Abstract
BACKGROUND:
Blood or bone marrow transplantation (BMT) survivors with frailty are at a higher risk of subsequent mortality. Longitudinal trends in the frailty state are not known and could help identify vulnerable subpopulations at risk of subsequent adverse events.
METHODS:
This study included a cohort of 470 autologous and allogeneic BMT recipients who had survived ≥2 years after BMT and completed a baseline questionnaire (t1) at a median of 7.3 years after BMT and a follow-up questionnaire (t2) 13.2 years after t1. The main outcome was change in frailty state between t1 and t2. Frailty phenotype was defined as exhibiting ≥3 of the following characteristics: clinically underweight, exhaustion, low energy expenditure, slow walking speed, and muscle weakness. The following categories of change in frailty state were evaluated: worsened, improved, and stable.
RESULTS:
Of the 470 participants, 36.4% were aged ≥60 years at t1, and 50.6% were men. The prevalence of frailty increased from 4.8% at t1 to 9.6% at t2. Worsening was observed in 18.8% of patients, and improvement was reported in 9.7%. Pre-BMT exposure to vincristine (odds ratio [OR], 2.1; 95% CI, 1.3–3.39) was associated with worsening. Female sex (OR, 1.5; 95% CI, 0.93–2.4) was associated with a trend toward worsening. Pre-BMT exposure to vincristine (OR, 2.79; 95% CI, 1.44–5.43), a history of chronic graft-versus-host disease (OR, 2.58; 95% CI, 1.2–5.5), and grade 3 and 4 chronic health conditions at t1 (OR, 2.1; 95% CI, 1.08–4.33) were associated with frailty at t2.
CONCLUSIONS:
In a cohort of BMT survivors who were followed longitudinally for a median of 20.6 years from BMT, the frailty status worsened for approximately20% over a 13-year timespan. BMT survivors who are at risk for worsening frailty could benefit from targeted interventions.
Keywords: cohort study, frailty, hematopoietic stem cell transplantation, longitudinal trajectory, long-term survivors
INTRODUCTION
Recent studies in young patients with cancer report a higher prevalence of frailty compared with unaffected individuals,1–3 suggesting accelerated aging.1,3 In our previous study, we reported that nonelderly blood or bone marrow transplantation (BMT) survivors were 8.4 times more likely to be frail compared with age-matched and sex-matched siblings. Furthermore, frail BMT survivors were at 2.7-fold higher risk of subsequent mortality compared with nonfrail survivors.3 Although the dynamic nature of frailty has been described in community-dwelling elderly,4,5 there is a paucity of information regarding the trajectory of frailty in BMT survivors. We addressed this gap by evaluating transitions in the frailty state over a period of 13 years in a cohort of BMT survivors enrolled in the BMT Survivor-Study (BMTSS). We hypothesized that BMT survivors have dynamic trends in frailty, and that pretransplantation, transplantation-related, and post-transplantation factors would identify subpopulations with a persistent and worsening frailty trajectory over time. This would identify patients who could benefit from interventions.
MATERIALS AND METHODS
Study Population
BMTSS is a retrospective cohort of patients who received BMT at the City of Hope, the University of Minnesota, or the University of Alabama at Birmingham between 1974 and 1998 and survived ≥2 years after transplantation. Participants completed a BMTSS questionnaire6; the validity of the BMTSS questionnaire to accurately report survivors’ health conditions has been demonstrated.7
To be eligible for this report, BMT survivors had to have completed the BMTSS questionnaire between 2000 and 2004 (t1) and again between 2013 and 2017 (t2). after a median interval of 13.2 years (range, 8.5–18.8 years). Study participants were aged ≥18 years at t1. The median time was 7.3 years between BMT and t1 and 20.6 years between BMT and t2. The human subjects committees at the participating institutions approved the protocol, and informed consent was provided according to the Declaration of Helsinki. Of the 770 t1 participants who were alive and eligible for participation at t2, 484 (63%) completed the questionnaire at t2. Frailty phenotype could not be estimated in 9 patients at t1 and 5 patients at t2, yielding 470 evaluable patients. Compared with non-participants, t2 participants were more likely to be white (86% vs 60%; P < .0001) and older at BMT (median age, 34 vs 28 years; P < .0001), were more likely to have undergone autologous BMT (43% vs 34%; P = .02), and were less likely to have acute leukemia or myelodysplastic syndrome (31% vs 41%; P = .001).
