Abstract
Abnormal coagulation dynamics, including disseminated intravascular coagulopathy, pulmonary embolism, venous thromboembolism and risk of thrombosis are often associated with the severity of COVID-19. However, very little is known about the contribution of platelets in above pathogenesis. In order to decipher the pathophysiology of thrombophilia in COVID-19, we recruited severely ill patients from ICU, based on the above symptoms and higher D-dimer levels, and compared these parameters with their asymptomatic counterparts. Elevated levels of platelet-derived microparticles and platelet-leukocyte aggregates suggested the hyperactivation of platelets in ICU patients. Strikingly, platelet transcriptome analysis showed a greater association of IL-6 and TNF signalling pathways in ICU patients along with higher plasma levels of IL-6 and TNFα. In addition, upregulation of pathways like blood coagulation and hemostasis, as well as inflammation coexisted in platelets of these patients. Further, the increment of necrotic pathway and ROS-metabolic processes in platelets was suggestive of its procoagulant phenotype in ICU patients. This study suggests that higher plasma IL-6 and TNFα may trigger platelet activation and coagulation, and in turn aggravate thrombosis and hypercoagulation in severe COVID-19 patients. Therefore, the elevated IL-6 and TNFα, may serve as potential risk factors for platelet activation and thrombophilia in these patients.
Keywords: Platelet transcriptome, IL-6, TNFα, Platelet activation, Coagulation, Thrombophilia, COVID-19
Abbreviations: SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; COVID-19, Coronavirus disease 2019; ARDS, acute respiratory disorder symptom
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a newly emerged member of the β-coronaviruses, a positive sense single-stranded RNA virus and is responsible for causing an acute respiratory disorder symptom (ARDS) in patients with Coronavirus Disease 2019 (COVID-19). In ARDS, accumulation of fluid prevents alveoli from filling with enough air, leading to a condition where less oxygen reaches bloodstream, or hypoxemia. Inflammation and intravascular clot formation in the lung are associated with pathogenesis of ARDS in COVID-19 [1], [2], [3]. These patients often develop multiorgan failure and thrombotic complications, including myocardial infarction and ischemic stroke [3], [4], [5], [6], [7].
Although contribution of platelets in the development of COVID-19 pathophysiology is not yet clear, but several studies have described its link with other viral diseases. Platelets express a broad range of receptors, including Toll-like receptors (TLRs), C-type lectin receptors, and nucleotide-binding and oligomerization domain-like receptors, that are known to help the cells recognize viral pathogens such as dengue, HIV-1, and influenza [8], [9], [10]. However, it is yet unclear whether SARS-CoV-2 binds to platelets and regulates their functions. A recent study describes no detectable levels of SARS-CoV-2 in platelets from patients with COVID-19 [11]. Although the study did report elevated platelet activation alongside platelet-leukocyte aggregates in these patients. Platelet activation often occurs when these cells respond to invading viruses and mediate immune responses directly through interaction with leukocytes [12], [13], [14]. On the other hand, cytokines including IL-6 and TNFα are also known to trigger platelet activation [15], [16]. The inflammatory and infectious illnesses are frequently associated with prothrombotic responses during viral infections. However, very little is known about the contribution of platelets in SARS-CoV-2 pathogenesis.
One recent study described that the hyperactivation of platelets [17] is associated with elevated thromboxane generation in COVID-19 patients [11]. In addition to abnormal coagulation dynamics, higher levels of D-dimers along with elevated fibrinogen, and von Willebrand factor (VWF) were observed in a large pool of COVID-19 patients [18], [19], [20]. Studies have reported D-dimer as a biomarker for thrombotic complications in disease severity and mortality in these patients [21].
The mechanism of platelet activation and development of thrombotic complications, including thrombophilia, in COVID-19 is largely unknown. While analysing whole platelet transcriptome a recent study has reported the elevation of certain gene transcripts in COVID-19 patients compared with healthy controls, but the global transcriptome changes were mostly similar in both ICU and non-ICU patients with COVID-19 [11]. Using the whole platelet transcriptome analysis, we here describe a large number of differentially expressed transcripts along with the elevated pathways like hemostasis, thrombosis, IL-6 and TNF signalling in severely ill ICU patients, as compared to non-ICU asymptomatic patients with COVID-19, from a local hospital in Delhi NCR, India. Our data also report that the high plasma levels of IL-6 and TNFα may trigger platelet activation and coagulation in severely ill COVID-19 patients.
2. Materials and methods
2.1. Study subjects
The Institutional Ethics Committee (IEC) of Regional Centre of Biotechnology (RCB), Faridabad and ESIC Medical College and Hospital, Faridabad approved the human study, reference numbers RCB-IEC-H-27. All study volunteers were recruited during the year 2020. Methods were performed in accordance with the relevant guidelines and regulations of the IEC. We collected limited volume (total 3 ml) of whole blood in ACD anticoagulant vials from asymptomatic [inclusion criteria: COVID-19 positive (PCR detection), without symptoms like sore throat, fever and tiredness, and released from hospital after primary treatment; exclusion criteria: any other major infections or problem such as kidney or lung], and severely symptomatic [inclusion criteria: COVID-19 positive, admitted in ICU, on ventilator support for >3 days, oxygen saturation level 90–75%; exclusion criteria: oxygen saturation < 75% or not stable, Hb <8–10, acute kidney failure or under dialysis, haven't undergone convalescent plasma therapy] COVID-19 patients (age between 18 and 75 yrs.). All the ICU patients received anti-inflammatory drug like Dexamethasone (6 mg/day for 7–10 days) according to WHO guideline. Due to exploratory nature of the study, we recruited n = 20 in each group. A written informed consent was received from all participants. Few healthy control individuals (n = 10, COVID-19 negative and no recent history of this disease) were recruited at RCB. Platelets, PBMCs and plasma were isolated. Platelet pellet was used for transcriptome analysis and plasma was analysed to estimate levels of cytokines and thrombotic factors. The protocol was approved from the Institutional Biosafety Committee (IBSC) of RCB, Faridabad, and the assays were performed according to safety guidelines.
