Significance
Stem cells maintain tissue homeostasis. We identified a factor, Yun, required for proliferation of normal and transformed intestinal stem cells in adult Drosophila. Yun acts as a scaffold to stabilize the Prohibitin (PHB) complex previously implicated in various cellular and developmental processes and diseases. The Yun/PHB complex acts downstream of EGFR/MAPK signaling and affects the levels of E2F1 to regulate intestinal stem cell proliferation. The role of the PHB complex in cell proliferation is evolutionarily conserved. Our results provide insight into the underlying mechanisms of how stem cell proliferation is properly controlled during tissue homeostasis and tumorigenesis.
Keywords: Yun, Prohibitin, intestinal stem cell, E2F1, Drosophila
Abstract
Stem cells constantly divide and differentiate to maintain adult tissue homeostasis, and uncontrolled stem cell proliferation leads to severe diseases such as cancer. How stem cell proliferation is precisely controlled remains poorly understood. Here, from an RNA interference (RNAi) screen in adult Drosophila intestinal stem cells (ISCs), we identify a factor, Yun, required for proliferation of normal and transformed ISCs. Yun is mainly expressed in progenitors; our genetic and biochemical evidence suggest that it acts as a scaffold to stabilize the Prohibitin (PHB) complex previously implicated in various cellular and developmental processes and diseases. We demonstrate that the Yun/PHB complex is regulated by and acts downstream of EGFR/MAPK signaling. Importantly, the Yun/PHB complex interacts with and positively affects the levels of the transcription factor E2F1 to regulate ISC proliferation. In addition, we find that the role of the PHB complex in cell proliferation is evolutionarily conserved. Thus, our study uncovers a Yun/PHB-E2F1 regulatory axis in stem cell proliferation.
Stem cells in adult tissues constantly proliferate to produce differentiated progeny to replenish lost cells, thereby maintaining tissue homeostasis. Disruption of the balanced control of stem cell proliferation and differentiation can lead to either excessive proliferation or precocious differentiation, eventually leading to various diseases and conditions, including cancer and precocious aging (1–3). Therefore, identifying the mechanisms underlying stem cell proliferation is critical to understand homeostasis control and for the development of potential therapeutics to treat human diseases including cancer.
The adult Drosophila intestine has proven to be an excellent model to study the regulation of stem cell and tumorigenesis. Mammalian and Drosophila intestines share marked similarities in terms of development, cellular makeup, and genetic control (4–8). Drosophila intestinal stem cells (ISCs) are scattered along the basement membrane of the adult midgut epithelium (9, 10) and divide asymmetrically to produce differentiating enteroblasts (EBs). One of the Notch ligands, Delta, is specifically expressed in ISCs, while the Notch receptor is expressed in ISCs and EBs (termed progenitors collectively). Activation of the Notch pathway in EBs trigger their differentiation into either absorptive enterocytes (ECs) or secretory enteroendocrine cells (EEs) depending on their signaling environments (9, 11–13). Moreover, recent studies show that EE cells may not be generated from EBs but directly from ISCs or EE progenitor cells (EEPs) (14–16).
Numerous studies have shown that the proliferation and differentiation of ISCs under physiological and stressed conditions are regulated by many signaling pathways including the Notch, JAK/STAT, and EGFR pathways (see reviews by refs. 7, 17–19 and references therein). In particular, the EGFR pathway integrates multiple signals to regulate ISC proliferation and differentiation. Defective EGFR signaling inhibits ISC proliferation and tissue regeneration, whereas ectopic EGFR activation promotes ISC proliferation and tumorigenesis (20–23). EGFR signaling is also required for the initiation of DNA endoreplication during EB/EC differentiation and exclusion of damaged ECs during regeneration (24, 25). Although mitosis and endoreplication are distinct processes, they share a common G1-S regulatory network, mastered by the conserved transcription factor E2F1 (26–28). Interestingly, E2F1 is posttranscriptionally regulated by EGFR signaling for the initiation of DNA endoreplication during regeneration (25). However, how E2F1 is differentially regulated by EGFR signaling in these two different processes is not well understood.
Prohibitins (PHBs) are members of the conserved SPFH superfamily (29–31). PHB1 was first identified by its antiproliferative activity upon ectopic expression, which was later attributed to its 3′ untranslated region instead of the PHB protein itself (32, 33). The PHB complex contains two homologous members: PHB1 and PHB2. PHB1 and PHB2 are ubiquitously expressed and are present in the mitochondria, the nucleus, cytosol, and the lipid rafts of the plasma membrane. A number of studies have described a role of the PHB complex within the mitochondria, where it forms a supramacromolecular structure at the inner membrane of the mitochondria acting as a scaffold (or a chaperone) for proteins and lipids regulating mitochondrial metabolism. The PHB complex has been implicated in various cellular and developmental processes and diseases, such as mitochondrial respiration, signaling, and mitophagy depending on its cellular localization (see reviews by refs. 30, 31, 34 and references therein). Disruption of the PHB genes has effects ranging from decreased replicative lifespan in yeast, to larval arrest in Drosophila, and to embryonic lethality in mice (35, 36). However, it remains unexplored whether they play a role in intestinal stem cell regulation in Drosophila.
Results
Yun Is a Positive Regulator of ISC Proliferation.
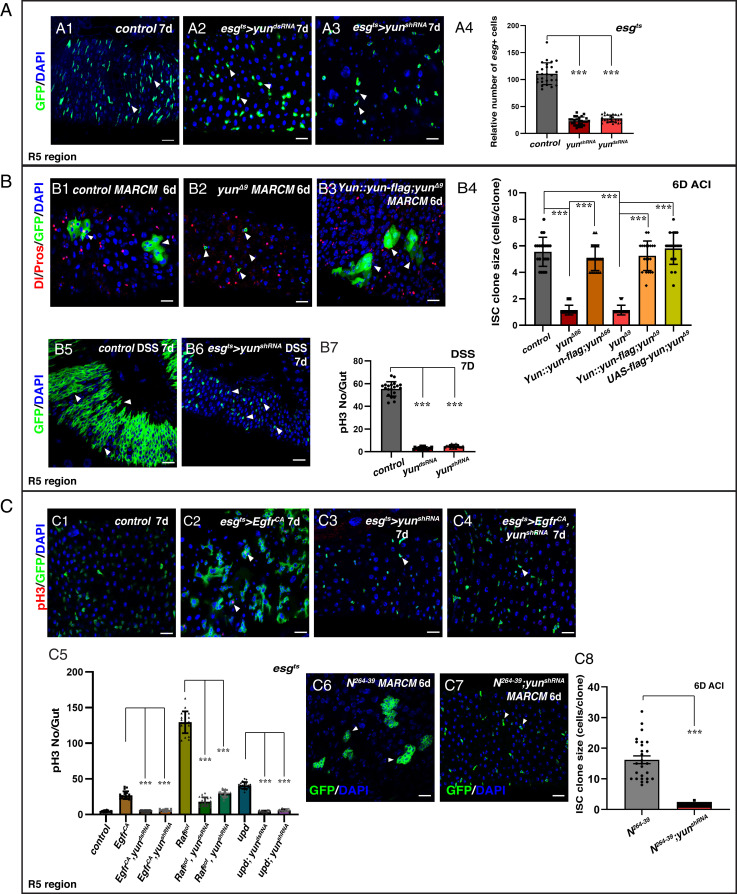
To identify factors that regulate the maintenance, proliferation, and/or differentiation of ISCs, we carried out a large-scale RNA interference (RNAi) screen using the esgGal4, UAS-GFP, tubGal80ts (esgts) driver in the posterior midgut (Methods). From the screen, one RNAi line targeting CG7705 showed a severe reduction in the number of esg+ cells (Fig. 1A). To exclude the possibility of an off-target effect, we generated a different short hairpin RNA that showed a similar phenotype (Fig. 1A and SI Appendix, Fig. S1A). CG7705 encodes a protein of 592 amino acids without any known domains or motifs that we named Yun (‘luck’ in Chinese) (SI Appendix, Fig. S1A). We note that during this work, an independent study named CG7705 as diamond (dind) and showed that it plays a role in mitosis of larval brain cells and for male meiosis (37) but did not examine its role in ISCs. The knockdown efficacy of the yun RNAi lines was confirmed by qRT-PCR and Western blots (SI Appendix, Fig. S1B). Next, as esgGal4 drives expression in both ISCs and EBs, we used the ISC-specific DlGal4 driver to examine the role of yun in ISCs. Depletion of yun significantly inhibited ISC proliferation, indicating that Yun is required for ISC proliferation (SI Appendix, Fig. S1C). Finally, we used the ISC lineage tracing Flp-out (F/O) (FLPase [flipase]-out) technique to examine ISC-traced lineage with reduced yun activity. Traced control ISC lineages almost fully covered the intestinal epithelium, while depletion of yun significantly inhibited ISC proliferation (SI Appendix, Fig. S1D).