Change in Frailty State
A frailty phenotype was constructed as reported previously.3 Briefly, frailty phenotype was defined as exhibiting ≥3 of the following characteristics: clinically underweight, exhaustion, low energy expenditure, slow walking speed, and muscle weakness. Participants who reported ≥3 of these 5 indices were classified as frail, and those who reported 1 or 2 indices were classified as prefrail. We created the following 3 categories of change in frailty state from t1 to t2: worsened (nonfrail→prefrail/frail, prefrail→frail, frail→frail), improved (frail→prefrail/nonfrail, prefrail→nonfrail), and stable (nonfrail→nonfrail, prefrail→prefrail).
Statistical Analyses
The objective of this study was to identify predictors of worsened phenotype, as well as predictors of frailty at t2, using multivariable logistic regression. The following variables were evaluated in univariate analysis: age at questionnaire (<60 vs ≥60 years), race/ethnicity (non-Hispanic whites vs other), socioeconomic status (annual household income of <$60,000 or <college-level education vs all others), smoking status (ever smoked vs never smoked), primary cancer diagnosis (acute leukemia, lymphoma, chronic myeloid leukemia, other), type of transplantation and the presence of chronic graft-versus-host disease (GvHD) (autologous BMT and allogeneic BMT without chronic GvHD vs allogeneic BMT with chronic GvHD), the use of total body irradiation (TBI) in conditioning (yes vs no), the presence of grade 3 or 4 chronic health conditions (yes vs no), the conditioning regimen used (cyclophosphamide and TBI; cyclophosphamide, etoposide, and TBI; etoposide and TBI; cyclophosphamide and etoposide; busulfan and cyclophosphamide; others), and pretransplantation therapeutic exposures (cytarabine, etoposide, vincristine, methotrexate, bleomycin, anthracyclines, alkylating agents, cisplatin, radiation). Because similar odds of frailty have been observed in survivors of allogeneic hematopoietic stem cell transplantation (HCT) without chronic GvHD and survivors of autologous HCT,3 the 2 were combined into 1 group for the analysis (autologous BMT and allogeneic BMT without chronic GvHD vs allogeneic BMT with chronic GvHD). Variables that were significant in the univariate analysis at P < .1 were included in the multivariable analysis using backward selection. Results of the final adjusted model are presented. Two-sided tests with P < .05 were considered statistically significant. Analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Demographic and clinical characteristics of the 470 study participants are provided in Table 1. The median time was 7.3 years between transplantation and t1 and 20.6 years between transplantation and t2. The median age at HCT was 34 years (range, 0–61 years) and was 42.9 years (range, 18.5–67.4 years) at t1 and 56.5 years (range, 31.0–80.0 years) at t2. Overall, 49.4% of participants were women, 85.3% were non-Hispanic whites, 48.5% had a college-level education or greater, 43% had an annual household income ≤$60,000, and 34.7% reported current or past smoking. In total, 57.7% of participants underwent allogeneic HCT. Among the allogeneic HCT recipients, 15.7% reported chronic GvHD. Pre-HCT receipt of vincristine was reported by 40.6% and 76.8% of participants received TBI-based conditioning.
TABLE 1.