2.2. Materials
Human PF4 ELISA kit (R&D Systems, USA), Human IL-6 and TNFα CBA Flex Set (BD Biosciences, USA), Human Coagulation factor II (F2) ELISA kit, (Cloud Clone Corp. Wuhan China), Kaolin-APTT kit (Diagnostica Stago Inc., USA), Anti-human CD41a PE conjugated antibody (BD Pharmingen, USA).
2.3. Platelet transcriptome analysis
RNA purification, library preparation: Total RNA extracted using Trizol was purified using Qiagen RNeasy mini kit (Qiagen, USA) and the mRNA was enriched using NEBNext Poly (A) mRNA magnetic isolation module (NEB, USA) according to the manufacturer's protocol. The purified mRNAs were used for preparation of sequencing library using NEBNext® UltraTM II RNA Library Prep Kit for Illumina (NEB, USA). In brief, the enriched mRNAs were primed with random oligos and chemically fragmented to inserts of ~200 nucleotides, which were then reverse-transcribed and converted to double-stranded DNA (dsDNA). The dsDNA was ligated to loop adapters and ligation products amplified by PCR. The quality of the final DNA library was assessed using Agilent High Sensitivity D1000 ScreenTape System in a 4150 TapeStation System (Agilent). The prepared library was sequenced in Illumina HiSeq. The mean number of sequencing reads (mean ± standard deviation [SD]) from asymptomatic and ICU patients (n = 9 for each) were 34.3 ± 4.2 million and 37.0 ± 8.5 million respectively. The RNASeq was performed by the Clevergene Biocorp Pvt. Ltd., Bangaluru, India. Data analysis: The RNASeq data was analysed by count based analysis protocol. The quality of raw data was verified using FastQC and MultiQC software. The processed reads were mapped to Human reference genome (GRCh38.p7) using Spliced Transcripts Alignment to a Reference (STAR)-v2 aligner. An average of 92.6% of the reads aligning to the reference genome. Normalization of the read count was performed using DESeq2 which takes sequence depth and RNA composition differences among samples into account, followed by computational differential gene expression analysis. Pathway analysis: The differentially expressed genes were shortlisted at P-value <0.05. We performed pathways analysis of upregulated and downregulated genes using Metascape (https://metascape.org/gp/index.html#/main/step1), a gene annotation and analysis software. We have used R software (version 3.6.3) for analysis. We have also performed STRING, (Search Tool for Recurring Instances of Neighboring Genes; v11.0; https://string-db.org/) analysis to predict functional associations between proteins.
2.4. D-dimer assay
D-dimer levels were measured in plasma samples using kit Diazyme Laboratories, Poway, CA, USA, according to manufacturer's protocol.
2.5. Platelet isolation and microparticles measurement
Platelet rich plasma (PRP) was separated from whole blood by centrifugation at 44g for 15 min. Platelet-free plasma was obtained by 2 sequential centrifugations: PRP at 1500g for 7 min followed by platelet-poor plasma (PPP) at 1500g for 15 min. Platelet-derived MPs were measured using flow cytometry after labelling with anti-CD41 PE antibody as mentioned [22]. Platelet pellet was used for whole transcriptome analysis. Gating strategy is described in supplementary Fig. S1.
2.6. Platelet - leukocyte aggregate
PBMCs, isolated from healthy individuals and COVID-19 patients were labelled with anti-CD41 PE and anti-CD45 V500 (BD Biosciences, San Jose, CA, USA) and measured using flow cytometry. Data was analysed using FlowJo software (Tree star, Ashland, USA). Gating strategy is described in supplementary Fig. S2.
2.7. CBA for quantifying cytokines
Cytokines, such as IL-6 and TNFα, were measured in plasma using cytometric bead array (CBA) and analysed by FCAP array software (BD Biosciences, San Jose, CA, USA). According to manufacturer's protocol, 25 μl of the plasma sample was added to 25 μl of reaction mixture to measure the levels of cytokines as mentioned in our work [23]. Gating strategy is described in supplementary Fig. S3.
2.8. Thrombin assay
Plasma thrombin was measured using ELISA kit (Cloud Clone Corp, USA). According to manufacturer's protocol, plasma was mixed with PBS (1:1vol). 100 μl of sample was incubated for 1 h at 37 °C with plate sealer. Supernatant was removed and reagent A was added to wells and incubated for 1 h at 37 °C. After wash, 100 μl of working solution of detection reagent B was added and incubated for 30 min at 37 °C. After wash, 90 μl substrate solution was added to each well and incubated for 20 min. As reaction time was over, 50 μl stop solution was added O.D. was taken at 450 nm using Elisa plate reader (Spectra Max i3x molecular device, California, USA).
2.9. Activated partial thromboplastin time (aPTT)
aPTT was measured using Kaolin-aPTT kit (Diagnostica Stago Inc., USA). 100 μl plasma and equal volume of reagent 1 were incubated for 3 min at 37 °C. 100 μl of 0.025 M calcium chloride solution was added and clot time was measured using Coagulometer.
