015 to 2019. This figure appears in color at www.ajtmh.org.
For low ABER districts, SPR was possibly a better indicator of malaria transmission and therefore comparisons were made between API and SPR. The API was plotted on the x axis and the SPR on the y axis with threshold limits of 0.9 (mid value of min-max) and 1.1 (mid value of min-max), respectively (Figure 3). Comparison between API and SPR indicates that most of the districts fell in Quadrant III with low API and low SPR (including low ABER districts, i.e., West Kameng, Tawang, and East Sikkim). Of these three districts, West Kameng district had highest SPR, almost at par with Chirang and Kokrajhar, located in the Quadrant IV and thus indicates that malaria was prevalent in this district and needed to be controlled.
Figure 3.
Quadrant analysis between average API and SPR from 2015 to 2019. This figure appears in color at www.ajtmh.org.
Comparison of malariometric indices.
As per framework of malaria elimination in India 2016–2030,7 API was considered the primary criterion to categorize Indian states for elimination. The districts reporting API ≥ 1 were considered in category 2 (pre-elimination) or category 3 (intensified control phase). Kokrajhar and Udalguri districts of Assam had an average API ≥ 1 from 2015 to 2019 (Supplemental Figure 1). Comparing year-wise API, in the year 2015, three districts of Assam viz. Chirang, Kokrajhar, and Udalguri reported API of > 2 whereas in the year 2019 none of the border districts reported API of > 0.5—this was very encouraging (Supplemental Figure 1). Nearly 80% reduction in API had been observed in 2019 as compared with 2015 in all districts except for Alipurduar district of West Bengal, where API increased but still has very few cases (from 0.2 in 2015 to 0.3 in 2019). While comparing API of the year 2019 to the API of 2018, district Kokrajhar of Assam and Alipurduar of West Bengal showed 181% (from 0.2 in 2018 to 0.5 in 2019) and 131% (from 0.1 in 2018 to 0.3 in 2019) increase respectively (Supplemental Figure 1). The average ABER of Tawang (1.4%), West Kameng (6.9%), Udalguri (9.5%), and East Sikkim (8.2%) districts reported was below 10%, whereas in the year 2019 Tawang (1.1%), West Kameng (6.7%), and East Sikkim (8.3%) reported ABER below 10% (Supplemental Figure 2). All other districts either showed stable or increasing ABER over the years. Hence, the districts with low ABER may need improved surveillance before it may be concluded that malaria is declining in them.
Planning and implementation of malaria elimination can be improved using SPR as it is a strong predictor of malaria transmission.17 In the case of low ABER, SPR is a more reliable malaria transmission indicator than API because it can provide a better indication of parasite load in the community. The average SPR of Udalguri district of Assam was highest (2%), though all border districts had shown declining trend of SPR from 2015 to 2019 (Supplemental Figure 2). Plasmodium falciparum percentage is the relative proportion of P. falciparum infection and its high value indicates possibility of higher morbidity and mortality. The Chirang district of Assam reported > 70% average P. falciparum percentage followed by Kokrajhar (∼68%) and Udalguri (∼56%). All districts except Udalguri showed decreasing trend in P. falciparum percentage but in Udalguri district it increased significantly from ∼25% in 2017 to 78% in 2018 (Supplemental Figure 2).
Like SPR, SFR is also less dependent on ABER and gives strong indication of parasite load in community. The average SFR of Udalguri district of Assam was highest (1.2) followed by Chirang (0.6) and Kokrajhar (0.5) districts. All border districts had shown declining trend of SFR from 2015 to 2019 (Supplemental Figure 2). AFI is the proportion of total positives for P. falciparum infection in the total population under malaria surveillance. The average AFI of Udalguri district of Assam was highest (1.0) followed by Kokrajhar (0.9) and Chirang (0.8) districts. All other border districts had shown lower AFI in 2019 as compared with 2015. Comparing AFIs of 2019 and 2018, districts Kokrajhar and Alipurduar showed an increase by 100% and 300%, respectively (Supplemental Figure 1). Hence, the above discussed malaria indicators present a heterogeneous picture of malaria transmission in the Bhutan-bordering districts of India. The analysis indicates that district-specific mitigation plans may need to be devised after assessing all malaria indicators for all these.