Demographic and Clinical Characteristics of the 470 Study Participants
| Characteristic | No. of Participants (%) |
|---|---|
| Age at questionnaire, y | |
| <40 | 46 (9.8) |
| 40–59 | 253 (53.8) |
| ≥60 | 171 (36.4) |
| Diagnosis | |
| Acute myeloid leukemia | 109 (23.2) |
| Acute lymphoid leukemia | 39 (8.3) |
| Chronic myelogenous leukemia | 117 (24.9) |
| Non-Hodgkin lymphoma/Hodgkin lymphoma | 137 (29.8) |
| Other diagnosesa | 68 (14.0) |
| Sex | |
| Men | 238 (50.6) |
| Race/ethnicity | |
| Non-Hispanic White | 401 (85.3) |
| Type of transplantation | |
| Autologous | 199 (42.6) |
| Allogeneic | 271 (57.7) |
| Presence of chronic GvHD (among allogeneic BMT recipients) | |
| Allogeneic BMT with chronic GvHD | 74 (15.7) |
| Educationb | |
| ≤High school | 70 (14.9) |
| Some college/training | 171 (36.4) |
| ≥College | 228 (48.5) |
| Annual household incomeb | |
| ≤$60,000 | 202 (43) |
| Ever smokerb | |
| Yes | 163 (34.7) |
| Conditioning regimen | |
| Cyclophosphamide + TBI | 181 (38.5) |
| Cyclophosphamide + TBI + etoposide | 111 (23.6) |
| Etoposide + TBI | 50 (10.6) |
| Cyclophosphamide + etoposide | 39 (8.3) |
| Busulfan + cyclophosphamide | 36 (7.7) |
| Other | 53 (11.3) |
| TBI | |
| Yes | 361 (76.8) |
| Pre-BMT chemotherapy | |
| Anthracycline | 280 (61.1) |
| Steroid | 207 (45.2) |
| Vincristine | 186 (40.6) |
| Alkylating agents | 184 (40.2) |
| Cytarabine | 174 (37) |
| Methotrexate | 101 (22.1) |
| Etoposide | 70 (15.3) |
| Bleomycin | 63 (13.8) |
| Cisplatin | 44 (9.6) |
| Pre-BMT radiation | |
| Yes | 73 (15.9) |
Abbreviations: BMT, blood or bone marrow transplantation; GvHD, graft-versus-host disease; TBI, total body irradiation.
Other diagnoses included aplastic anemia (n = 23), multiple myeloma (n = 17), breast cancer (n = 11), chronic lymphocytic leukemia (n = 7), adrenoleukodystrophy (n = 3), Ewing sarcoma (n = 1), neuroblastoma (n = 10), Hurler syndrome (n = 1), severe combined immunodeficiency (n = 1), severe osteopetrosis (n = 1), Fanconi anemia (n = 1), and systemic sclerosis (n = 1).
Data were missing for the variables education (n = 1), annual household income (n = 29), and ever smoker (n = 4).
Prevalence of Frailty and Prefrailty
Although the prevalence of prefrailty was comparable between t1 (13.8%) and t2 (16.1%; P = .4), the prevalence of frailty increased from 4.8% at t1 to 9.6% at t2 (P = .03) (Fig. 1A). Worsening was observed in 18.8% of survivors, whereas improvement was observed in 9.7% (Fig. 1B).
Figure 1.
