Abstract
Oxidative damage can lead to severe ovarian dysfunctions and even premature ovarian failure. Nrf2, a significant transcription factor that regulates the oxidative stress response of cells, declines with age. Daphnetin, as a kind of natural Chinese herbal medicine, can activate Nrf2 and further promote the antioxidant defense of cells. However, whether Daphnetin treatment can protect ovary from premature ovarian failure and the specific mechanism involved are not understood. This study aimed to investigate the protective function of Daphnetin against the ovarian aging induced by D-galactose in wild-type and Nrf2−/− mice. Female C57BL/6 mice with Wild-type and Nrf2−/− were divided into five groups separately and the premature ovarian failure model were established by D-galactose and then Daphnetin and VE were given for treatment. After 42 days, ovaries tissue and serum were collected for biochemical determination, H&E staining, Immunohistochemical staining and western blot analysis. In the WT-POF group, ovarian function was broke, and the expression of the ovarian senescence-associated protein P16 and the level of oxidative stress were significantly increased, while the expression of the anti-senescence protein klotho was significantly decreased. In addition, the expression of Nrf2 and the antioxidases GCLC, HO-1 and NQO1 were decreased, but TXNIP and NLRP3 were significantly increased. Furthermore, the characteristics of premature ovarian failure were more significant in Nrf2 knockout mice than in wild-type mice, especially the expression of NLRP3 and TXNIP. Moreover, daphnetin, an Nrf2 activator, rescued d-gal-induced POF in a dose-dependent manner, while the protective effect was weakened or even lost in Nrf2 knockout mice. Our results suggested that daphnetin is likely to be a candidate drug for premature ovarian failure treatment and it is mostly possible referred to the molecular mechanism of increasing Nrf2 expression and inhibiting NLRP3 activation in the ovarian aging process.
Keywords: premature ovarian failure, Nrf2, NLRP3, Txnip, daphentin
Introduction
Premature ovarian failure (POF), the current term for ovarian dysfunction called premature ovarian insufficiency (POI), occurs in women under 40 years of age whose follicle-stimulating hormone (FSH) level is > 40 IU/ml and is accompanied by secondary amenorrhea, infertility and low estrogen symptoms (Perry et al., 2013; Hewlett and Mahalingaiah, 2015). Epidemiological surveys have shown that the prevalence of POF in the population is approximately 1–3%, and in amenorrheic patients, it is approximately 2–10% (Rafique et al., 2012). In recent years, the incidence of POF has been increasing year by year because of factors such as the environment and the increase in women’s work intensity. Clinically, POF is a highly heterogeneous female disease with extremely complex etiology, and risk factors include genetics, autoimmune, metabolic disorders and iatrogenic diseases, which contribute to the difficulty of treatment (Qin et al., 2015; Kirshenbaum and Orvieto, 2019; Christianson et al., 2020). Hormone replacement therapy (HRT) is now being abandoned for some female patients because of the potential risk of endometrial, breast cancer and thrombotic diseases (Deady, 2004). The ovarian stem cell treatment method is still under investigation (Wang et al., 2013).
Previous studies have indicated that oxidative damage in the ovary can lead to severe ovarian dysfunctions (Pandey et al., 2018). As age increases, the increase in free radical production in the ovaries and the reduction in antioxidants diminish the capability of the ovaries to scavenge free radicals (Bandyopadhyay et al., 2003). Nuclear factor erythroid-2 related factor 2 (Nrf2) is a critical transcription factor that regulates the oxidative stress reaction of cells. Meanwhile, it also acts as a central regulator to maintain the redox equilibrium of cells and the body. Nrf2 can protect cells from damage caused by reactive oxygen species (ROS) and keep them in a stable state by regulating the expression of complex downstream antioxidant enzymes, such as glutamate cysteine ligase catalytic (GCLC), NAD(P)H dehydrogenase quinine 1 (NQO1) and heme oxygenase-1 (HO-1) (Latella, 2018; Tonelli et al., 2018). Studies have shown that Nrf2 knockouts can contribute to an increase in the prevalence of age-related diseases, such as cancers, kidney damage, pulmonary vascular remodeling, liver damage and coronary heart disease, and could be associated with reduced life expectancy and premature aging (Volonte et al., 2013; Duarte et al., 2017). In addition, overexpression of senescence-associated protein P16 protein can induce premature senescence of cells, while the anti-senescence protein klotho inhibits ovarian and endothelial cell senescence by activating the Nrf2 pathway (Romero et al., 2019; Chen et al., 2021). A number of recent preclinical reports have shown that the application of antioxidants such as Nrf2 activators can scavenge excessive ROS and decrease inflammatory responses by suppressing NLRP3 inflammasome activation (Wang et al., 2016; Xu et al., 2019). Moreover, studies have shown that inhibition of NLR family pyrin domain containing 3 (NLRP3) inflammasome activation prevents ovarian aging (Navarro-Pando et al., 2021). Currently, the Nrf2-related oxidative stress theory about the mechanism underlying POF has been discussed extensively, but the specific mechanism is not yet clear (Yan et al., 2018; Li et al., 2019).
It is well known that cellular damage caused by ROS continuously generated during cell metabolism is one of the fundamental causes of senescence in the body, and maintaining appropriate antioxidants and free radical scavengers can prolong life span (Lee and Wei, 2001). Treatment of mice with D-gal can lead to excessive production of ROS and the accumulation of advanced glycation end products (AGEs) in the ovaries, damaging ovarian physiological function. Because the accelerated aging process in the aging model induced by D-gal is highly similar to the process that occurs during human aging, researchers have used this approach to establish a POF model in mice (Song et al., 1999) (Semba et al., 2010). However, the role and mechanism of the Nrf2 signaling pathway in D-gal-induced POF have not been clarified. Daphnetin (Daph) acts as an activator of Nrf2, enhancing the antioxidant defenses of liver, kidney and heart cells through activating the Nrf2 pathway (Fan et al., 2020; Lv et al., 2020). In this study, we detected that the Nrf2/TXNIP/NLRP3 axis was regulated in a D-gal-induced POF model. Furthermore, activation of Nrf2 and inhibition of NLRP3 by Daph significantly protected against D-gal-induced POF.
Materials and Methods
Reagents
D-gal (D-galactose, C6H12O6) was acquired from Sigma-Aldrich Chemical Co. (Switzerland), assay: ≥99%. Daphnetin (C9H6O2) was obtained from Sigma-Aldrich Chemical Co. (United States), assay: ≥97%. VE (Vitamin E, C25H5O2) was obtained from Dalian Meilun Biotechnology (China), assay: ≥98%. Sodium chloride injection was acquired from Jilin Dubang Pharmaceutical Co., Ltd. Jilin, China. Ethanol absolute was obtained from Tianjin Xinbote Chemical Co., Ltd. Tianjin, China.
Animals and Treatment
Female C57BL/6 mice aged 6–8 weeks were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Nrf2-deficient mice (Nrf2−/−) were supplied by the Jackson Laboratory (Bar Harbor, ME, United States). Food and water were provided via free choice. Firstly, mice were randomly divided into 4 independent groups (5 mice per group): saline, D-gal: 200 mg/kg/d, 400 mg/kg/d and 600 mg/kg/d. All mice were subcutaneous injected daily for 42 days to determine the appropriate D-gal concentration for the POF induction. Then, mice were randomly divided into the following five groups (5 mice per group, and every group mice were injected daily for 42 days): Negative control group: subcutaneous injection of saline; D-gal group: subcutaneous injection of D-gal 600 mg/kg/d; Daph group (low- or high-does): intraperitoneal injection of Daph (15 or 30 mg/kg/d) after subcutaneous injection of D-gal; Positive control group: oral administration of Vitamin E (VE) (30 mg/kg/d) after subcutaneous injection of D-gal. Female Nrf2 −/− mice were also divided into five groups following the above method. All animals involved in the study were approved by the Animal Health and Research Ethics Committee of Jilin University.