Fig. 1.
Yun is required for ISC proliferation. (A) Yun is required for progenitor proliferation. Quantification of the relative number of esg+ cells in control and yun-depleted intestines (A4). Mean ± SD is shown. ***P < 0.001. (B) Yun is required for ISC proliferation and regeneration. Quantification of ISC clone size of different genotypes (B4). Mean ± SD is shown. ***P < 0.001. (B7) Quantification of the number of pH3 in intestines treated with DSS. Mean ± SD is shown. ***P < 0.001. (C) Yun sustains the proliferation of transformed stem cells. Quantification of the number of pH3 in different genotypes (C5). Mean ± SD is shown. ***P < 0.001. The proliferation of Notch clones (C6) is inhibited by yun depletion (C7). (C8) Quantification of the size of ISC clones. Mean ± SD is shown. ***P < 0.001 (Scale bars, 20 μm).
To confirm the results obtained with RNAi, we generated two null yun deletion mutants, yunΔ66 and yunΔ9 (SI Appendix, Fig. S1A). yun is essential for viability, as homozygous mutant died after the third instar larval stage (SI Appendix, Fig. S2). Consistent with the role of Yun in ISC proliferation, ISC clones of yun null mutants generated using the Mosaic analysis with a repressible cell marker (MARCM) technique did not proliferate, resulting in 1 to 2 cell clones (Fig. 1B and SI Appendix, Fig. S1E) (38). Furthermore, we were able to rescue the ISC proliferation phenotype of yun deletion mutants using either a yun overexpression construct or a Flag-tagged form of yun under its endogenous promoter (Fig. 1B and SI Appendix, Fig. S1E). Altogether, our studies demonstrate that Yun is intrinsically required for ISC proliferation under normal conditions.
Next, we examined whether cell death is the cause of proliferation defects in the absence of yun. No increased apoptosis was observed in yun-deficient progenitors (SI Appendix, Fig. S3 A–C). Furthermore, we tested whether expression of the antiapoptotic baculovirus protein p35 could rescue yun-deficient progenitors (39). Consistent with previous reports, expression of p35 increased clone sizes in wild-type (WT) intestines (40–42). Ectopic expression of p35 only partially increased clone sizes of yun mutants, indicating that the reduced clone size associated with loss of yun is mainly due to proliferation defects but not apoptosis (SI Appendix, Fig. S3D). Altogether, these data indicate that yun-deficient progenitors do not die but cannot proliferate.
Interestingly, although the proliferation capability of yun mutant ISCs is greatly reduced, large Dl− mature or differentiating ECs and EE cells could be occasionally observed in yun mutant MARCM clones (SI Appendix, Figs. S1F and S4). Furthermore, although depletion of yun in progenitors decreased the number of EB cells, in which Notch signaling is activated, EB cell identity does not appear to be affected by yun depletion (SI Appendix, Fig. S1G). These data suggest that Yun is mainly required for ISC proliferation but not progeny differentiation and that the low frequency of differentiated yun mutant cells is likely due to reduced ISC proliferation.
Yun Is Required for Tissue Regeneration and Transformed Stem Cell Proliferation.
ISCs undergo rapid proliferation to produce differentiated progeny during regeneration following tissue damage (43). As Yun is required for ISC proliferation under normal conditions, we examined whether Yun is also involved in tissue regeneration. Control progenitors undergo rapid proliferation to regenerate damaged intestinal epithelium after dextran sulfate sodium salt (DSS) treatment, while depletion of yun in progenitors completely blocked tissue regeneration (Fig. 1B). Consistent with this, neither yun mutant clones regenerated intestines treated with DSS (SI Appendix, Fig. S1H). These data indicate that Yun is also required for tissue regeneration after acute intestinal damage.
Next, we examined whether yun is required for the proliferation of transformed stem cells/cancer stem cells. We generated different models of transformed stem cells by activating either EGFR or JAK/STAT signaling pathways or by blocking Notch signaling (9, 10, 20, 21, 44). In all cases, simultaneous knockdown of yun in these tumor types dramatically suppressed the division of transformed stem cells (Fig. 1C and SI Appendix, Fig. S5).
Yun Is Mainly Expressed in Progenitors and EE Cells in Intestines.
To determine where Yun is expressed, we generated Yun-specific antibodies, transgenic flies carrying Flag-tagged Yun at its C terminus under its endogenous promoter, and a driver line under the control of the yun promoter (yunGal4) and used these reagents to examine the expression pattern and subcellular localization of Yun (SI Appendix, Figs. S6 and S7). Double labeling of Flag and Yun antibodies showed that they colocalized, and knockdown of yun by RNAi could effectively decrease Yun protein levels in progenitors (SI Appendix, Fig. S6). Yun is almost uniformly localized in the cytosol and nucleus of diploid cells (progenitors and EE cells) but not in polyploid ECs (SI Appendix, Figs. S6 and S7). Yun expression in progenitors and EE cells was confirmed by double labeling of Yun with different cell-type–specific drivers (SI Appendix, Fig. S8). No obvious defects were observed after yun was depleted in EEs (SI Appendix, Fig. S9). These data indicate that the expression of Yun in progenitors is consistent with its requirement in ISC proliferation.
Interestingly, we noticed that the levels of Yun protein in progenitors increased with age (SI Appendix, Fig. S10A), suggesting that an increased level of Yun in older flies may be involved in intestine dysplasia, which is known to occur in older flies (45). Consistent with this model, ectopic expression of yun in progenitors resulted in a modest increase in the number of progenitors (SI Appendix, Fig. S10B).
We also noticed that Yun protein is partially localized to the mitochondria, suggesting that Yun might regulate ISC proliferation by affecting mitochondrial functions (SI Appendix, Fig. S11 A–D). To test this, we examined various aspects of mitochondrial-related functions, such as the morphology and maturation of mitochondria, production of ATP, mitoUPR, apoptosis, autophagy, and lipid metabolism in the absence of yun. However, as Yun did not affect these processes (SI Appendix, Figs. S11–S14), the proliferation defects observed in yun-defective progenitors are unlikely to be due to mitochondrial dysfunction.
Yun Associates with the PHB Complex.