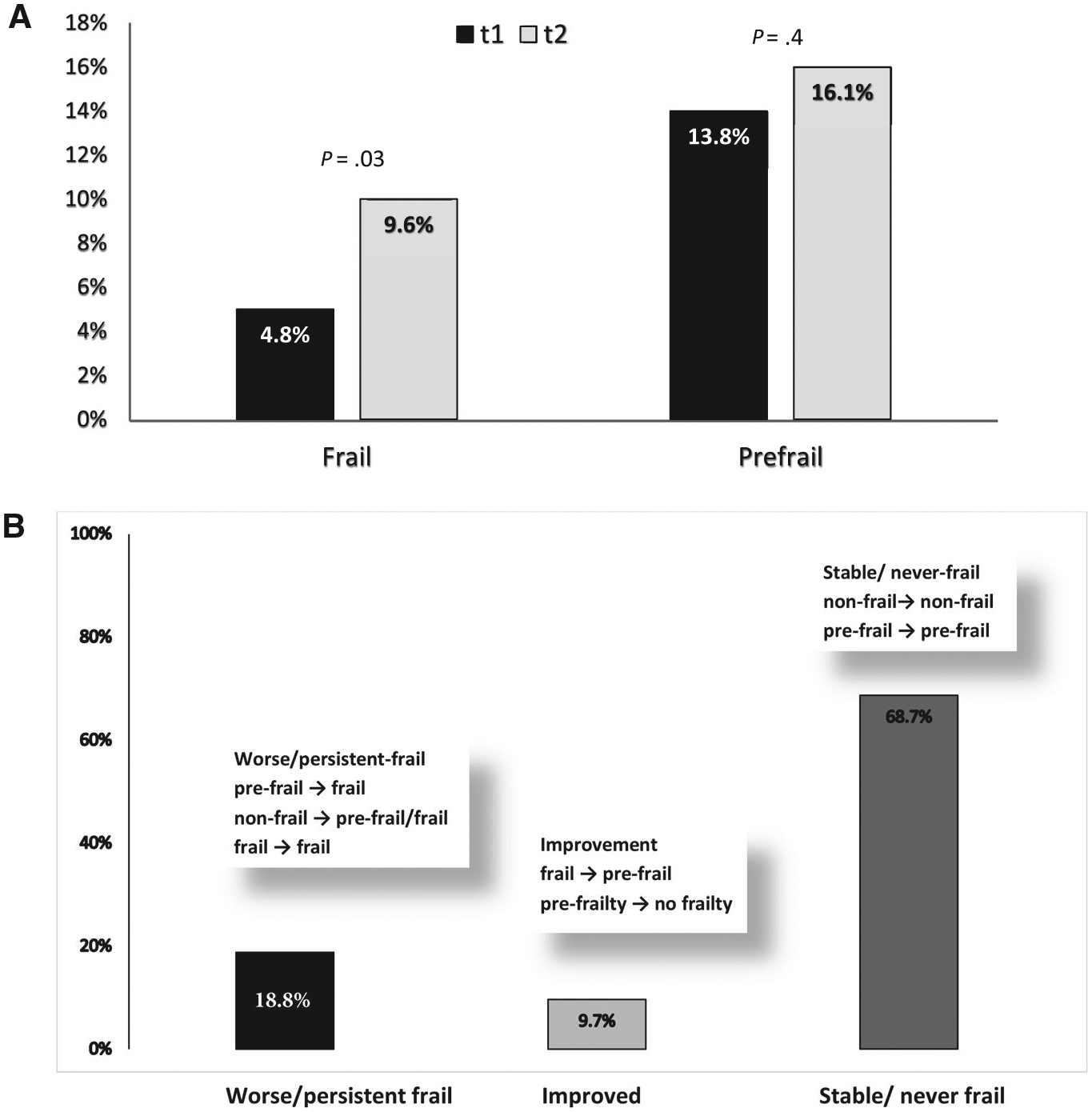
(A) The prevalence of frailty and prefrailty at t1 (the time of the baseline questionnaire) and t2 (the time of the follow-up questionnaire) is illustrated along with (B) the change in frailty status over time.
Predictors of Worsened Frailty Status
In multivariable analysis, survivors who had pre-BMT exposure to vincristine (reference category, no vincristine exposure) were at 2.1 times higher odds (95% CI, 1.3–3.39; P = .002) of worsening. Women exhibited a trend toward higher odds of worsening compared with men (odds ratio [OR], 1.5; 95% CI, 0.93–2.4; P = .09) (Table 2).
TABLE 2.
