Significance
Dinucleoside tetraphosphate alarmones function in bacteria as precursors to 5′-terminal nucleoside tetraphosphate (Np4) caps, becoming incorporated at high levels into RNA during stress and thereby influencing transcript lifetimes. However, little is known about how these noncanonical caps are removed as a prelude to RNA degradation. Here, we report that the RNA pyrophosphohydrolase RppH assumes a leading role in decapping those transcripts under conditions of disulfide stress and that it recognizes Np4-capped 5′ ends by an unexpected mechanism, generating a triphosphorylated RNA intermediate that must undergo further deprotection by RppH to trigger degradation. These findings help to explain the uneven distribution of Np4 caps on bacterial transcripts and have important implications for how gene expression is reprogrammed in response to stress.
Keywords: diadenosine tetraphosphate, Ap4A, Np4A, noncanonical cap, X-ray structure
Abstract
Dinucleoside tetraphosphates, often described as alarmones because their cellular concentration increases in response to stress, have recently been shown to function in bacteria as precursors to nucleoside tetraphosphate (Np4) RNA caps. Removal of this cap is critical for initiating 5′ end-dependent degradation of those RNAs, potentially affecting bacterial adaptability to stress; however, the predominant Np4 decapping enzyme in proteobacteria, ApaH, is inactivated by the very conditions of disulfide stress that enable Np4-capped RNAs to accumulate to high levels. Here, we show that, in Escherichia coli cells experiencing such stress, the RNA pyrophosphohydrolase RppH assumes a leading role in decapping those transcripts, preferring them as substrates over their triphosphorylated and diphosphorylated counterparts. Unexpectedly, this enzyme recognizes Np4-capped 5′ ends by a mechanism distinct from the one it uses to recognize other 5′ termini, resulting in a one-nucleotide shift in substrate specificity. The unique manner in which capped substrates of this kind bind to the active site of RppH positions the δ-phosphate, rather than the β-phosphate, for hydrolytic attack, generating triphosphorylated RNA as the primary product of decapping. Consequently, a second RppH-catalyzed deprotection step is required to produce the monophosphorylated 5′ terminus needed to stimulate rapid RNA decay. The unconventional manner in which RppH recognizes Np4-capped 5′ ends and its differential impact on the rates at which such termini are deprotected as a prelude to RNA degradation could have major consequences for reprogramming gene expression during disulfide stress.
Messenger RNA (mRNA) degradation is an important mechanism for controlling gene expression in all organisms. mRNA lifetimes directly impact protein synthesis by limiting the number of times a transcript can be translated by ribosomes. The half-lives of distinct messages in the same cell can vary widely, from seconds to an hour in bacteria and from minutes to days in vertebrates, yet the mechanisms that govern these differences remain poorly understood.
In Escherichia coli, the essential endoribonuclease RNase E plays a central role in controlling the decay rates of most mRNAs (1). This regulatory enzyme gains access to its cleavage sites in RNA either directly or in a 5′ end-dependent manner. The latter pathway requires prior conversion of the 5′ triphosphate to a monophosphate by a two-step process involving a diphosphorylated intermediate whose β-phosphate is removed by the RNA pyrophosphohydrolase RppH, a member of the Nudix hydrolase superfamily (2–4). The resulting monophosphorylated 5′ end accelerates endonucleolytic cleavage by binding selectively to a pocket on the surface of RNase E and facilitating access by this enzyme to internal cleavage sites (5, 6). Other Nudix hydrolases, many only distantly related to E. coli RppH, appear to play an analogous role in deprotecting mRNA 5′ ends in disparate prokaryotic and eukaryotic organisms (7–12).
Hundreds of E. coli mRNAs are degraded by this 5′ end-dependent mechanism (3, 13), their susceptibility being determined in large measure by the 5′-terminal substrate preferences of RppH and RNase E. Both require at least two unpaired nucleotides at the RNA 5′ end and prefer three or more (14, 15). In addition, RppH, which can convert both triphosphorylated and diphosphorylated 5′ ends to monophosphates, favors substrates whose second nucleotide is a purine rather than a pyrimidine (4, 14). This nucleotide binds in a cleft near the active site and helps to position the β-phosphate for hydrolytic attack in the catalytic center (16). By contrast, the 5′-terminal sequence dependence of RNase E is negligible (15). Both RppH and RNase E assemble with other E. coli proteins to form distinct multimeric complexes but remain highly active in the absence of their respective protein partners (DapF for RppH and polynucleotide phosphorylase, RhlB, and enolase for RNase E) (1, 17–19).
Recently, we have reported high levels of a novel 5′-terminal RNA modification, a nucleoside tetraphosphate (Np4) cap (Fig. 1A), in E. coli cells experiencing stress (20). Acquired by incorporation of a dinucleoside 5′, 5′′′-P1, P4-tetraphosphate (Ap4A, Gp4A, Cp4A, or Up4A) during transcription initiation by RNA polymerase (21), these caps directly affect 5′ end-dependent RNA degradation. Dinucleoside tetraphosphates (Np4As) were first described over 50 y ago as byproducts of transfer RNA aminoacylation and are present in all realms of life (22–24), yet their biological function as cap precursors was only recently recognized. Increases in their abundance are correlated with many interesting bacterial phenotypes including several related to pathogenesis (25–28). Np4As are often characterized as alarmones because their cellular concentration rises dramatically in response to certain stresses, especially disulfide stress induced by cysteine cross-linkers like cadmium and diamide (29, 30), which arrest growth. The same stress conditions greatly increase the cellular concentration of Np4-capped RNAs, raising their abundance to such an extent that they come to represent 4 to 76% of most primary transcripts (20). As a result of molecular symmetry (Np4A = Ap4N) and the mechanism of cap acquisition, both the cap nucleotide and the first transcribed nucleotide of Np4-capped RNAs can be A, G, C, or U.
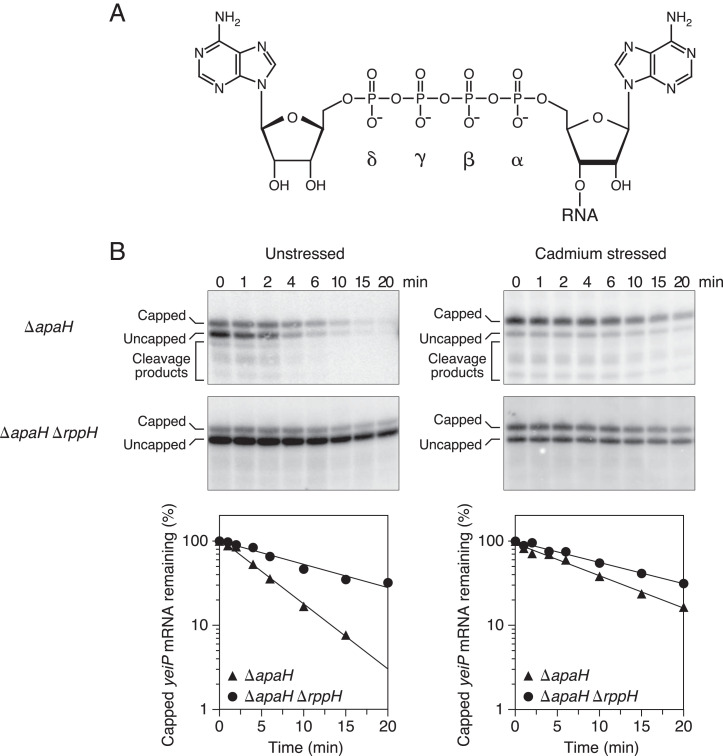
Fig. 1.
Effect of disulfide stress on RppH activity in E. coli. (A) Structure of the 5′ end of Ap4-capped yeiP mRNA. Only the cap and the first transcribed nucleotide of this A-initiated RNA are shown. The α-, β-, γ-, and δ-phosphates of the cap are labeled. (B) Rate of loss of capped yeiP mRNA after arresting transcription in E. coli mutants lacking ApaH and/or RppH. (Top) Northern blots. Cultures of the indicated strains (ΔapaH or ΔapaH ΔrppH) growing in MOPS-glucose medium either were treated with cadmium chloride (cadmium stressed; Right) or were not (unstressed; Left), and equal amounts of total RNA extracted at time intervals after inhibiting transcription were cleaved site-specifically with a deoxyribozyme targeting yeiP (SI Appendix, Table S3) and analyzed by boronate gel electrophoresis and blotting with a radiolabeled yeiP probe. (Bottom) Graphs. The amount of capped yeiP mRNA that remained was plotted semilogarithmically as a function of time, and best-fit lines were calculated by linear regression. First-order rate constants for the disappearance of capped yeiP mRNA were determined from the slopes of these lines. Representative experiments are shown.
Cap removal is a critical first step in the degradation of Np4-capped transcripts via the 5′ end-dependent pathway. Both in vitro and in E. coli, Np4-capped RNA can be deprotected by either of two enzymes, ApaH or RppH (20, 31), which also function as Np4A hydrolases (32–34). Of the two, ApaH appears to be the principal Np4-decapping enzyme in unstressed E. coli cells. Its inactivation by cysteine cross-linkers enables Np4-capped transcripts to accumulate to a high cellular concentration during disulfide stress, a phenomenon recapitulated in unstressed apaH deletion mutants, which lack this enzyme altogether (20). By contrast, although RppH contributes to deprotecting Np4-capped RNA in E. coli under ordinary growth conditions, deleting the rppH gene is not sufficient to cause Np4-capped RNAs to rise to measurable levels. Despite the importance of ApaH and RppH for governing the lifetimes of Np4-capped transcripts, nothing is known about their substrate specificity as decapping enzymes, which could have a major impact on gene expression during stress.