Oestrous Cycles
Oestrous cycles were monitored through vaginal smear after D-gal treatment for consecutive 12 days to evaluate the oestrous cycle stages of the mice (5 mice per group). Vaginal lavage sample were diluted with sodium chloride injection at an appropriate concentration. Then the cells were distributed evenly on a clean slide through Centrifugal smear dyeing machin (CYTOPRO, Cytoprp 7,621), fixed in Ethanol absolute for 10 s and stained with Wright-Giemsa Stain (Baso Diagnostics Inc. Zhuhai, China). Vaginal cells were analysed by light microscopy in terms of morphological criteria. We assessed the length of each cycle as the length of time between two consecutive occurrences of oestrus.
Serum Preparation and Hormones and Oxidative Stress Level Measurement
Blood samples were collected by retro-orbital bleeding while mice were during the dioestrus, and then, samples were allowed to clot at room temperature for 60 min. At last, the samples were centrifuged at 2,500 rpm for 15 min to harvest serum, which were stored at −80°C. The commercially available ELISA kits FSH (ml001876) and E2 (ml063198) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. and were used to measure the serum follicle-stimulating hormone (FSH) and estradiol (E2) levels of samples. The levels of oxidative stress in ovaries, including the total superoxide dismutase (SOD) activity and L-Glutathione (GSH) level, were measured by using commercially available kits for SOD (A001-3-2) and GSH (A005-1-2) (Jiancheng Bioengineering Institute, Nanjing, China). All steps were performed according to the manufacturer’s instructions.
Hematoxylin and Eosin Staining and Follicle Number Counting
The right ovaries (5 mice per group) were embedded in paraffin after being fixed in 4% paraformaldehyde overnight. The tissues were cut into 4-μm-thick sections for H&E staining. Only follicles with a visible oocyte nucleus were counted to avoid repeat counting. The follicle classification system was used: primordial follicles were identified as those containing a primary oocyte in the center and surrounded by a monolayer of flattened granulosa cells; primary follicles were characterised by the presence of an enlarged oocyte surrounded by one layer of columnar granulosa cells and with red-stained zona pellucida between the egg and the oocyte; secondary follicles containing more than one layer of columnar granulosa cells and no visible antrum surrounding the oocyte; antral follicles have follicular fluid or a single large antral space; and atretic follicles were identified as those entring into a degenerative process and the follicles were replaced by fibrous connective tissue.
Immunohistochemical Staining
The paraffin sections were sequentially prepared according to the following instructions: dewaxing of the sections, antigen retrieval, blocking with endogenous peroxidase, blocking with 10% normal rabbit serum and incubation at room temperature for 30 min. The primary antibodies anti-p16 (380,928) (Zhengneng Biotechnology), anti-klotho 382,164) (Zhengneng Biotechnology), anti-TXNIP (ab188865) (Abcam) and anti-NLRP3 (19771-1-AP) (Proteintech) were added to the slices at appropriate dilution concentrations and incubated at 4°C overnight. They were then rinsed with PBS for 5 min 3 times and incubated with biotin-labeled secondary antibody for 30 min at 37°C. And then, they were rinsed with PBS for 5 min 3 times again. The SP Rabbit & Mouse HRP Kit (DAB) was developed for 10 min, rinsed thoroughly with PBS, counterstained, dehydrated, transparent, and mounted. Tissue sections were observed and photographed with a microscope, and Image-Pro Plus 6.0 software was used for semi-quantification. Staining was quantified by measuring the immunoreactive area (IA) in μm2 and the integrated optical density (IOD). The staining intensity (SI) for each image was calculated as SI = IOD/IA.
Western Blot Analysis
Protein expression was analyzed by western blot as described previously. Equal amounts of ovarian protein lysate from each group were added to the lanes of gels for electrophoresis and then transferred to a polyvinylidene fluoride membrane. Then, the membrane was blocked with 5% nonfat milk at room temperature for 1 h, eluted with PBST and incubated with primary antibodies against Nrf2 (16396-1-AP) (Proteintech), GCLC (12601-1-AP) (Proteintech), NQO1 (ab80588) (Abcam), HO-1 (ab189491) (Abcam), p16 (380,928) (Zhengneng Biotechnology) and klotho 382,164) (Zhengneng Biotechnology) at appropriate dilutions overnight at 4°C. The membranes were washed 3 times using PBST and then incubated in secondary antibody for 1 h. The membranes were detected by ECL and analyzed by Image Lab 4.0.1.
Statistical Analysis
All the studies and the results’ analyses were repeated at least 3 times. The data are shown as the mean ± SEMs and were analyzed using SPSS 19.0 (IBM). The experimental data were compared by one-way analysis of variance (ANOVA). A p value <0.05 was considered statistically significant. *p < 0.05 and **p < 0.01 compared with the negative control group; # p < 0.05 and ## p < 0.01 compared with the D-gal (600 mg/kg/d) group.
Results
High-Dose D-Gal (600 mg/kg/day)-Induced POF Mouse Model
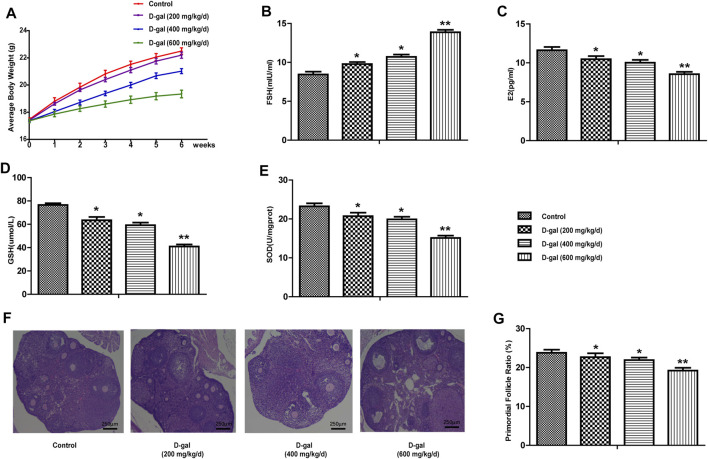
In this study, we first chose 200, 400 and 600 mg/kg/d D-gal to induce ovarian aging in mice. As shown in Figure 1A, the mice in the high-dose D-gal group gradually showed signs of decreased activity, reduced food intake, depilation, poor mental state, and slow weight gain; this phenomenon was not observed in the other three D-gal groups. In Figure 1B, compared with the control group, the serum FSH was significantly increased in the 600 mg/kg/d-dose D-gal group. Moreover, serum E2 levels, the expression levels of GSH and SOD in the ovarian tissues showed the opposite trend compared to FSH (Figures 1C–E). In addition, Figures 1F,G indicate that the proportion of primordial follicles in the D-gal group (high-dose group) was more significantly reduced. Therefore, in this study, we selected 600 mg/kg/d of D-gal as the appropriate concentration to establish a mouse model of POF.
FIGURE 1.