2.10. PF4 Immunoassay
Plasma PF4 was measured using kit from (R&D Systems Minneapolis, USA). Plasma diluted in PBS (1:10 vol) was incubated with 100 μl of assay diluent RD-1-15 in each well for 2 h at room temperature. Supernatant was removed and wells were washed. Human PF4 conjugate was incubated for 2 h. After wash, substrate solution was added to each well and incubated for 30 min. 50 μl stop solution was added and O.D. was measured at 450 nm using Elisa plate reader.
2.11. Western blotting
Platelet lysates from patients and healthy individuals were processed for western blotting of P-ERK (T202/Y204), ERK1, P-p38 (T180/Y182), p38 and β-Actin (Cell Signalling, USA) as described in our work [22].
2.12. Statistical analysis
Data from at least three experiments are presented as mean ± SEM. Statistical differences among experimental sets were analysed by one-way ANOVA. Graph Pad Prism version 8.0 software was used for data analysis and P-values <0.05 were considered statistically significant.
3. Results
3.1. High D-dimer level in severe ICU patients with COVID-19
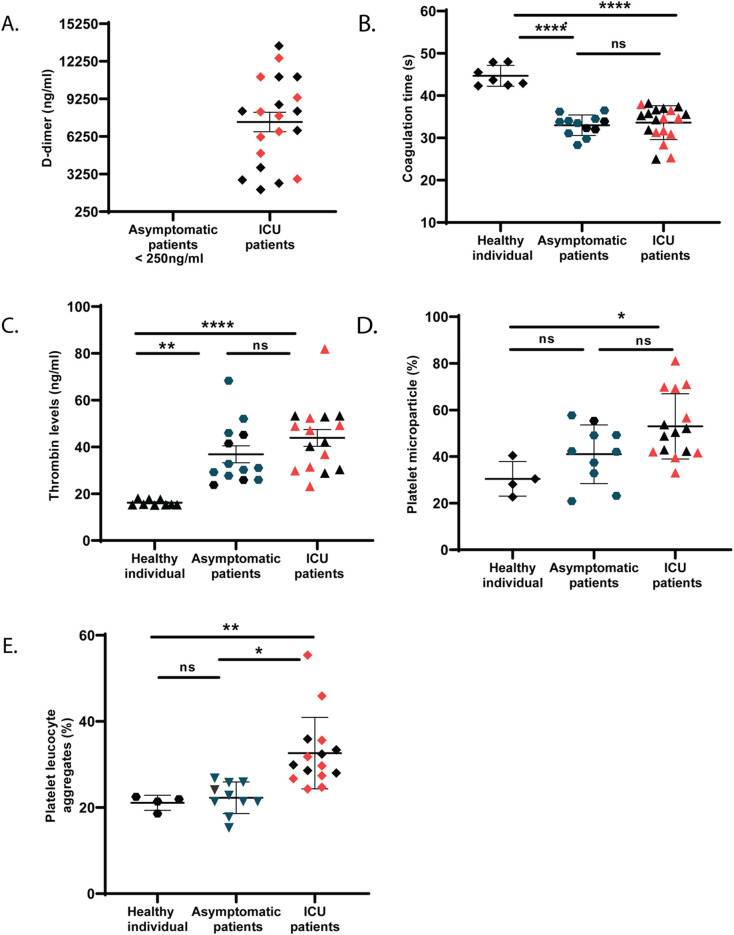
In line with previously published reports [21], we observed elevated plasma D-dimer in severe patients with COVID-19 who were in ICU on ventilator support for at least 3 days and had blood oxygen saturation levels between 75 and 90%. D-dimer is formed in a sequential reaction where thrombin first cleaves soluble fibrinogen to fibrin monomer, and the monomers then forms polymer. Fibrin polymer, together with platelets, forms clot at the site of injury to stop bleeding, in a physiological process called hemostasis. In diseased condition, circulating clots called emboli, are formed. The fibrin polymers of stable clots or circulating emboli are finally cleaved by plasmin and D-dimers are generated. An elevated D-dimer level in plasma is an indicator of clot disorders, including pulmonary embolism (PE), characterized by small emboli in the lung, which lead to breathing troubles and chest pain in these patients. Our data show that asymptomatic patients positive for COVID-19 (n = 20, 17 males and 3 females, average age 50) had normal D-dimer levels, unlike the COVID-19 positive ICU patients (n = 20, 13 males and 7 females, average age 57), <250 ng/ml vs 7409 ng/ml (Fig. 1A). We tested the activated partial thromboplastin time (aPTT) that determines the ability to form clots. We observed similar aPTT in plasma from both ICU and asymptomatic patients (Fig. 1B), although the thrombin level in plasma showed slightly higher trend in ICU patients than asymptomatic counterparts (Fig. 1C). Thus, this observation raises further questions about how ICU patients are susceptible for thrombo-embolic and thrombo-coagulative complications compared to asymptomatic patients with COVID-19. We observed hyperactivation of platelets, measured by elevated trends in platelet microparticle generation (Fig. 1D) and increased platelet-leukocyte aggregates in peripheral blood (Fig. 1E) of ICU patients as compared to asymptomatic counterparts, thus indicating the need of a detailed investigation of platelets in such patients. We performed whole transcriptome analysis of platelets from ICU and asymptomatic COVID-19 patients.
Fig. 1.