Because ApaH is inactivated in disulfide-stressed cells (20), we have now examined the importance of RppH for deprotecting Np4-capped transcripts under those conditions and found that it remains active. Biochemical and crystallographic studies have revealed unexpected differences in the recognition of capped versus triphosphorylated RNAs by this enzyme, resulting in distinct substrate preferences and reaction products. These studies suggest an explanation for the strikingly uneven distribution of Np4 caps on cellular transcripts and provide key insights into the mechanism by which Np4-capped transcripts are degraded when such caps are abundant.
Results
Effect of Disulfide Stress on RppH Activity in E. coli.
Two E. coli pyrophosphohydrolases, ApaH and RppH, have each been shown to remove Np4 caps from RNA 5′ ends, both in vitro and in vivo (20, 31). In E. coli, ApaH appears to be the predominant source of Np4 decapping activity under ordinary growth conditions, where it is so efficient that Np4-capped RNAs are virtually undetectable. Np4-capped transcripts are able to accumulate to significant levels under conditions that inactivate ApaH, such as disulfide stress, or in mutant strains (ΔapaH) that lack this enzyme altogether (20). To determine whether RppH remains active during disulfide stress, we induced this condition in isogenic E. coli mutants lacking either ApaH alone or both ApaH and RppH, blocked further RNA synthesis, and measured the rate at which Np4-capped transcripts were lost. In principle, any difference between the rates in these two strains can be attributed to the action of RppH.
As a model RNA, we chose the E. coli yeiP transcript, which encodes a paralog of the translation elongation factor EF-P and is highly capped in wild-type E. coli cells experiencing disulfide stress and in unstressed ΔapaH cells (20). We first determined the rate of yeiP decapping by RppH in the absence of stress. Log-phase cultures of ΔapaH and ΔapaH ΔrppH cells were treated with rifampicin to arrest transcription, and total RNA was harvested at time intervals thereafter. Capped and uncapped cellular transcripts were then separated by electrophoresis through a polyacrylamide matrix modified with boronate side chains that selectively retard the migration of capped RNAs by transiently forming a covalent adduct with the vicinal diol of the cap nucleoside (35). Distinct bands representing capped and uncapped yeiP mRNA were subsequently detected by Northern blotting (Fig. 1 B, Left). By subtracting the rate of disappearance of the capped yeiP transcript in the ΔapaH ΔrppH strain from the rate in the isogenic ΔapaH strain, we calculate that the first-order rate constant for yeiP decapping by RppH is 0.115 ± 0.008 min−1 in unstressed E. coli cells (SI Appendix, Table S1).
We then repeated these measurements in the same E. coli strains after treatment with cadmium chloride to induce disulfide stress (Fig. 1 B, Right). Under these conditions, the rate constant for yeiP decapping by RppH was calculated to be 0.031 ± 0.004 min−1 (SI Appendix, Table S1). Thus, RppH is 27 ± 4% as active during cadmium stress as it is in unstressed cells. We conclude that RppH retains the ability to deprotect Np4-capped transcripts in E. coli under disulfide stress conditions that completely inactivate ApaH. The presence of yeiP decay intermediates resulting from 5′ monophosphate-dependent cleavage by RNase E (36) in ΔapaH but not ΔapaH ΔrppH cells (Fig. 1B) shows that RNase E also remains active under these stress conditions.
Requirements for Unpaired Nucleotides at the 5′ End.
Because RppH not only contributes to the deprotection of Np4-capped RNA under normal growth conditions but also appears to be the primary decapping enzyme during disulfide stress, knowing the substrate preferences of this enzyme is crucial for understanding the diverse rates at which capped transcripts are degraded in E. coli. To examine those preferences, we employed a set of structurally unambiguous RNA substrates similar to those that had previously been used to investigate the specificity of RppH when removing pyrophosphate from triphosphorylated RNA (11, 14, 37). Synthesized by in vitro transcription in the presence of a specific Np4A (synonymous with Ap4N due to molecular symmetry), each substrate comprised an Np4-capped 5′-terminal RNA segment that was not base paired followed by two 3′-terminal stem-loops, one of which bore a fluorescein tag in its apical loop (Fig. 2A and SI Appendix, Fig. S1A). Another Np4-capped RNA containing a third stem-loop (Ap4A8XL; SI Appendix, Fig. S1B) was included in every reaction as an internal standard. To compare rates of decapping, each RNA substrate was combined with Ap4A8XL and RppH, and reaction samples were quenched with ethylenediaminetetraacetate (EDTA) at various times thereafter. The substrates and reaction products were then separated by boronate gel electrophoresis and visualized by fluorescence.
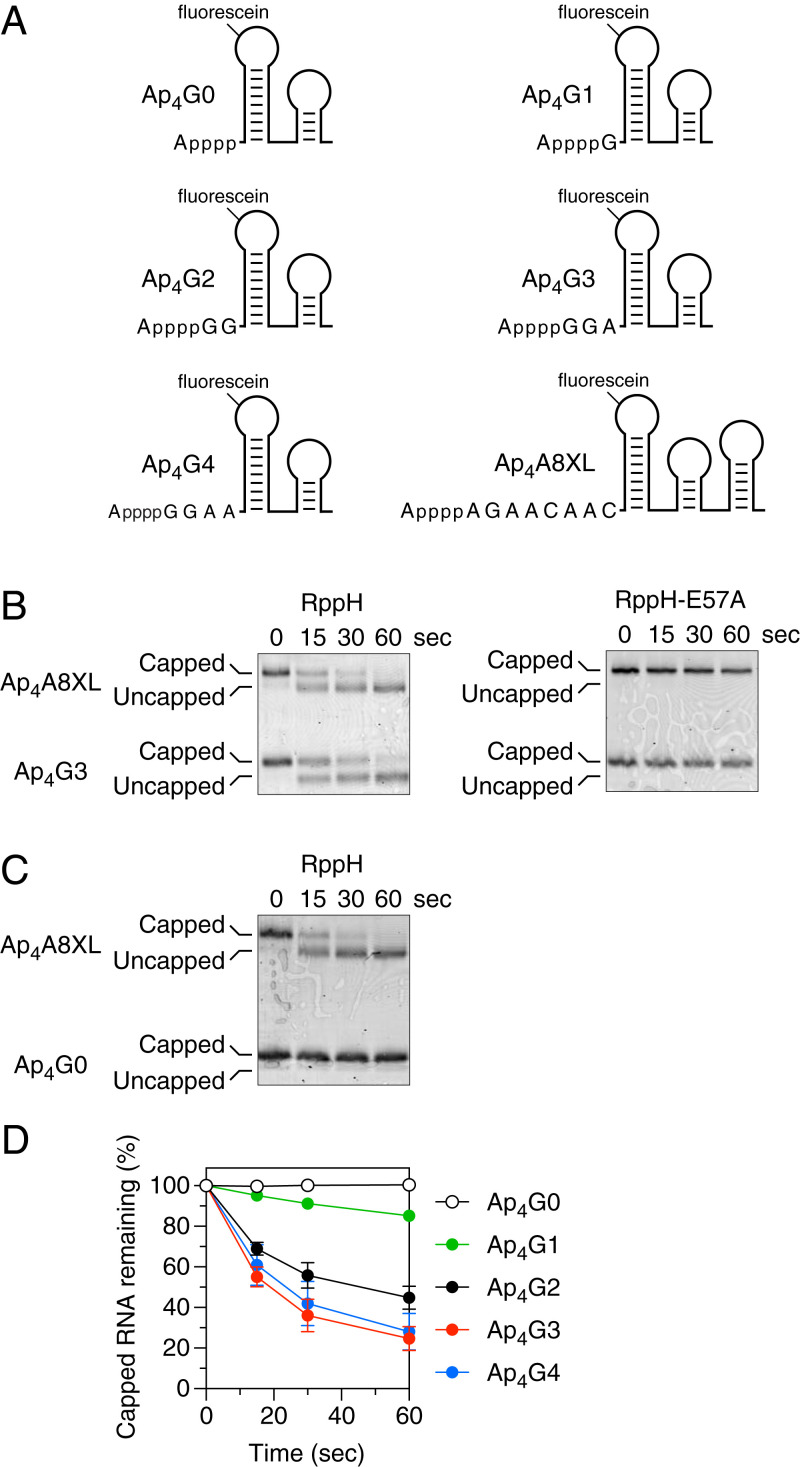
Fig. 2.
Effect of the number of unpaired 5′-terminal nucleotides on decapping by RppH in vitro. (A) 5′-terminal sequence and expected secondary structure of Ap4G0, Ap4G1, Ap4G2, Ap4G3, Ap4G4, and Ap4A8XL. Each bore a 5′-terminal adenosine tetraphosphate cap and a fluorescein label at the top of the first stem-loop. In each RNA name, the first uppercase letter indicates the identity of the cap nucleotide, the second uppercase letter indicates the identity of the first transcribed nucleotide, and the second numeral indicates the number of unpaired transcribed nucleotides at the 5′ end. Truncated derivatives of Ap4G4 (Ap4G3, Ap4G2, Ap4G1, and Ap4G0) lacked one to four nucleotides from the 3′ boundary of the unpaired 5′-terminal RNA segment. (B) Decapping of Ap4G3. Ap4G3 was mixed with Ap4A8XL and treated with either active RppH (30 nM; Left) or catalytically inactive RppH-E57A (30 nM; Right), and the decapping of each RNA was monitored as a function of time by boronate gel electrophoresis and fluorescence. (C) Decapping of Ap4G0. Ap4G0 was mixed with Ap4A8XL, and their decapping by active RppH (30 nM) was monitored as a function of time. (D) Rate of Ap4G4, Ap4G3, Ap4G2, Ap4G1, and Ap4G0 decapping. Cap removal by active RppH was monitored as in A and B, and the percentage of capped RNA remaining was calculated at each time point from the ratio of capped substrate to decapped product. Each time point is the average of three or more independent measurements. Error bars correspond to standard deviations. Time courses for decapping of the internal standard Ap4A8XL by RppH are graphed in SI Appendix, Fig. S2A.