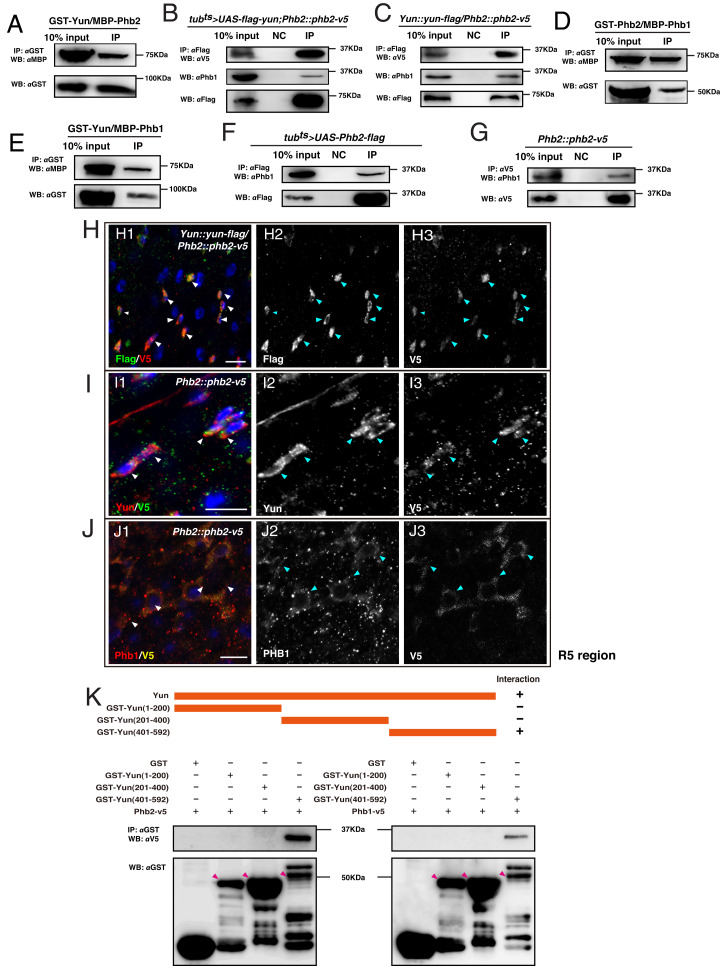
To address how Yun regulates ISC proliferation, we performed coimmunoprecipitation (co-IP) and liquid chromatography–tandem mass spectrometry experiments to identify Yun-interacting proteins. Among the candidate interactors, we identified Prohibitin 2 (Phb2) (SI Appendix, Fig. S15 A–C) (29, 30). Follow up detailed glutathione S-transferase immunoprecipitation (GST IP) experiments confirmed that Yun associates with Phb2 in vitro and that both endogenous and overexpressed Yun interact with endogenous and overexpressed Phb2 in vivo, respectively, suggesting that Phb2 is a Yun-interacting protein (Fig. 2 A–C). As Phb2 forms a complex with Phb1 (encoded by l(2)37Cc in Drosophila), we examined whether Yun, Phb2, and Phb1 form a tertiary complex. Strikingly, both Yun and Phb2 interact with Phb1 by in vitro GST IP assays (Fig. 2 D and E). Furthermore, in vivo co-IP results showed that either endogenous or overexpressed Yun and Phb2 interact with Phb1, indicating that Yun, Phb1, and Phb2 form a ternary complex (Fig. 2 B, C, F, and G). Consistent with this, endogenous Yun, Phb2, and Phb1 are expressed in the same intestinal cells, and colocalization between them was often observed (Fig. 2 H–J). Altogether, these data suggest that Yun forms a complex with Phb1 and Phb2 (the Yun/PHB complex).
Fig. 2.
The formation of the Yun/PHB complex. (A) GST-Yun associates with MBP-Phb2. Overexpressed (B) and endogenous Yun (C) associates with endogenous Phb1 and Phb2 in vivo. (D) GST-Phb2 associates with MBP-Phb1. (E) GST-Yun associates with MBP-Phb1. Overexpressed (F) and endogenous Phb2 (G) associates with endogenous Phb1 in vivo. (H and I) Endogenous Yun and endogenous Phb2 are coexpressed and colocalize. (J) Endogenous Phb1 and Phb2 are coexpressed and colocalize. (K) Phb2 and Phb1 interact with the C terminus of Yun (Scale bars, 10 μm).
Next, we performed a series of co-IP experiments to define the interaction domain between Yun and the PHB complex. Using various purified truncated GST-tagged Yun proteins to precipitate endogenous Phb1 and Phb2, we found that the C terminus of Yun is essential for their association, while the N terminus and central parts are dispensable for the interaction (Fig. 2K and SI Appendix, Fig. S15D). These data indicate that Yun associates with Phb1 and Phb2 through its C terminus.
The PHB Complex Is Required for Normal and Transformed Stem Cell Proliferation.
To determine the expression pattern and subcellular localization of Phb1 and Phb2, we used a Phb1-specific antibody and a transgenic Phb2 reporter with a V5 tag at its C terminus under its endogenous promoter (Methods). In contrast to the prevalent idea that the PHB complex is ubiquitously expressed in eukaryotic cells (31), both Phb1 and Phb2 are specifically expressed in progenitors and EE cells but not in ECs in the intestinal epithelium, as observed for Yun, supporting the idea that Phb1 and Phb2 work together with Yun (SI Appendix, Fig. S16A).
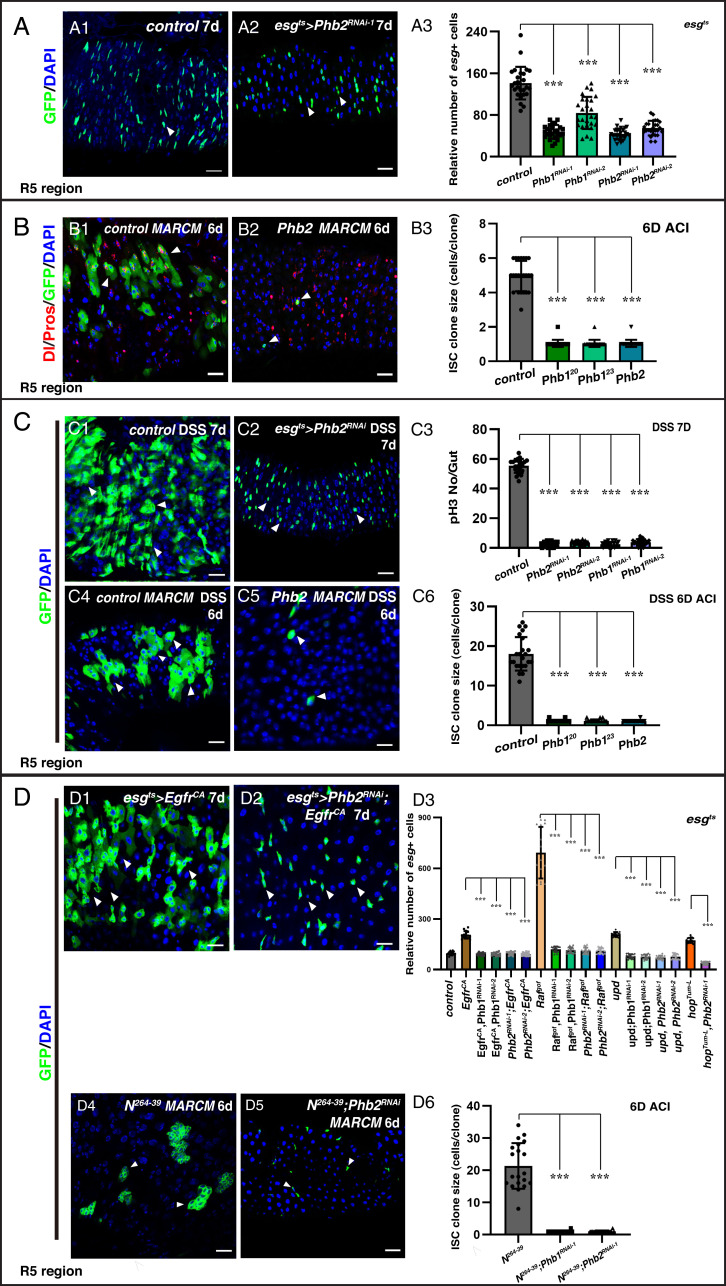
Next, we analyzed the role of Phb2 and Phb1 in ISC proliferation. Depletion of Phb2 using two effective RNAi lines resulted in significant decrease in the number of progenitors (Fig. 3A and SI Appendix, Fig. S16 B–D). Furthermore, knockdown of Phb2 using the F/O lineage tracing technique produced 1- to 2-cell lineages, as observed for yun (SI Appendix, Fig. S16E). In addition, we generated a null mutant of Phb2, Phb2Δ and observed that Phb2Δ mutant ISC clones contained only one cell (Fig. 3B). Similarly, depletion of Phb1 in progenitors using two different RNAi constructs and knockdown of Phb1 using the F/O technique also effectively inhibited ISC proliferation (Fig. 3A and SI Appendix, Fig. S16 B–E). In addition, we performed MARCM clonal analysis of two Phb1 mutants, Phb120 and Phb123. Almost all ISC clones in both Phb1 mutants contained only one cell, indicating that Phb1 is also essential for ISC proliferation (Fig. 3B and SI Appendix, Fig. S16F). Finally, as observed for Yun, overexpression of both Phb1 and Phb2 resulted in a slight increase in the number of progenitors (SI Appendix, Fig. S17). Additionally, no obvious defects were detected upon deletion of the PHB complex in EEs, as that of yun depletion (SI Appendix, Figs. S9 and S18).