D-dimer and thrombin levels and aPTT in plasma of COVID-19 patients. (A) D-dimer level (B) activated partial thromboplastin time (aPTT) and (C) thrombin levels were measured in plasma of patients (severe or ICU and asymptomatic) with COVID-19 and healthy individuals (as reference). (D) Platelet microparticles and (E) platelet-leukocyte aggregates were measured from peripheral blood using flow cytometry. Gating strategy is described in Figs. S1 and S2. Each dot represents individual mean from triplicate reading. Data are the mean ± SEM. One-way ANOVA was used to compare between the groups, *P < 0.05, ** < 0.01, ****P < 0.0001 and ns = non-significant. The red dots (in case of ICU patients) and dark green dots (asymptomatic) represents the individuals (n = 9 each) used for platelet transcriptome analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Altered platelet transcriptome reveals elevated procoagulant and prothrombotic properties of platelets in ICU patients
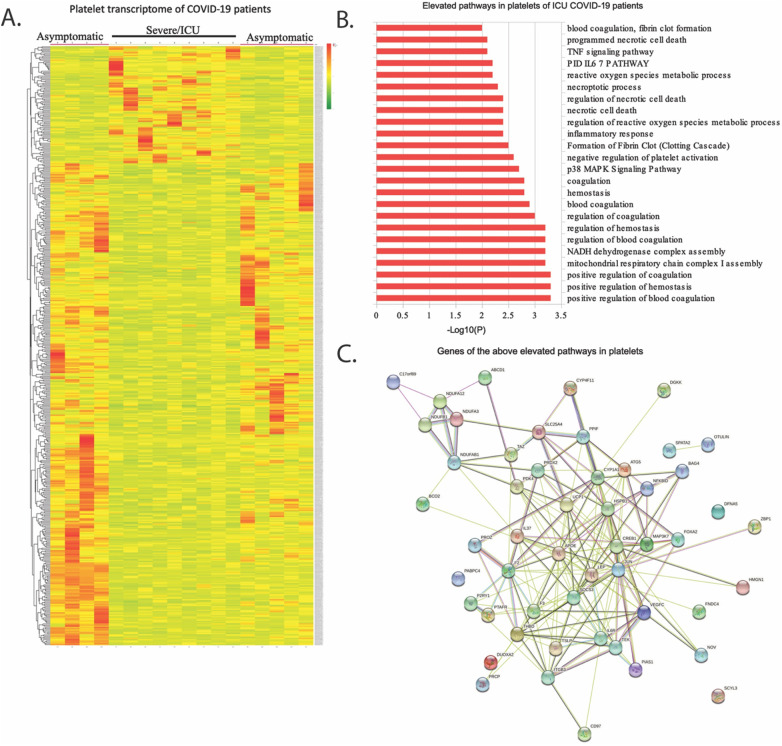
A heatmap generated from the list of differentially expressed transcripts showed a clear distinction between the platelet transcriptome from severe and asymptomatic patients (n = 9 in each group, patients were selected arbitrarily from Fig. 1A (Fig. 2A). A total of 1274 gene transcripts were altered in ICU patients when compared to asymptomatic at P-value <0.05. About 598 gene transcripts were upregulated and 676 were downmodulated in ICU patients compared to asymptomatic (Table S1). Using gene annotation and analysis that generate enrichment clusters of biological pathways, we show that the upregulated transcripts are enriched in nearly 211 pathways, including hemostasis and blood coagulation, IL-6 and TNF signalling pathways, ROS metabolic processes and necrotic pathways (Fig. 2B, Table S2); while the downregulated transcripts were enriched in around 131 pathways, including Notch signalling, in ICU patients as compared to asymptomatic counterparts (Table S3). Further, the above upregulated pathways (Fig. 2A) were analysed using STRING that revealed a unique interaction among the above proteins and factors, including IL6R, JUN, CREB1, SOCS3 and MAP3K (Fig. 2C) involved in IL-6 and TNF signalling mechanism. This suggests that plasma levels of these cytokines may influence the platelet activation in these patients.
Fig. 2.
Platelet transcriptome of COVID-19 patients. Total RNA from platelets of ICU and asymptomatic COVID-19 patients (n = 9 for each) was sequenced in Illumina HiSeq. (A) A heatmap generated from the list of differentially expressed transcripts showing a clear distinction between the platelet transcriptome from two groups. (B) Table shows some of the relevant pathways from total 211 pathways analysed from 598 differentially upregulated transcripts in ICU patients compared to asymptomatic. Rest of the pathways are described in Table S2. A similar list of 131 pathways from 676 downmodulated transcripts is described in Table S3. (C) Schematic represents the interaction network among the all gene transcripts described in pathways described in above Fig. B.
Platelet transcriptome of COVID-19 patients. Total RNA from platelets of ICU and asymptomatic COVID-19 patients (n = 9 for each) was sequenced in Illumina HiSeq. (A) A heatmap generated from the list of differentially expressed transcripts showing a clear distinction between the platelet transcriptome from two groups. (B) Table shows some of the relevant pathways from total 211 pathways analysed from 598 differentially upregulated transcripts in ICU patients compared to asymptomatic. Rest of the pathways are described in Table S2. A similar list of 131 pathways from 676 downmodulated transcripts is described in Table S3. (C) Schematic represents the interaction network among the all gene transcripts described in pathways described in above Fig. B.