The first capped substrate tested was Ap4G3, which contained three unpaired 5′-terminal nucleotides, the first of which was G (Fig. 2A). RppH was able to efficiently decap both Ap4G3 and Ap4A8XL, whereas catalytically inactive RppH-E57A was unable to remove the cap from either substrate, as expected (Fig. 2B). Because a 5′-terminal stem-loop is known to prevent RppH-catalyzed pyrophosphate removal from triphosphorylated RNA (3, 14), we next examined whether such a structure also inhibits Np4 cap removal by this enzyme. A substrate with no unpaired nucleotides at the 5′ end, Ap4G0, was completely resistant to RppH, while Ap4A8XL in the same reaction mixture was readily decapped (Fig. 2C). To determine the number of unpaired 5′-terminal nucleotides required for decapping by RppH, we tested three more Ap4-capped substrates containing one, two, or four unpaired nucleotides there (Ap4G1, Ap4G2, and Ap4G4, respectively). RppH reacted slowly with Ap4G1, substantially faster with Ap4G2, and even more rapidly with Ap4G4, whose reactivity resembled that of Ap4G3 (Fig. 2D). The internal standard Ap4A8XL was efficiently decapped in every reaction (SI Appendix, Fig. S2A). We conclude that RppH requires at least one unpaired nucleotide at the 5′ end for Np4 cap removal and prefers two or more. These requirements differ from those observed for pyrophosphate removal from triphosphorylated RNA, where RppH was found to require at least two unpaired 5′-terminal nucleotides and to prefer three or more (14).
Effect of Nucleotide Identity on Decapping.
The requirement for at least one unpaired nucleotide at the 5′ end of capped substrates raised the possibility that RppH might be sensitive to the identity of that nucleotide. We therefore compared the reactivity of four Ap4-capped substrates (Ap4A4, Ap4G4, Ap4C4, and Ap4U4; SI Appendix, Fig. S1C) that differed in sequence only at the first of four unpaired 5′-terminal nucleotides (i.e., at the first transcribed nucleotide). RppH showed a marked preference for decapping substrates that bore a purine rather than a pyrimidine there (Fig. 3A and SI Appendix, Fig. S2B). Little discrimination was observed between A and G or between U and C at that position. The preference for a purine at the 5′ terminus of Np4-capped substrates is reminiscent of the nucleobase preference of this enzyme at the second nucleotide of triphosphorylated substrates (14).
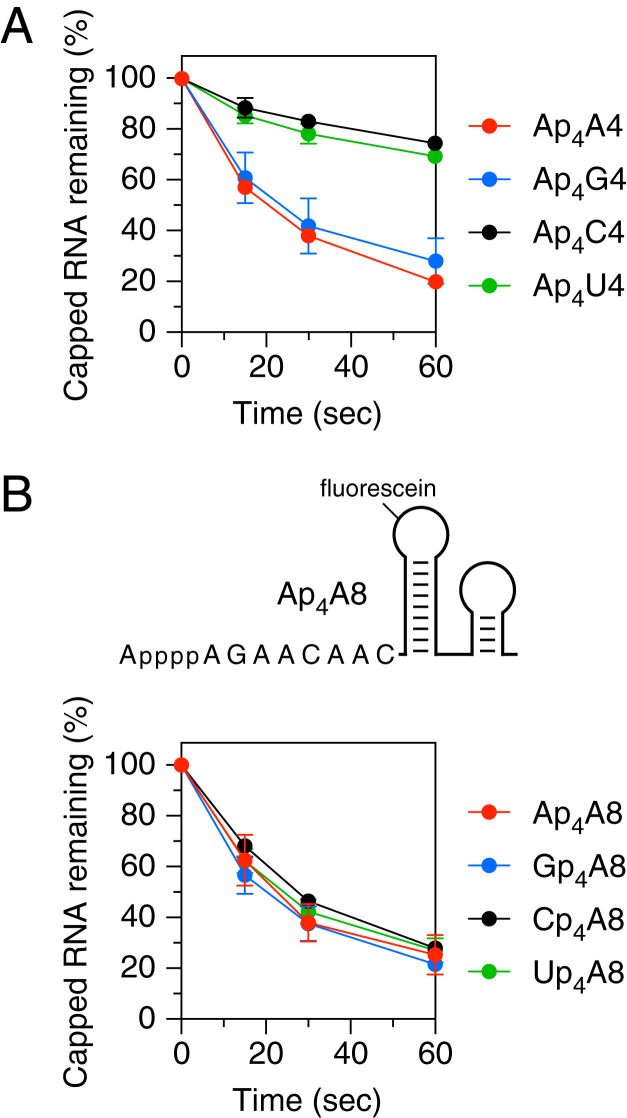
Fig. 3.
Effect of nucleotide identity on decapping by RppH in vitro. (A) Reactivity of substrates differing in the first transcribed nucleotide. The decapping of Ap4A4, Ap4G4, Ap4C4, and Ap4U4 by RppH was monitored as in Fig. 2. (B) Reactivity of substrates differing in the cap nucleotide. (Top) 5′-terminal sequence and expected secondary structure of Ap4A8. (Bottom) Decapping of Ap4A8, Gp4A8, Cp4A8, and Up4A8 by RppH. The 5′-terminal sequences of these substrates are shown in SI Appendix, Fig. S1. Corresponding time courses for decapping of the internal standard Ap4A8XL by RppH are graphed in SI Appendix, Fig. S2 B and C. Each time point is the average of three or more independent measurements. Error bars correspond to SDs.
Because Ap4, Gp4, Cp4, and likely Up4 caps have been observed in E. coli (20), we also investigated whether RppH discriminates between different types of caps (Ap4A8, Gp4A8, Cp4A8, and Up4A8; SI Appendix, Fig. S1D). No significant disparities were observed in its reactivity with substrates that differed only in the identity of the cap nucleobase (Fig. 3B and SI Appendix, Fig. S2C).
Influence of the 5′-Terminal Transcribed Nucleotide in E. coli.
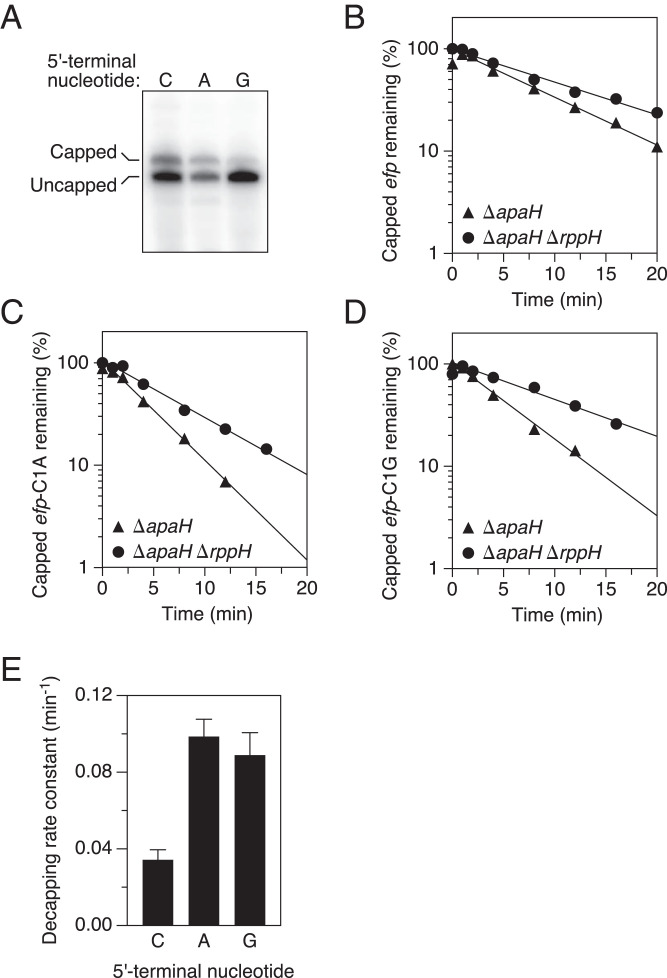
To determine whether the 5′-terminal nucleotide preference of RppH observed in vitro is also evident in vivo, we examined the influence of the first nucleotide of efp mRNA on its rate of decapping by RppH in E. coli. This transcript, which encodes the translation elongation factor EF-P, naturally begins with C at the 5′ end, as does its capped counterpart in ΔapaH cells (20). Plasmids encoding wild-type efp mRNA or either of two efp point mutants in which the 5′-terminal pyrimidine had been replaced with a purine (efp-C1A or efp-C1G) were introduced into an isogenic pair of ΔapaH and ΔapaH ΔrppH strains lacking the chromosomal copy of the efp gene. Log-phase cultures growing without stress were then treated with rifampicin, and the rate constant for RppH-mediated decapping of efp mRNA was calculated from the difference in the rate of disappearance of the Np4-capped transcript in the two strains, as determined by boronate gel electrophoresis and Northern blotting.
The three efp transcripts were Np4-capped to varying degrees in ΔapaH cells (Fig. 4A), and their rates of deprotection by RppH also differed substantially. Both of the 5′-terminal purine substitution mutants were decapped much faster than the wild-type, C-initiated transcript (Fig. 4 B–D). The rate constant for decapping by RppH in E. coli was 0.099 ± 0.009 min−1 for efp-C1A and 0.089 ± 0.012 min−1 for efp-C1G versus only 0.034 ± 0.005 min−1 for wild-type efp, a nearly threefold acceleration (Fig. 4E and SI Appendix, Table S1). The influence of the first transcribed nucleotide on the rate of efp decapping by RppH in vivo, where the enzyme forms a heteromeric complex with the diaminopimelate epimerase DapF (17–19), was in full agreement with the 5′-terminal sequence preference observed for decapping by monomeric RppH in vitro, evidence that DapF does not alter the substrate specificity of decapping by RppH.