Fig. 3.
Phb2 and Phb1 are required for ISC proliferation. (A) Phb2 and Phb1 are required for progenitor proliferation. Quantification of the esg+ cells (ROI) in flies with indicated genotypes (A3). Mean ± SD is shown. ***P < 0.001. Control (B1) and Phb2Δ (B2) ISC MARCM clones. (B3) Quantification for ISC clone size with indicated genotypes. Mean ± SD is shown. ***P < 0.001. (C) The PHB complex is required for tissue regeneration. Control (C1) and Phb2-depleted (C2) intestines with DSS treatment. (C3) Quantification of the number of pH3 and esg+ cells in indicated intestines with DSS treatment. Mean ± SD is shown. ***P < 0.001. (C6) Quantification of the size of ISC clones in indicated intestines after DSS treatment. Mean ± SD is shown. ***P < 0.001. (D) The PHB complex sustains the proliferation of different transformed stem cells. Quantification of the relative number of esg+ cells in different genotypes. Mean ± SD is shown. ***P < 0.001. The proliferation of Notch (D4) clones is inhibited by Phb depletion. (D6) Quantification of ISC clone size with indicated genotypes. Mean ± SD is shown. ***P < 0.001 (Scale bars, 20 μm).
As observed for Yun, Phb1 and Phb2 are mainly required for ISC proliferation but not progeny differentiation, as we could observe marked large Dl− cells mature or differentiating ECs and EE cells in Phb mutant MARCM clones (SI Appendix, Figs. S16F and S19). Also, EB cell identity (or Notch signaling activation) was not affected in Phb-depleted intestines (SI Appendix, Fig. S16G). Finally, no apoptosis was observed, and ectopic expression of p35 did not rescue proliferation defects of Phb mutants, indicating that Phb-deficient progenitors do not die but cannot proliferate (SI Appendix, Fig. S20). Altogether, these data indicate that like Yun, the PHB complex is required for ISC proliferation under physiological conditions.
Next, we examined whether the PHB complex is also required for tissue regeneration. Phb-depleted progenitors and clones of Phb mutants could not proliferate to regenerate the intestine epithelium after DSS treatment, indicating that the PHB complex is required for tissue regeneration after acute intestinal damage (Fig. 3C and SI Appendix, Fig. S21). Consistent with these results, proliferation of different types of transformed stem cells was also suppressed by Phb1 or Phb2 depletion (Fig. 3D and SI Appendix, Figs. S22 and S23). Collectively, these data demonstrate that like Yun, the PHB complex sustains the proliferation of normal and different types of transformed stem cells.
In addition, we noticed that like Yun, Phb1 and Phb2 are partially localized to the mitochondria, suggesting that the PHB complex might regulate ISC proliferation by affecting mitochondrial functions (SI Appendix, Fig. S24 A and B). To test this, we then examined various aspects of mitochondrial-related functions as for Yun in the absence of Phb1/2. However, neither loss of the PHB complex affects these processes, indicating that the proliferation defects observed in Phb-defective progenitors are unlikely to be due to mitochondrial dysfunction (SI Appendix, Figs. S25–S29). Together, our data indicate that the regulation of stem cell proliferation by the Yun/PHB complex is likely to be due to its function outside of the mitochondria.
Yun Acts as a Scaffold for the PHB Complex to Regulate ISC Proliferation.
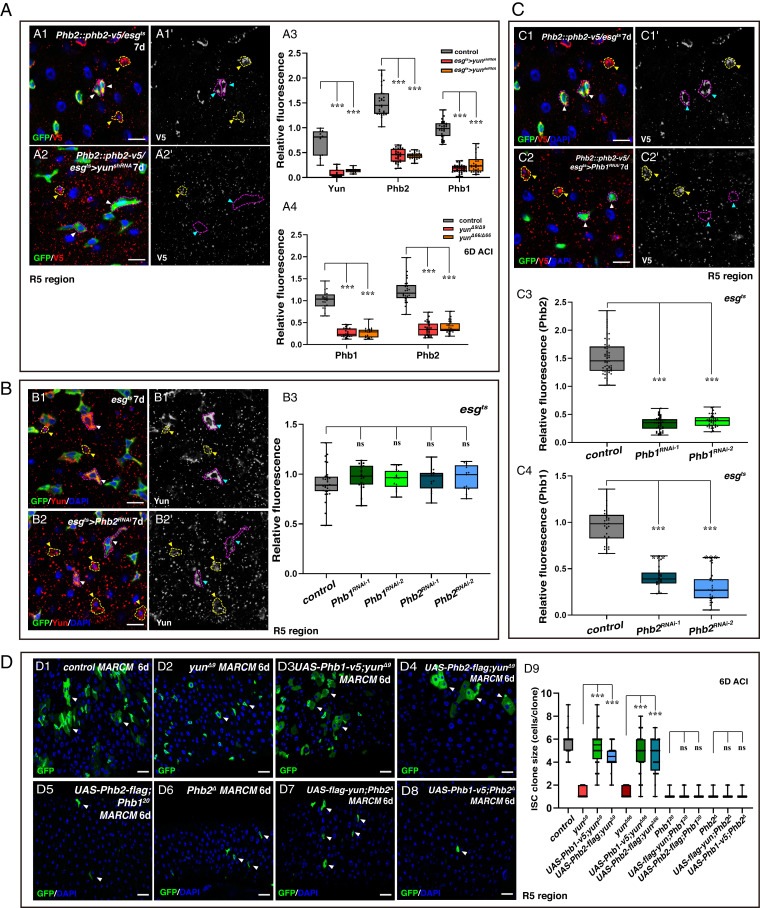
To further investigate the functional relationship between Yun, Phb1, and Phb2, we analyzed the state of Phb1 and Phb2 in the absence of Yun. Strikingly, Phb2 was almost undetectable in yun-depleted progenitors (Fig. 4A and SI Appendix, Fig. S30A). Consistent with this, Phb2 was also greatly diminished in clones of both yun mutants (Fig. 4A and SI Appendix, Fig. S30A). Similar results were observed when Phb1 was examined in the absence of yun (Fig. 4A and SI Appendix, Fig. S30A). The decreased levels of Phb proteins in the absence of Yun may be a result of dispersed distribution of Phb proteins or reduced levels of Phb transcripts, directly or indirectly. Interestingly, the levels of Yun protein remained largely unchanged in the absence of Phb (Fig. 4B and SI Appendix, Fig. S30 B and C). In addition, the levels of Phb2 were dramatically diminished in Phb1-depleted progenitors and in Phb1 mutant clones (Fig. 4C and SI Appendix, Fig. S30D). Similarly, in both Phb2-depleted progenitors and Phb2 mutant clones, Phb1 levels were significantly reduced (Fig. 4C and SI Appendix, Fig. S30E). Altogether, these data suggest that Yun recruits and stabilizes the PHB complex while the PHB complex is dispensable for Yun levels, and that Phb1 and Phb2 are interdependent.