3.3. Elevated plasma IL-6 and TNFα relate to higher activation state of platelets in ICU patients
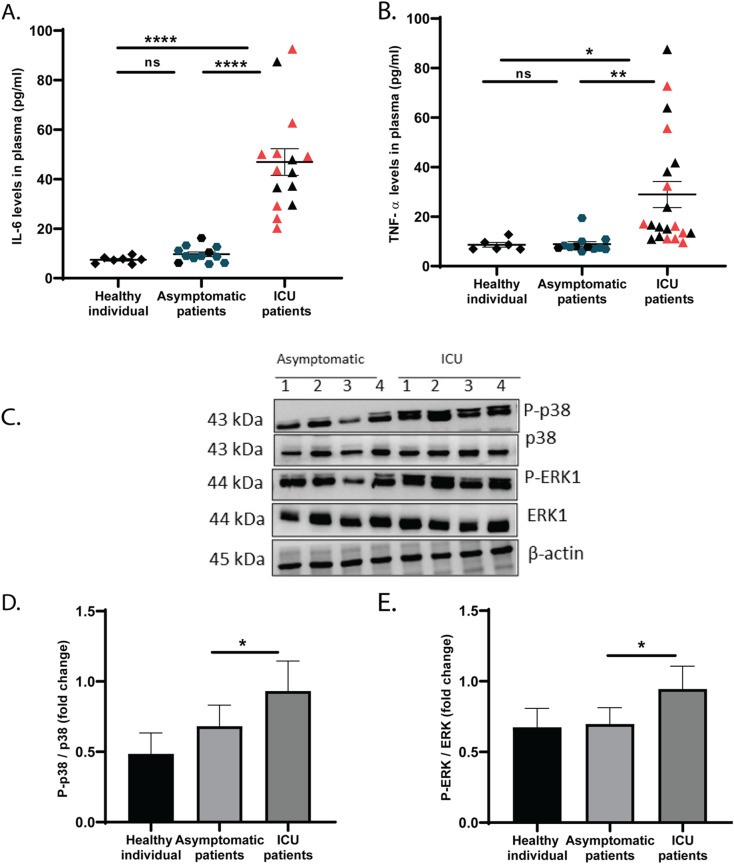
Since the IL-6 and TNF pathways and related receptor and signalling molecules, including IL6R, JUN, CREB1, SOCS3 and MAP3K were increased in platelets of ICU patients, we therefore measured cytokines in plasma. Even though the ICU patients received anti-inflammatory drug like Dexamethasone, an elevated level of pro-inflammatory cytokines such as IL-6 and TNFα was measured in plasma of these individuals compared to asymptomatic counterparts (Fig. 3A–B). Since IL-6 and TNFα are known stimulators to platelets [15], [16], we measured platelet activation in these patients. The severe patients displayed an elevated expression of phosphorylated p38 and ERK (Fig. 3C–E), indicating a higher activation of platelets in ICU patients than asymptomatic COVID-19 patients.
Fig. 3.
Cytokines and platelet microparticles in plasma of COVID-19 patients. (A) IL-6 and (B) TNFα were measured in plasma of above patients using CBA Array. Gating strategy is described in Fig. S3. Each dot represents individual value. The red dots (in case of ICU patients) and dark green dots (asymptomatic) represents the individuals (n = 9 each) used for platelet transcriptome analysis. Data are the mean ± SEM. One-way ANOVA was used to compare between the groups, *P < 0.05, ** < 0.01, ****P < 0.0001 and ns = non-significant. (C) Western blotting of platelet lysates for phospho/non-phospho p38 and ERK from 4 representative images in each asymptomatic and ICU patient. Densitometry data of MAPKs were normalized with β-actin and presented as fold change of phospho/non-phospho (D) p38 and (E) ERK from asymptomatic (n = 6), ICU patients (n = 9 and 8 for p38 and ERK respectively). Unpaired t-test was used to compare between the groups, *P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our study suggests a possible role of elevated plasma cytokines in activation of platelets and development of related complications, including thrombophilia, in critically ill COVID-19 patients, as depicted in schematic Fig. 4 . The upregulation of IL-6 and TNF pathways alongside elevated levels of related receptor and signalling molecules including IL6R, JUN, CREB1, SOCS3 and MAP3K in platelets coexisted with high plasma levels of IL-6 and TNFα in these patients. Moreover, a predominant elevation of pathways relevant to hemostasis, thrombosis and inflammation coexisted with increased reactive oxygen species (ROS)-associated metabolic processes in platelets, strongly suggesting their hyperactivation in ICU patients. Further, the increased expression of signalling adaptor molecules like MAP kinases confirmed the hyperactivation of platelets in critically ill COVID-19 patients. Several studies have reported that severe COVID-19 infection leads to the elevation of coagulative and thrombotic factors like D-dimer, fibrinogen and VWF in the circulation [18], [19], [20]. In line with this, we observed higher levels of circulating D-dimer in severe (ICU) patients with COVID-19 than their asymptomatic counterparts. The higher plasma D-dimer correlates with pulmonary clots in COVID-19 patients. These could be the platelet aggregates that developed into pulmonary clots. A recent study using autopsy lung specimens of COVID-19 victims demonstrated platelet aggregates in the interalveolar capillaries and smaller vessels [24].
Fig. 4.
