Abstract
Background
Although carotid sonographic features have been used as predictors of recurrent stroke, few large-scale studies have explored the use of machine learning analysis of carotid sonographic features for the prediction of recurrent stroke.
Methods
We retrospectively collected electronic medical records of enrolled patients from the data warehouse of China Medical University Hospital, a tertiary medical center in central Taiwan, from January 2012 to November 2018. We included patients who underwent a documented carotid ultrasound within 30 days of experiencing an acute first stroke during the study period. We classified these participants into two groups: those with non-recurrent stroke (those who has not been diagnosed with acute stroke again during the study period) and those with recurrent stoke (those who has been diagnosed with acute stroke during the study period). A total of 1,235 carotid sonographic parameters were analyzed. Data on the patients' demographic characteristics and comorbidities were also collected. Python 3.7 was used as the programming language, and the scikit-learn toolkit was used to complete the derivation and verification of the machine learning methods.
Results
In total, 2,411 patients were enrolled in this study, of whom 1,896 and 515 had non-recurrent and recurrent stroke, respectively. After extraction, 43 features of carotid sonography (36 carotid sonographic parameters and seven transcranial color Doppler sonographic parameter) were analyzed. For predicting recurrent stroke, CatBoost achieved the highest area under the curve (0.844, CIs 95% 0.824–0.868), followed by the Light Gradient Boosting Machine (0.832, CIs 95% 0.813–0.851), random forest (0.819, CIs 95% 0.802–0.846), support-vector machine (0.759, CIs 95% 0.739–0.781), logistic regression (0.781, CIs 95% 0.764–0.800), and decision tree (0.735, CIs 95% 0.717–0.755) models.
Conclusion
When using the CatBoost model, the top three features for predicting recurrent stroke were determined to be the use of anticoagulation medications, the use of NSAID medications, and the resistive index of the left subclavian artery. The CatBoost model demonstrated efficiency and achieved optimal performance in the predictive classification of non-recurrent and recurrent stroke.
Keywords: machine learning, carotid sonographic features, recurrent stroke, acute stroke, CatBoost model
Introduction
Stroke is the second most common cause of death and a leading cause of disability worldwide (1). It is a heterogeneous syndrome with two major types: ischemic, which accounts for ~60–85% of all cases, and hemorrhagic. The common pathogenesis of both types involves atherosclerosis and hypertension (2, 3). Stroke may lead to a wide range of complications including neurological disorders, infections, mobility dysfunction, thromboembolism, and emotional disorders (4). Among these complications, recurrent stroke is considered the most catastrophic: patients with recurrent stroke often become trapped in a vicious cycle and experience rapid degradation of their functions (5, 6).
Studies have focused on the exploration and identification of risk factors for recurrent stroke, including left atrial enlargement (7); blood biomarkers (8, 9); elevated von Willebrand factor levels (10); and clinical factors such as components of metabolic syndrome (11), a history of coronary heart disease (12), frequent rehabilitation (13), and plaque and perfusion being visible in magnetic resonance imaging (MRI) (14–17). In addition to risk factors, risk scores—including the total small vessel disease score (18), simple point scores (19), the CHA2DS2VASc Score, the Essen Stroke Risk Score, and the ABCD3 serial score (20–22)—have been proposed for evaluating patients at risk of recurrent stroke. In studies that have investigated the components of these risk scores (18–22), imaging components have been reported to be as important as clinical components. Regarding the ABCD3 serial score, Kiyohara et al. discovered that adding intracranial arterial stenosis could further improve the score's predictive value for recurrent stroke (22). Although computed tomography and MRI are sensitive and accurate neuroimaging tools, they are often expensive and dependent on practitioners' interpretation skills, and their utilization rates vary widely (23, 24). Carotid Doppler sonography provides a low-cost, low-risk, and highly portable alternative modality for evaluating the vessels of patients with acute stroke (25). Although individual components, such as significant stenosis (>60%) determined by a patient's internal carotid artery (ICA)/common carotid artery (CCA) peak systolic velocity (PSV) ratio, have been reported to be effective carotid Doppler sonographic indicators (26), few large-scale studies have explored the use of machine learning analysis of carotid sonographic features for predicting recurrent stroke. We conducted a retrospective cohort study involving the collection of clinical and Doppler parameters and the application of machine learning models to differentiate between recurrent and non-recurrent stroke. We employed the CatBoost and Light Gradient Boosting Machine (LGBM) machine learning algorithms, which are seldom employed in medical studies. We also compared the performance of the random forest, support vector machine (SVM), decision tree, Logistic Regression, CatBoost, and LGBM algorithms.
Methods
Data Collection and Study Design
The electronic medical records of the enrolled subjects were retrospectively collected from the data warehouse of China Medical University Hospital (CMUH), a tertiary medical center in central Taiwan, from January 2012 to November 2018. The records contained longitudinal electronic demographic information, laboratory data, International Classification of Diseases (ICD) coding, records of medical procedures, and medical imaging (including computed tomography, MRI, ultrasounds, and nuclear imaging) for all inpatients and outpatients of CMUH. Most treatments by CMUH, especially those for catastrophic illnesses, were covered by Taiwan's National Health Insurance, and the medical payments were thus under strict supervision by the National Health Insurance Administration. This study was approved by the Research Ethics Committee of CMUH (CMUH109-REC2-035).
Participants and Definitions
We included patients who were diagnosed with acute first stroke and who received a documented carotid ultrasound within 30 days of the acute stroke during the study period. Initially, we enrolled 10,822 patients. Patients who lacked a documented report of their carotid sonography or who lacked complete DICOM SR data for the carotid sonography were excluded. Each patient's carotid and transcranial color-coded sonographic parameters were collected and examined. Carotid sonography was performed on a GE Vivid 7 system (GE Healthcare, Milwaukee, WI, USA) with a 3–10 MHZ linear array transducer linear 9L probe.
The enrolled patients were classified into two groups: those who experienced non-recurrent stroke (stroke once; those who has not been diagnosed with acute stroke again) and those who experienced recurrent stroke (those who has been diagnosed with another acute stroke during study period; Figure 1).
Figure 1.
Flow chart.
In this study, acute stroke was defined according to the National Health Insurance Administration's definition of catastrophic illness ICD-9 and ICD-10 codes for acute stroke, including occlusion and stenosis, hemorrhagic strokes, transient ischemic attacks (TIAs) and related syndromes, stroke syndromes, and other cerebral vascular diseases (Supplementary Table 1). Comorbidities considered in this study included hypertension, diabetes mellitus, hyperlipidemia, end-stage renal disease, atrial fibrillation, heart failure, liver cirrhosis, and cancer, which were also defined based on ICD coding in the CMUH data warehouse.