Fig. 4.
Effect of the identity of the first transcribed nucleotide on decapping by RppH in E. coli. (A) Presence of capped efp, efp-C1A, and efp-C1G mRNA in unstressed cells lacking ApaH. Total RNA extracted from unstressed E. coli cells lacking the chromosomal apaH and efp genes and containing a plasmid that encoded efp, efp-C1A, or efp-C1G mRNA was cleaved site-specifically with a deoxyribozyme targeting efp (SI Appendix, Table S3), and capped plasmid-encoded transcripts were detected by boronate gel electrophoresis and blotting. (B–D) Rate of loss of capped efp, efp-C1A, and efp-C1G mRNA, respectively, after arresting transcription in E. coli mutants lacking ApaH and/or RppH as well as the chromosomal efp gene. Cultures of the indicated strains growing without stress in MOPS-glucose medium were treated with rifampicin, and equal amounts of total RNA extracted at time intervals thereafter were analyzed as in A. The amount of capped efp, efp-C1A, or efp-C1G mRNA that remained was plotted semilogarithmically as a function of time, and best-fit lines were calculated by linear regression. Representative experiments are shown. (E) Rate constants for decapping of efp, efp-C1A, and efp-C1G mRNA by RppH in E. coli. First-order rate constants for the disappearance of capped efp, efp-C1A, and efp-C1G mRNA were determined from the slopes of the best-fit lines in B–D, and the rate constant for RppH-mediated decapping of each mRNA was calculated by subtracting the rate constant for the disappearance of the capped transcript in ΔapaH ΔrppH cells from the corresponding rate constant in ΔapaH cells.
Structure of RppH Bound to Ap4A.
By analogy to the regiospecificity of Ap4A hydrolysis by RppH, which cuts dinucleoside tetraphosphates asymmetrically (34), RppH would be predicted to release the cap of Np4-capped RNAs as either a nucleoside triphosphate or a nucleoside monophosphate. A nucleoside triphosphate (NTP) product would be expected if Np4-capped RNA binds to RppH in the same manner as triphosphorylated RNA, which is cleaved between the α- and β-phosphates to release pyrophosphate (3). However, the substrate preferences of RppH as a decapping enzyme differ from those observed for its reaction with triphosphorylated and diphosphorylated RNAs (4, 14); in particular, cap removal requires one fewer unpaired nucleotide at the 5′ end and prefers a purine as the first transcribed nucleotide rather than as the second transcribed nucleotide. These findings suggest that the positioning of capped RNA bound to RppH may be shifted by one nucleotide relative to the positioning of triphosphorylated and diphosphorylated RNA substrates.
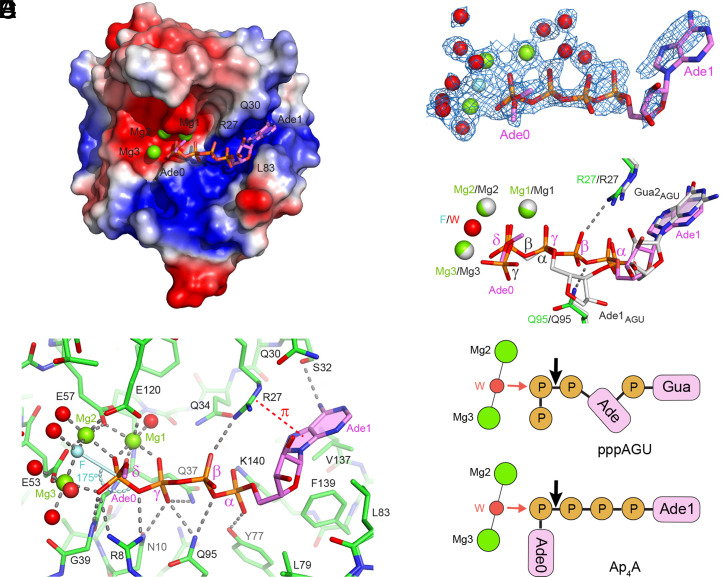
To visualize the interaction of RppH with the 5′ end of Np4-capped RNA, we determined the X-ray crystal structure of this enzyme bound to Ap4A at 1.6 Å resolution (SI Appendix, Table S2). The structure was determined in the precleavage state with a catalytically competent enzyme, whose activity was inhibited by adding fluoride ions that replace the reactive water molecule in the catalytic center. In the structure, the tetraphosphate moiety of Ap4A splays out on a positively charged surface of the protein (Fig. 5A), extending from the catalytic center where three Mg2+ ions are present to a semiopen cleft where one of the adenines (Ade1) is sandwiched between R27 and Q30 above and L83, V137, and F139 below (Fig. 5B). The side chain of R27 forms a cation-π interaction with the nucleobase of Ade1 but does not hold it tightly in the cleft, as evidenced by its partial electron density map (Fig. 5C). The other adenosine (Ade0) is disordered in the structure. The tetraphosphate bridge connecting the two nucleosides accepts 10 hydrogen bonds from seven amino-acid residues that line the binding path, including three each formed by the γ- and δ-phosphates and two each formed by the α- and β-phosphates (Fig. 5B). In addition, the δ-phosphate coordinates all three Mg2+ ions, and the γ-phosphate contacts Mg1 (SI Appendix, Fig. S3). Together, these interactions position the substrate optimally for catalysis.
Fig. 5.
Structure of RppH bound to Ap4A. (A) Overall structure of the complex, with RppH in electrostatic surface representation and Ap4A in violet sticks. Mg2+ ions are shown as green spheres. Note the alternative conformations of Ade0 as represented by the location of its C5′ carbon atom. (B) Details of Ap4A binding. The four phosphates of Ap4A are labeled with Greek letters. Green spheres, Mg2+. Cyan sphere (F), fluoride. Red spheres, water molecules that coordinate Mg2+or fluoride. Cyan stick, direction of in-line attack on the δ-phosphate. Curved cyan dashed line, angle of in-line attack by fluoride on the δ-phosphorus atom to displace the bridging oxygen atom. Gray dashed lines, hydrogen bonds and coordination bonds. Red dashed line, cation-π interaction. Only one of the possible locations of the Ade0 C5′ carbon atom is depicted. Note that the spheres are not intended to represent the actual sizes of ions and water molecules. (C) Refined 2Fo-Fc electron density map (1 σ level, blue mesh) shown with the refined model of Ap4A and bound Mg2+ ions. (D) View of the complexes formed by RppH with Ap4A (green and violet) and the triphosphorylated RNA oligonucleotide pppAGU (gray) after all-atom superposition of the corresponding structures [PDB identifiers: 7SP3 (current work) and 4S2Y (16)]. The substrates, magnesium ions, and respective nucleophiles (F, fluoride; W, water) are shown along with the RppH residues (R27 and Q95) that form hydrogen bonds (dashed lines) with the β-phosphate of Ap4A. (E) Schematic representation of substrates bound to RppH, vertically aligned according to the structural superposition in D. (Top) pppAGU; (Bottom) Ap4A. Mg2+ ions (green circles) coordinate (thin black lines) a nucleophilic water molecule (W, red circle). Phosphates (P) are depicted as brown circles and nucleosides as magenta rectangles. Red and black arrows represent in-line nucleophilic attack and the site of phosphoanhydride cleavage, respectively. In the bottom schematic, the fluoride ion used to trap the crystallized enzyme-substrate complex in a catalytically active conformation is replaced by a water molecule normally present there.
The structure readily explains the catalytic mechanism for asymmetrical Ap4A cleavage. The electron density peak positioned between Mg2 and Mg3 and assigned to a fluoride ion corresponds to a water molecule in the noninhibited structure (16). This nucleophile is located 2.9 Å from the phosphorus atom of the δ-phosphate at an angle (175°) that is ideal for in-line attack to sever the δ-phosphate from the γ-phosphate (Fig. 5B). Cleavage there by water would yield AMP and ATP as products, as previously reported (34).
Comparison of this structure to that of RppH bound to the 5′-triphosphorylated RNA oligonucleotide pppAGU (16) revealed striking similarities, including the absence of any appreciable change in protein conformation, as well as important differences. Specifically, Ade1 of Ap4A replaces the second nucleoside (Gua2AGU) of pppAGU in the cleft, the α-phosphate of Ap4A replaces the phosphodiester linkage between Gua2AGU and the preceding nucleoside, and the γ- and δ-phosphates of Ap4A replace the α- and β-phosphates of the RNA ligand (Fig. 5 D and E). As a result, the 5′-terminal nucleoside of pppAGU (Ade1AGU) is replaced nonisosterically by the β-phosphate of Ap4A. This phosphate forms two new hydrogen bonds with RppH residues R27 and Q95 that could not be formed by the ribose of Ade1AGU. Thus, the four phosphates of Ap4A maximize the number of interactions with RppH and, together with Ade1, effectively replace the two 5′-terminal nucleotides of the RNA ligand, thereby positioning the δ-phosphate of Ap4A in the catalytic center, where the β-phosphate of pppAGU would have been.
Products of Decapping by RppH.
Because Ap4A is symmetrical, its complex with RppH does not alone reveal the bound orientation of Np4-capped RNA. However, the preference of RppH for Np4-capped substrates that begin with a purine suggests that the first transcribed nucleotide of capped RNA replaces Ade1 of Ap4A in the cleft, where R27 could engage in a stronger cation-π interaction with a purine than with a pyrimidine (Fig. 5B). Likewise, the indifference of RppH to the identity of the cap nucleoside suggests that the cap replaces Ade0, whose disordered structure in the complex of Ap4A with RppH implies a lack of stable base-specific interactions with the protein. Binding Np4-capped RNA in this manner would position its δ-phosphate for hydrolytic attack in the catalytic center. If so, the deprotection of such substrates by RppH should release the cap nucleotide as a nucleoside monophosphate (NMP), not an NTP, so as to generate a triphosphorylated RNA product that could then react further with RppH to produce a monophosphorylated RNA susceptible to rapid degradation by RNase E.