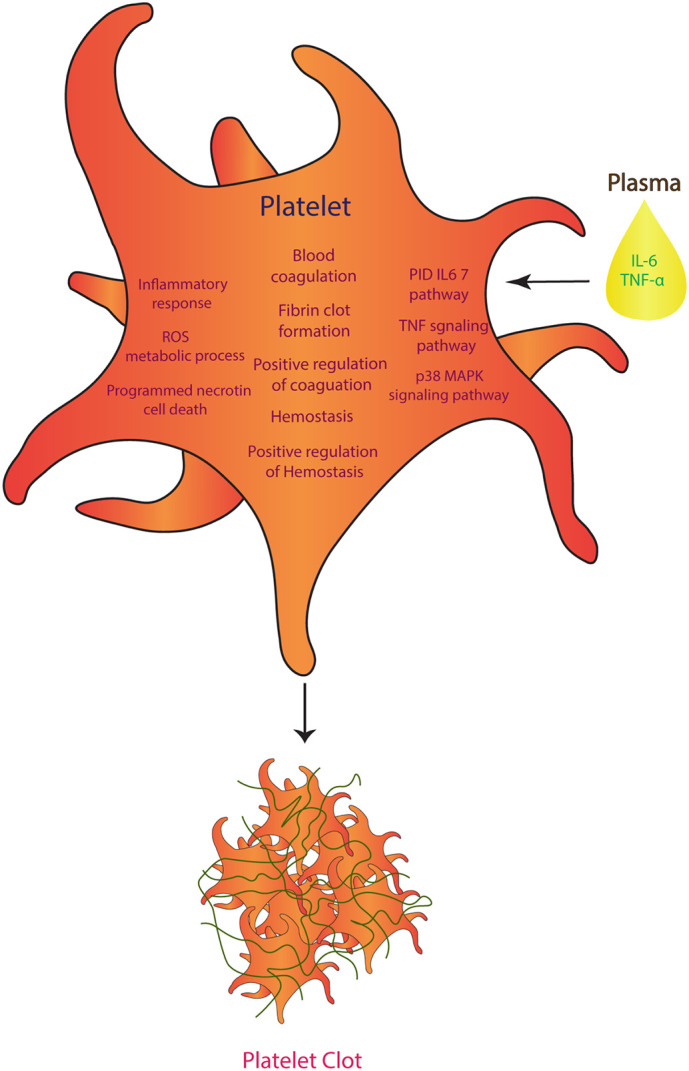
Schematic showing mechanism of platelet activation in severely ill COVID-19 patients. An elevated plasma IL-6 and TNFα coexisted with the upregulated pathways like IL-6 and TNF signalling, pro-coagulation and thrombosis, inflammation and ROS metabolism in platelets of ICU patients. Thus, suggesting that these cytokines may be the risk factor for platelet activation and related malady like thrombophilia in these patients.
Platelet aggregation and clot formation are associated with maladies like pulmonary embolism (PE), deep vein thrombosis (DVT), venous thromboembolism (VET), disseminated intravascular coagulation (DIC), and ischemic stroke as the causes of morbidity and mortality in severely ill COVID-19 patients [6], [25], [26], [27]. As reported by others [28], [29], we also observed shorter aPTT and higher thrombin in plasma of these COVID-19 patients compared to healthy individuals, confirming the crucial association of prothrombic complications with this disease. However, we observed comparable values of above parameters between ICU and asymptomatic patient groups, further indicating the plausibility of differential activation of platelets between these groups of COVID-19 patients.
In order to investigate platelet activation, we performed a thorough transcriptome analysis. Our data reveal upregulation of pathways associated with blood coagulation, hemostasis, and inflammation in platelets of ICU patients with severe forms of COVID-19, as compared to asymptomatic counterparts. The elevated transcripts of thrombin, tissue factor, collagen, thrombopoietin, complement factor H, VEGF and L1CAM suggest a state of hyperactivation in platelets of ICU patients. This was further supported by upregulated IL-6 and TNF pathways alongside elevated expressions of related receptor and signalling molecules including IL6R, JUN, CREB1, SOCS3 and MAP3K in these cells. High plasma levels of both IL-6 and TNFα also suggested possible involvement of these cytokines in platelet activation in severely ill COVID-19 patients. Several studies have described that elevated plasma levels of IL-6 lead to thrombotic complications in COVID-19 patients [30], [31], [32]. It has been postulated that IL-6 can mediate formation of stable clot by modulating platelet function and enhancing thrombin-induced platelet activation [15], [31]. IL-6 can not only activate megakaryocytes [33] but also directly upregulate the expression of several factors including VWF [34], fibrinogen [35] and tissue factor [36], indicating a prothrombotic role of this cytokine in the development of hypercoagulability in severe COVID-19 patients. IL-6 - IL6R mediated activation pathway involves signalling molecules like MAP3K and SOCS3 [37], [38]. Therefore, the elevated expression of IL6R, JUN, PIAS1 and SOCS3 transcripts in platelets suggests a direct regulatory role of IL-6 in the activation of platelets of severely ill COVID-19 patients compared to asymptomatic counterparts. Furthermore, our data also showed that elevated levels of plasma TNFα coexisted with the increased expression of CREB1, JUN, MAP3K7, VEGFC and SOCS3, the molecules of TNF signalling pathway, in platelets of the severely ill COVID-19 patients, indicating the involvement of this cytokine in platelet activation. It has been suggested that interaction of TNFα and TNFR [39] plays an important role in platelet activation and aggregation [40] through arachidonic acid pathway [16]. TNF supplementation to mice caused platelet activation and thrombocytopenia [41]. Extensive studies have reported the TNFα mediated platelet activation. Our data also suggest that elevated TNFα can be another trigger for platelet activation in severely ill COVID-19 patients.