The primary outcome of our study was recurrent stroke, and we established a model for predicting a patient's risk of recurrent stroke after their first episode of acute stroke.
Data Preprocessing
Data preprocessing was required to ensure the performance of our model. We applied feature extraction and data normalization when preprocessing the collected clinical, demographic, and sonographic variables.
Feature Extraction
To identify significant features, three types of feature extraction were utilized in our study. First, we used Pearson correlation coefficients to determine the strength of the linear relationships between the carotid sonographic parameters and the clinical variables. The carotid sonographic parameters with Pearson correlation coefficients < 0.1 were not considered. Second, we used least absolute shrinkage and selection operator (lasso) regression, a shrinkage and variable selection method for regression models, to eliminate less representative features and select more representative features (27). Last, we used the statistical significance to determine significant features, which the carotid sonographic parameters with p-value < 0.05 were considered. Only the features selected using the Pearson correlation, lasso regression methods and statistical significance method were included in our training dataset.
Data Normalization
Because the units of the sonographic variables varied, data scaling was required for normalization. We applied standard deviation (SD) normalization, which is a method commonly used when a dataset contains a few non-extreme outliers. We calculated the mean and SD values of the training data and scaled the values to ensure that the mean of all the values was 0 and the SD was 1. The formula used to calculate the z-score was
where x is the original datum, μ is the mean, and σ is the SD.
Data Balancing
The ratio of patients with non-recurrent stroke to those with recurrent stroke was ~3:1. Therefore, the number of fault samples and the number of positive training samples were imbalanced, and the algorithm tended to ignore small classes and concentrate on the accurate classification of the large classes, resulting in a weaker model with limited predictive ability. To overcome the imbalanced nature of the data, we applied class weight balancing and balanced bagging methods in our training models. When class weight balancing methods are applied, if the sample size of a category is high, then it is assigned a low weight, and vice versa (28). Balanced bagging, which involves bootstrapping or applying sampling techniques to the original data n times with replacements to create training sets, also improves a model's classification accuracy and reduces data imbalance (29).
Machine Learning Models
Six machine learning models were used in this study: random forest, SVM, Logistic Regression, decision tree, CatBoost, and LGBM.
Random forest, an ensemble learning technique, involves the aggregation of a large number of decision trees (30). Each individual tree in the random forest provides a class prediction based on a given number mtry of randomly selected features (31). Random forests produce less variance compared with single decision trees and produce predictions more accurate than those of any of the individual trees.
SVMs are linear supervised classifiers capable of performing binary and multiclass classification on a dataset (32). In an SVM, each data point is an n-dimensional vector. According to the margin maximization principle, the SVM chooses the most appropriate hyperplane to maximize the distance from the hyperplane to the nearest data point on each side (33).
Logistic Regression, a linear regression model, converts the log-odds of input variables to a predicted probability of outcome.
Decision trees, non-parametric supervised learning tools, are treelike structures consisting of a root node, condition or leaf nodes, and associated branches (34). The end of each branch that does not split anymore represents a potential outcome. The probability model with the maximum likelihood of attaining a desirable outcome among the decision trees was considered the most effective prediction model.
CatBoost is a gradient boosting framework that employs oblivious decision trees as base predictors; it is an open-source software library developed by Yandex (35). For each level of each decision tree, decision rules containing feature indices and threshold values are collected, which eventually form a collection of disjoint subsets of feature vectors. The collections of feature vectors function as a prediction model. CatBoost reduces overfitting and improves the quality of a model (36).
LGBM is another gradient boosting algorithm and an implementation of ensemble learning. LGBM uses a leaf-wise algorithm to grow trees vertically; a leaf that most reduces the loss is chosen to split (37). The main function of LGBM is to create large gradients, which contribute more to information gain (38).
Statistical Analysis
Baseline sociodemographic and clinical characteristics are displayed as the mean ± SD. Categorical variables are expressed as absolute and percent frequencies.
The Python 3.7 software package and scikit-learn toolkit were employed, and the defaults were applied for the training of the random forest, SVM, LogisticRegression, decision tree, CatBoost, and LGBM algorithms. We used the Gaussian radial basis function as the kernel function in our SVM model, and the regularization parameter (C) was 1.0. For the random forest and decision tree algorithms, 10 decision trees were used. For the Logistic Regression algorithm, we added a penalty term (known as the L2 norm or L2 penalty) to the loss function. For the CatBoost algorithm, 1,000 decision trees and six hidden layers were used. For the LGBM, 100 decision trees and 31 hidden layers were used.
We used the following evaluation metrics of sensitivity, specificity, accuracy, and area under the receiver operating characteristic curve (AUC) to evaluate the performance of the machine learning algorithms in this study:
where TP denotes true positives; FP, false positives; TN, true negatives; and FN, false negatives. Precision was denoted by TP/(TP + FP), and recall was denoted by TP/(TP + FN).
We used stratified k-fold cross-validation to estimate the accuracy of the models. The data were first stratified and then split into k portions. In each k iteration, one portion was used as the test set, and the remaining k – 1 portions were used as training sets. Then, the model was fit to the training sets, and the performance of the model on the test set was evaluated. This procedure was repeated until each of the k subsets had served as the validation set. The average of the k performance measurements on the k validation sets was the cross-validated performance (39). In this study, we used stratified 10-fold cross-validation to estimate the accuracy, as generally recommended (40). The Shap algorithm was used to measure the contribution of features to predicting “non-recurrence” and “recurrence.” Shapley Additive explanation proposed by Lundberg and Lee is a method of explaining predictions based on the optimal Shapley value of game theory (41).