DISCUSSION
Malariometric indices are critical components of surveillance, monitoring, and evaluation of malaria incidence and control. These indices can suggest necessity for preventative and control efforts, as well as highlight when control actions are efficacious. Though each indicator has its limitations, monitoring and evaluating malaria programs may need assessment of multiple indicators to understand the malaria situation on the ground. Our analysis of 10 Bhutan-bordering districts showed a declining trend in APIs between 2015 and 2019 with none reporting API more than 0.5 in 2019. Similarly, with the exception of Udalguri where percentage of P. falciparum cases increased from ∼24% in 2017 to ∼77% in 2018, the P. falciparum percentage exhibited a downward trend in all districts. Overall, some coverage enhancement in low ABER districts (with substantial SPR) may be required for a better overall assessment of border malaria in the context of India and Bhutan. There may be several interrelated factors that drive the heterogeneous malaria epidemiology in the regions discussed. These include:
1. Malaria vectors: The primary malaria vectors in the region are Anopheles minimus and An. baimaii (An. dirus species D) and are linked to high malaria prevalence. Anopheles minimus, an endophillic, endophagic, and highly anthropophilic species that breeds in foothills and seepage water streams and is abundant in the region all year. It can bite humans all night long, with peak biting hours being between 01:00 and 04:00 hours.18 Anopheles baimaii is usually confined to a closed forest thus making it a complex mosquito to control.19 Anopheles baimaii displays high anthropophilicity with an ability to bite both indoors and outdoors.20 District-level distribution of malaria vectors and peak biting time is shown in Supplemental Figure 3.21–29 Both major vector species, An. minimus and An. baimaii, are yet susceptible to insecticides such as dichlorodiphenyltrichloroethane (DDT), malathion, and deltamethrin.21,30
2. Population movement: This may be one of the main drivers of malaria transmission. Malaria control in mobile and migrant communities is difficult because of the differences in administrative structures and the functioning of health systems in India and Bhutan. There is likely a constant danger of influx of malaria cases from high-endemic to low-endemic areas. Cross international border migrations for pilgrimage, tourism, picnics, retail, and labor is a major concern. Four national highways of NH-317A (Alipurduar), NH-127C (Chirang), NH-127A (Baksa), and NH-127D (Baksa) and one state highway (SH-4) connect India and Bhutan. Routine surveillance of transitory populations is an un-addressed need for malaria control in the region.13
3. Socioeconomic conditions: The majority of people in the study districts of Assam and Arunachal Pradesh live in rural settings, with urban populations of less than 25%. Except for East Sikkim, which had ∼84% percent literacy rate, the majority of studied districts reported literacy rates between 59% and 76%.31 Hence, both rural setting and low education levels (poor health awareness) may contribute to sustained malaria transmission indirectly. It is also worth noting that as a result of inadequate housing and sanitation, a lack of proper surface water drainage, and the usage of unprotected water reservoirs may contribute to an increase in malaria transmission.32 It is possible that because of the low literacy rates among rural community, many information, education and communication activities such as posters, pamphlets, hoardings, and electronic media have little or no impact. A study conducted in Paraguay and Sri Lanka revealed negative and significant effect of the malaria rate on educational attainment.33
4. Climatic conditions: These are favorable for perennial transmission of both P. vivax and P. falciparum in the Indo–Bhutan districts studied here.34,35 Supplemental Figure 3 depicts district-level climate, which includes temperature, rainfall and humidity in the study area. Thus, perennial mosquito breeding occurs in study areas as a result of the hot and humid climate that is supported by the numerous hill streams and tributaries.9 Because of heavy rainfall, the villages in forest fringe are hard to reach and remain inaccessible during rainy season when malaria is at its peak. These climatic conditions together build up a favorable condition for malaria transmission almost all year.
For malaria elimination in India and Bhutan adjacent malarious areas are of common concern among nations. The countries on the verge of malaria elimination usually get sporadic cases along international borders that are adjacent to the countries having higher malaria incidence. In this case there have been efforts by the two countries and by WHO to address the issue of cross-border malaria. In November 2019, a road map was developed for cross-border collaboration in a WHO meeting on India–Bhutan malaria problem. Major components of this were strengthened surveillance via sharing of real-time data using technology-based platforms such as dashboard, screening of population at border districts, diagnosis and treatment irrespective of nationality, investigation of cases/foci, and importantly reporting of private sector data by India.13,36,37 Other collaborative areas that were identified include synchronized deployment of vector control activities and IEC activities.38 The Ministerial Declaration on Accelerating and Sustaining Malaria Elimination in November 2017,39 the Regional Action Plan 2017–2030, and a framework for a South Asia sub-regional cross-border collaboration network were the key steps taken by both countries and WHO to eliminate the disease.40
Based on our results, the districts of Udalguri, Kokrajhar, Chirang and West Kameng may be a cause of concern for cross-border malaria and hence India and Bhutan may consider further joint measures to address these. It is vital that cross-border collaborations take into cognizance the dynamic epidemiological and entomological situations along the Indo–Bhutan districts studied here. This will enable their health systems to deploy tailor-made strategies and actions in these porous border areas. As a joint responsibility, both nations need to mitigate the importation/exportation of malaria cases. Our work here highlights key facets involved in cross-border malaria and can help in devising tailor-made interventional strategies that benefit both the nations.