Establish a mouse model of POF. (A) The body weight changes of mice in control and D-gal group (low, middle and high dose) were recorded during the process of inducing the POF model. (B,C) The serum FSH and E2 levels were tested in four groups. (D,E) The levels of GSH and SOD in the ovarian tissues were tested. (F,G) The proportion of primordial follicle. All data are shown as means ± SEMs, n = 5. Statistical significance: *p < 0.05, **p < 0.01.
Nrf2 Knockout Significantly Damages the Ovarian Function and Antioxidant Capacity of POF Mice
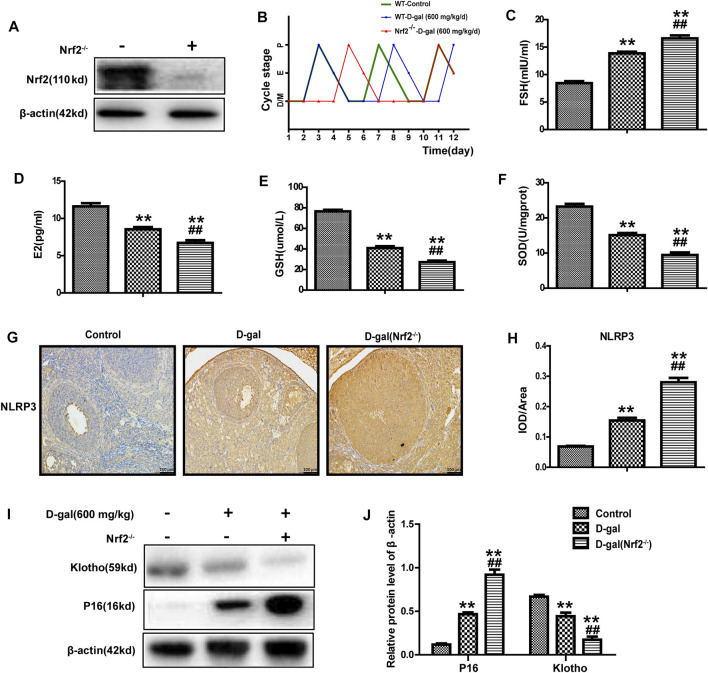
Studies have shown that Nrf2 plays a crucial role in premature ovarian failure. Next, as mentioned above, we chose D-gal (600 mg/kg/day) to construct a POF model via subcutaneous injection in wild-type and Nrf2−/− mice. Immunoblotting identified the genotypes of Nrf2−/− mice (Figure 2A). Oestrous cycles were gained through a daily vaginal smear. The results showed that the control group exhibited regular oestrous cycles about 4 days, and the using of D-gal could lengthen the cycle from 4 to 5 days; however, this phenomenon was more significant in Nrf2−/− mice with an obviously increased length of the dioestrus period (Figure 2B). Compared with the wild-type mice, D-gal more significantly regulated the levels of serum FSH and E2 in the Nrf2−/− mice (Figures 2C,D). Figures 2E,F show that GSH and SOD levels were lower in the Nrf2−/− group. Moreover, D-gal-induced NLRP3 and P16 were much higher in Nrf2−/− mice, while klotho was much lower (Figures 2G–J). These results indicated that Nrf2 plays significant effect in ovarian function of POF mice.
FIGURE 2.
Nrf2 knockout significantly damaged the ovarian function and antioxidant capacity of POF mice. (A) Immunoblotting analysis of Nrf2 protein. (B) Using vaginal cytology evaluating the oestrous cycle and days spent in pro-oestrus (P), oestrus (E), metoestrus (M), and dioestrus (D) over the course of 12 consecutive days. (C,D) The serum FSH and E2 levels were tested. (E,F) The levels of GSH and SOD in ovarian tissues. (G,H) NLRP3 protein expression was analyzed after immunohistochemistry. (I,J) The level of P16 and klotho were analyzed. The data in B-H are shown as means ± SEMs, n = 5. The data in A, I-J are shown as means ± SEMs, n = 3. Statistical significance: **p < 0.01, ## p < 0.01.
Daph Protects Ovarian Function and Follicular Development in POF Mice
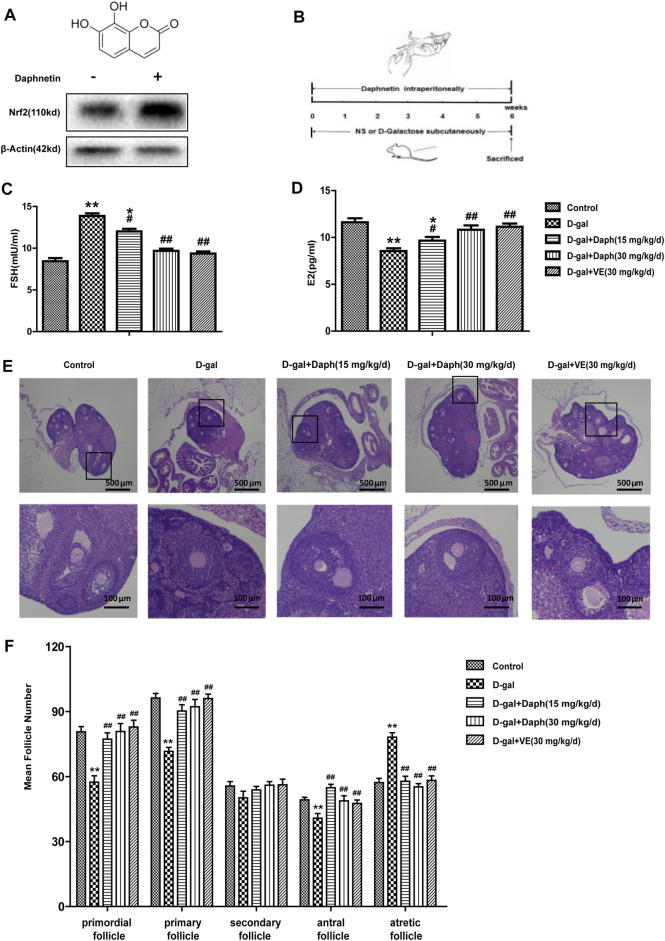
Previous results indicated that Nrf2 has a vital effect on POF. Next, we further explored the protective effect of Daph, a Nrf2 activator, on D-gal-induced POF. As shown in Figure 3A, Daph increased Nrf2 expression in ovaries. Furthermore, Daph (15 mg/kg/d) significantly decreased serum FSH levels and increased E2 levels, while its 30 mg/kg/d dose and VE almost restored FSH and E2 to the levels of the control group, indicating that Daph significantly rescued the ovarian function injury caused by D-gal (Figures 3C,D). After H&E staining, the follicles were classified and counted based on the methods previously described (Figure 3E). The results showed that D-gal treatment reduced the proportion of primordial follicles, primary follicles and antral follicles, and increased the proportion of atretic follicles (Figure 3F). In contrast, the follicle counts of the 15 mg/kg/d-dose Daph treatment group increased significantly at different stages of maturation, and the 30 mg/kg/d-dose Daph and VE almost fully demonstrated its protective effect on follicle development, although secondary follicles have not obvious change (Figure 3F). The above results showed that Daph can protect ovarian function and follicular development in POF mice in a concentration dependent-manner.
FIGURE 3.