Results
Patient Population and Demographics
A total of 2,411 patients were enrolled in this study. Of these, 1,896 were classified into the non-recurrent stroke cohort, and 515 were classified into the recurrent stroke cohort (Figure 1). The mean ages of the non-recurrent stroke and recurrent stroke cohorts were 66.18 ± 12.67 years (range: 24–98 years) and 67.63 ± 13.14 years (range: 27–96 years), respectively. Regarding gender, 61.66 and 62.14% of the patients in the non-recurrent stroke and recurrent stroke cohorts, respectively, were men. Strokes involving occlusion and stenosis were the most common and accounted for 75.84 and 80.19% of patients' strokes in the non-recurrent stroke and recurrent stroke cohorts, respectively. TIAs and related conditions were the second most common stroke type, accounting for 8.81 and 6.80% of the patients' strokes in the non-recurrent stroke and recurrent stroke cohorts, respectively. The prevalence of each comorbidity was higher among patients in the recurrent stroke cohort than among patients with non-recurrent stroke: of the patients with recurrent stroke, 72.04% had hypertension, 45.24% had diabetes mellitus, 27.96% had hyperlipidemia, 4.08% had end-stage renal disease, 8.54% had atrial fibrillation, 12.43% had heart failure, 8.35% had cancer, and 36.50% had a body mass index (BMI) > 25. Furthermore, 57.12 and 74.76% of the patients in the non-recurrent stroke and recurrent stroke cohorts, respectively, took dihydropyridine derivatives; 91.67 and 97.09% of patients in the non-recurrent stroke and recurrent stroke cohorts, respectively took antiplatelet medications; and 62.29 and 73.98% of the patients in the non-recurrent stroke and recurrent stroke cohorts, respectively, had taken HMG-COA inhibitors (Table 1).
Table 1.
Clinical characteristics in 2,411 study patients.
| Non-recurrent stroke (%) | Recurrent stroke (%) | p-value | |
|---|---|---|---|
| Study patients | N = 1,896 | N = 515 | |
| Men | 1,169 (61.66%) | 320 (62.14%) | 0.843 |
| Age | 66.18 ± 12.67 (24–98) | 67.63 ± 13.14 (27–96) | <0.05 |
| Stroke type | |||
| Occlusion and stenosis | 1,438 (75.84%) | 413 (80.19%) | <0.05 |
| Hemorrhage | 144 (7.59%) | 30 (5.83%) | 0.169 |
| TIA and related syndrome | 167 (8.81%) | 35 (6.80%) | 0.144 |
| Stroke syndrome | 51 (2.69%) | 8 (1.55%) | 0.139 |
| Others cerebral vascular disease | 96 (5.06%) | 29 (5.63%) | 0.606 |
| Comorbidity | |||
| Hypertension | 1,227 (64.72%) | 371 (72.04%) | <0.05 |
| Diabetes mellitus | 682 (35.97%) | 233 (45.24%) | <0.001 |
| Hyperlipidemia | 503 (26.53%) | 144 (27.96%) | 0.516 |
| End stage renal disease | 10 (0.53%) | 21 (4.08%) | <0.0001 |
| Atrial fibrillation | 81 (4.27%) | 44 (8.54%) | <0.001 |
| Heart failure | 123 (6.49%) | 64 (12.43%) | <0.0001 |
| Liver cirrhosis | 36 (1.90%) | 7 (1.36%) | 0.412 |
| Cancer | 113 (5.96%) | 43 (8.35%) | 0.051 |
| BMI > 25 | 675 (35.60%) | 188 (36.50%) | 0.862 |
| Medicine after first stroke | |||
| Angiotensin II receptor blockers (ARBs) | 753 (39.72%) | 287 (55.73%) | <0.0001 |
| Dihydropyridine derivatives | 1,083 (57.12%) | 385 (74.76%) | <0.0001 |
| Anti-coagulant | 665 (35.07%) | 353 (68.54%) | <0.0001 |
| Anti-platelet | 1,738 (91.67%) | 500 (97.09%) | <0.0001 |
| HMG-COA inhibitors | 1,181 (62.29%) | 381 (73.98%) | <0.0001 |
| NSAID | 761 (40.14%) | 312 (60.58%) | <0.0001 |
*Values are expressed as the mean ± SD.
HTN, hypertention; DM, diabetes mellitus; ESRD, end stage renal disease.
Selected Features
The algorithms identified 36 carotid sonographic parameters and seven transcranial color-coded sonographic parameter as features. Supplementary Table 2 summarizes the results of the feature selection process of sonographic parameters; bold type denotes the selected features. In addition, 18 clinical variables, namely age, gender, stroke type, hypertension, diabetes mellitus, hyperlipidemia, end-stage renal disease, atrial fibrillation, heart failure, liver cirrhosis, cancer, and types of medications, were identified as features. In total, this study involved the analysis of 65 features.
Performance of Models in Predicting Non-recurrent and Recurrent Stroke
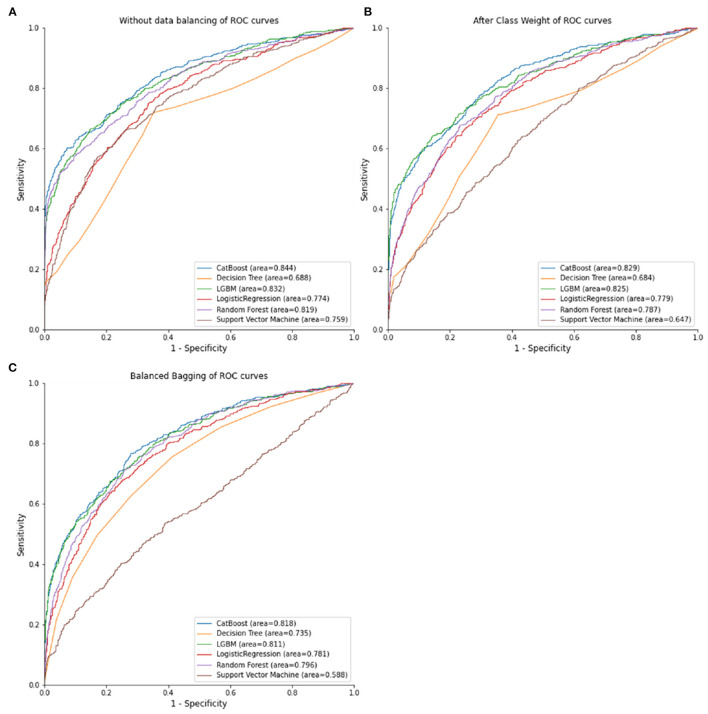
Table 2 listed the predictive performance of the random forest, SVM, LogisticRegression, decision tree, CatBoost, and LGBM models. The best AUC achieved was 0.844 (0.824–0.868) by the CatBoost model with no balancing method, exceeding the AUC of 0.818 (0.797–0.843) achieved by the CatBoost model with class weight balancing and the AUC of 0.829 (0.814–0.849) achieved by the CatBoost model with balanced bagging. The random forest model (AUC = 0.819, 0.802–0.846), CatBoost model (AUC = 0.844, 0.824–0.868), and LGBM model (AUC = 0.832, 0.813–0.851) resulted in higher AUCs without balancing methods than when class weight balancing or balanced bagging were employed. The Logistic Regression model (AUC = 0.781, 0.764–0.800) and decision tree model (AUC = 0.735, 0.717–0.755) resulted in higher AUCs with balanced bagging methods than when either of non-balancing or class weight balancing methods were employed. Figure 2 illustrates the receiver operating characteristic curve of the random forest, SVM, Logistic Regression, decision tree, CatBoost, and LGBM models in combination with the various data balancing methods.