Predictors of Frailty: Univariate and Multivariate Analyses
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Predictor | OR (95% CI) | P | OR (95% CI) | P |
| Worsened | ||||
| Age at questionnaire, y | ||||
| ≥60 vs <60 | 1.25 (0.78–1.99) | .35 | ||
| Race/ethnicity | ||||
| Other vs non-Hispanic White | 1.02 (0.45–2.29) | .97 | ||
| Socioeconomic status | ||||
| Annual income <$60,000/<college level vs annual income ≥$60,000/≥college level | 1.25 (0.74–2.12) | .40 | ||
| Time between first and second questionnaire | 0.95 (0.82–1.11) | .54 | ||
| Smoking status | ||||
| Ever smoked vs never smoked | 0.97 (0.60–1.56) | .90 | ||
| Sex | ||||
| Men | 1.00 | 1.00 | .09 | |
| Women | 1.31 (0.83–2.08) | .12 | 1.5 (0.93–2.4) | |
| Primary cancer diagnosis | ||||
| Acute leukemia | 1.00 | |||
| Lymphoma | 1.04 (0.59–1.81) | .90 | ||
| Chronic myeloid leukemia | 0.47 (0.23–0.93) | .03 | ||
| Other | 1.21 (0.6–2.42) | .60 | ||
| Conditioning regimen | ||||
| Cyclophosphamide + TBI | 1.00 | |||
| Cyclophosphamide + TBI + etoposide | 1.41 (0.79–2.53) | .24 | ||
| Etoposide + TBI | 1.02 (0.45–2.3) | .97 | ||
| Cyclophosphamide + etoposide | 1.39 (0.6–3.2) | .44 | ||
| Busulfan + cyclophosphamide | 1.54 (0.66–3.59) | .32 | ||
| Others | 0.59 (0.23–1.5) | .27 | ||
| TBI, yes vs no | 0.94 (0.55–1.6) | .81 | ||
| Chronic GvHD at t1 | ||||
| Allogeneic BMT with chronic GvHD vs allogeneic BMT with no chronic GvHD or autologous BMT | 1.43 (0.79–2.57) | .24 | ||
| Pre-BMT therapeutic exposure, yes vs no | ||||
| Vincristine | 1.95 (1.22–3.11) | < .005 | 2.1 (1.3–3.39) | .002 |
| Cytarabine | 1.01 (0.63–1.62) | .98 | ||
| Etoposide | 1.27 (0.69–2.35) | .44 | ||
| Methotrexate | 1.03 (0.59–1.79) | .92 | ||
| Bleomycin | 1.35 (0.72–2.54) | .35 | ||
| Anthracyclines | 1.8 (1.09–2.99) | .02 | ||
| Alkylating agents | 0.96 (0.6–1.55) | .88 | ||
| Cisplatin | 0.76 (0.33–1.77) | .53 | ||
| Radiation | 1.58 (0.88–2.83) | .13 | ||
| Grade 3–4 conditions at t1, yes vs no | 1.24 (0.68–2.25) | .48 | ||
| Frailty at t2 | ||||
| Age at questionnaire, y | ||||
| ≥60 vs <60 | 1.19 (0.63–2.22) | .60 | ||
| Race/ethnicity | ||||
| Other vs non-Hispanic White | 1.02 (0.35–3.01) | .97 | ||
| Socioeconomic status | ||||
| Annual income <$60,000/<college level vs annual income ≥$60,000/≥college level | 1.14 (0.56–2.31) | .72 | ||
| Time between BMT and questionnaire: t2 | 1.01 (0.96–1.07) | .71 | ||
| Smoking status | ||||
| Ever smoked vs never smoked | 1.11 (0.58–2.13) | .75 | ||
| Sex | ||||
| Men | ||||
| Women | 1.13 (0.61–2.09) | .70 | ||
| Primary cancer diagnosis | ||||
| Acute leukemia | 1.00 | |||
| Lymphoma | 1.0 (0.48–2.08) | 0.99 | ||
| Chronic myeloid leukemia | 0.44 (0.17–1.15) | .09 | ||
| Other | 1.05 (0.41–2.68) | .91 | ||
| Conditioning regimen | ||||
| Cyclophosphamide + TBI | ||||
| Cyclophosphamide + TBI + etoposide | 1.06 (0.48–2.36) | .88 | ||