Protective effects of Daph on the ovarian function and follicular development. (A) The structure of Daph monomer and immunoblotting analysis of Nrf2 protein expression after Daph was administrated. (B) Construct mice model according to the method as shown. (C,D) Serum FSH and E2 were tested in the control, D-gal, Daph and VE groups. (E,F) Follicles in five groups were observed after H&E staining and the numbers of follicles at different stages of maturation were summarized. The data in C-F are shown as means ± SEMs, n = 5. The data in A are shown as means ± SEMs, n = 3. Statistical significance: *p < 0.05, **p < 0.01, # p < 0.05, ## p < 0.01.
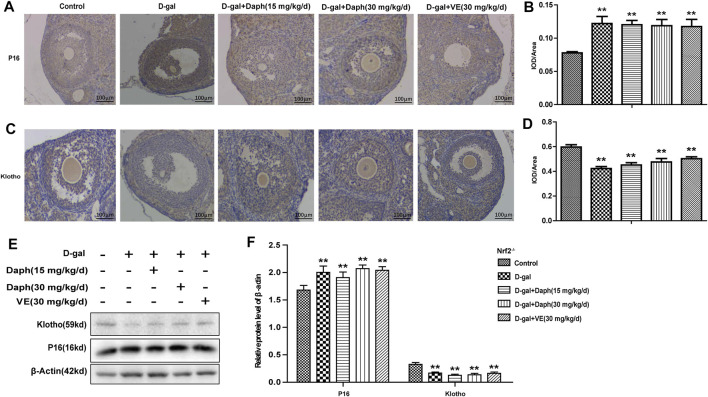
Effects of Daph on P16 and Klotho Protein Expression
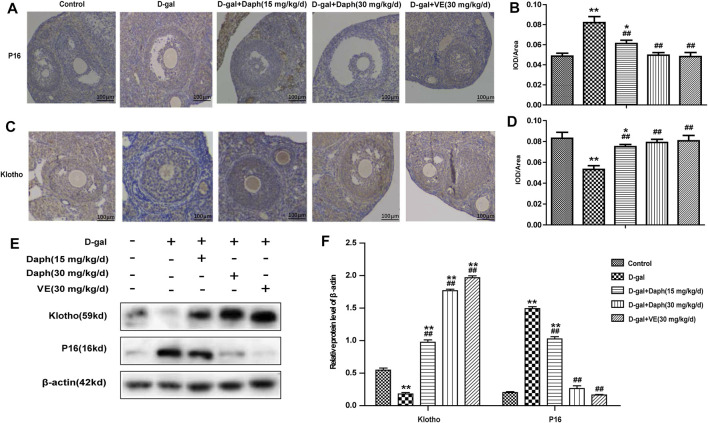
Next, immunohistochemistry was performed to examine the expression of p16 and klotho in ovarian tissues. P16 was mostly located in follicular granulosa cells and oocytes. D-gal treatment led to a noteworthy increase in p16 protein expression compared with the control group, which was reversed by Daph and VE treatment (Figures 4A,B). Studies have shown that klotho participates in the development of follicles as an anti-senescence protein. The level of klotho protein were significantly lower in the D-gal group but partially increased in the Daph and VE group (Figures 4C,D). Consistent with the immunohistochemistry results, western blotting also confirmed the same tendency (Figures 4E,F), which indicated that Daph has a protective effect on cell senescence in a POF mouse model.
FIGURE 4.
Effects of Daph on p16 and klotho expression. (A,C) The IHC staining showed cellular locations of p16 and klotho proteins in different groups. (B,D) The p16 and klotho expressions were quantitatively analyzed. (E,F) Expressions of p16 and klotho were verified by western blot. The data in (A–D) are shown as means ± SEMs, n = 5. The data in (E–F) are shown as means ± SEMs, n = 3. Statistical significance: *p < 0.05, **p < 0.01, ## p < 0.01.
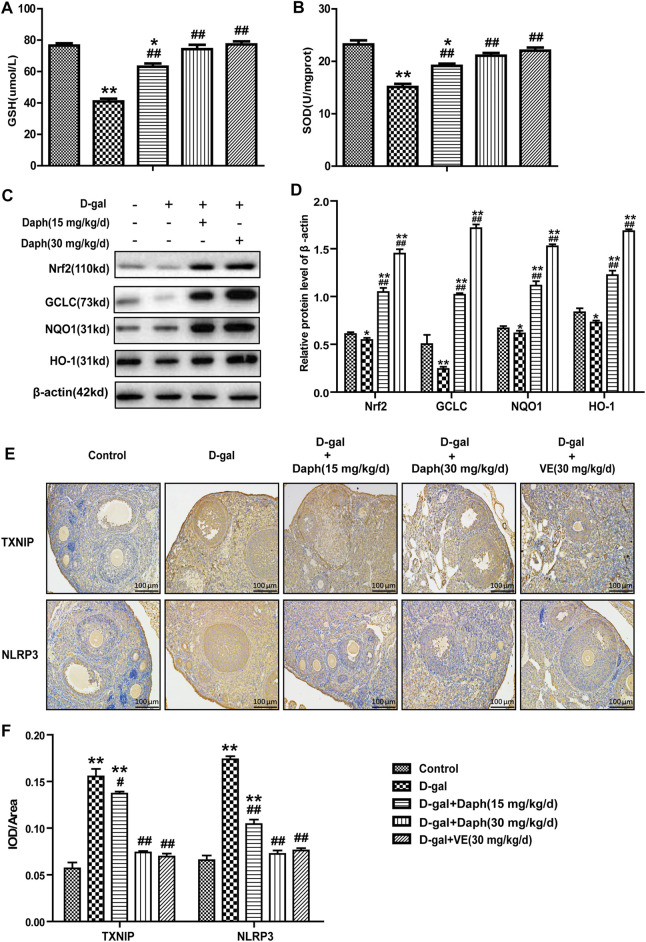
Effect of Daph on Oxidative Stress-Related Pathways in the Ovary
Compared to the control group, the total SOD enzyme activity and antioxidant GSH were significantly reduced by D-gal treatment in ovarian tissues but were partially rescued by Daph and VE treatment (Figures 5A,B). Next, we further researched the possible mechanisms involved in the effects of Daph on D-gal-induced POF. As shown in Figures 5C,D, expression of Nrf2 and its downstream antioxidant enzymes GCLC, HO-1 and NQO1 were markedly decreased in the D-gal group but upregulated by Daph treatment in a dose-dependent manner. As mentioned above, the NLRP3 inflammasome has an important effect on ovarian aging, and high TXNIP protein expression indicates oxidative damage to cells. As shown in Figures 5E,F, TXNIP and NLRP3 protein expressions were markedly increased in the D-gal group versus the control group but were significantly decreased by Daph and VE treatment.
FIGURE 5.
Effect of Daph on oxidative stress-related pathways in the ovary. (A,B) The total SOD enzyme activity and the GSH level were measured in ovarian tissues. (C) The levels of Nrf2, GCLC, NQO1 and HO-1 protein expression in ovarian tissues were observed via Western blotting. (D) The protein expression levels were quantitatively analyzed. (E,F) TXNIP and NLRP3 protein expression in the five groups using IHC staining. All data are shown as means ± SEMs, n = 5, besides (C,D) which data are shown as means ± SEMs, n = 3. Statistical significance: *p < 0.05, **p < 0.01, # p < 0.05, ## p < 0.01.