Table 2.
Comparison of the predictive performance for six models (cross-validated data).
| Model | Method | Sensitivity | Specificity | Accuracy | AUC |
|---|---|---|---|---|---|
| (CIs 95%) | (CIs 95%) | (CIs 95%) | (CIs 95%) | ||
| RF | - | 0.070 (0.049–0.091) | 1.000 (1.000–1.000) | 0.801 (0.797–0.805) | 0.819 (0.802–0.846) |
| SVM | - | 0.216 (0.184–0.247) | 0.973 (0.968–0.978) | 0.811 (0.805–0.818) | 0.759 (0.739–0.781) |
| LR | - | 0.305 (0.256–0.353) | 0.958 (0.948–0.969) | 0.819 (0.809–0.829) | 0.774 (0.759–0.793) |
| DT | - | 0.155 (0.116–0.194) | 0.997 (0.994–1.000) | 0.817 (0.811–0.824) | 0.688 (0.686–0.733) |
| CatBoost | - | 0.441 (0.394–0.488) | 0.994 (0.990–0.998) | 0.876 (0.867–0.885) | 0.844 (0.824–0.868) |
| LGBM | - | 0.421 (0.381–0.461) | 0.982 (0.977–0.987) | 0.862 (0.855–0.870) | 0.832 (0.813–0.851) |
| RF | Class weight | 0.678 (0.632–0.722) | 0.762 (0.742–0.782) | 0.744 (0.726–0.762) | 0.787 (0.766–0.818) |
| SVM | Class weight | 0.060 (0.038–0.082) | 0.996 (0.993–0.999) | 0.796 (0.791–0.801) | 0.647 (0.617–0.683) |
| LR | Class weight | 0.678 (0.636–0.720) | 0.735 (0.707–0.763) | 0.723 (0.703–0.743) | 0.779 (0.762–0.798) |
| DT | Class weight | 0.717 (0.664–0.769) | 0.624 (0.606–0.641) | 0.644 (0.627–0.661) | 0.684 (0.674–0.726) |
| CatBoost | Class weight | 0.522 (0.484–0.560) | 0.928 (0.912–0.943) | 0.841 (0.832–0.851) | 0.829 (0.814–0.849) |
| LGBM | Class weight | 0.493 (0.467–0.519) | 0.954 (0.943–0.965) | 0.856 (0.845–0.866) | 0.825 (0.808–0.843) |
| RF | Balanced bagging | 0.604 (0.558–0.649) | 0.814 (0.790–0.838) | 0.769 (0.751–0.788) | 0.796 (0.770–0.823) |
| SVM | Balanced bagging | 0.474 (0.432–0.516) | 0.675 (0.636–0.714) | 0.632 (0.602–0.662) | 0.588 (0.563–0.620) |
| LR | Balanced bagging | 0.687 (0.640–0.734) | 0.736 (0.706–0.765) | 0.725 (0.707–0.744) | 0.781 (0.764–0.800) |
| DT | Balanced bagging | 0.497 (0.463–0.531) | 0.829 (0.815–0.842) | 0.758 (0.752–0.764) | 0.735 (0.717–0.755) |
| CatBoost | Balanced bagging | 0.606 (0.555–0.656) | 0.845 (0.823–0.867) | 0.794 (0.780–0.808) | 0.818 (0.797–0.843) |
| LGBM | Balanced bagging | 0.592 (0.559–0.625) | 0.851 (0.835–0.866) | 0.796 (0.786–0.805) | 0.811 (0.793–0.833) |
Figure 2.
ROC curves of combinations of different data balancing methods and machine learning algorithms. (A) No data balancing. (B) After class weight. (C) balanced bagging.
Based on the models' calculated accuracy, the CatBoost model without a balancing method exhibited the optimal performance in predicting the patients' risk of recurrent stroke (accuracy = 0.844), followed by the LGBM without balancing methods (accuracy = 0.832), the LGBM model without balancing methods (accuracy = 0.839), and the CatBoost model with class weight balancing (Accuracy = 0.829). Regarding specificity, the random forest with no balancing methods achieved the highest specificity (1.000).
The performance of each training model, as judged by sensitivity, was inadequate without the application of balancing methods. After data balancing using the class weight or balanced bagging methods, the sensitivity of random forest, Logistic Regression, decision tree, CatBoost, and LGBM models increased; the decision tree model with class weight balancing achieved a sensitivity of 0.617.
Since CatBoost model performed best among these models, we also compare the carotid sonographic features, clinical characteristics and combination between non-recurrent stroke and recurrent-stroke in CatBoostt model. Figure 3 showed AUC of combination was 0.837, AUC of carotid sonographic features was 0.809, and AUC of clinical parameters was 0.723.
Figure 3.
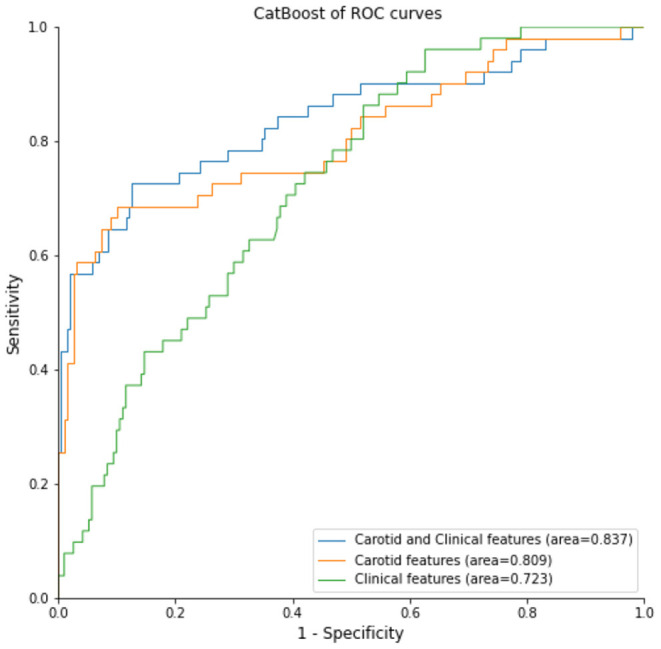
CatBoost model for different settings: clinical data, sonographic data, and combination of clinical and sonographic data for recurrent stroke prediction.