Elevated levels of platelet-derived microvesicles and platelet-leukocyte aggregates in circulation alongside increased expression of MAP kinases in platelets confirm the hyperactivation of platelets in COVID-19 patients in ICU compared to asymptomatic counterparts. A recent study has also reported elevated platelet activation in correlation to a differentially expressed gene pool in ICU patients than non-ICU counterpart in COVID-19 infection [11]. Therefore, our findings on platelet-hyperactivation seem to be a common phenotype in severe COVID-19 illness [11], [42]. A recent study also described similarities in the differentially expressed genes in platelets between COVID-19 and other viral infection like influenza A/H1N1, suggesting the presence of a convergent mechanism driving platelet activation in these diseases [11].
Although our study has several limitations as described below, the platelet transcriptome data provides new insights into the mechanism of hyperactivation of platelets in severely ill COVID-19 patients. Upregulated IL-6 and TNF pathways along with elevated prothrombotic and coagulation factors in platelet coexist with high plasma levels of IL-6 and TNFα, suggesting cytokine-mediated platelet activation and related clinical complications like thrombophilia in severe COVID-19 patients.
This study for the first time describes that upregulation of IL-6 and TNF signalling pathways in platelets along with the elevated levels of these cytokines in plasma of severely ill COVID-19 patients. The study also provides evidence that elevated levels of these cytokines may be the risk factor for platelet activation and related maladies, like thrombophilia, in these individuals.
5. Limitations of the study
-
•
Due to restrictions, the study could not recruit the predicted sample size. The small sample volume of 3 ml was obtained from both severely ill and asymptomatic COVID-19 patients. The small sample volume also limited our study to platelet gene analysis and a few assays.
-
•
Other relevant clinical information, apart from plasma D-dimer level and oxygen saturation level in blood, remained unavailable for all patients.
-
•
We were also limited to gene sequencing data as we dependent on vendor for this service.
The following are the supplementary data related to this article.
Supplementary figures
All differentally expressed transcripts at p<0.05.
GO pathways of 598 upregulated transcripts.
GO pathways of 676 downmodulated transcripts.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GEO Submission (GSE181439) [NCBI tracking system #22252094].
CRediT authorship contribution statement
SK, AS and JK performed experiments. SK analysed data. AKP, NV and SD recruited patients and collected clinical data. SB and PG designed and supervised the study and analysed the data. SD, SB and PG conceptualized the study and wrote the manuscript. All authors have read, edited and approved the final manuscript.
Declaration of competing interest
There are no financial conflicts of interest to disclose. The authors declare no competing interests.
Acknowledgements
This study is supported by grants: BT/PR22881 from the Department of Biotechnology (DBT), Govt. of India; and CRG/000092 from the Science and Engineering Research Board, Govt. of India to PG and SB. Authors acknowledge Mr. Abhaydeep Pandey, Regional Centre for Biotechnology, Faridabad, India for transcriptome data analysis; and Dr. Arundhati Tiwari, Department of Biochemistry, Institute of Medical Science, Banaras Hindu University, Varanasi, India for editing the manuscript. Study acknowledges the participation of patients and volunteers in this study.
Editor: Mohandas Narla
References
- 1.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcari L., Luciani M., Cacciotti L., Musumeci M.B., Spuntarelli V., Bentivegna E., Camastra G., Ansalone G., Santini C., Martelletti P., Volpe M., De Biase L. CHA2DS2-VASc score in patients with COVID-19 pneumonia and its relationship with biomarkers of thrombosis, inflammation and myocardial injury. Blood Coagul. Fibrinolysis. 2021 doi: 10.1097/MBC.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 3.Luciani M., Bentivegna E., Spuntarelli V., Lamberti P.Amoriello, Guerritore L., Chiappino D., Nalli G., Proietta M., Porto F.Del, Martelletti P., Sesti G. Coinfection of tuberculosis pneumonia and COVID-19 in a patient vaccinated with Bacille Calmette-Guerin (BCG): case report. SN Compr. Clin. Med. 2020:1–4. doi: 10.1007/s42399-020-00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jager H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Bertuzzi A., Sandri M.T., Barco S., Humanitas C.-T.F. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boilard E., Pare G., Rousseau M., Cloutier N., Dubuc I., Levesque T., Borgeat P., Flamand L. Influenza virus H1N1 activates platelets through FcgammaRIIA signaling and thrombin generation. Blood. 2014;123:2854–2863. doi: 10.1182/blood-2013-07-515536. [DOI] [PubMed] [Google Scholar]
- 9.Chao C.H., Wu W.C., Lai Y.C., Tsai P.J., Perng G.C., Lin Y.S., Yeh T.M. Dengue virus nonstructural protein 1 activates platelets via toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auerbach D.J., Lin Y., Miao H., Cimbro R., Difiore M.J., Gianolini M.E., Furci L., Biswas P., Fauci A.S., Lusso P. Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9569–9574. doi: 10.1073/pnas.1207314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraemer B.F., Campbell R.A., Schwertz H., Cody M.J., Franks Z., Tolley N.D., Kahr W.H., Lindemann S., Seizer P., Yost C.C., Zimmerman G.A., Weyrich A.S. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas M.R., Storey R.F. The role of platelets in inflammation. Thromb. Haemost. 2015;114:449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 15.Kerr R., Stirling D., Ludlam C.A. Interleukin 6 and haemostasis. Br. J. Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 16.Pignatelli P., De Biase L., Lenti L., Tocci G., Brunelli A., Cangemi R., Riondino S., Grego S., Volpe M., Violi F. Tumor necrosis factor-alpha as trigger of platelet activation in patients with heart failure. Blood. 2005;106:1992–1994. doi: 10.1182/blood-2005-03-1247. [DOI] [PubMed] [Google Scholar]