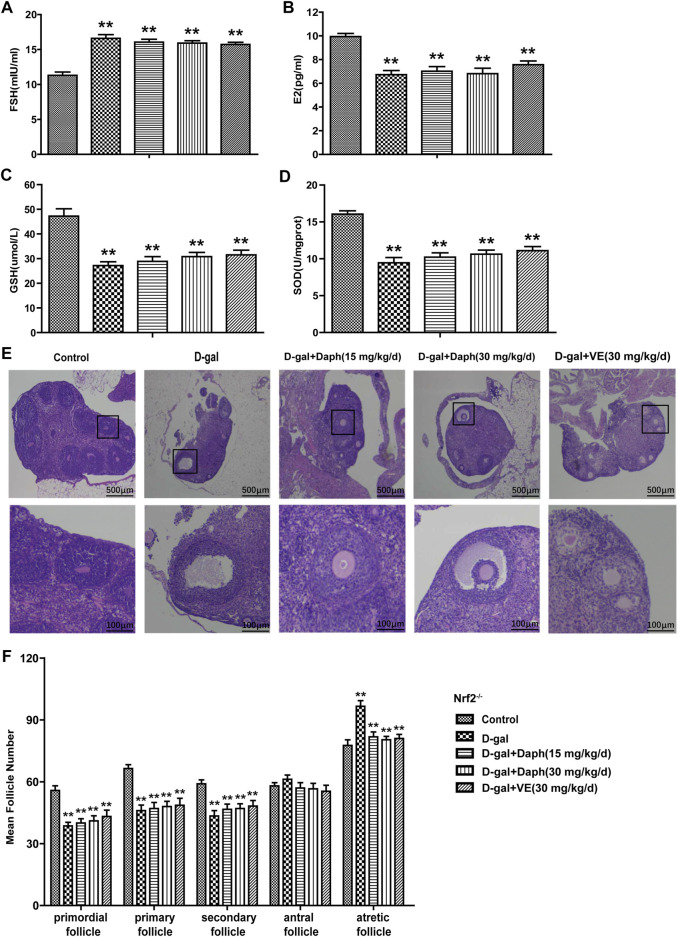
Knockout of Nrf2 Abolished the Protective Capability of Daph for Ovarian Function and Follicular Development
As shown in Figures 6A,B, Daph could not rescue serum hormones regulation induced by D-gal in Nrf2 knockout mice, including FSH upregulation and E2 downregulation. Meanwhile, GSH and SOD depletion induced by D-Gal could not be reversed by daphnetin treatment (Figures 6C,D ). Furthermore, compared with the control group, Daph did not increase the proportion of primordial or primary follicles or decrease the proportion of atretic follicles after D-gal treatment in Nrf2−/− mice (Figures 6E,F). These results demonstrated that the protective capability of Daph for ovarian function and follicular development was abolished in Nrf2 knockout mice.
FIGURE 6.
Knockout of Nrf2 abolished the protective capability of Daph for ovarian function and follicular development. (A,B) The serum FSH and E2 levels were tested in the five groups in Nrf2−/− mice. (C,D) GSH and SOD expression in ovarian tissues was tested. (E) Follicles were observed after H&E staining. (F) Summarizing the numbers of follicles at different developmental stages. All data are shown as means ± SEMs, n = 5. Statistical significance: **p < 0.01.
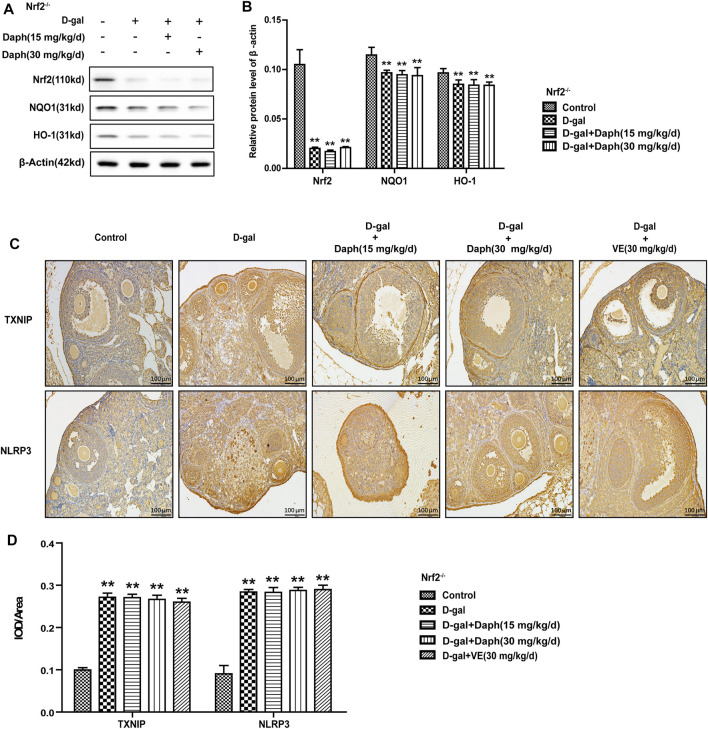
Knockout of Nrf2 Abolished the Regulatory Capability of Daph for Ovarian Senescence-Associated Protein Expression
In Figures 7A–D, compared to the control group, Daph and VE could not recover ovarian senescence-associated protein expression from increased P16 or decreased klotho by D-gal in Nrf2−/− mice. Consistent with these immunohistochemistry results, western blot analysis also verified this trend (Figures 7E,F). These results indicated that Daph lost the capability of recovering ovarian senescence-associated protein expression after Nrf2 knockout.
FIGURE 7.
Knockout of Nrf2 abolished the regulatory capability of Daph for ovarian senescence-associated protein expression. (A–D) The IHC staining showed the p16 and klotho expressions in the five groups in Nrf2−/− mice. (E,F) Expressions of p16 and klotho were measured through western blot. The data in (A–D) are shown as means ± SEMs, n = 5. The data in (E,F) are shown as means ± SEMs, n = 3. Statistical significance: **p < 0.01.
Knockout of Nrf2 Abolished the Adjustive Power of Daph on Oxidative Stress-Related Pathways
In Nrf2−/− mice, the levels of Nrf2 and its antioxidases (NQO1 and HO-1) were significantly decreased, while Daph treatment did not save this tendency (Figures 8A,B). Daph and VE did not reverse the increased expression of the oxidative stress-related factors TXNIP or NLRP3 induced by D-gal in Nrf2−/− mice (Figures 8C,D).
FIGURE 8.
Knockout of Nrf2 abolished the adjustive power of Daph on oxidative stress-related pathways. (A,B) The Nrf2, NQO1 and HO-1 protein expression levels in ovarian tissues were tested by Western blotting. (C,D) TXNIP and NLPR3 protein expression level analysis via immunohistochemistry. The data in (A,B) are shown as means ± SEMs, n = 3. The data in (C,D) are shown as means ± SEMs, n = 5. Statistical significance: **p < 0.01.
Discussion
For human body, ROS are the critical factors in aging, and the decreasing of Nrf2 level is also a key contributor. Mechanically, Nrf2 can upregulate associated genes combating oxidative stress, and the loss of Nrf2 can accelerate the aging process (Schmidlin et al., 2019). However, the function and the specific mechanism of Nrf2 in the POF are not known and which are the points of this study. Daphnetin, an activator of Nrf2, is derived from a natural Chinese herbal medicine and enhances the antioxidant defense of liver, kidney and heart cells via activating the Nrf2 pathway (Zhang et al., 2020) (Lv et al., 2020). However, its function in ovary aging remains unclear, which will be further clarified in this study.