Top 10 Significant Features Correlated With Recurrent Stroke in the CatBoost Model
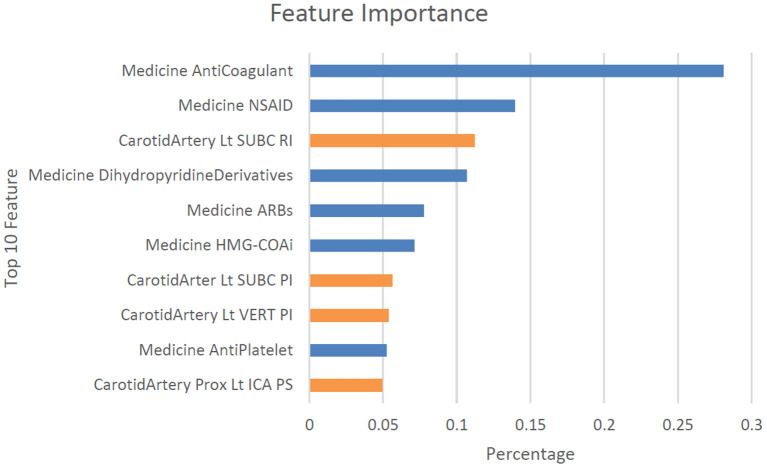
In this study, the CatBoost model with no balancing methods exhibited the best and the most stable performance, with an AUC of 0.844 (0.824–0.868), an accuracy of 0.876 (0.867–0.885), a sensitivity of 0.441 (0.394–0.488, no balancing method), and a specificity of 0.994 (0.990–0.998). We further analyzed the details of the CatBoost algorithm. The confusion matrix of the CatBoost model indicated that the numbers of patients with true positive and true negative results were 227 and 1,885, respectively, in our cross validated data set (Table 3). We also explored significant features identified by the CatBoost model for optimally predicting recurrent stroke. The top 10 most significant features were the use of anticoagulation medications, the use of NSAID medications, the resistive index (RI) of the left subclavian artery, the use of dihydropyridine derivatives medications, the use of ARBs medications, the use of HMG-COAi medications, the PI of the left subclavian artery, the PI of the left vertebral artery, the use of anti-platelet medications, and the PSV (peak systolic velocity) of the left proximal internal carotid artery (Figure 4).
Table 3.
Confusion matrix for the CatBoost without balancing method prediction.
| Predicted | |||
|---|---|---|---|
| No | Yes | ||
| True | No | 1,885 | 11 |
| Yes | 288 | 227 | |
Figure 4.
Top 10 features observed in the CatBoost model without balancing method for classifying stroke once and recurrent stroke. Dist, distal; Lt, left, Rt, right; CCA, common carotid artery; ICA, internal carotid artery; ECA, external carotid artery; SUBC, subclavian artery; VERT, vertebral artery; BA, basilar artery; Prox, proximal; TCD, transcranial Doppler; PI, pulse index; RI, resistive index; TAMEAN, intensity-weighted mean frequency; EDV, end-diastolic velocity; PSV, peak systolic velocity.
Discussion
In this study, we adopted machine learning algorithms to analyze potential clinical and sonographic risk factors for recurrent stroke among patients with acute stroke. We first evaluated the patients' carotid sonographic parameters using a large-scale CatBoost model and identified key features associated with an increased risk of recurrent stroke, including the use of anticoagulation medications, the use of NSAID medications, the resistive index (RI) of the left subclavian artery, the use of dihydropyridine derivatives medications, the use of ARBs medications, the use of HMG-COAi medications, the PI of the left subclavian artery, the PI of the left vertebral artery, the use of antiplatelet medications, and the PSV (peak systolic velocity) of the left proximal internal carotid artery. The CatBoost model demonstrated efficiency and achieved optimal performance in predicting non-recurrent and recurrent stroke on the basis of carotid Doppler sonographic parameters.
The significant correlation between the use of anticoagulation medications and recurrent stroke is reasonable because patients who have already experienced a stroke commonly use anticoagulation medications, especially those who had stroke of embolic events (42). Besides, our findings might also imply that cardiac or cryptogenic embolism would play a role in recurrent stroke (43, 44). Among the top 10 features correlated with recurrent stroke, four were carotid ultrasonographic parameters that require further investigation. Patients' vessel diameter; plaque; PSV; PI; RI; and end-diastolic velocity (EDV) of the left and right external carotid artery, internal carotid artery, subclavian artery, basilar artery, CCA, and vertebral artery are components commonly examined in standard carotid Doppler sonographic exams (45). However, most previous research has focused on the effects on stroke risk exerted by specific carotid sonographic features such as occlusion of the middle cerebral artery (MCA) (46); high-intensity signals of symptomatic arteries (47), carotid arteries, M1 segments of the MCA (48), and P2 segments of posterior cerebral arteries (PCAs) (48); the presence of carotid plaque (48, 49); ICA/CCA PSV ratios (50); decreased poststenotic PSV (51); and poststenotic arterial narrowing (51). To the best of our knowledge, this is the first study to involve the large-scale investigation of all carotid sonographic parameters. The correlation powers of the PI and RI of certain carotid arteries with recurrent stroke were greater than indices of carotid arteries stenosis, including PSV and percentage of stenosis, in our machine learning models. The degree of a patient's stenosis could be determined by their intima thickening and residual diameter/total diameter in grayscale ultrasound and PSV, ICA/CCA PSV, and ICA EDV in color Doppler ultrasound (52–54). Each patient's PI was calculated using the formula PI = (PSV – EDV)/MV, and the RI was calculated using RI = PSV – EDV/PSV (55). Previous studies have demonstrated that stroke risk is positively correlated with degree of stenosis in patients with symptomatic carotid stenosis (56). However, in the present study, we observed that the PI and RI of individual subclavian, vertebral, and internal carotid arteries were more positively correlated with recurrent stroke than stenosis was. These study results provide valuable clinical information because each carotid sonography was performed within 30 days of the respective patient's acute stroke (46). Our findings are consistent with those of Barnett et al. that ~20 and 45% of strokes in the symptomatic and asymptomatic carotid arteries with 70–99% stenosis, respectively, are unrelated to carotid stenosis (57). Because the complex machine learning algorithms employed in this study are black boxes, the variables explored should be interpreted as powerful indicators for differential diagnosis but not as casual factors (58). Furthermore, this study employed a cohort design; whether the PI and RI of individual subclavian, vertebral, and internal carotid arteries can be used to predict recurrent stroke must be investigated in future studies.