Fig. 4.
Yun acts as a scaffold in the Yun/PHB complex. (A) Yun affects the levels of Phbs. (A3) Quantification of the relative fluorescence intensity (IOD) of Yun and Phbs. Mean ± SD is shown. ***P < 0.001. (A4) Quantification of the IOD of Phbs. Mean ± SD is shown. ***P < 0.001. (B) The PHB complex doesn’t affect the levels of Yun. (B3) Quantification of the IOD of Yun. Mean ± SD is shown. (C) The levels of Phbs are interdependent. (C3) Quantification of the IOD of Phb2. Mean ± SD is shown. ***P < 0.001. (C4) Quantification of the IOD of Phb1. Mean ± SD is shown. ***P < 0.001. (D) Yun acts genetically upstream of the PHB complex. (D9) Quantification of the clone size indicated. Mean ± SD is shown. ***P < 0.001 [Scale bars, 10 μm (A–C) and 20 μm (D)].
To further test the functional relationships between Yun and the PHB complex, we performed a series of rescue experiments. Strikingly, overexpression of either Phb1 or Phb2 significantly rescued proliferation defects observed in yun mutant clones, largely restoring them to WT (Fig. 4D and SI Appendix, Fig. S31). These data indicate that Yun genetically functions upstream of the PHB complex to regulate ISC proliferation, and ectopic expression of Phb1/2 could bypass the requirement for Yun. Meanwhile, ectopic expression of yun could not rescue the proliferation defects observed in Phb-depleted intestines and clones of Phb mutants (Fig. 4D and SI Appendix, Figs. S31 and S32). Finally, neither overexpression of Phb2 in Phb1 mutant clones nor ectopic expression of Phb1 in Phb2 mutant clones rescued the proliferation defects of Phb1 and Phb2 mutants, respectively (Fig. 4D and SI Appendix, Fig. S33). Collectively, these data demonstrate that Yun genetically functions upstream of Phb1 and Phb2, while Phb1 and Phb2 function at the same level to regulate ISC proliferation.
The Yun/PHB Complex Is Regulated by EGFR/MAPK Signaling.
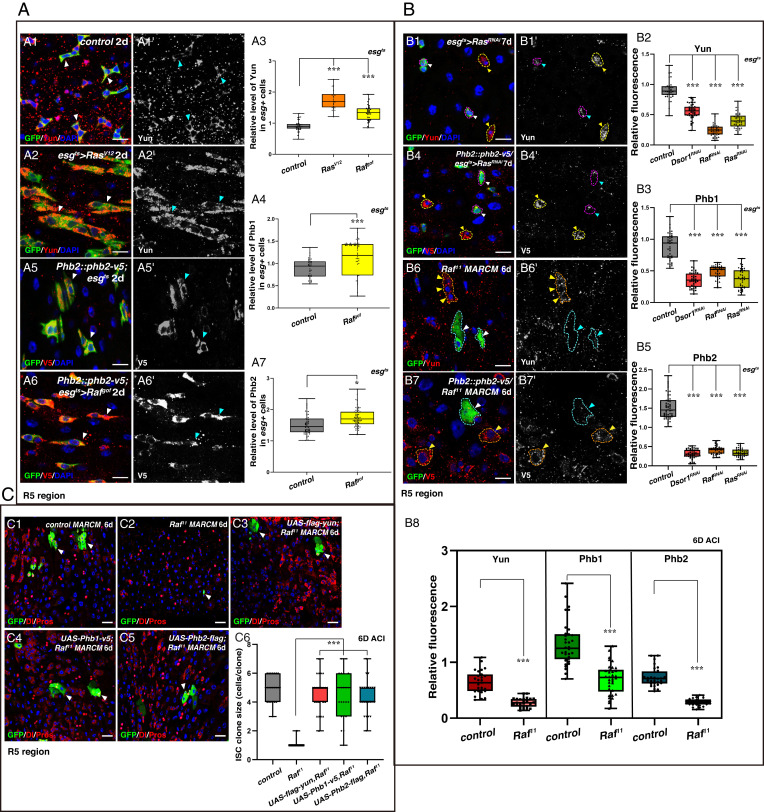
As EGFR signaling is critical to ISC proliferation, we tested whether this pathway regulates the Yun/PHB complex (20, 21). Levels of Yun protein were significantly increased in progenitors with ectopic EGFR/MAPK activation (Fig. 5A and SI Appendix, Fig. S34A). In addition, the levels of both Phb1 and Phb2 were also significantly increased in Rafgof-expressing progenitors (Fig. 5A and SI Appendix, Fig. S34B). Furthermore, when EGFR/MAPK signaling was suppressed in progenitors using RNAi constructs against different key components of the EGFR/MAPK signaling pathway as well as in null Raf mutant cells, the levels of Yun, Phb1, and Phb2 were greatly diminished (Fig. 5B and SI Appendix, Fig. S34 C–F). Together, these data show that the Yun/PHB complex is positively regulated by EGFR/MAPK signaling.
Fig. 5.
The Yun/PHB complex is regulated by EGFR signaling. (A) The Yun/PHB complex is positively regulated by EGFR signaling. (A3) Quantification for the IOD of Yun. Mean ± SD is shown. ***P < 0.001. (A4) Quantification of the IOD of Phb1. Mean ± SD is shown. ***P < 0.001. (A7) Quantification of the IOD of Phb2. Mean ± SD is shown. *P < 0.05. (B) The levels of the Yun/PHB complex are significantly diminished upon inhibition of EGFR signaling. (B2) Quantification of the IOD of Yun. Mean ± SD is shown. ***P < 0.001. (B3) Quantification of the IOD of Phb1. Mean ± SD is shown. ***P < 0.001. (B5) Quantification of the IOD of Phb2. Mean ± SD is shown. ***P < 0.001. (B8) Quantification of the IOD of Yun and Phbs. Mean ± SD is shown. ***P < 0.001. (C) The Yun/PHB complex acts downstream of EGFR signaling. (C6) Quantification of the clone size indicated (C1 to C5). Mean ± SD is shown. ***P < 0.001 [Scale bars, 10 μm (A and B) and 20 μm (C)].
Next, we examined whether the Yun/PHB complex functions downstream of EGFR/MAPK signaling to regulate ISC proliferation. Strikingly, ectopic expression of either Yun, Phb1, or Phb2 significantly rescued ISC proliferation defects observed in Raf mutant cells (Fig. 5C). Collectively, these data show that the Yun/PHB complex acts downstream of EGFR/MAPK signaling to regulate ISC proliferation.
The Yun/PHB Complex Acts through E2F1 to Regulate ISC Proliferation.