| Etoposide + TBI | 0.84 (0.27–2.62) | .76 | ||
| Cyclophosphamide + etoposide | 1.42 (0.49–4.11) | .52 | ||
| Busulfan + cyclophosphamide | 1.93 (0.75–5.29) | .20 | ||
| Others | 0.38 (0.08–1.69) | .20 | ||
| TBI, yes vs no | 0.81 (0.4–1.64) | .56 | ||
| Chronic GvHD at t1 | ||||
| Allogeneic BMT with no chronic GvHD or autologous BMT | 1.00 | 1.00 | .01 | |
| Allogeneic BMT with chronic GvHD | 2.39 (1.19–4.81) | .01 | 2.58 (1.21–5.5) | |
| Chronic health conditions at t1 | ||||
| Grade ≤2 | 1.00 | 1.00 | .04 | |
| Grade 3 or 4 | 2.14 (1.08–4.26) | .03 | 2.1 (1.02–4.33) | |
| Pre-BMT therapeutic exposure, yes vs no | ||||
| Cytarabine | 0.83 (0.43–1.6) | .58 | ||
| Etoposide | 0.86 (0.35–2.13) | .75 | ||
| Methotrexate | 1.04 (0.5–2.19) | .91 | ||
| Bleomycin | 1.45 (0.64–3.28) | .38 | ||
| Anthracyclines | 1.26 (0.65–2.41) | .50 | ||
| Alkylating agents | 1.03 (0.55–1.95) | .92 | ||
| Cisplatin | 0.94 (0.32–2.75) | .90 | ||
| Radiation | 1.89 (0.91–3.94) | .09 | ||
| Vincristine, yes vs no | 2.55 (1.35–4.82) | .004 | 2.79 (1.44–5.43) | .002 |
Abbreviations: BMT, blood or bone marrow transplantation; GvHD, graft-versus-host disease; t1, the time of the baseline questionnaire (a median of 7.3 years after BMT); t2, the time of the follow-up questionnaire (a median of 13.2 years after BMT); TBI, total body irradiation.
Predictors of Frailty at t2
Allogeneic BMT recipients with chronic GvHD (OR, 2.58; 95% CI, 1.21–5.5; P = .01; reference category, autologous BMT/allogeneic BMT without chronic GvHD), those with grade 3 and 4 chronic health conditions (OR, 2.1; 95% CI, 1.01–4.33; P = .04; reference category, grade 0–2 chronic health conditions), and those with pre-BMT exposure to vincristine (OR, 2.79; 95% CI, 1.44–5.43; P = .002; reference category, no vincristine exposure) were at higher odds of frailty at t2 (Table 2). Next, we evaluated the domains of frailty in participants with and without prior exposure to vincristine. Three domains—weakness (6.4% vs 2.2% in those with or without exposure to vincristine, respectively; P = .02), exhaustion (43.5% vs 34.7%, respectively; P = .05), and low energy expenditure (40.8% vs 30.3%, respectively; P = .02)—were more prevalent in participants who had prior exposure to vincristine.
DISCUSSION
In our study, the prevalence of frailty doubled among BMT survivors over a period of 13 years. Furthermore, almost 20% of survivors demonstrated worsening over this period. Patients who had prior exposure to vincristine were at a higher odds of worsening. Women showed a trend toward higher odds of worsening. Nonetheless, approximately 74% of patients were not frail or prefrail at t2. Placing these findings in the context of the higher risk of subsequent mortality among frail BMT survivors indicates an urgent need for early identification and intervention8–11 to mitigate adverse events.