Regarding the machine learning algorithms, we believe we are the first to adopt the CatBoost model in the risk assessment of patients with acute stroke, and our study demonstrated that the CatBoost model exhibited high performance in predicting recurrent stroke. CatBoost is a powerful machine learning algorithm suitable for datasets with many categorical variables (59). CatBoost is commonly utilized in the fields of business (60), financial assessments (61), Medicare fraud detection (62), environmental science (63, 64), and public science (36). According to our review of the literature, in the field of medicine, the random forest model has retained a competitive edge and is often superior in the prediction and classification of medical conditions compared with traditional logistic regression methods and machine learning methods such as neural networks, SVMs, and decision trees (65–68). In our study, the CatBoost model outperformed the random forest model in classifying non-recurrent and recurrent stroke. Although its performance in other hospital settings has not been explored, our results indicate that CatBoost may be considered when selecting machine leaning models to apply for treating acute stoke or elsewhere in the medical field.
This study has several limitations. First, it is a single-center-based retrospective study, and external validation would be required to determine machine learning models' suitability for the risk assessment of other patients with acute stroke. Second, this was a retrospective cohort study and carotid ultrasonographic features were collected in the first time of stroke. Thus, the top 10 features identified in the study should be interpreted as predicative or classification factors rather than as casual factors. Further large-scale prospective cohort studies are necessary to investigate the predictive value of these features. Third, due to incomplete carotid Doppler sonography and missing values, only ~22% of 10,822 patients with acute stroke were enrolled in the study. Possible enrollment bias and baseline bias may have influenced the results. Fourth, information regarding the infarct areas of the patients was unavailable in the database employed in the study. Therefore, although we have reported four significant sonographic parameters related to brain vessels, whether the individual vessels overlapped with infarct areas or same infarct territory of high-risk artery were not analyzed in this study. Finally, in this study, further analysis about using TOAST classification for cerebral ischemic cases or dual antiplatelet agents among the recurrent event group have not been performed.
In conclusion, this study revealed that the CatBoost model is efficient and achieved optimal performance in predicting non-recurrent and recurrent stroke. The flow parameters of the carotid ultrasound, PI and RI, are more useful in differentiating between non-recurrent and recurrent stroke compared with other carotid ultrasonographic parameters.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of CMUH (CMUH109-REC2-035). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
S-YL and C-HK: conception/design. C-HK: provision of study materials. All authors have contributed significantly, agreement with the content of the manuscript, collection and/or assembly of data, data analysis, interpretation, manuscript writing, and final approval of manuscript.
Funding
This study was supported in part by China Medical University Hospital (DMR-110-089, DMR-111-090, and DMR-111-091); Ministry of Science and Technology (MOST 110-2321-B-039-003). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.804410/full#supplementary-material
References
- 1.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. (2019) 5:1–22. 10.1038/s41572-019-0118-8 [DOI] [PubMed] [Google Scholar]
- 2.Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. (2011) 13:200–7. 10.1007/s11906-011-0195-x [DOI] [PubMed] [Google Scholar]
- 3.Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. (2016) 94:634. 10.2471/BLT.16.181636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke. (2000) 31:1223–9. 10.1161/01.STR.31.6.1223 [DOI] [PubMed] [Google Scholar]
- 5.Saengsuwan J, Suangpho P, Tiamkao S. Knowledge of stroke risk factors and warning signs in patients with recurrent stroke or recurrent transient ischaemic attack in Thailand. Neurol Res Int. (2017) 2017:8215726. 10.1155/2017/8215726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arima H, Chalmers J. Progress: prevention of recurrent stroke. J Clin Hypertens. (2011) 13:693–702. 10.1111/j.1751-7176.2011.00530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogata T, Matsuo R, Kiyuna F, Hata J, Ago T, Tsuboi Y, et al. Left atrial size and long-term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc. (2017) 6:e006402. 10.1161/JAHA.117.006402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo J, Alvarez-Sabín J, Martínez-Vila E, Montaner J, Sobrino T, Vivancos J, et al. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol. (2009) 256:217–24. 10.1007/s00415-009-0058-4 [DOI] [PubMed] [Google Scholar]
- 9.Segal HC, Burgess AI, Poole DL, Mehta Z, Silver LE, Rothwell PM. Population-based study of blood biomarkers in prediction of subacute recurrent stroke. Stroke. (2014) 45:2912–7. 10.1161/STROKEAHA.114.005592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams SR, Hsu FC, Keene KL, Chen WM, Dzhivhuho G, Rowles 3rd JL, et al. Genetic drivers of von Willebrand factor levels in an ischemic stroke population and association with risk for recurrent stroke. Stroke. (2017) 48:1444–50. 10.1161/STROKEAHA.116.015677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X, Liu H, Zhang X, Zhang H, Qin X, Ji X. Metabolic syndrome, its components, and diabetes on 5-year risk of recurrent stroke among mild-to-moderate ischemic stroke survivors: a multiclinic registry study. J Stroke Cerebrovasc Dis. (2016) 25:626–34. 10.1016/j.jstrokecerebrovasdis.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Zhao X, Wang C, Liu L, Ding Y, Akbary F, et al. Prediction factors of recurrent ischemic events in one year after minor stroke. PLoS ONE. (2015) 10:e0120105. 10.1371/journal.pone.0120105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng YY, Shu JH, Hsu HC, Liang Y, Chang ST, Kao CL, et al. The impact of rehabilitation frequencies in the first year after stroke on the risk of recurrent stroke and mortality. J Stroke Cerebrovasc Dis. (2017) 26:2755–62. 10.1016/j.jstrokecerebrovasdis.2017.06.047 [DOI] [PubMed] [Google Scholar]