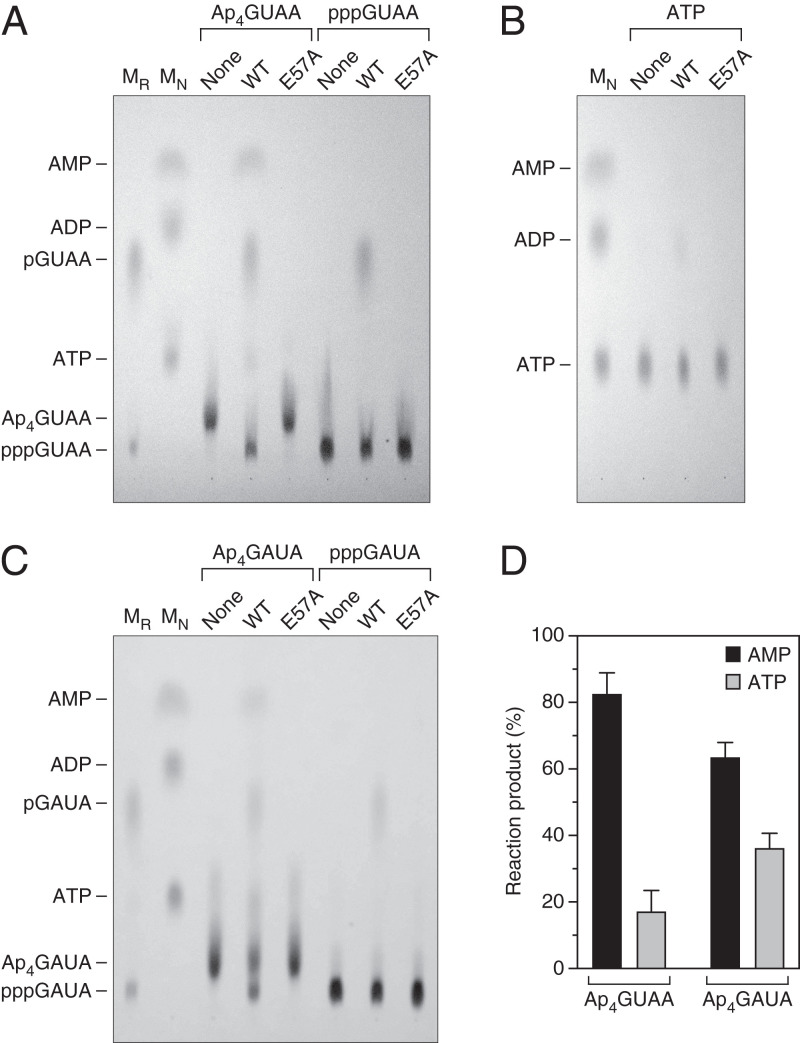
To test this hypothesis, we examined the RppH reaction products of the Np4-capped RNA tetramer Ap4GUAA. We chose a substrate of this length to ensure that the substrate and each of the possible reaction products would have distinct chromatographic mobilities on phosphoethyleneimine (PEI)-cellulose. As determined by thin-layer chromatography and ultraviolet (UV) shadowing, RppH released the cap of Ap4GUAA primarily as AMP while generating an initial RNA product that comigrated with triphosphorylated GUAA and that was subsequently converted by RppH to monophosphorylated GUAA (Fig. 6A). Little (<20%) of the cap was released as ATP. To rule out the possibility that much of the cap released as ATP was quickly converted to AMP, we tested the reactivity of ATP with RppH under the same conditions and found it to be largely inert (Fig. 6B), consistent with prior observations (38). Moreover, what little ATP did react was converted primarily to ADP rather than AMP. As expected, Ap4GUAA did not react with catalytically inactive RppH-E57A.
Fig. 6.
Products of RppH-mediated decapping in vitro. (A) Ap4GUAA. Ap4GUAA and pppGUAA were examined by thin-layer chromatography on fluorescent PEI-cellulose plates before (None) and after treatment with wild-type RppH (WT) or catalytically inactive RppH (E57A). An equimolar mixture (MN) of ATP, ADP, and AMP and a separate mixture (MR) of pGUAA and pppGUAA were included as markers. Substrates and products were detected by UV shadowing. (B) ATP. The slow reaction of ATP with RppH was examined by thin-layer chromatography as in A. (C) Ap4GAUA. The products of the reaction of Ap4GAUA and pppGAUA with RppH were examined by thin-layer chromatography as in A, except that pGAUA and pppGAUA were substituted as RNA markers (MR). (D) Products of decapping. The percentage of the Ap4GUAA and Ap4GAUA cap released by RppH as AMP or ATP was calculated by quantifying the AMP and ATP spots and determining their ratio. Each value is the average of two to three measurements. Error bars correspond to standard deviations. P = 0.0002 for Ap4GUAA and 0.02 for Ap4GAUA.
To ensure that the positioning of Ap4GUAA on the surface of RppH was not biased by its nucleotide sequence, which might favor binding of the 5′-terminal purine in the enzyme cleft, we tested another capped substrate, Ap4GAUA, in which the first two transcribed nucleotides were both purines. Despite the presence of a purine at both of these positions, this substrate also yielded AMP as the principal mononucleotide product (Fig. 6 C and D). Together, these findings suggest that RppH deprotects all Np4-capped transcripts by releasing the cap primarily as a nucleoside monophosphate, thereby generating triphosphorylated RNA as the initial reaction product regardless of the 5′-terminal RNA sequence.
Finally, the reactivity of Ap4GUAA and Ap4GAUA with RppH was compared to that of their triphosphorylated and diphosphorylated counterparts by monitoring the reaction of each as a function of time. The Ap4-capped substrates were the most reactive of all, irrespective of the RNA sequence (SI Appendix, Fig. S4). Among the less reactive substrates, the diphosphorylated RNAs reacted faster than the triphosphorylated RNAs, a finding consistent with our previous observations (4, 18).
Discussion
It was long believed that only eukaryotic RNAs are capped at the 5′ end. However, the recent discovery of 5′ caps on bacterial transcripts and their impact on RNA degradation has transformed thinking about the 5′-terminal regulatory events that govern RNA function in bacteria (10, 20, 39). The most abundant types of bacterial caps yet reported are the Np4 caps observed in E. coli during disulfide stress, when the Np4A hydrolase and decapping enzyme ApaH becomes inactivated (20). Our findings now show that, under these conditions, the RNA pyrophosphohydrolase RppH assumes a leading role in RNA decapping, triggering the degradation of myriad Np4-capped transcripts in that organism. By a combination of enzyme kinetics and X-ray crystallography, we have conducted a systematic investigation of capped substrate recognition by this enzyme in vitro and in vivo and discovered that it binds Np4-capped 5′ ends in an unexpected manner to generate RNA products whose 5′ phosphorylation state (primarily triphosphorylated) is unlike that of any product yet described for any decapping enzyme. Np4-capped transcripts are the most reactive RNA substrates of RppH yet identified, exceeding the reactivity of their triphosphorylated (31) and even their diphosphorylated counterparts. These insights are key to understanding how bacterial mRNAs are degraded and their lifetimes are regulated during stress.
Previously determined structures of RppH bound to triphosphorylated and diphosphorylated RNA have revealed that the enzyme binds the second nucleotide of these uncapped substrates in a cleft, where that nucleobase is held in place by cation-π and hydrophobic interactions on either side that favor a purine there (14, 16). By doing so, RppH positions the β-phosphate for hydrolytic attack promoted by magnesium ions in the catalytic center, generating monophosphorylated RNA and either pyrophosphate or orthophosphate, respectively, as reaction products. By analogy, one might have expected an Np4-capped substrate to be bound in a similar manner. However, the distinct substrate preferences of RppH when catalyzing the deprotection of Np4-capped rather than uncapped substrates suggest otherwise. In particular, instead of requiring at least two unpaired 5′-terminal nucleotides, the second of which should ideally be a purine (14), RppH requires only one unpaired nucleotide at the 5′ end of Np4-capped substrates and prefers for that 5′-terminal nucleotide to be a purine. This one-nucleotide shift in specificity suggests that RppH binds the first transcribed nucleotide rather than the second transcribed nucleotide of Np4-capped RNA in the cleft to form a complex that resembles RppH bound to Ap4A. As a result, the δ-phosphate, instead of the β-phosphate, is positioned for hydrolytic attack. Corroborating this conclusion, the reaction of Ap4-capped RNAs with RppH releases the cap primarily as AMP rather than ATP, even when the capped substrate is designed to increase the potential for the second nucleotide to bind in the cleft. A third possible mode of interaction, in which the cap nucleotide binds in the cleft, is ruled out by the indifference of the enzyme to the identity of that nucleotide and by the release of the cap principally as AMP.