Sex differences in frailty phenotype have been described previously in community-dwelling12,13 and cancer populations.1 The etiology remains unclear, but differential influences on muscle mass by sex hormones has been postulated.1 We also identified an association between grade 3 and 4 chronic health conditions at t1 and the subsequent development of frailty at t2. It has been demonstrated that the presence of serious chronic health conditions adversely affects physical characteristics that may result in frailty.1,14
We did not observe an effect of age on frailty. In contrast, in a large, community-dwelling younger cohort (n = 493,737; aged 37–73 years), it was observed that the prevalence of frailty increased with age (from 3% to 5% in women and from 2% to 5% in men).15 This supports the hypothesis that therapeutic exposures and post-BMT complications constitute a substantial stressor, placing younger BMT survivors at higher risk for frailty.3
To our knowledge, this is the first study to identify pre-BMT exposure to vincristine as a risk factor for a worsening or persistent frailty state. The prevalence of frailty was 14.5% versus 6.2% (P = .003) in participants who had pre-BMT exposure to vincristine versus those without vincristine exposure. Prior exposure to vincristine was more frequent in patients with acute lymphocytic leukemia and lymphoma. We also evaluated frailty characteristics in patients who were exposed to vincristine versus those who were not exposed and observed a higher prevalence of weakness, low energy expenditure, and exhaustion in those with prior exposure to vincristine. This likely represents the sequelae of vincristine-related neuropathy/muscle weakness leading to low energy expenditure in these patients, presenting yet another opportunity for intervention.
The current study identifies vulnerable populations at higher risk for subsequent frailty, providing evidence for targeted intervention even before the onset of frailty. These interventions may be in the form of physical therapy, exercise, and nutrition, preferably as individually tailored, multicomponent interventions.16
This study needs to be placed within context of its limitations. Our study design required patients to be alive at t1 and t2 and thus could be subject to survival bias. Therefore, the prevalence of worsening may be underestimated. This possibly could be evaluated in a prospective cohort with timed and frequent measurements of frailty status. We did not evaluate predictors of improvement in frailty status because of the smaller numbers in this group (9.7%). This study is limited by the relatively small number of events; therefore, the associations need to be interpreted with caution. Also, although we previously demonstrated7 that there is concordance between outcomes abstracted from medical records and self-reported outcomes, these analyses relied on self-reported measures, which are subject to reporting and recall bias. There are differences between our constructs and the clinical constructs used by Fried et al.13 Details of our construct3 compared with the construct by Fried et al13 are included in our prior report and are included in Table 3. Furthermore, our cohort included patients who underwent transplantation between 1974 and 1998. There have been significant changes in transplantation strategies over the past 2 decades. Thus, although it is important to study and report on survivors who were followed for an extended period, changes in practice necessitate the assessment of patients who undergo transplantation in the contemporary era as well.3
TABLE 3.
Frailty Phenotype
| Indices | BMTSS Questionnaire | Codea | Fried Criteria (Fried 200113) | Codea |
|---|---|---|---|---|
| Low lean muscle mass | BMI calculated from self-reported weight and height (kg/m2) | 1 = <18.5 kg/m2, 0 = ≥18.5 kg/m2 | Unintentional weight loss in prior y | 1 = ≥10 lbs or loss of ≥5% of body weight |
| Exhaustion | BMI 18 kg/m2: “Feeling weak in parts of your body” | 0 = Not at all or a little bit; 1 = moderate, quite a bit, or extremely | CES-D scale: Two possible questions, “How often in the last week did you feel this way:” 1) “I felt that everything I did was an effort” and 2) “I could not get going” | 0 = Rarely or none of the time (<1 d), 1 = some or a little of the time (1–2 d), 2 = moderate amount of the time (3–4 d), or 3 = most of the time (1 = participants answering “2” or “3”) |
| Low energy expenditure | “On how many of the past 7 d did you exercise or do sports at least 20 min that made you sweat or breathe hard (eg, dancing, jogging, basketball etc)” | 1 = <2 d; 0 = ≥2 d | Based on the short version of the Minnesota Leisure Time Activity questionnaire, asking about activities and sports; Kcals per wk expended are calculated using standardized algorithm | Men: 1 = <383 Kcals of physical activity per wk; Women: 1 = <270 Kcals of physical activity per wk |
| Slowness | “Over the last 2 y, how long (if at all) has your health limited you in: 1) walking uphill or climbing a few flights of stairs; 2) walking 1 block | 1 = Either item limited >3 mo; 0 = other | Time to walk 15 feet | Men: 1 = height ≤173 cm, ≥7 s or height >173 cm, ≥6 s; Women: 1 = height ≤159 cm, ≥7 s or height >159 cm, ≥6 s |
| Weakness | Weakness or inability to move arm(s) | 1 = Yes, 0 = no | Grip strength | Cutoff for grip strength (kg): Men: BMI ≤24 kg/m2, ≤29-kg cutoff; BMI 24.1–26 kg/m2, ≤30-kg cutoff; BMI 26.1–28 kg/m2, ≤30-kg cutoff; BMI >28 kg/m2, ≤32-kg cutoff; Women: BMI ≤23 kg/m2, ≤17-kg cutoff; BMI 23.1–26 kg/m2, ≤17.3-kg cutoff; BMI 26.1–29 kg/m2, ≤18-kg cutoff; BMI >29 kg/m2, ≤21-kg cutoff (1 = grip strength ≤ cutoff) |
Abbreviations: BMT, bone marrow transplantation; BMTSS, BMT Survivor Study; BSI, Brief Symptom Inventory; CES-D, Center for Epidemiological Studies-Depression Scale.