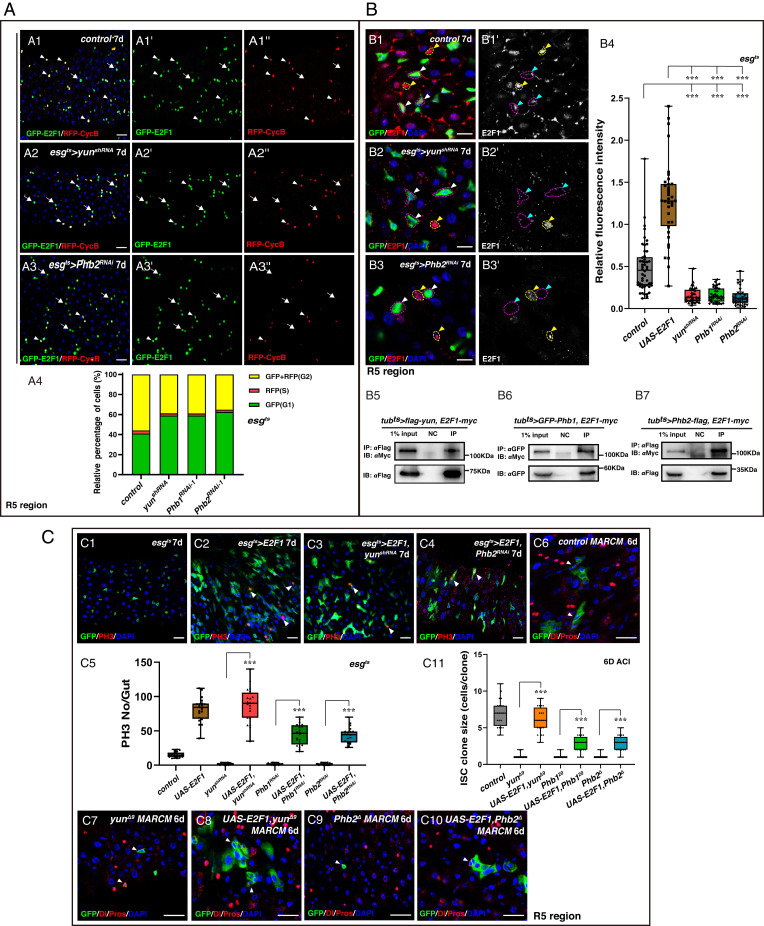
How does the Yun/PHB complex regulate ISC proliferation? We used the Fly-FUCCI (fluorescent ubiquitination–based cell cycle indicator) to track the cell cycle pattern in yun/Phb-defective progenitors (46). Consistent with a previous report, progenitors are normally arrested in either G1 or G2 phase under physiological conditions (Fig. 6A) (47). However, depletion of yun/Phb increased the fraction of progenitors in G1 and decreased the fraction in S and G2 phases, indicating that the Yun/PHB complex may regulate cell cycle progression in progenitors (Fig. 6A and SI Appendix, Fig. S35A).
Fig. 6.
The Yun/PHB complex acts through E2F1. (A) Cell cycle arrests in upon yun/Phb depletion. (A4) Quantification of the percentage of cells per frame. n = 20. (B) The levels of E2F1 are significantly diminished upon yun/Phb depletion. (B4) Quantification of the IOD of E2F1. Mean ± SD is shown. n ≥ 50. ***P < 0.001. (B5 to B7) The Yun/PHB complex associates with E2F1 in vivo. (C) E2F1 acts downstream of the Yun/PHB complex. (C5) Quantification of the number of pH3. Mean ± SD is shown. ***P < 0.001. (C11) Quantification of the clone size. Mean ± SD is shown. ***P < 0.001 [Scale bars, 20 μm (A and C) and 10 μm (B)].
The transcription factor E2F1 plays an important role in the regulation of cell proliferation by controlling the expression of many target genes (28). Thus, we examined whether E2F1 participates in the Yun/PHB-mediated ISC proliferation regulation. E2F1 could be detected in most progenitors; however, the levels of E2F1 protein in all yun-depleted progenitors and yun mutant clones were significantly diminished, suggesting that Yun affects E2F1 levels (Fig. 6B and SI Appendix, Figs. S35B and S36B). The levels of E2F1 were also dramatically decreased in yunΔ9 wing discs (SI Appendix, Fig. S37). The levels of E2F1 were also dramatically reduced in the absence of Phb (Fig. 6B and SI Appendix, Figs. S35B and S36). Furthermore, the expression of the E2F1 downstream target PCNA (proliferating cell nuclear antigen) was abolished in the absence of the Yun/PHB complex (SI Appendix, Fig. S38). The notion that the Yun/PHB complex affects the levels of E2F1 protein was further supported by the finding that the levels of E2F1 protein are significantly increased upon ectopic expression of the Yun/PHB complex (SI Appendix, Fig. S39). Next, we explored how the Yun/PHB complex regulates E2F1 levels. Interestingly, we found that the Yun/PHB complex interacts with E2F1 when overexpressed, suggesting that the Yun/PHB complex may affect E2F1 levels directly in vivo (Fig. 6B). Furthermore, we found the levels of E2F1 transcripts were significantly reduced upon yun/Phb depletion, indicating that the Yun/PHB complex also regulates the levels of E2F1 transcripts directly or indirectly (SI Appendix, Fig. S40).
We further tested whether the reduction of E2F1 was responsible for the ISC proliferation defects observed in the absence of the Yun/PHB complex. Consistently, ectopic expression of E2F1/Dp promotes ISC proliferation (Fig. 6C and SI Appendix, Fig. S35C) (25, 47). Overexpression of E2F1/Dp in yun/Phb-depleted progenitors significantly rescued the proliferation defects observed in yun/Phb-depleted intestines, respectively, with esgts > E2F1, yun/PhbRNAi intestines resembling esgts > E2F1 intestines (Fig. 6C and SI Appendix, Fig. S35C). Consistently, the number of progenitors undergoing mitosis was also significantly increased by simultaneous expression of E2F1/Dp in yun/Phb-depleted intestines, to similar levels as those of esgts > E2F1 intestines (Fig. 6C and SI Appendix, Fig. S35C). These data indicate that E2F1 functions downstream of the Yun/PHB complex to regulate ISC proliferation. Furthermore, ectopic expression of E2F1/Dp in either yun/Phb mutant cells significantly promoted the proliferation of these mutant cells (Fig. 6C and SI Appendix, Fig. S35D). Moreover, simultaneous depletion of Rbf (Retinoblastoma-family protein), the negative regulator of E2F1, in yun/Phb-depleted progenitors could also significantly promote ISC proliferation in yun/Phb-depleted intestines, albeit weaker than those of E2F1/Dp ectopic expression (SI Appendix, Fig. S35E). Interestingly, ectopic expression of only one of the E2F1 downstream targets, PCNA, was not sufficient to rescue the proliferation defects in the absence of yun, indicating that it likely requires multiple E2F1 downstream targets acting together to restore proliferation defects in the absence of the Yun/PHB complex (SI Appendix, Fig. S41). Taken together, these data show that the Yun/PHB complex acts through E2F1 to sustain ISC proliferation.
Evolutionarily Conserved Function of the PHB Complex in Cell Proliferation.
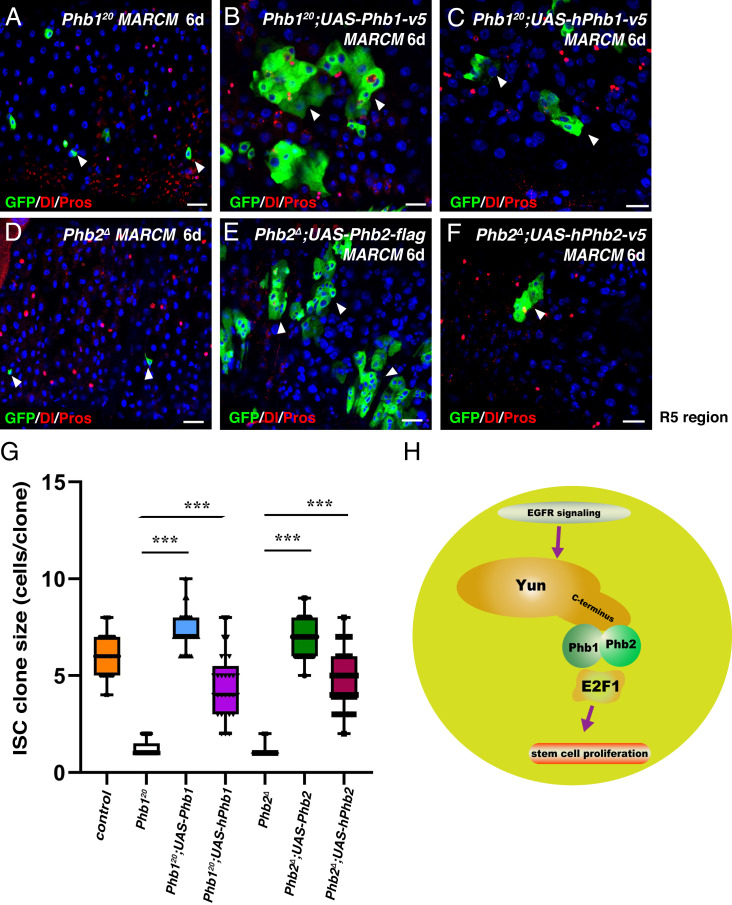
We could not identify a clear ortholog of Yun in vertebrates; however, Phb1 and Phb2 are conserved. Thus, we decided to investigate whether human Phb1 and Phb2 play a role in cell proliferation. First, as observed for its fly counterpart, ectopic expression of human Phb1 (hPhb1) or Phb2 (hPhb2) significantly rescued the proliferation defects observed in Phb1 and Phb2 mutant clones, respectively (Fig. 7 A–G). Furthermore, hPhb1 did not rescue the proliferation defects observed in Phb2 mutant clones, and vice versa (SI Appendix, Fig. S42). Second, we examined whether the human PHB complex is required for the proliferation of human cancer cell lines. Depletion of either hPhb1 or hPhb2 significantly inhibited the proliferation of human colorectal cancer cell line HCT116 and human cervical cancer cell line HeLa, indicating that the human PHB complex is required for proliferation of different human cancer cells (SI Appendix, Fig. S43). Furthermore, sphere formation of these cancer cell lines was almost completely inhibited by hPhb1 or hPhb2 depletion. Collectively, these data indicate that the role of the PHB complex in cell proliferation is evolutionarily conserved.