- 14.Lyu J, Ma N, Tian C, Xu F, Shao H, Zhou X, et al. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc Neurol. (2019) 4:129–34. 10.1136/svn-2018-000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke. (2012) 43:1013–7. 10.1161/STROKEAHA.111.637421 [DOI] [PubMed] [Google Scholar]
- 16.Kang DW, Latour L, Chalela J, Dambrosia J, Warach S. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology. (2004) 63:2261–5. 10.1212/01.WNL.0000147295.50029.67 [DOI] [PubMed] [Google Scholar]
- 17.Shi Z, Li J, Zhao M, Zhang X, Degnan AJ, Mossa-Basha M, et al. Progression of plaque burden of intracranial atherosclerotic plaque predicts recurrent stroke/transient ischemic attack: a pilot follow-up study using higher-resolution MRI. J Magn Reson Imaging. (2021) 54:560–70. 10.1002/jmri.27561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology. (2017) 88:2260–7. 10.1212/WNL.0000000000004042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weimar C, Benemann J, Michalski D, Müller M, Luckner K, Katsarava Z, et al. Prediction of recurrent stroke and vascular death in patients with transient ischemic attack or nondisabling stroke: a prospective comparison of validated prognostic scores. Stroke. (2010) 41:487–93. 10.1161/STROKEAHA.109.562157 [DOI] [PubMed] [Google Scholar]
- 20.Andersen SD, Gorst-Rasmussen A, Lip GY, Bach FW, Larsen TB. Recurrent stroke: the value of the CHA2DS2VASc score and the essen stroke risk score in a nationwide stroke cohort. Stroke. (2015) 46:2491–7. 10.1161/STROKEAHA.115.009912 [DOI] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Brazzelli M, Chappell FM, Miranda H, Shuler K, Sandercock PA, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. (2015) 85:373–80. 10.1212/WNL.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyohara T, Kamouchi M, Kumai Y, Ninomiya T, Hata J, Yoshimura S, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short-and long-term risks of stroke after transient ischemic attack. Stroke. (2014) 45:418–25. 10.1161/STROKEAHA.113.003077 [DOI] [PubMed] [Google Scholar]
- 23.Martinez G, Katz JM, Pandya A, Wang JJ, Boltyenkov A, Malhotra A, et al. Cost-effectiveness study of initial imaging selection in acute ischemic stroke care. J Am Coll Radiol. (2021) 18:820–33. 10.1016/j.jacr.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puhr-Westerheide D, Froelich MF, Solyanik O, Gresser E, Reidler P, Fabritius MP, et al. Cost-effectiveness of short-protocol emergency brain MRI after negative non-contrast CT for minor stroke detection. Eur Radiol. (2021) 2021:8222. 10.1007/s00330-021-08222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrnes KR, Ross CB. The current role of carotid duplex ultrasonography in the management of carotid atherosclerosis: foundations and advances. Int J Vasc Med. (2012) 2012:187872. 10.1155/2012/187872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haq S, Mathur M, Singh J, Kaur N, Sibia RS, Badhan R. Colour Doppler evaluation of extracranial carotid artery in patients presenting with acute ischemic stroke and correlation with various risk factors. J Clin Diagn Res. (2017) 11:TC01–5. 10.7860/JCDR/2017/25493.9541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranstam J, Cook J. LASSO regression. J Br Surg. (2018) 105:1348–1348. 10.1002/bjs.1089525855820 [DOI] [Google Scholar]
- 28.Johnson JM, Khoshgoftaar TM. Survey on deep learning with class imbalance. J Big Data. (2019) 6:27. 10.1186/s40537-019-0192-5 [DOI] [Google Scholar]
- 29.Hakim L, Sartono B, Saefuddin A. Bagging Based Ensemble Classification Method on Imbalance Datasets. Bogor Regency: Repositories-Dept. of Statistics, IPB University; (2017). p. 670–6. [Google Scholar]
- 30.Couronné R, Probst P, Boulesteix AL. Random forest versus logistic regression: a large-scale benchmark experiment. BMC Bioinformat. (2018) 19:1–14. 10.1186/s12859-018-2264-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breiman L. Random forests. Mach Learn. (2001) 45:5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 32.Noble WS. What is a support vector machine? Nat Biotechnol. (2006) 24:1565–7. 10.1038/nbt1206-1565 [DOI] [PubMed] [Google Scholar]
- 33.Suykens JA, Vandewalle J. Least squares support vector machine classifiers. Neural Proces Lett. (1999) 9:293–300. 10.1023/A:101862860974211972910 [DOI] [Google Scholar]
- 34.Sarker IH, Colman A, Han J, Khan AI, Abushark YB, Salah K. Behavdt: a behavioral decision tree learning to build user-centric context-aware predictive model. Mob Netw Appl. (2020) 25:1151–61. 10.1007/s11036-019-01443-z [DOI] [Google Scholar]
- 35.Hancock JT, Khoshgoftaar TM. CatBoost for big data: an interdisciplinary review. J big data. (2020) 7:1–45. 10.1186/s40537-020-00369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang P, Lin Z, Teng S, Zhang G, Guo L, Zhang W. Catboost-based framework with additional user information for social media popularity prediction. In: MM '19: Proceedings of the 27th ACM International Conference on Multimedia. (2019). p. 2677–81. 10.1145/3343031.3356060 [DOI] [Google Scholar]
- 37.Alzamzami F, Hoda M, El Saddik A. Light gradient boosting machine for general sentiment classification on short texts: a comparative evaluation. IEEE Access. (2020) 8:101840–58. 10.1109/ACCESS.2020.299733027295638 [DOI] [Google Scholar]
- 38.Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, et al. Lightgbm: A highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. (2017) 30:3146–54. [Google Scholar]
- 39.Berrar D. Cross-validation. Encycl Bioinformat Computat Biol. (2018) 1:542–5. 10.1016/B978-0-12-809633-8.20349-X [DOI] [Google Scholar]
- 40.Govindarajan M, Chandrasekaran R. Evaluation of k-nearest neighbor classifier performance for direct marketing. Expert Syst Appl. (2010) 37:253–8. 10.1016/j.eswa.2009.04.055 [DOI] [Google Scholar]
- 41.Lundberg SM, Lee SI. A unified approach to interpreting model predictions. In: NIPS'17: Proceedings of the 31st International Conference on Neural Information Processing Systems. (2017). p. 4768–77. [Google Scholar]