Supplemental Material
ACKNOWLEDGMENTS
We thank the National Vector Borne Diseases Control Programme (NVBDCP) for providing data. This paper bears the NIMR publication screening committee approval no. RIC-02/2021. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental figures appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization , 2020. World Malaria Report 2020, 20 Years of Global Progress & Challenges. Geneva, Switzerland: WHO. Available at: https://www.who.int/publications/i/item/9789240015791. Accessed March 20, 2021. [Google Scholar]
- 2. World Health Organization , 2021. Countries and Territories Certified Malaria-free by WHO. Geneva, Switzerland: WHO. Available at: https://www.who.int/teams/global-malaria-programme/elimination/countries-and-territories-certified-malaria-free-by-who. Accessed March 20, 2021. [Google Scholar]
- 3. World Health Organization , 2011. Bhutan Malaria Control Programme Review: A Report. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/204831. [Google Scholar]
- 4. Wangdi K Penjor K Tobgyal, Lawpoolsri S Price RN Gething PW Gray DJ Da Silva Fonseca E Clements ACA , 2021. Space–time clustering characteristics of malaria in Bhutan at the end stages of elimination. Int J Environ Res Public Health 18: 5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vector-borne Diseases Control Programme, Department of Public Health Ministry of Health Royal Government of Bhutan , 2020. Strategic Plan for Elimination of Malaria and Prevention of Re-introduction in Bhutan 2020–2025. Available at: https://apmen.org/sites/default/files/all_resources/Strategic%20Plan%20for%20Elimination%20%26%20Prevention%20of%20Re-Introduction_Bhutan%20%282020-2025%29.pdf.
- 6. Ministry of Health, Royal Government of Bhutan , 2020. Annual Health Bulletin 2020. Available at: http://www.moh.gov.bt/wp-content/uploads/ict-files/2017/06/health-bulletin-Website_Final.pdf. Accessed October 16, 2020.
- 7. National Vector Borne Disease Control Programme , 2016. National Framework for Malaria Elimination in India (2016–2030). Available at: https://nvbdcp.gov.in/WriteReadData/l892s/National-framework-for-malaria-elimination-in-India-2016%E2%80%932030.pdf. Accessed October 16, 2020.
- 8. National Vector Borne Disease Control Programme , 2020. Malaria Situation. Available at: https://nvbdcp.gov.in/WriteReadData/l892s/70838173921597401184.pdf. Accessed October 3, 2020.
- 9. Sarma DK Mohapatra PK Bhattacharyya DR Chellappan S Karuppusamy B Barman K Senthil Kumar N Dash AP Prakash A Balabaskaran Nina P , 2019. Malaria in north-east India: importance and implications in the era of elimination. Microorganisms 7: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dev V Sharma VP Barman K , 2015. Mosquito-borne diseases in Assam, north-east India: current status and key challenges. WHO South-East Asia J Public Health 4: 20–29. [DOI] [PubMed] [Google Scholar]
- 11. Saikia NJ , 2017. Malaria: an epidemic in Assam. Asian J Sci Technol 8: 5079–5083. [Google Scholar]
- 12. Wangchuk S Gyeltshen S Dorji K Wangdi T Dukpa T Namgay R Dorjee S Tobgay T Chaijaroenkul W Na-Bangchang K , 2019. Malaria elimination in Bhutan: asymptomatic malaria cases in the Bhutanese population living in malaria-risk areas and in migrant workers from India. Rev Inst Med Trop Sao Paulo 61: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization, South-East Asia , 2019. Meeting on Cross Border Collaboration on Malaria Elimination Along the India-Bhutan border. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/331933 Accessed October 16, 2020. [Google Scholar]
- 14. Quantum GIS Development Team , 2021. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. Available at: https://qgis.org/en/site/.
- 15. Bioquest AAT, Inc , 2019. Quest Graph™ Kolmogorov-Smirnov (K-S) Test Calculator. Available at: https://www.aatbio.com/tools/kolmogorov-smirnov-k-s-test-calculator. Accessed March 24, 2021.
- 16. Cohen AA Dhingra N Jotkar RM Rodriguez PS Sharma VP Jha P , 2010. The Summary Index of Malaria Surveillance (SIMS): a stable index of malaria within India. Popul Health Metr 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bi Y Hu W Liu H Xiao Y Guo Y Chen S Zhao L Tong S , 2012. Can slide positivity rates predict malaria transmission? Malar J 11: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dev V Sharma VP , 2013. The Dominant Mosquito Vectors of Human Malaria in India, Anopheles Mosquitoes - New Insights into Malaria Vectors. Sylvie Manguin IntechOpen 2013. Available at: https://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors/the-dominant-mosquito-vectors-of-human-malaria-in-india.