- 17.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., Limami Y., Zaid N., Sadki K., Ben El Haj R., Mahir W., Belayachi L., Belefquih B., Benouda A., Cheikh A., Langlois M.A., Cherrah Y., Flamand L., Guessous F., Boilard E. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin D.O., Jensen A., Khan M., Chin J., Chin K., Saad J., Parnell R., Awwad C., Patel D. Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg. Infect. Dis. 2020;26:1941–1943. doi: 10.3201/eid2608.201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Gandet F.Fagot, Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Angles-Cano E., Sattler L., Mertes P.M., Meziani F., Group C.T. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., Navalesi P., Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z., Chen X., Chen S., Yu K., Huang Z., Hu B. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal R., Annarapu G.K., Pandey A., Chawla S., Ojha A., Gupta A., Cruz M.A., Seth T., Guchhait P. Hemoglobin interaction with GP1balpha induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica. 2015;100:1526–1533. doi: 10.3324/haematol.2015.132183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhasym A., Annarapu G.K., Saha S., Shrimali N., Gupta S., Seth T., Guchhait P. Neutrophils develop rapid proinflammatory response after engulfing hb-activated platelets under intravascular hemolysis. Clin. Exp. Immunol. 2019;197:131–140. doi: 10.1111/cei.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullen P.D., Cho J.H., Miller J.L., Husain A.N., Pytel P., Krausz T. A descriptive and quantitative immunohistochemical study demonstrating a Spectrum of platelet recruitment patterns across pulmonary infections including COVID-19. Am. J. Clin. Pathol. 2021;155:354–363. doi: 10.1093/ajcp/aqaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thachil J. What do monitoring platelet counts in COVID-19 teach us? J. Thromb. Haemost. 2020;18:2071–2072. doi: 10.1111/jth.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thachil J., Cushman M., Srivastava A. A proposal for staging COVID-19 coagulopathy. Res. Pract. Thromb. Haemost. 2020;4:731–736. doi: 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D., C.C.T.H.o.t.S.S.C.o.t.I.S.o.T. Subcommittee on Perioperative Haemostasis., Scientific and Standardization Committee communication Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 31.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J. Thromb. Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstein S.A. Cytokines, platelet production and hemostasis. Platelets. 1997;8:93–104. doi: 10.1080/09537109709169324. [DOI] [PubMed] [Google Scholar]
- 34.Burstein S.A., Peng J., Friese P., Wolf R.F., Harrison P., Downs T., Hamilton K., Comp P., Dale G.L. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells. 1996;14(Suppl. 1):154–162. doi: 10.1002/stem.5530140720. [DOI] [PubMed] [Google Scholar]
- 35.Amrani D.L. Regulation of fibrinogen biosynthesis: glucocorticoid and interleukin-6 control. Blood Coagul. Fibrinolysis. 1990;1:443–446. [PubMed] [Google Scholar]
- 36.Neumann F.J., Ott I., Marx N., Luther T., Kenngott S., Gawaz M., Kotzsch M., Schomig A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 1997;17:3399–3405. doi: 10.1161/01.atv.17.12.3399. [DOI] [PubMed] [Google Scholar]
- 37.Kandasamy K., Mohan S.S., Raju R., Keerthikumar S., Kumar G.S., Venugopal A.K., Telikicherla D., Navarro J.D., Mathivanan S., Pecquet C., Gollapudi S.K., Tattikota S.G., Mohan S., Padhukasahasram H., Subbannayya Y., Goel R., Jacob H.K., Zhong J., Sekhar R., Nanjappa V., Balakrishnan L., Subbaiah R., Ramachandra Y.L., Rahiman B.A., Prasad T.S., Lin J.X., Houtman J.C., Desiderio S., Renauld J.C., Constantinescu S.N., Ohara O., Hirano T., Kubo M., Singh S., Khatri P., Draghici S., Bader G.D., Sander C., Leonard W.J., Pandey A. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11 doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pullamsetti S.S., Seeger W., Savai R. Classical IL-6 signaling: a promising therapeutic target for pulmonary arterial hypertension. J. Clin. Invest. 2018;128:1720–1723. doi: 10.1172/JCI120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar J., Zosmer A., Hod M., Elder M.G., Sullivan M.H. The regulation of platelet aggregation in vitro by interleukin-1beta and tumor necrosis factor-alpha: changes in pregnancy and in pre-eclampsia. Thromb. Haemost. 1997;78:1255–1261. [PubMed] [Google Scholar]
- 40.Soslau G., Morgan D.A., Jaffe J.S., Brodsky I., Wang Y. Cytokine mRNA expression in human platelets and a megakaryocytic cell line and cytokine modulation of platelet function. Cytokine. 1997;9:405–411. doi: 10.1006/cyto.1996.0182. [DOI] [PubMed] [Google Scholar]
- 41.Tacchini-Cottier F., Vesin C., Redard M., Buurman W., Piguet P.F. Role of TNFR1 and TNFR2 in TNF-induced platelet consumption in mice. J. Immunol. 1998;160:6182–6186. [PubMed] [Google Scholar]
- 42.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pao C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
All differentally expressed transcripts at p<0.05.
GO pathways of 598 upregulated transcripts.
GO pathways of 676 downmodulated transcripts.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GEO Submission (GSE181439) [NCBI tracking system #22252094].