Abstract
Simple Summary
Photoprotection reduces invasive melanoma incidence and mortality, but not all sun protection modalities are created equal. Dermatologists have long debated the pros and cons of photoprotective clothing and sunscreen, but few studies compare the effectiveness of these two modalities head-to-head. This study uses both in vitro and in vivo techniques to compare the ultraviolet radiation (UVR) protective capacity of four modern textiles and two commercially available, broad-spectrum sunscreens.
Abstract
Ultraviolet radiation (UVR) exposure is the most important modifiable risk factor for skin cancer development. Although sunscreen and sun-protective clothing are essential tools to minimize UVR exposure, few studies have compared the two modalities head-to-head. This study evaluates the UV-protective capacity of four modern, sun-protective textiles and two broad-spectrum, organic sunscreens (SPF 30 and 50). Sun Protection Factor (SPF), Ultraviolet Protection Factor (UPF), Critical Wavelength (CW), and % UVA- and % UVB-blocking were measured for each fabric. UPF, CW, % UVA- and % UVB-blocking were measured for each sunscreen at 2 mg/cm2 (recommended areal density) and 1 mg/cm2 (simulating real-world consumer application). The four textiles provided superior UVR protection when compared to the two sunscreens tested. All fabrics blocked erythemogenic UVR better than the sunscreens, as measured by SPF, UPF, and % UVB-blocking. Each fabric was superior to the sunscreens in blocking full-spectrum UVR, as measured by CW and % UVA-blocking. Our data demonstrate the limitations of sunscreen and UV-protective clothing labeling and suggest the combination of SPF or UPF with % UVA-blocking may provide more suitable measures for broad-spectrum protection. While sunscreen remains an important photoprotective modality (especially for sites where clothing is impractical), these data suggest that clothing should be considered the cornerstone of UV protection.
Keywords: photoprotective clothing, photoprotection, sun protection factor (SPF), ultraviolet protection factor (UPF), critical wavelength (CW), skin cancer, melanoma
1. Introduction
1.1. UVB and UVA Cause Skin Cancer via Cyclobutane Pyrimidine Dimers (CPDs)
Exposure to ultraviolet radiation (UVR) remains the most important and most modifiable risk factor for the development of skin cancer [1,2]. As skin cancer incidence rises worldwide, minimization of exposure to UVR is critical for reducing the morbidity, mortality, and cost associated with skin cancer [3]. To achieve this, the American Academy of Dermatology recommends sun avoidance, application of broad-spectrum sunscreen (SPF 30 or higher), and use of hats and protective clothing [4]. Since avoidance of UVR is not always possible, sunscreen and sun-protective clothing are essential features of a multi-pronged approach to skin cancer prevention. Recent data from Queensland, Australia underscore the fact that photoprotection is effective. Aitken et al. showed a decline in invasive melanoma incidence and mortality in individuals under age 40 years in Queensland [5]. The steepest decline was in those born after 1980, the decade in which photoprotection education initiatives such as the “Slip” (on a shirt), “Slop” (on sunscreen), and “Slap” (on a hat) campaign were implemented [5,6].
Both UVB (280–315 nm) and UVA (315–400 nm) radiation are carcinogenic. Of note, UVC (100–280 nm) is the most damaging type of UVR but is completely attenuated by ozone in the atmosphere before reaching the earth’s surface. The UV portion of solar radiation that reaches the earth’s surface comprises 5% UVB and 95% UVA radiation. UVB is a higher energy radiation but is less able to penetrate through the skin than the longer wavelengths of UVA. UVB causes erythema of skin and has historically been implicated as the main contributor to the development of skin cancer [7]. UVB is directly absorbed by DNA bases resulting in the formation of cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts that can result in mutations if not repaired [7]. UVA causes pigment darkening or tanning of the skin and is associated with photoaging [8]. Recent work shows that UVA has a much greater role than previously understood in skin cancer development due to its ability to induce reactive oxygen and nitrogen species, and ultimately generate CPDs hours after UVA exposure [9,10,11,12].