- 19. Sharma VP , 2012. Continuing challenge of malaria in India. Curr Sci 102: 678–682. [Google Scholar]
- 20. Dev V , 2020. Vector Biology and Control: an Update for Malaria Elimination Initiative in India. New Delhi, India: The National Academy of Sciences. [Google Scholar]
- 21. Raghavendra K, Velamuri PS, Verma V, Elamathi N, Barik TK, Bhatt RM, Dash AP, 2017. Temporo-spatial distribution of insecticide-resistance in Indian malaria vectors in the last quarter-century: need for regular resistance monitoring and management. J Vector Borne Dis 54: 111–130. [PubMed] [Google Scholar]
- 22. Government of Arunachal Pradesh , 1996. Arunachal Pradesh District Gazetteers. East Kameng, West Kameng. Available at: http://www.arunachalpradesh.gov.in/east-kameng-west-kameng-tawang-districts. Accessed October 16, 2020.
- 23. Dhiman S, Yadav K, Rabha B, Goswami D, Hazarika S, Tyagi V, 2016. Evaluation of insecticides susceptibility and malaria vector potential of anopheles annularis s.l. and anopheles vagus in Assam, India. PLoS One 11: e0151786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prakash A Mohapatra PK Srivastava VK , 1996. Vector incrimination in Tamulpur primary health centre, district Nalbari, lower Assam during malaria outbreak 1995. Indian J Med Res 103: 146–149. [PubMed] [Google Scholar]
- 25. Nath DC Mwchahary DD , 2012. Malaria prevalence in forest and non-forest areas of Kokrajhar district of Assam. Public Health 2012: 142037. [Google Scholar]
- 26. Prakash A, Sarma DK, Bhattacharyya DR, Mohapatra PK, Bhattacharjee K, Das K, Mahanta J, 2010. Spatial distribution and r-DNA second internal transcribed spacer characterization of Anopheles dirus (Diptera: Culicidae) complex species in north-east India. Acta Trop 114: 49–54. [DOI] [PubMed] [Google Scholar]
- 27. Mittal PK Wijeyaratne P Pandey S , 2004. Status of insecticide resistance of malaria, Kala-azar and Japanese encephalitis vectors in Bangladesh, Bhutan, India and Nepal (BBIN). Environ Health Proj Act Rep 129: 44–48. [Google Scholar]
- 28. Subbarao SK Nanda N Rahi M Raghavendra K , 2019. Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar J 18: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tananchai C Tisgratog R Juntarajumnong W Grieco JP Manguin S Prabaripai A Chareonviriyaphap T , 2012. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasit Vectors 5: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yadav RS, Raghavendra K, Velamuri PS, Verma V, Uragayala S, Dev V. Vector Biology and Control – An Update for Malaria Elimination Initiative in India. Allahabad, India: National Academy of Sciences, 129–148. [Google Scholar]
- 31. Census of India , 2011. District Census Hand Book. Available at: https://censusindia.gov.in/2011census/dchb/DCHB.html. Accessed October 16, 2020.
- 32. Martens P, Hall L, 2000. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis 6: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucas AM , 2010. Malaria eradication and educational attainment: evidence from Paraguay and Sri Lanka. Am Econ J Appl Econ 2: 46–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yadav K Dhiman S Rabha B Saikia P Veer V , 2014. Socio-economic determinants for malaria transmission risk in an endemic primary health centre in Assam, India. Infect Dis Poverty 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mutheneni SR Upadhyayula SM Kadiri MR Nishing K , 2014. Malaria prevalence in Arunachal Pradesh—a northeastern state of India. Am J Trop Med Hyg 91: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rahi M Sharma A , 2020. For malaria elimination India needs a platform for data integration. BMJ Glob Health 5: e004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahi M Chaturvedi R Das P Sharma A , 2021. India can consider integration of three eliminable disease control programmes on malaria, lymphatic filariasis, and visceral leishmaniasis. PLoS Pathog 17: e1009492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rahi M Ahmad SS Sharma A , 2021. Coverage enhancement and community empowerment via commercial availability of the long-lasting nets for malaria in India. Public Health Pract 2: 100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization, Regional Office for South-East Asia , 2018. WHO Regional Committee for South-East Asia – Report of the Seventy-first Session. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/310929. [Google Scholar]
- 40. World Health Organization, Regional Office for South-East Asia , 2017. Regional Action Plan 2017–2030. Towards 0. Malaria-Free South-East Asia Region. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/272389. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.