What explains the distinct binding modes of Np4-capped and uncapped substrates? For one thing, triphosphorylated and diphosphorylated RNAs would be unreactive if the 5′-terminal nucleotide, rather than the second nucleotide, were to bind in the cleft, as their phosphate chains would then be too short to reach the nucleophilic water molecule in the catalytic center. By contrast, although Np4-capped RNAs could, in principle, bind productively in any of three ways, their decapping by RppH appears to rely primarily on only one of these binding modes, in which the first transcribed nucleotide docks in the cleft. The structure of RppH bound to Ap4A and the influence of the number of unpaired 5′-terminal nucleotides on reactivity suggest likely explanations for this preference. When bound to RppH, an adenylate nucleotide and the γ- and δ-phosphates of Ap4A (a structural analog of Np4-capped RNA) isosterically replace the second nucleotide and the α- and β-phosphates of triphosphorylated RNA and make similar contacts with the enzyme and magnesium ions (Fig. 5D). The key difference between these enzyme-substrate complexes is the β-phosphate of Ap4A, which replaces the first nucleoside of triphosphorylated RNA and is uniquely able to accept hydrogen bonds from the positively charged side chain of R27 to a negatively charged nonbridging oxygen and from the side chain of Q95 to a bridging oxygen. These energetically favorable interactions would be lost if the second transcribed nucleotide of Np4-capped RNA were to bind in the cleft. These interactions likely also explain the greater reactivity of Np4-capped RNA as an RppH substrate compared to both triphosphorylated (31) and diphosphorylated RNA. The failure of Np4-capped substrates to bind RppH in the opposite orientation with the cap in the cleft may be related to the substantially greater reactivity of substrates bearing additional unpaired nucleotides downstream of the nucleotide in the cleft, which implies favorable interactions of those nucleotides with the enzyme that would be sacrificed if the cap were to occupy the cleft.
The mechanism by which E. coli RppH recognizes Np4 caps is likely to be replicated in nearly all α-, β-, γ-, and ε-proteobacteria, including many important pathogens, as the active-site residues of RppH that interact with such caps and with Mg2+ are highly conserved in those species (14). It remains to be determined whether nonhomologous RNA pyrophosphohydrolases from δ-proteobacteria like Bdellovibrio bacteriovorus (8) and Firmicutes like Bacillus subtilis (9) can recognize Np4 caps.
Besides its crucial role in Np4 cap removal during stress, E. coli RppH has been shown to react in vitro with certain additional types of RNA caps and cap analogs but not with others. Our insights into the various modes of substrate binding by this enzyme now make it possible to explain those differences in reactivity and to predict the effect of RNA sequence and structure on the rate at which those caps are removed. For example, diadenosine polyphosphates bearing three to six bridging phosphates have been tested as substrates for RppH, and all but Ap3A are reactive (34). In the case of Ap4A, Ap5A, and Ap6A, ATP is always one of the reaction products, a finding consistent with the structure reported here for Ap4A bound to RppH, in which adenine binding in the cleft positions the δ-phosphate beside a nucleophile poised for attack. Lacking a δ-phosphate, Ap3A would be too short to react. Similarly, the eukaryotic cap analog m7Gp3G is unreactive, whereas m7Gp3-capped RNA reacts with RppH to produce m7GDP and monophosphorylated RNA (40), presumably because the second transcribed nucleotide of this capped RNA can bind in the cleft and position the β-phosphate for hydrolytic attack. The same rationale undoubtedly explains why RppH reacts slowly with RNA bearing an NAD or NADH cap to yield nicotinamide mononucleotide and monophosphorylated RNA but does not react with NAD or NADH themselves (10, 12, 34, 41), which each contain only two bridging phosphates. Because transcripts that are m7Gp3 capped or NAD(H) capped are expected to resemble triphosphorylated RNA in their productive mode of binding, we predict that their efficient decapping by RppH requires at least two unpaired 5′-terminal nucleotides and prefers a purine at the second position. Therefore, the mechanism by which RppH recognizes Np4-capped RNAs and positions them in the active site is probably unique among both capped and uncapped transcripts.
The 5′ end-dependent degradation pathway is a key contributor to RNA turnover in E. coli (3). The rate at which transcripts are degraded in this manner depends both on whether or not they are capped and on the activity and specificity of each enzyme in the pathway. In unstressed wild-type cells, where the Np4A hydrolase and decapping enzyme ApaH is active, Np4A levels are very low and Np4-capped transcripts are undetectable (20). Under these conditions, the RNA substrates available for 5′-terminal deprotection are predominantly triphosphorylated and diphosphorylated, and RppH acts not as a decapping enzyme but as an RNA pyrophosphohydrolase that converts their 5′ ends to monophosphates (3, 4). Uncapped RppH substrates of this kind are most reactive if they contain at least two, and preferably three or more, unpaired 5′-terminal nucleotides and bear a purine at the second position, characteristics that also enable ready cleavage of their monophosphorylated reaction products by the endonuclease RNase E (14, 15). What little Np4-capped RNA is made undergoes rapid decapping by ApaH to produce a diphosphorylated decay intermediate that must then react with RppH to generate the monophosphorylated 5′ end favored by RNase E (20). During disulfide stress, ApaH inactivation allows the cellular concentration of Np4As and Np4-capped transcripts to increase markedly (20) and forces RppH to assume responsibility for Np4 cap removal. Therefore, the initial RNA product of decapping is primarily triphosphorylated under these conditions. As a result of the one-nucleotide shift in RppH specificity, decapping of these transcripts by this enzyme requires only one unpaired 5′-terminal nucleotide and prefers two or more while favoring RNAs that begin with a purine (Figs. 2–4). Nevertheless, to attain a 5′ phosphorylation state vulnerable to rapid cleavage by RNase E, which remains active under these conditions, the triphosphorylated products of decapping must react a second time with RppH to convert their 5′ ends to monophosphates, a reaction with distinct substrate preferences (14). As a consequence, the identities of both the first and second transcribed nucleotide appear to be critical for governing rates of RppH-mediated deprotection of Np4-capped transcripts, whose susceptibility to 5′ end-dependent degradation during disulfide stress is expected to be maximized by the presence of two unpaired 5′-terminal purines followed by at least one more unpaired nucleotide of any kind.
A recent survey of 14 E. coli RNAs showed that the steady-state percentage of each that was Np4 capped when ApaH was inactivated by cadmium stress was 33 to 76% for A-initiated transcripts, 36 to 72% for C-initiated transcripts, and 26% for the lone U-initiated transcript examined yet was only 4 to 8% for G-initiated transcripts (20). Moreover, changing the first transcribed nucleotide of yeiP mRNA from A to G reduced the capped fraction of that transcript from 76 to 5%. Consistent with those observations, we now find in ΔapaH cells that the steady-state percentage of Np4-capped efp mRNA is only slightly affected by changing the 5′-terminal transcribed nucleotide from C (25 ± 3%) to A (21 ± 1%) but falls to just 4 ± 1% when that nucleotide is changed to G (Fig. 4A). The percentage of an RNA that is capped at steady state depends on the combined effects of multiple cellular processes, especially the ratio of the rates of cap acquisition and cap removal. Rates of cap acquisition should depend on the availability of cap precursors (Np4As = Ap4Ns) relative to the NTP with which they compete for 5′-terminal incorporation during transcription initiation by RNA polymerase (21). In cadmium-stressed E. coli, the molar ratio of Ap4G to GTP (0.26) is lower than the ratio of Ap4C to CTP (0.73) and Ap4U to UTP (0.68) and much lower than the ratio of Np4A to ATP (2.6, where N = A, G, C, or U) (42). Furthermore, our findings now show that RppH decaps G- and A-initiated transcripts three to five times faster than C- and U-initiated transcripts (Figs. 3A and 4E). The combination of a low cap-acquisition rate and a high decapping rate probably explains the small percentage of G-initiated transcripts that are Ap4-capped at steady state. By contrast, the much higher rate of cap acquisition by A-initiated RNAs, whose synthesis can begin with any Np4A (21), likely counterbalances their rapid decapping by RppH, allowing a substantial percentage of A-initiated transcripts to be Np4-capped at steady state. Similarly, the ample fraction of C- and U-initiated transcripts that are Ap4-capped at steady state can be attributed to their intermediate rate of synthesis and slow rate of decapping.
The removal of Np4 caps appears to be crucial for governing E. coli mRNA lifetimes during disulfide stress. By controlling rates of RppH-dependent decapping and degradation, the substrate recognition mechanism described here may help cells to recover from this stress by increasing the production of key stress-response proteins and reducing the synthesis of other proteins of less immediate importance. The ability to reset levels of gene expression under these perilous conditions may be critical for survival.
Materials and Methods
Strains and Plasmids.
Measurements of 5′ cap levels and decapping rates in E. coli were performed in isogenic derivatives of the K-12 strain BW25113 (43) bearing in-frame deletions of the apaH and rppH coding regions, either individually or in combination (20). The strains used to analyze yeiP mRNA contained plasmid pYeiP1 to facilitate detection of that mRNA by increasing its cellular concentration (36). The strains used to analyze efp, efp-C1A, and efp-C1G mRNA contained plasmid pEfp1, pEfp1-C1A, or pEfp1-C1G [derivatives of plasmid pBR322fd (13) encoding efp mRNA or a variant thereof] and lacked the chromosomal efp gene, which was deleted by P1 transduction from the efp::kan strain of the Keio collection (JW4107) followed by excision of the kan gene (44).
Rates of Decapping by RppH in E. coli.
E. coli cells were grown to mid-log phase (an optical density of 0.3 at 650 nm) at 37 °C in MOPS-glucose medium (45) before arresting transcription with rifampicin (200 μg/mL). For measurements made during cadmium stress, the mid-log phase cells were treated with CdCl2 (0.2 mM) for 90 min before adding rifampicin. Total RNA was extracted at time intervals after inhibiting transcription, and equal amounts of RNA (10 μg) were cut with a 10–23 deoxyribozyme (DZyeiP69 or DZefp87; SI Appendix, Table S3) specific for yeiP or efp mRNA, subjected to electrophoresis on a boronate gel to separate capped from uncapped RNA, and analyzed by Northern blotting (20). Band intensities were graphed semilogarithmically as a function of time, and first-order rate constants for the disappearance of capped yeiP or efp mRNA were obtained from the slope of the best-fit line as determined by linear regression. The rate constant for RppH-mediated decapping in E. coli was then calculated by subtracting the rate constant for the disappearance of the capped transcript in ΔapaH ΔrppH cells from the corresponding rate constant in ΔapaH cells.
RNA Synthesis by In Vitro Transcription.