Score ≥3 = frail; score 1 or 2 = prefrail.
In a cohort of patients followed longitudinally for a median of 20.6 years after BMT, with measurement of frailty state at 2 timepoints 13.2 years apart, we observed worsening in approximately 20% of the patients. The current results identify high-risk subpopulations that could benefit from targeted interventions.14,17
FUNDING SUPPORT
This study was supported by grants from the National Cancer Institute (R01 CA078938), and the Leukemia and Lymphoma Society (R6502-16 to Smita Bhatia).
CONFLICTS OF INTEREST DISCLOSURES
Mukta Arora reports grants from Pharmacyclics, Kadmon, and Syndax and personal fees from Fate Therapeutics, outside the submitted work. Daniel J. Weisdorf reports grants from Incyte and personal fees from FATE Therapeutics, outside the submitted work. Stephen J. Forman reports grants and other support from Mustang Bio outside the submitted work and is a member of the board of Lixte Biotechnology. The remaining authors made no disclosures.
Footnotes
Presented at the 2019 American Society of Hematology (ASH) Annual Meeting; December 7–10, 2019; Orlando, Florida.
REFERENCES
- 1.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayek S, Gibson TM, Leisenring WM, et al. Prevalence and predictors of frailty in childhood cancer survivors and siblings: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2020;38:232–247. doi: 10.1200/JCO.19.01226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2:1277–1286. doi: 10.1001/jamaoncol.2016.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55:539–549. doi: 10.1159/000211949 [DOI] [PubMed] [Google Scholar]
- 5.Thompson MQ, Theou O, Adams RJ, Tucker GR, Visvanathan R. Frailty state transitions and associated factors in South Australian older adults. Geriatr Gerontol Int. 2018;18:1549–1555. doi: 10.1111/ggi.13522 [DOI] [PubMed] [Google Scholar]
- 6.Armenian SH, Sun CL, Francisco L, et al. Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): a report from the BMT Survivor Study. Bone Marrow Transplant. 2012;47:283–290. doi: 10.1038/bmt.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419 [DOI] [PubMed] [Google Scholar]
- 8.Arrieta H, Rezola-Pardo C, Gil SM, et al. Effects of multicomponent exercise on frailty in long-term nursing homes: a randomized controlled trial. J Am Geriatr Soc. 2019;67:1145–1151. doi: 10.1111/jgs.15824 [DOI] [PubMed] [Google Scholar]
- 9.Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17:426–433. doi: 10.1016/j.jamda.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 10.Ng TP, Feng L, Nyunt MS, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128:1225–1236.e1. doi: 10.1016/j.amjmed.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 11.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. doi: 10.1001/jama.1990.03440220053029 [DOI] [PubMed] [Google Scholar]
- 12.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontology A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- 15.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493,737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34:25–38. doi: 10.1016/j.cger.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]