Fig. 7.
Conserved functions of the PHB complex. (A–C) Expression of fly Phb1 (B) and hPhb1 (C) rescues the defects of Phb120 mutant (A). (D–F) Expression of fly Phb2 (E) and hPhb2 (F) significantly rescues the defects observed in Phb2Δ mutant (D). (G) Quantification of clone size indicated. Mean ± SD is shown. ***P < 0.001. (H) Model of the Yun/PHB complex in ISC proliferation regulation (Scale bars, 20 μm).
Discussion
Proliferation and differentiation of adult stem cells must be tightly controlled to maintain tissue homeostasis and prevent tumorigenesis. However, how stem cell proliferation is properly controlled and in particular how the cell cycle is regulated in stem cells is not fully understood. Here, we identify Yun as an ISC proliferation regulator from a large-scale RNAi screen. Loss of yun function in progenitors restricts them from proliferating, such that they remain in a quiescent state under normal conditions and during tissue regeneration following acute tissue damage. We show that Yun acts as a scaffold for the PHB complex and that the Yun/PHB complex is regulated by EGFR signaling and functions through E2F1 to sustain proliferation of normal stem cells for tissue homeostasis/regeneration and transformed stem cells in tumorigenesis.
The Yun/PHB-E2F1 Regulatory Pathway in Stem Cell Proliferation.
In addition to EGFR signaling, the levels of the Yun/PHB complex are also elevated upon activation of JAK/STAT signaling or in the absence of Notch, indicating that the Yun/PHB complex may also be regulated by JAK/STAT and Notch signaling directly or indirectly (SI Appendix, Figs. S44 and S45). Previous studies and our data show that EGFR signaling acts downstream of JAK/STAT, Notch, and Wnt signaling in ISC proliferation and that ectopic expression of yun/Phb could rescue proliferation defects in the absence of EGFR signaling. Therefore, we propose that EGFR signaling is the major upstream of signal of the Yun/PHB complex, although we cannot fully exclude the possibilities that it may also be regulated by the other signaling pathways directly or indirectly (20, 22, 48, 49). The EGFR/MAPK pathway and E2F1 are differentially required for stem cell proliferation (mitosis) and differentiation (endoreplication) (20–22, 25). How E2F1 is differentially regulated during these two processes is not clear. The regulation of E2F1 levels by EGFR/MAPK signaling has been proposed to be due to increased translation or/and increased protein stability, possibly involving some unknown cytoplasmic factors (25). The identification of the Yun/PHB complex may account for the differential regulation of E2F1 by EGFR/MAPK signaling. The Yun/PHB complex is expressed in progenitors and mediates EGFR/MAPK signaling for ISC proliferation but not progeny differentiation, indicating that the Yun/PHB complex is more specifically required for ISC proliferation. Interestingly, a previous study identified another target of EGFR signaling, the transcription factor Sox100B/dSox9B, which has a critical role in progeny differentiation (40), indicating that the control of the ISC proliferation and progeny differentiation by EGFR/MAKP signaling is likely differentially mediated by different effectors.
The levels of E2F1 protein, along with the expression of PCNA-GFP, were significantly diminished in yun/Phb-defective progenitors or imaginal wing discs, suggesting that Yun affects E2F1 levels. Our biochemical analysis show that the Yun/PHB complex associates with E2F1 in vivo, indicating that the Yun/PHB complex interacts and stabilizes E2F1 protein to regulate ISC proliferation. Furthermore, ectopic expression of single components of the Yun/PHB complex increased E2F1 protein levels, which were further increased when two or three components of the Yun/PHB complex were coexpressed, supporting the notion that the Yun/PHB complex regulates E2F1 protein levels. Moreover, ectopic expression of E2F1/Dp significantly restored ISC proliferation in yun/Phb-defective intestines. Consistently, knockdown of the negative regulator of E2F1, Rb, also restored ISC proliferation in these yun/Phb-defective intestines, albeit at a weaker level than that of E2F1/Dp overexpression. Together, these data demonstrate that E2F1 acts downstream of the Yun/PHB complex for ISC proliferation. Interestingly, overexpressing PCNA alone is not sufficient to restore ISC proliferation in yun-depleted intestines, indicating that collective activation of multiple downstream targets of the E2F1/Dp complex are required to restore ISC proliferation. It has been previously proposed that PHB1 binds to Rb and functions as a negative regulator of E2F1-mediated transcription (50–53). These studies contrast with previous work suggesting that the PHB complex is required for cell proliferation (35, 36, 54–57). Our in vivo study uncovered how E2F1 is differential regulated by EGFR/MAPK signaling and acts downstream of the Yun/PHB complex in ISC proliferation to maintain tissue homeostasis under normal and stress conditions and during tumorigenesis in Drosophila (Fig. 7H), which is in striking contrast to the proposed antiproliferation role of PHB1.
Conserved Function of the PHB Complex in Cancer Cell Proliferation.
Unlike Yun, Phb1 and Phb2 are conserved and have been reported to form a complex that localizes to the nucleus, plasma membrane, and mitochondria in mammalian cells (30, 58). In mitochondria, the PHB complex functions as chaperones in mitochondria in some cell types (29, 58, 59). Although in flies, the Yun/PHB complex is partially localized in mitochondria in progenitors, our results indicate that the ISC proliferation defects observed in yun/Phb-defective progenitors are unlikely due to their roles in mitochondria.
Human Phb1 and Phb2 could significantly restore ISC proliferation defects in Phb1- and Phb2-depleted intestines, respectively, and were required for the proliferation of different human cancer cell lines, indicating that the function of the PHB complex in proliferation is conserved. Finally, the observations that 1) Yun acts as a scaffold of PHBs for their proper function; 2) the Yun/PHB complex acts downstream of EGFR/MAPK signaling; and 3) PHBs and EGFR/MAPK signaling are evolutionarily conserved, suggest that a functional counterpart of Yun exists in mammals, which is different in primary sequence but possibly similar in structure.
Methods
Fly Lines and Husbandry.
Information about alleles and transgenes used in this study can be found either in FlyBase or as noted. Please refer to SI Appendix for detailed information.
Immunostainings and Fluorescence Microscopy.
Immunostainings and microscopy follow standard procedures as described in SI Appendix.