Previous researches have shown that using D-gal could contribute to an increase in ROS and AGE levels, an increase in granulosa cell apoptosis, and impaired follicular development. D-gal induces oxidative stress in the body, and the toxicity of galactose reduces the biological activity of FSH and inhibits the production of E2 by granulosa cells (Song et al., 1999; Semba et al., 2010). In terms of treatment, HRT is one of the recommended methods for the treatment of POF, but long-term estrogen therapy may increase the chance of endometrial cancer, breast cancer and thrombotic diseases in patients (Sullivan et al., 2016). In recent years, stem cell therapy for POF has also received increasing attention (Li et al., 2017; Mohamed et al., 2018). Despite these advances, the prevention and treatment of POF are still challenging because of the limitations of existing methods. In short, exploring the pathogenesis and available therapies are critical for preventing and treating POF. Our study successfully established a mice POF model induced by D-gal (600 mg/kg/day). We found that after using D-gal, the serum FSH level of mice increased, the E2 level decreased and the total SOD enzyme activity and antioxidant GSH were significantly lower. Moreover, the proportion of primordial follicles was more significantly reduced (Figure 1). Therefore, any approach inhibiting oxidative stress may potentially display the prevention and treatment of POF.
Nrf2 can bind to AREs in the promoter region of the Nrf2 target gene, which can also be an adaptive response to oxidative stress to remove ROS through continuous enzymatic reactions by activating downstream factors of Nrf2, such as GCLC, NQO1 and HO-1. Previous studies have pointed out that aging may reduce the antioxidant capacity of rat ovaries by regulating downstream genes of Nrf2. In our study, D-gal treatment significantly downregulated the expression of Nrf2 and its downstream antioxidant enzymes GCLC, HO-1 and NQO1 (Figure 5), as well as the anti-senescence protein klotho (Figure 4), but significantly upregulated senescence-associated protein P16 expression in the ovary, suggesting that D-gal damaged the antioxidant capacity of the ovary and may be related to POF. To further verify the protective role of Nrf2 in POF, we produced D-gal-induced POF in wild-type and Nrf2−/− mice. As shown in Figure 2, the characteristics of oxidative stress and POF induced by D-gal, such as FSH, E2, GSH and SOD were more significant in Nrf2−/− mice than in wild-type mice. The results above indicated that Nrf2 knockout significantly damaged the ovarian function and antioxidant capacity of POF mice. In the past decade, the NLRP3 inflammasome has been extensively investigated (Kelley et al., 2019). Studies have shown that NLRP3 is a tripartite protein that consists of the scaffold protein, the adaptor protein ASC and caspase-1 (Schroder and Tschopp, 2010). Previous studies have demonstrated that Nrf2 reverses the adjustment of NLRP3 inflammasome activity by inhibiting the priming step (Liu et al., 2017). The decline in the transcription factor Nrf2 could contribute to NLRP3 inflammasome activation during aging (Zhang et al., 2015; Schmidlin et al., 2019). Moreover, inhibition of the NLRP3 inflammasome could prevent ovarian aging. Our results demonstrated that D-gal-induced NLRP3 and senescence-associated protein P16 were much higher in Nrf2−/− mice, while the anti-senescence protein klotho was much lower (Figures 2G–J), suggesting that Nrf2 could be of great value in protection against D-gal-induced POF by inhibiting NLRP3.
Next, we tested the protective effect of Daph on D-gal-induced POF. We found that Daph treatment protected ovarian function and follicular development in POF mice (Figure 3). Moreover, the ovarian senescence-associated protein P16 and the level of oxidative stress were significantly decreased, while the anti-senescence protein klotho was significantly increased (Figure 4). In addition, the expression of Nrf2 and antioxidases such as GCLC, HO-1, NQO1 was increased, and the expression of TXNIP and NLRP3 was significantly decreased (Figure 5), which confirmed that Daph significantly rescued the POF caused by D-gal. And, we summarized the main experimental results by Daph’s effects in Table 1. However, these effects of Daph were weakened or even lost after Nrf2 knockout, and the most obvious were the effects on the expression of TXNIP and NLRP3 (Figure 6 to Figure 8 and Table 2). Therefore, our results suggested that Daph protects against D-gal-induced POF through activating Nrf2 and then inhibiting the NLRP3 inflammasome.
TABLE 1.
Daph’ effect in WT mice.
| Parameters | Control group | D-gal (600 mg/kg/d) group | D-gal + Daph (15 mg/kg/d) group | D-gal + Daph (30 mg/kg/d) group | D-gal + VE (30 mg/kg/d) group |
|---|---|---|---|---|---|
| FSH (mIU/ml) | 8.382 ± 0.349 | 13.868 ± 0.314 | 12.012 ± 0.315 | 9.686 ± 0.261 | 9.354 ± 0.262 |
| E2 (pg/ml) | 11.814 ± 0.397 | 8.548 ± 0.300 | 9.625 ± 0.4.6 | 10.820 ± 0.460 | 11.140 ± 0.343 |
| GSH (umol/L) | 76.536 ± 1.516 | 40.946 ± 1.771 | 63.118 ± 2.041 | 74.268 ± 2.783 | 77.300 ± 1.882 |
| SOD (U/mgprot) | 23.240 ± 0.774 | 15.106 ± 0.604 | 19.134 ± 0.420 | 21.092 ± 0.515 | 22.030 ± 0.615 |
| P16 a | 0.283 ± 0.012 | 1.387 ± 0.009 | 0.963 ± 0.285 | 0.320 ± 0.010 | 0.267 ± 0.012 |
| Klotho a | 0.690 ± 0.015 | 0.227 ± 0.009 | 0.997 ± 0.047 | 1.557 ± 0.044 | 1.627 ± 0.044 |
| Nrf2 a | 0.581 ± 0.050 | 0.275 ± 0.040 | 1.039 ± 0.089 | 1.444 ± 0.047 | — |
| GCLC a | 0.478 ± 0.071 | 0.222 ± 0.022 | 0.931 ± 0.051 | 1.836 ± 0.073 | — |
| NQO1 a | 0.653 ± 0.033 | 0.211 ± 0.034 | 1.365 ± 0.085 | 1.573 ± 0.150 | — |
| HO-1 a | 1.056 ± 0.050 | 0.689 ± 0.040 | 1.427 ± 0.077 | 1.560 ± 0.052 | — |
| TXNIP (IOD/Aera) | 0.057 ± 0.006 | 0.155 ± 0.008 | 0.137 ± 0.002 | 0.074 ± 0.002 | 0.070 ± 0.003 |
| NLRPE (IOD/Aera) | 0.066 ± 0.005 | 0.174 ± 0.003 | 0.104 ± 0.005 | 0.072 ± 0.004 | 0.076 ± 0.003 |
: Relative protein level of β-actin.
TABLE 2.