To prepare double-stranded DNA templates for synthesizing Ap4G4 and related substrates by in vitro transcription, pairs of complementary oligodeoxynucleotides (200 pmol each; SI Appendix, Table S3) were annealed and then extended with the Klenow fragment of DNA polymerase (five units; New England Biolabs) and deoxynucleoside triphosphates (0.5 mM each) in a solution (20 µL) containing Tris⋅HCl, pH 7.9 (10 mM), NaCl (50 mM), MgCl2 (10 mM), and dithiothreitol (1 mM) (37). The fully double-stranded products were phenol extracted, ethanol precipitated, and dissolved in water. Templates for the synthesis of Ap4A8XL, Ap4A4, and the four Np4A8 RNAs contained a T7 ϕ2.5 promoter; the other templates all contained a T7 ϕ6.5 promoter (46). Capped RNAs were synthesized by in vitro transcription of the double-stranded DNA template (0.25 pmol/µL) for 8 h at 37 °C with T7 RNA polymerase (5 units/µL, New England Biolabs) in the presence of Ap4A, Gp4A, Cp4A, or Up4A at a concentration (1 to 2 mM) ≥5-fold higher than that of the NTP with which it was competing for 5′-terminal incorporation (0.1 to 0.4 mM). Each reaction mixture also contained other NTPs (ATP, GTP, and/or CTP) for internal incorporation (1 mM), fluorescein-12-UTP (0.1 mM), Tris⋅HCl, pH 7.9 (40 mM), MgCl2 (6 mM), spermidine (2 mM), dithiothreitol (10 mM), and rRNasin (0.25 units/μL, Promega) in a final volume of 40 µL. The resulting transcripts were purified by gel electrophoresis and elution of the band of interest.
Ap4GUAA and Ap4GAUA were synthesized by in vitro transcription of the double-stranded DNA template (2 pmol/µL; SI Appendix, Table S3) for 3 h at 37 °C with T7 RNA polymerase (0.1 µg/µL) in a solution containing Tris⋅HCl, pH 8.0 (100 mM), MgCl2 (20 mM), spermidine (2 mM), dithiothreitol (40 mM), Ap4G (3 mM), ATP (6 mM), and UTP (3 mM) (47). Their triphosphorylated, diphosphorylated, and monophosphorylated counterparts (pppGUAA, ppGUAA, pGUAA, pppGAUA, ppGAUA, and pGAUA) were prepared in the same manner, except that Ap4G was replaced by GTP, GDP, or GMP (3 mM), respectively. The products of transcription were purified by anion-exchange chromatography on a 1-mL MonoQ (5/50) column (GE Healthcare). RNA was eluted with a 25-mL 0 to 50% gradient of 2 M triethylammonium bicarbonate buffer, pH 8.5, at a flow rate of 1 mL/min, and the desired RNA fractions were frozen and lyophilized. The dried RNAs were dissolved in water, and their concentration was determined spectrophotometrically. The identity of each RNA product was confirmed by mass spectrometry (Bruker UltraFlex MALDI-TOF). Ap4GUAA, pppGUAA, ppGUAA, pGUAA, Ap4GAUA, pppGAUA, ppGAUA, and pGAUA have calculated masses of 1,816.97, 1,487.76, 1,407.79, 1,327.81, 1,816.97, 1,487.76, 1,407.79, and 1,327.81 Da, respectively. Their observed masses were 1,818.00 ([M+H]+), 1,488.11, 1,407.68, 1,327.50, 1,818.51 ([M+H]+), 1,489.32 ([M+H]+), 1,407.66, and 1,327.70, respectively.
RppH Purification.
For the in vitro decapping assays, E. coli RppH and RppH-E57A, each bearing an amino-terminal hexahistidine tag, were produced in E. coli from plasmids pPlacRppH6 and pPlacRppH6-E57A, respectively, purified by affinity chromatography on TALON beads, and stored at −80 °C in a buffer containing HEPES, pH 7.5 (10 mM), NaCl (300 mM), and glycerol (50% vol/vol) (3, 14).
Structure determination was performed with a truncated variant of E. coli RppH that bore an additional serine residue at the amino terminus and lacked carboxyl-terminal residues 159 through 176, which were replaced by two alanines (16, 18). This variant, RppHt, retained enzymatic activity but yielded better crystals than the full-length protein. It was produced as a fusion with amino-terminal tandem decahistidine and SUMO tags by using a T7 RNA polymerase–based expression system in E. coli strain BL21(DE3). The recombinant protein was purified by affinity chromatography on a HisTrap FF column (GE Healthcare), and the tags were cleaved off by a His-tagged variant of ULP1 protease, leaving an extra serine residue at the amino terminus. The affinity tags and the protease were removed by passage through a HisTrap FF column, and RppHt was further purified by ion-exchange chromatography on a HiTrap SP column and gel filtration on Superdex 75 (GE Healthcare).
Rates of Decapping by RppH In Vitro.
To compare rates of RppH-mediated decapping in vitro, each Np4-capped transcript to be tested (750 fmol) was combined with Ap4A8XL (500 fmol) and prewarmed to 37 °C for 3 min in a buffer containing HEPES, pH 7.5 (20 mM), MgCl2 (10 mM), NaCl (50 mM), dithiothreitol (1 mM), rRNasin (10 units; Promega), and glycerol (1% vol/vol) in a volume of 34.1 µL. An unreacted sample (7 µL) was removed and mixed with loading buffer (7 µL; 95% formamide, 20 mM EDTA, pH 8, containing bromophenol blue). RppH was freshly diluted for each reaction into a high-salt buffer containing HEPES, pH 7.5 (20 mM), MgCl2 (10 mM), NaCl (300 mM), dithiothreitol (1 mM), and glycerol (10% vol/vol), and the diluted enzyme (7.9 µL) was added to the preheated reaction mixture to achieve a final concentration of 30 nM. Reaction samples (8.5 µL) were quenched with loading buffer (8.5 µL) after 15, 30, and 60 s, and all four samples were subjected to electrophoresis on a denaturing gel containing 13.7% acrylamide:bisacrylamide (19:1), 0.3% 3-acrylamidophenylboronic acid, 7 M urea, and 0.1 M Tris-acetate, pH 9.0 (35, 48). The fluorescein-labeled RNAs were then detected with a Typhoon Trio imager (GE Healthcare) and quantified with ImageQuant TL software. The percentage of each RNA that was capped at each time point was calculated from the ratio of band intensities (capped versus uncapped) and then normalized to the percentage that was capped at time 0.
Structure Determination by X-ray Crystallography.
RppHt was prepared in a solution of 20 mM sodium acetate, pH 5.0, 100 mM NaCl, and 1 mM dithiothreitol. Crystals of RppHt were grown in sitting drop format, typically against 0.4 mL of reservoir solution at 20 °C for 4 d. Crystals were grown from a mixture comprising 1 μL of 1 mM protein and 2 μL of reservoir solution [0.4 M (NH4)2SO4, 10% (wt/vol) PEG3350, and 10% glycerol]. To soak Ap4A into the crystals, they were transferred to 1 µL of soaking solution containing 0.1 M sodium cacodylate, pH 6.0, 0.2 M Na2SO4, 25 mM NaF, 15% PEG3350, 10% glycerol, and 1 mM Ap4A. After incubating the crystals for 20 min, they were transferred to a fresh 1-μL drop of the same solution and cross-linked by incubation over a 2-μL drop of 50% glutaraldehyde for 30 min. The crystals were transferred to a 2-μL drop of 50 mM MOPS, pH 7.0, 25 mM MgCl2, 25 mM NaF, 1 mM Ap4A, 30% (vol/vol) pentaerythritol propoxylate 5/4 PO/OH, and 15% PEG3350 for 20 min and then mounted and frozen in a cryostream without additional cryoprotector.
Diffraction data were collected at 100 K at the home Rigaku X-ray source and on beamline 24ID-C of the Advanced Photon Source (Argonne National Laboratory). Data were processed by using the X-ray Detector Software (XDS) suite (49). The crystal structure was solved by molecular replacement using the structure of E. coli RppH (Protein Data Bank [PDB] code 4S2W) as a search model and the Phaser-MR implementation in PHENIX (50). The models were rebuilt manually in COOT (51) and refined in PHENIX. The ligand, water molecules, and ions were added at the late stages of refinement based on the Fo-Fc and 2Fo-Fc electron density maps. Three density map peaks in the catalytic center were assigned to Mg2+ ions based on their octahedral coordination geometry and coordination distances in the range of 1.9 to 2.2 Å, which are characteristic of these cations.
Products of Decapping by RppH.
To characterize the products of RppH-mediated decapping, either Ap4GUAA, pppGUAA, Ap4GAUA, or pppGAUA (6 nmol) was prewarmed to 37 °C for 2 min in a buffer containing HEPES, pH 7.5 (20 mM), MgCl2 (10 mM), NaCl (50 mM), and dithiothreitol (1 mM), resulting in a total volume of 6 µL. For each reaction, RppH or catalytically inactive RppH-E57A was freshly diluted into a high-salt buffer containing HEPES, pH 7.5 (20 mM), MgCl2 (10 mM), NaCl (300 mM), and dithiothreitol (1 mM) to achieve an enzyme concentration of 14 µM, and the diluted enzyme (1 µL) was added to the prewarmed substrate, resulting in a final enzyme concentration of 2 µM. The reactions were quenched after 8 min by adding 0.7 µL EDTA (220 mM), and the products were analyzed by thin-layer chromatography on fluorescent PEI-cellulose plates (Millipore Sigma) developed with 0.4 M ammonium sulfate, pH 5.5. Spots visualized by UV shadowing were photographed and quantified by using ImageJ software.