Data Analysis.
pH3 numbers were scored manually under a Zeiss Imager Z2/LSM780 microscope. The number of intestines scored is indicated in the text. Statistical analysis was done using the Student’s t test. nsP > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Supplementary Material
Acknowledgments
We are grateful to Yu Cai, Xiaolin Bi, Steven Hou, Rongwen Xi, Xiaohang Yang, Lei Xue, Margret Ho, Jianquan Ni, Gyeong Hun Baeg, Chao Tong, Jose Pastor, Ruoxi Wang, Developmental Studies Hybridoma Bank, Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center, Fly stocks of National Institute of Genetics stock center, and Transgenic RNAi Project for reagents and stocks, and Drosophila Genetic Resource Consortium for complementary DNA clones. We also thank Francois Schweisguth, Steven Hou, Yu Cai, and Julie Secombe, Joshua Li, and Stephanie Mohr for suggestions, comments, and/or critical reading. N.P. is an investigator of the Howard Hughes Medical Institute. This work is supported by grants from Beijing Municipal Commission of Education (Grant No. KZ201910028040) and the National Natural Science Foundation of China (Grant Nos. 92054109, 31972893, and 31471384).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111711119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
Change History
May 6, 2022: The author affiliations have been updated.
References
- 1.Morrison S. J., Spradling A. C., Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radtke F., Clevers H., Self-renewal and cancer of the gut: Two sides of a coin. Science 307, 1904–1909 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Lin H., Cell biology of stem cells: An enigma of asymmetry and self-renewal. J. Cell Biol. 180, 257–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casali A., Batlle E., Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 4, 124–127 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Stainier D. Y. R., No organ left behind: Tales of gut development and evolution. Science 307, 1902–1904 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Edgar B. A., Intestinal stem cells: No longer immortal but ever so clever. EMBO J. 31, 2441–2443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gervais L., Bardin A. J., Tissue homeostasis and aging: New insight from the fly intestine. Curr. Opin. Cell Biol. 48, 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Jasper H., Intestinal stem cell aging: Origins and interventions. Annu. Rev. Physiol. 82, 203–226 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Micchelli C. A., Perrimon N., Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Ohlstein B., Spradling A., The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Yeung T. M., Chia L. A., Kosinski C. M., Kuo C. J., Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell. Mol. Life Sci. 68, 2513–2523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beebe K., Lee W. C., Micchelli C. A., JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 338, 28–37 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Ohlstein B., Spradling A., Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988–992 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Zeng X., et al. , Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep. 10, 1226–1238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biteau B., Jasper H., Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 7, 1867–1875 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., et al. , Transient Scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells. Nat. Cell Biol. 20, 152–161 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Colombani J., Andersen D. S., The Drosophila gut: A gatekeeper and coordinator of organism fitness and physiology. Wiley Interdiscip. Rev. Dev. Biol. 9, e378 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Jiang H., Tian A., Jiang J., Intestinal stem cell response to injury: Lessons from Drosophila. Cell. Mol. Life Sci. 73, 3337–3349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joly A., Rousset R., Tissue adaptation to environmental cues by symmetric and asymmetric division modes of intestinal stem cells. Int. J. Mol. Sci. 21, E6362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H., Grenley M. O., Bravo M.-J., Blumhagen R. Z., Edgar B. A., EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biteau B., Jasper H., EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045–1055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu N., et al. , EGFR, wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31–43 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Ma M., et al. , Wildtype adult stem cells, unlike tumor cells, are resistant to cellular damages in Drosophila. Dev. Biol. 411, 207–216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchon N., Broderick N. A., Kuraishi T., Lemaitre B., Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 8, 152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang J., et al. , EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat. Commun. 8, 15125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee L. A., Orr-Weaver T. L., Regulation of cell cycles in Drosophila development: Intrinsic and extrinsic cues. Annu. Rev. Genet. 37, 545–578 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Nevins J. R., E2F: A link between the Rb tumor suppressor protein and viral oncoproteins. Science 258, 424–429 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Nevins J. R., Transcriptional regulation. A closer look at E2F. Nature 358, 375–376 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Osman C., Merkwirth C., Langer T., Prohibitins and the functional compartmentalization of mitochondrial membranes. J. Cell Sci. 122, 3823–3830 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Mishra S., Ande S. R., Nyomba B. L. G., The role of prohibitin in cell signaling. FEBS J. 277, 3937–3946 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Signorile A., Sgaramella G., Bellomo F., De Rasmo D., Prohibitins: A critical role in mitochondrial functions and implication in diseases. Cells 8, E71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jupe E. R., Liu X. T., Kiehlbauch J. L., McClung J. K., Dell’Orco R. T., Prohibitin in breast cancer cell lines: Loss of antiproliferative activity is linked to 3′ untranslated region mutations. Cell Growth Differ. 7, 871–878 (1996). [PubMed] [Google Scholar]
- 33.McClung J. K., et al. , Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem. Biophys. Res. Commun. 164, 1316–1322 (1989). [DOI] [PubMed] [Google Scholar]
- 34.Wei Y., Chiang W. C., Sumpter R. Jr., Mishra P., Levine B., Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168, 224–238.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coates P. J., Jamieson D. J., Smart K., Prescott A. R., Hall P. A., The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr. Biol. 7, 607–610 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Supale S., et al. , Loss of prohibitin induces mitochondrial damages altering β-cell function and survival and is responsible for gradual diabetes development. Diabetes 62, 3488–3499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graziadio L., et al. , Phenotypic characterization of diamond (dind), a Drosophila gene required for multiple aspects of cell division. Chromosoma 127, 489–504 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Lee T., Luo L., Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Hay B. A., Wolff T., Rubin G. M., Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121–2129 (1994). [DOI] [PubMed] [Google Scholar]
- 40.Jin Z., et al. , The Drosophila ortholog of mammalian transcription factor Sox9 regulates intestinal homeostasis and regeneration at an appropriate level. Cell Rep. 31, 107683 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Reiff T., et al. , Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila. EMBO J. 38, e101346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthurton L., Nahotko D. A., Alonso J., Wendler F., Baena-Lopez L. A., Non-apoptotic caspase activation preserves Drosophila intestinal progenitor cells in quiescence. EMBO Rep. 21, e48892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H., et al. , Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H., Edgar B. A., EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biteau B., Hochmuth C. E., Jasper H., JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielke N., et al. , Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell Rep. 7, 588–598 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Zhang P., et al. , The Krüppel-like factor Cabut has cell cycle regulatory properties similar to E2F1. Proc. Natl. Acad. Sci. U.S.A. 118, e2015675118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou F., Rasmussen A., Lee S., Agaisse H., The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev. Biol. 373, 383–393 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel P. H., Dutta D., Edgar B. A., Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 17, 1182–1192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi B., Ko D., Ordonez-Ercan D., Chellappan S. P., A putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcription and induce apoptosis. Biochem. Biophys. Res. Commun. 312, 459–466 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Wang S., Nath N., Adlam M., Chellappan S., Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene 18, 3501–3510 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Wang S., Nath N., Fusaro G., Chellappan S., Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals. Mol. Cell. Biol. 19, 7447–7460 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S., Zhang B., Faller D. V., Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. EMBO J. 21, 3019–3028 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merkwirth C., et al. , Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 22, 476–488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schleicher M., et al. , Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J. Cell Biol. 180, 101–112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merkwirth C., Langer T., Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta 1793, 27–32 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Eveleth D. D. Jr., Marsh J. L., Sequence and expression of the Cc gene, a member of the dopa decarboxylase gene cluster of Drosophila: Possible translational regulation. Nucleic Acids Res. 14, 6169–6183 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ande S. R., Mishra S., Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem. Biophys. Res. Commun. 390, 1023–1028 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Artal-Sanz M., Tavernarakis N., Prohibitin and mitochondrial biology. Trends Endocrinol. Metab. 20, 394–401 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.