- 42.Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. (2018) 3:76–83. 10.1136/svn-2018-000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamel H, Healey JS. Cardioembolic stroke. Circ Res. (2017) 120:514–26. 10.1161/CIRCRESAHA.116.308407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang WY, Ovbiagele B, Lee M. Oral anticoagulants vs. antiplatelets in cryptogenic stroke with potential cardiac emboli: meta-analysis. Eur J Intern Med. (2021). 95:44–9. 10.1016/j.ejim.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 45.Nedelmann M, Stolz E, Gerriets T, Baumgartner RW, Malferrari G, Seidel G, et al. Consensus recommendations for transcranial color-coded duplex sonography for the assessment of intracranial arteries in clinical trials on acute stroke. Stroke. (2009) 40:3238–44. 10.1161/STROKEAHA.109.555169 [DOI] [PubMed] [Google Scholar]
- 46.Allendoerfer J, Goertler M, von Reutern GM. Prognostic relevance of ultra-early doppler sonography in acute ischaemic stroke: a prospective multicentre study. Lancet Neurol. (2006) 5:835–40. 10.1016/S1474-4422(06)70551-8 [DOI] [PubMed] [Google Scholar]
- 47.Babikian VL, Hyde C, Pochay V, Winter MR. Clinical correlates of high-intensity transient signals detected on transcranial Doppler sonography in patients with cerebrovascular disease. Stroke. (1994) 25:1570–3. 10.1161/01.STR.25.8.1570 [DOI] [PubMed] [Google Scholar]
- 48.Wada K, Kimura K, Minematsu K, Yasaka M, Uchino M, Yamaguchi T. Combined carotid and transcranial color-coded sonography in acute ischemic stroke. Eur J Ultrasound. (2002) 15:101–8. 10.1016/S0929-8266(02)00030-7 [DOI] [PubMed] [Google Scholar]
- 49.Singh AS, Atam V, Jain N, Yathish BE, Patil MR, Das L. Association of carotid plaque echogenicity with recurrence of ischemic stroke. N Am J Med Sci. (2013) 5:371. 10.4103/1947-2714.114170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes M, Keerthiraj B, Mahale AR, Kumar A, Dudekula A. Evaluation of carotid arteries in stroke patients using color Doppler sonography: a prospective study conducted in a tertiary care hospital in South India. Int J Appl Basic Med Res. (2016) 6:38–44. 10.4103/2229-516X.174007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaser T, Hofmann K, Buerger T, Effenberger O, Wallesch CW, Goertler M. Risk of stroke, transient ischemic attack, and vessel occlusion before endarterectomy in patients with symptomatic severe carotid stenosis. Stroke. (2002) 33:1057–62. 10.1161/01.STR.0000013671.70986.39 [DOI] [PubMed] [Google Scholar]
- 52.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis—Society of Radiologists in Ultrasound consensus conference. Ultrasound Q. (2003) 19:190–8. 10.1097/00013644-200312000-00005 [DOI] [PubMed] [Google Scholar]
- 53.Alexandrov AV, Vital D, Brodie DS, Hamilton P, Grotta JC. Grading carotid stenosis with ultrasound: an interlaboratory comparison. Stroke. (1997) 28:1208–10. 10.1161/01.STR.28.6.1208 [DOI] [PubMed] [Google Scholar]
- 54.Filis KA, Arko FR, Johnson BL, Pipinos II, Harris EJ, Olcott C 4th, et al. Duplex ultrasound criteria for defining the severity of carotid stenosis. Ann Vasc Surg. (2002) 16:413–21. 10.1007/s10016-001-0175-8 [DOI] [PubMed] [Google Scholar]
- 55.Moreira T, Michel P, Binaghi S, Hirt L. Risk factor impact on blood flow velocities and clinical outcomes of stented cervical and intracranial stenoses: preliminary observations. Clin Neurol Neurosurg. (2012) 114:922–9. 10.1016/j.clineuro.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 56.Rothwell PM, Gibson R, Warlow C. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. Stroke. (2000) 31:615–21. 10.1161/01.STR.31.3.615 [DOI] [PubMed] [Google Scholar]
- 57.Barnett HJ, Gunton RW, Eliasziw M, Fleming L, Sharpe B, Gates P, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. J Am Med Assoc. (2000) 283:1429–36. 10.1001/jama.283.11.1429 [DOI] [PubMed] [Google Scholar]
- 58.Rudin C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Machine Intell. (2019) 1:206–15. 10.1038/s42256-019-0048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prokhorenkova L, Gusev G, Vorobev A, Dorogush AV, Gulin A. CatBoost: unbiased boosting with categorical features. arXiv Preprint arXiv:1706.09516. (2017). [Google Scholar]
- 60.Jhaveri S, Khedkar I, Kantharia Y, Jaswal S. Success prediction using random forest, CatBoost, XGBoost and AdaBoost for kickstarter campaigns. In: 2019 3rd International Conference on Computing Methodologies and Communication (ICCMC). (2019). p. 1170–3. 10.1109/ICCMC.2019.8819828 [DOI] [Google Scholar]
- 61.Jabeur SB, Gharib C, Mefteh-Wali S, Arfi WB. CatBoost model and artificial intelligence techniques for corporate failure prediction. Technol Forecast Soc Change. (2021) 166:120658. 10.1016/j.techfore.2021.120658 [DOI] [Google Scholar]
- 62.Hancock J, Khoshgoftaar TM. Performance of CatBoost and XGBoost in medicare fraud detection 2020. In: 19th IEEE International Conference on Machine Learning and Applications (ICMLA). (2020). p. 572–9. 10.1109/ICMLA51294.2020.00095 [DOI] [Google Scholar]
- 63.Luo M, Wang Y, Xie Y, Zhou L, Qiao J, Qiu S, et al. Combination of feature selection and CatBoost for prediction: the first application to the estimation of aboveground biomass. Forests. (2021) 12:216. 10.3390/f12020216 [DOI] [Google Scholar]
- 64.Huang G, Wu L, Ma X, Zhang W, Fan J, Yu X, et al. Evaluation of CatBoost method for prediction of reference evapotranspiration in humid regions. J Hydrol. (2019) 574:1029–41. 10.1016/j.jhydrol.2019.04.085 [DOI] [Google Scholar]
- 65.Watanabe E, Noyama S, Kiyono K, Inoue H, Atarashi H, Okumura K, et al. Comparison among random forest, logistic regression, and existing clinical risk scores for predicting outcomes in patients with atrial fibrillation: a report from the J-RHYTHM registry. Clin Cardiol. (2021) 44:1305–15. 10.1002/clc.23688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng SY, Chuang YC, Kang TW, Tseng KH. Random forest can predict 30-day mortality of spontaneous intracerebral hemorrhage with remarkable discrimination. Eur J Neurol. (2010) 17:945–50. 10.1111/j.1468-1331.2010.02955.x [DOI] [PubMed] [Google Scholar]
- 67.Hsieh CH, Lu RH, Lee NH, Chiu WT, Hsu MH, Jack Li YC. Novel solutions for an old disease: diagnosis of acute appendicitis with random forest, support vector machines, and artificial neural networks. Surgery. (2011) 149:87–93. 10.1016/j.surg.2010.03.023 [DOI] [PubMed] [Google Scholar]
- 68.Daghistani T, Alshammari R. Comparison of statistical logistic regression and random forest machine learning techniques in predicting diabetes. J Adv Inform Technol. (2020) 11:78–83. 10.12720/jait.11.2.78-83 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.