UV-induced CPDs are more mutagenic than 6-4 photoproducts, likely due to their slower repair [13]. The most common UV-induced mutation in human skin cancer is the C>T transition. This mutation can be caused by the rapid deamination of cytosine (C) or 5-methylcytosine in CPDs, transforming them into uracil and thymidine, respectively. Error-free replication of the deaminated CPDs by DNA polymerase η then passes on C>T mutations to daughter cells [14]. The CPDs thereby cause skin cancer [15].
1.2. Sunscreens and Clothing Protect against UVR-Induced Mutagenesis
Active ingredients of sunscreens fall into two categories: inorganic (also known as mineral or physical) and organic (also referred to as chemical). Inorganic ingredients include zinc oxide (ZnO) and titanium dioxide (TiO2). New data suggest that these inorganic compounds primarily absorb UV radiation within the UVB and short UVA wavelengths and reflect radiation in the long UVA and visible wavelengths [16]. Organic UVR filters contain aromatic hydrocarbons that absorb photons in the UV spectrum and emit lower-energy, longer wavelength photons and/or heat that do not damage the skin [17]. Both inorganic and organic sunscreens prevent actinic keratoses (premalignant keratinocytic neoplasms) and squamous cell carcinoma [18,19,20]. Sunscreen is also effective in prevention of basal cell carcinoma and melanoma [18,21,22]. Furthermore, routine sunscreen application prevents photoaging [17].
Clothing provides protection by scattering and absorbing UVR. Mouse models have shown that sun-protective clothing can prevent skin cancer, but little human data exist in the literature [23]. However, since UVR is a causal agent in skin cancer, protection with clothing is likely to be dependent on the degree of protection provided from UVR. The degree of protection depends on the color, material, fiber, yarn and fabric structure [24]. Fabric structure is one of the most important factors, with the least porous material providing the greatest protection [25,26,27]. Synthetic fabrics have demonstrated the highest UV protection [28,29,30]. Dark colors absorb more UVR and thus provide higher protection than light colors [29,31,32,33]. Other factors that impact penetration of UVR through the clothing include: stretch, wetness, wear (from use or washing), color loss (bleaching), UVR-absorbing additives, and yarn morphology [34,35,36,37]. Although all clothing blocks some degree of UVR, some studies suggest many commonly worn fabrics may provide insufficient UVR protection [29,38,39].
1.3. Measures of UVR Protection
The sun protection factor (SPF) rating traditionally used in sunscreen labeling is measured by exposing small areas of skin of human subjects (Fitzpatrick skin types I–III) to simulated solar radiation for varying durations. The smallest dose of UVR that produces visible, well-circumscribed redness on tested skin is called the minimal erythemal dose (MED). SPF is the ratio of the MED with sunscreen uniformly applied at 2 mg/cm2 (MEDprotected) to that of skin without sunscreen (MEDunprotected):
| (1) |
Simply stated, SPF reflects the factor by which one can spend more time in the sun without getting burned. For example, SPF 30 would theoretically allow someone who would normally burn in 10 min to be exposed for 300 min before burning, assuming constant incident solar UVR energy.