Daph’ effect in Nrf2 Knockout mice.
| Parameters | Control group | D-gal (600 mg/kg/d) group | D-gal + Daph (15 mg/kg/d) group | D-gal + Daph (30 mg/kg/d) group | D-gal + VE (30 mg/kg/d)Group |
|---|---|---|---|---|---|
| FSH (mIU/ml) | 11.310 ± 0.481 | 16.594 ± 0.546 | 16.062 ± 0.411 | 15.920 ± 0.341 | 15.726 ± 0.308 |
| E2 (pg/ml) | 9.944 ± 0.265 | 6.702 ± 0.360 | 7.018 ± 0.395 | 6.814 ± 0.462 | 7.566 ± 0.319 |
| GSH (umol/L) | 47.224 ± 3.012 | 27.190 ± 1.557 | 28.870 ± 1.960 | 30.828 ± 1.705 | 31.542 ± 1.900 |
| SOD (U/mgprot) | 16.056 ± 0.447 | 9.464 ± 0.714 | 10.224 ± 0.576 | 10.632 ± 0.539 | 11.116 ± 0.540 |
| P16 a | 0.998 ± 0.252 | 2.088 ± 0.157 | 2.034 ± 0.165 | 2.083 ± 0.251 | 2.023 ± 0.150 |
| Klotho a | 0.316 ± 0.014 | 0.232 ± 0.002 | 0.226 ± 0.003 | 0.232 ± 0.013 | 0.238 ± 0.014 |
| Nrf2 a | 0.138 ± 0.027 | 0.042 ± 0.005 | 0.041 ± 0.003 | 0.045 ± 0.005 | — |
| NQO1 a | 0.148 ± 0.030 | 0.045 ± 0.005 | 0.042 ± 0.003 | 0.047 ± 0.006 | — |
| HO-1 a | 0.135 ± 0.025 | 0.042 ± 0.003 | 0.42 ± 0.002 | 0.046 ± 0.005 | — |
| TXNIP (IOD/Aera) | 0.100 ± 0.006 | 0.271 ± 0.010 | 0.271 ± 0.008 | 0.267 ± 0.010 | 0.260 ± 0.009 |
| NLRP3 (IOD/Aera) | 0.090 ± 0.020 | 0.284 ± 0.006 | 0.283 ± 0.012 | 0.288 ± 0.007 | 0.290 ± 0.011 |
: Relative protein level of β-actin.
Conclusion
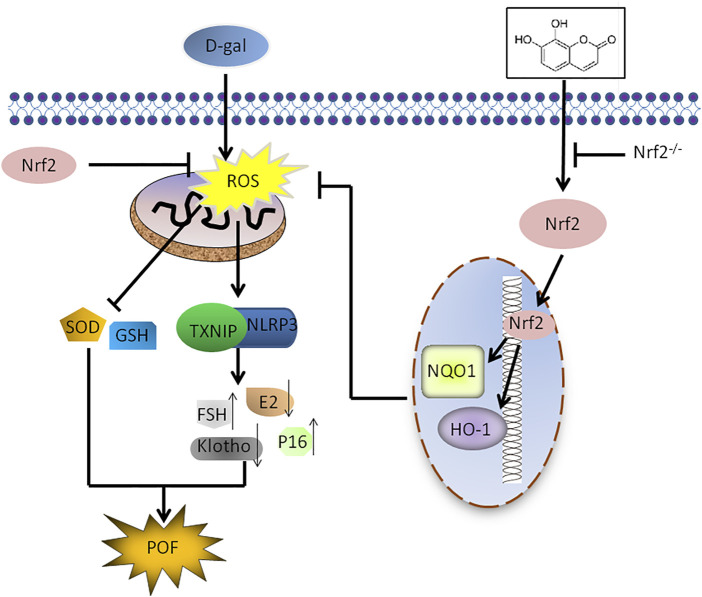
In summary, we revealed the substantial significance of upregulating Nrf2 to attenuate POF induced by D-Gal. This conclusion stems from three key findings. First, Nrf2 knockout mice were more susceptible to D-Gal-induced POF. Second, the protective effect of daphnetin on D-Gal-induced POF was achieved by activating the antioxidant signaling molecule Nrf2 and inhibiting TXNIP/NLRP3. Finally, treatment of Nrf2 KO mice with daphnetin nearly failed to rescue D-Gal-induced POF. Interestingly, we found the Nrf2/TXNIP/NLRP3 axis may be a promising target in preventing POF (Figure 9). Daph, clinically used to treat rheumatoid arthritis and coagulopathy, is likely to be a candidate drug for the treatment of POF. Further clinical trials are certainly worth investigating the safety and efficacy of daphnetin in POF.
FIGURE 9.
Scheme summarizing the protective effects of Nrf2 activation on D-gal-induced POF via inhibiting TXNIP/NLRP3 inflammasome activation. Under D-gal condition, Nrf2 downregulation increases the accumulation of ROS, and then increase TXNIP/NLRP3, p16 and FSH and decrease klotho, SOD, E2 and GSH, leading to POF. Furthermore, Daph inhibits the accumulation of ROS and the activation of TXNIP/NLRP3 inflammsome via activating Nrf2, has a protective effect on D-gal-induced premature ovarian failure.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Animal Health and Research Ethics Committee University.
Author Contributions
SZ and XC contributed to conception and design, and final approval of the manuscript. MZ, XY, and DL contributed to collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript. NM and ZW contributed to collection and assembly of data. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2018YFA0106902), the National Natural Science Foundation of China (Grant Nos 81902637 and 81970576) and the Natural Science Foundation of Jilin (No. 20200404078YY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Daph, Daphnetin; D-gal, D-galactose; FSH, follicle-stimulating hormone insufficiency; GCLC, glutamate cysteine ligase catalytic; HO-1, heme oxygenase-1; KO, knockout; NLRP3, NLR family pyrin domain containing 3; NQO1, NAD(P)H dehydrogenase quinone 1; Nrf2, Nuclear factor erythroid-2 related factor 2; POF, Premature ovarian failure; TXNIP, thioredoxin-interacting protein; VE, Vitamin E; WT, wild-type
References
- Bandyopadhyay S., Chakrabarti J., Banerjee S., Pal A. K., Goswami S. K., Chakravarty B. N., et al. (2003). Galactose Toxicity in the Rat as a Model for Premature Ovarian Failure: an Experimental Approach Readdressed. Hum. Reprod. 18, 2031–2038. 10.1093/humrep/deg414 [DOI] [PubMed] [Google Scholar]
- Chen K., Wang S., Sun Q. W., Zhang B., Ullah M., Sun Z. (2021). Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ. Res. 128, 492–507. 10.1161/CIRCRESAHA.120.317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson M. S., Wodi P., Talaat K., Halsey N. (2020). Primary Ovarian Insufficiency and Human Papilloma Virus Vaccines: a Review of the Current Evidence. Am. J. Obstet. Gynecol. 222, 239–244. 10.1016/j.ajog.2019.08.045 [DOI] [PubMed] [Google Scholar]
- Deady J. (2004). Clinical Monograph: Hormone Replacement Therapy. J. Manag. Care Pharm. 10, 33–47. 10.18553/jmcp.2004.10.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte T. L., Caldas C., Santos A. G., Silva-Gomes S., Santos-Gonçalves A., Martins M. J., et al. (2017). Genetic Disruption of NRF2 Promotes the Development of Necroinflammation and Liver Fibrosis in a Mouse Model of HFE-Hereditary Hemochromatosis. Redox Biol. 11, 157–169. 10.1016/j.redox.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Wei W., Huang J., Peng L., Ci X. (2020). Daphnetin Attenuated Cisplatin-Induced Acute Nephrotoxicity with Enhancing Antitumor Activity of Cisplatin by Upregulating SIRT1/SIRT6-Nrf2 Pathway. Front. Pharmacol. 11, 579178. 10.3389/fphar.2020.579178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M., Mahalingaiah S. (2015). Update on Primary Ovarian Insufficiency. Curr. Opin. Endocrinol. Diabetes Obes. 22, 483–489. 10.1097/MED.0000000000000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley N., Jeltema D., Duan Y., He Y. (2019). The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 20. 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum M., Orvieto R. (2019). Premature Ovarian Insufficiency (POI) and Autoimmunity-An Update Appraisal. J. Assist. Reprod. Genet. 36, 2207–2215. 10.1007/s10815-019-01572-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latella G. (2018). Redox Imbalance in Intestinal Fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 Connection. Dig. Dis. Sci. 63, 312–320. 10.1007/s10620-017-4887-1 [DOI] [PubMed] [Google Scholar]
- Lee H. C., Wei Y. H. (2001). Mitochondrial Alterations, Cellular Response to Oxidative Stress and Defective Degradation of Proteins in Aging. Biogerontology 2, 231–244. 10.1023/a:1013270512172 [DOI] [PubMed] [Google Scholar]