Comparisons of the reactivity of Ap4GUAA, pppGUAA, ppGUAA, Ap4GAUA, pppGAUA, and ppGAUA were performed identically, except that the reaction mixtures were scaled up 5.5-fold and samples (7 µL) were quenched after 0, 2, 4, 8, and 16 min. The extent of reaction at each time point was calculated from the molar ratio of substrate to RNA product(s), with correction for the difference in the extinction coefficients of the capped and uncapped RNAs.
Supplementary Material
Acknowledgments
This research was supported by a fellowship to R.L.P. (T32AI007180) and research grants to J.G.B. (R01GM035769) and A.S. (R01GM112940) from the NIH. The X-ray diffraction studies used the Northeastern Collaborative Access Team beamline (funded by NIH Grant P30GM124165) at the Advanced Photon Source operated by the Department of Energy Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117318119/-/DCSupplemental.
Data Availability
The atomic coordinates for the complex of RppH with Ap4A have been deposited in the PDB under accession no. 7SP3. All other data are included in the figures and SI Appendix.
References
- 1.Mackie G. A., RNase E: At the interface of bacterial RNA processing and decay. Nat. Rev. Microbiol. 11, 45–57 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Celesnik H., Deana A., Belasco J. G., Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol. Cell 27, 79–90 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deana A., Celesnik H., Belasco J. G., The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature 451, 355–358 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Luciano D. J., Vasilyev N., Richards J., Serganov A., Belasco J. G., A novel RNA phosphorylation state enables 5′ end-dependent degradation in Escherichia coli. Mol. Cell 67, 44–54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie G. A., Ribonuclease E is a 5′-end-dependent endonuclease. Nature 395, 720–723 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Callaghan A. J., et al. , Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature 437, 1187–1191 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Jiao X., Carr-Schmid A., Kiledjian M., The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc. Natl. Acad. Sci. U.S.A. 99, 12663–12668 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messing S. A., et al. , Structure and biological function of the RNA pyrophosphohydrolase BdRppH from Bdellovibrio bacteriovorus. Structure 17, 472–481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards J., et al. , An RNA pyrophosphohydrolase triggers 5′-exonucleolytic degradation of mRNA in Bacillus subtilis. Mol. Cell 43, 940–949 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahová H., Winz M. L., Höfer K., Nübel G., Jäschke A., NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519, 374–377 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Bischler T., et al. , Identification of the RNA pyrophosphohydrolase RppH of Helicobacter pylori and global analysis of its RNA targets. J. Biol. Chem. 292, 1934–1950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grudzien-Nogalska E., et al. , Structural and mechanistic basis of mammalian Nudt12 RNA deNADding. Nat. Chem. Biol. 15, 575–582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luciano D. J., et al. , Differential control of the rate of 5′-end-dependent mRNA degradation in Escherichia coli. J. Bacteriol. 194, 6233–6239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley P. L., Hsieh P. K., Luciano D. J., Belasco J. G., Specificity and evolutionary conservation of the Escherichia coli RNA pyrophosphohydrolase RppH. J. Biol. Chem. 290, 9478–9486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards J., Belasco J. G., Distinct requirements for 5′-monophosphate-assisted RNA cleavage by Escherichia coli RNase E and RNase G. J. Biol. Chem. 291, 5038–5048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasilyev N., Serganov A., Structures of RNA complexes with the Escherichia coli RNA pyrophosphohydrolase RppH unveil the basis for specific 5′-end-dependent mRNA decay. J. Biol. Chem. 290, 9487–9499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C. R., Kim M., Park Y. H., Kim Y. R., Seok Y. J., RppH-dependent pyrophosphohydrolysis of mRNAs is regulated by direct interaction with DapF in Escherichia coli. Nucleic Acids Res. 42, 12746–12757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao A., et al. , Structural and kinetic insights into stimulation of RppH-dependent RNA degradation by the metabolic enzyme DapF. Nucleic Acids Res. 46, 6841–6856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., et al. , DapF stabilizes the substrate-favoring conformation of RppH to stimulate its RNA-pyrophosphohydrolase activity in Escherichia coli. Nucleic Acids Res. 46, 6880–6892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luciano D. J., Levenson-Palmer R., Belasco J. G., Stresses that raise Np4A levels induce protective nucleoside tetraphosphate capping of bacterial RNA. Mol. Cell 75, 957–966 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luciano D. J., Belasco J. G., Np4A alarmones function in bacteria as precursors to RNA caps. Proc. Natl. Acad. Sci. U.S.A. 117, 3560–3567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finamore F. J., Warner A. H., The occurrence of P1, P4-diguanosine 5′-tetraphosphate in brine shrimp eggs. J. Biol. Chem. 238, 344–348 (1963). [PubMed] [Google Scholar]
- 23.Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K., Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem. Biophys. Res. Commun. 24, 91–97 (1966). [DOI] [PubMed] [Google Scholar]
- 24.Lee P. C., Bochner B. R., Ames B. N., Diadenosine 5′,5”'-P1,P4-tetraphosphate and related adenylylated nucleotides in Salmonella typhimurium. J. Biol. Chem. 258, 6827–6834 (1983). [PubMed] [Google Scholar]
- 25.Ismail T. M., Hart C. A., McLennan A. G., Regulation of dinucleoside polyphosphate pools by the YgdP and ApaH hydrolases is essential for the ability of Salmonella enterica serovar typhimurium to invade cultured mammalian cells. J. Biol. Chem. 278, 32602–32607 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Hansen S., Lewis K., Vulić M., Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli. Antimicrob. Agents Chemother. 52, 2718–2726 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monds R. D., et al. , Di-adenosine tetraphosphate (Ap4A) metabolism impacts biofilm formation by Pseudomonas fluorescens via modulation of c-di-GMP-dependent pathways. J. Bacteriol. 192, 3011–3023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji X., et al. , Alarmone Ap4A is elevated by aminoglycoside antibiotics and enhances their bactericidal activity. Proc. Natl. Acad. Sci. U.S.A. 116, 9578–9585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varshavsky A., Diadenosine 5′, 5′′′-P1, P4-tetraphosphate: A pleiotropically acting alarmone? Cell 34, 711–712 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N., AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37, 225–232 (1984). [DOI] [PubMed] [Google Scholar]
- 31.Hudeček O., et al. , Dinucleoside polyphosphates act as 5′-RNA caps in bacteria. Nat. Commun. 11, 1052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guranowski A., Jakubowski H., Holler E., Catabolism of diadenosine 5′,5′′′-P1,P4-tetraphosphate in procaryotes. Purification and properties of diadenosine 5′,5′′′-P1,P4-tetraphosphate (symmetrical) pyrophosphohydrolase from Escherichia coli K12. J. Biol. Chem. 258, 14784–14789 (1983). [PubMed] [Google Scholar]
- 33.Plateau P., Fromant M., Brevet A., Gesquière A., Blanquet S., Catabolism of bis(5′-nucleosidyl) oligophosphates in Escherichia coli: Metal requirements and substrate specificity of homogeneous diadenosine-5′,5′''-P1,P4-tetraphosphate pyrophosphohydrolase. Biochemistry 24, 914–922 (1985). [DOI] [PubMed] [Google Scholar]
- 34.Bessman M. J., et al. , The gene ygdP, associated with the invasiveness of Escherichia coli K1, designates a Nudix hydrolase, Orf176, active on adenosine (5′)-pentaphospho-(5′)-adenosine (Ap5A). J. Biol. Chem. 276, 37834–37838 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Igloi G. L., Kössel H., Affinity electrophoresis for monitoring terminal phosphorylation and the presence of queuosine in RNA. Application of polyacrylamide containing a covalently bound boronic acid. Nucleic Acids Res. 13, 6881–6898 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards J., Luciano D. J., Belasco J. G., Influence of translation on RppH-dependent mRNA degradation in Escherichia coli. Mol. Microbiol. 86, 1063–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh P. K., Richards J., Liu Q., Belasco J. G., Specificity of RppH-dependent RNA degradation in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 110, 8864–8869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao A., Vasilyev N., Kaushik A., Duan W., Serganov A., Principles of RNA and nucleotide discrimination by the RNA processing enzyme RppH. Nucleic Acids Res. 48, 3776–3788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y. G., Kowtoniuk W. E., Agarwal I., Shen Y., Liu D. R., LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat. Chem. Biol. 5, 879–881 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song M. G., Bail S., Kiledjian M., Multiple Nudix family proteins possess mRNA decapping activity. RNA 19, 390–399 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conyers G. B., Bessman M. J., The gene, ialA, associated with the invasion of human erythrocytes by Bartonella bacilliformis, designates a nudix hydrolase active on dinucleoside 5′-polyphosphates. J. Biol. Chem. 274, 1203–1206 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Coste H., Brevet A., Plateau P., Blanquet S., Non-adenylylated bis(5′-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J. Biol. Chem. 262, 12096–12103 (1987). [PubMed] [Google Scholar]
- 43.Datsenko K. A., Wanner B. L., One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba T., et al. , Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neidhardt F. C., Bloch P. L., Smith D. F., Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman T. M., Wang G., Huang F., Superior 5′ homogeneity of RNA from ATP-initiated transcription under the T7 φ2.5 promoter. Nucleic Acids Res. 32, e14 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasilyev N., Serganov A., Preparation of short 5′-triphosphorylated oligoribonucleotides for crystallographic and biochemical studies. Methods Mol. Biol. 1320, 11–20 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Ivanov A. E., Larsson H., Galaev I. Y., Mattiasson B., Synthesis of boronate-containing copolymers of N,N-dimethylacrylamide, their interaction with poly(vinyl alcohol) and rheological behaviour of the gels. Polymer (Guildf.) 45, 2495–2505 (2004). [Google Scholar]
- 49.Kabsch W., XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates for the complex of RppH with Ap4A have been deposited in the PDB under accession no. 7SP3. All other data are included in the figures and SI Appendix.