- Li J., Mao Q., He J., She H., Zhang Z., Yin C. (2017). Human Umbilical Cord Mesenchymal Stem Cells Improve the reserve Function of Perimenopausal Ovary via a Paracrine Mechanism. Stem Cel Res Ther 8, 55. 10.1186/s13287-017-0514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Wang J., Wang X., Sun J., Li Z. (2019). Icariin Exerts a Protective Effect against D-Galactose Induced Premature Ovarian Failure via Promoting DNA Damage Repair. Biomed. Pharmacother. 118, 109218. 10.1016/j.biopha.2019.109218 [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang X., Ding Y., Zhou W., Tao L., Lu P., et al. (2017). Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal. 26, 28–43. 10.1089/ars.2015.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Zhu C., Wei W., Lv X., Yu Q., Deng X., et al. (2020). Enhanced Keap1-Nrf2/Trx-1 axis by Daphnetin Protects against Oxidative Stress-Driven Hepatotoxicity via Inhibiting ASK1/JNK and Txnip/NLRP3 Inflammasome Activation. Phytomedicine 71, 153241. 10.1016/j.phymed.2020.153241 [DOI] [PubMed] [Google Scholar]
- Mohamed S. A., Shalaby S. M., Abdelaziz M., Brakta S., Hill W. D., Ismail N., et al. (2018). Human Mesenchymal Stem Cells Partially Reverse Infertility in Chemotherapy-Induced Ovarian Failure. Reprod. Sci. 25, 51–63. 10.1177/1933719117699705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Pando J. M., Alcocer-Gómez E., Castejón-Vega B., Navarro-Villarán E., Condés-Hervás M., Mundi-Roldan M., et al. (2021). Inhibition of the NLRP3 Inflammasome Prevents Ovarian Aging. Sci. Adv. 7, eabc7409. 10.1126/sciadv.abc7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A. K., Gupta A., Tiwari M., Prasad S., Pandey A. N., Yadav P. K., et al. (2018). Impact of Stress on Female Reproductive Health Disorders: Possible Beneficial Effects of Shatavari (Asparagus racemosus). Biomed. Pharmacother. 103, 46–49. 10.1016/j.biopha.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Perry J. R., Corre T., Esko T., Chasman D. I., Fischer K., Franceschini N., et al. (2013). A Genome-wide Association Study of Early Menopause and the Combined Impact of Identified Variants. Hum. Mol. Genet. 22, 1465–1472. 10.1093/hmg/dds551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Jiao X., Simpson J. L., Chen Z. J. (2015). Genetics of Primary Ovarian Insufficiency: New Developments and Opportunities. Hum. Reprod. Update 21, 787–808. 10.1093/humupd/dmv036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique S., Sterling E. W., Nelson L. M. (2012). A New Approach to Primary Ovarian Insufficiency. Obstet. Gynecol. Clin. North. Am. 39, 567–586. 10.1016/j.ogc.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A., San Hipólito-Luengo Á., Villalobos L. A., Vallejo S., Valencia I., Michalska P., et al. (2019). The angiotensin-(1-7)/Mas Receptor axis Protects from Endothelial Cell Senescence via Klotho and Nrf2 Activation. Aging Cell 18, e12913. 10.1111/acel.12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin C. J., Dodson M. B., Madhavan L., Zhang D. D. (2019). Redox Regulation by NRF2 in Aging and Disease. Free Radic. Biol. Med. 134, 702–707. 10.1016/j.freeradbiomed.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Tschopp J. (2010). The Inflammasomes. Cell 140, 821–832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- Semba R. D., Nicklett E. J., Ferrucci L. (2010). Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? J. Gerontol. A. Biol. Sci. Med. Sci. 65, 963–975. 10.1093/gerona/glq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Bao M., Li D., Li Y. M. (1999). Advanced Glycation in D-Galactose Induced Mouse Aging Model. Mech. Ageing Dev. 108, 239–251. 10.1016/s0047-6374(99)00022-6 [DOI] [PubMed] [Google Scholar]
- Sullivan S. D., Sarrel P. M., Nelson L. M. (2016). Hormone Replacement Therapy in Young Women with Primary Ovarian Insufficiency and Early Menopause. Fertil. Steril 106, 1588–1599. 10.1016/j.fertnstert.2016.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli C., Chio I. I. C., Tuveson D. A. (2018). Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 29, 1727–1745. 10.1089/ars.2017.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte D., Liu Z., Musille P. M., Stoppani E., Wakabayashi N., Di Y. P., et al. (2013). Inhibition of Nuclear Factor-Erythroid 2-related Factor (Nrf2) by Caveolin-1 Promotes Stress-Induced Premature Senescence. Mol. Biol. Cel 24, 1852–1862. 10.1091/mbc.E12-09-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yu L., Sun M., Mu S., Wang C., Wang D., et al. (2013). The Therapeutic Potential of Umbilical Cord Mesenchymal Stem Cells in Mice Premature Ovarian Failure. Biomed. Res. Int. 2013, 690491. 10.1155/2013/690491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang H., Qian C., Tang J., Zhou W., Liu X., et al. (2016). 3-(2-Oxo-2-phenylethylidene)-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one (Compound 1), a Novel Potent Nrf2/ARE Inducer, Protects against DSS-Induced Colitis via Inhibiting NLRP3 Inflammasome. Biochem. Pharmacol. 101, 71–86. 10.1016/j.bcp.2015.11.015 [DOI] [PubMed] [Google Scholar]
- Xu L. L., Wu Y. F., Yan F., Li C. C., Dai Z., You Q. D., et al. (2019). 5-(3,4-Difluorophenyl)-3-(6-methylpyridin-3-yl)-1,2,4-oxadiazole (DDO-7263), a Novel Nrf2 Activator Targeting Brain Tissue, Protects against MPTP-Induced Subacute Parkinson's Disease in Mice by Inhibiting the NLRP3 Inflammasome and Protects PC12 Cells against Oxidative Stress. Free Radic. Biol. Med. 134, 288–303. 10.1016/j.freeradbiomed.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Yan Z., Dai Y., Fu H., Zheng Y., Bao D., Yin Y., et al. (2018). Curcumin Exerts a Protective Effect against Premature Ovarian Failure in Mice. J. Mol. Endocrinol. 60, 261–271. 10.1530/JME-17-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Davies K. J. A., Forman H. J. (2015). Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 88, 314–336. 10.1016/j.freeradbiomed.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yao J., Wu Z., Zou K., Yang Z., Huang X., et al. (2020). Chondroprotective and Antiarthritic Effects of Daphnetin Used In Vitro and In Vivo Osteoarthritis Models. Life Sci. 240, 116857. 10.1016/j.lfs.2019.116857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.