The ultraviolet protection factor (UPF) rating traditionally used for clothing labeling requires a laboratory spectrophotometer to measure UVR transmittance (the fraction of UVR that is transmitted through the clothing). Although related, SPF and UPF are not equivalent [40]. In contrast to SPF measurements (that utilize UVB-induced erythema as the readout), UPF objectively measures all wavelengths of solar simulated light transmitted through the clothing and then applies weighting constants to mathematically recapitulate SPF testing. These constants (known as the erythemal effectiveness function and solar spectral irradiance) heavily weight UPF toward the UVB wavelengths (Figure 1A) because these are the primary wavelengths that induce erythema in the skin. The UPF of a garment represents how much erythemally-weighted UVR is transmitted through the clothing. UPF values are the ratio of erythemally-weighted UVR detected with or without a specimen (clothing) between the light source and detector:
| (2) |
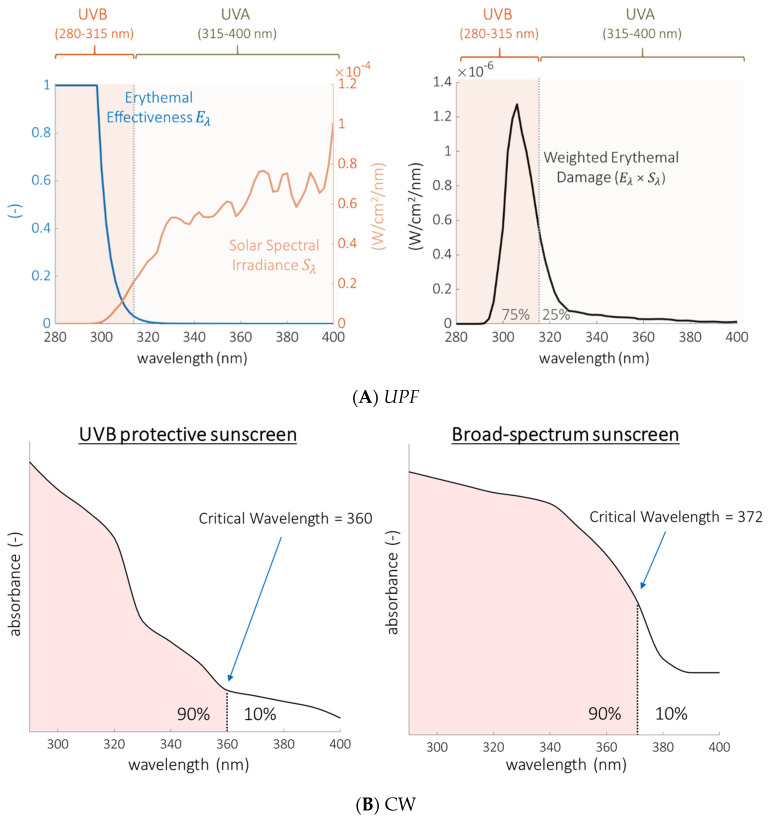
Figure 1.
Ultraviolet protection factor (UPF) is primarily a measure of UVB protection whereas critical wavelength (CW) is a measure of the degree of broad-spectrum protection. (A) UPF is a mathematical function designed to recapitulate sun protection factor (SPF) from a laboratory measurement of transmittance and is weighted toward the UVB portion of the spectrum. Left: Plots of the erythemal effectiveness function (Eλ) and the solar spectral irradiance (Sλ) over the UVR spectral range. Right: The product of Eλ and Sλ has a peak that lies predominantly (75%) within the UVB range (280–315 nm). Very little of the UPF function comes from wavelengths longer than 360 nm, where the UVR intensity is highest but the erythemal effectiveness is near zero [48]. (B) Visual representation of the CW as the wavelength below which 90% of the total absorbance (area under the curve) in the UVR region is contained. Left: Hypothetical sunscreen that primarily blocks UVB radiation has a critical wavelength below the 370 nm threshold required by the FDA to be labeled broad-spectrum. Right: Hypothetical sunscreen with improved UVA blocking performance meets the broad-spectrum criterion. A sunscreen could meet the CW criterion of 370 nm without comprehensively blocking UVA radiation.
Eλ is the relative erythemal spectral effectiveness, a constant that adjusts for the ability of each wavelength (λ) to generate cutaneous erythema [41]. Sλ is the solar spectral irradiance (Wm−2 nm−1), a constant that represents sun intensity at each wavelength at noon in Albuquerque, New Mexico [42]. Tλ is the average measured spectral transmittance of the specimen and Δλ is the measured wavelength interval (nm).
Both of these measures represent the combined effect of incident dose and a biological response. This biological response, slight reddening, is related to discomfort but it is blistering sunburn that is related to skin cancer [43,44]. Neither SPF or UPF rating systems take into consideration the fact that non-erythema-inducing UVR wavelengths are carcinogenic. Nor do they take into consideration the fact that erythema and skin cancer have different dose-dependencies. One measure currently used to address this deficit in evaluating the performance of sunscreen is the critical wavelength.
The critical wavelength (CW, λc) is calculated from the measured absorbance of a sunscreen across the entire UV spectrum and is intended to provide an objective quantification of how well a sunscreen reduces exposure to both UVB and UVA wavelengths [45]. Absorbance (A), is the negative of the (base ten) logarithm of transmittance (T):
| (3) |
For example, at a given wavelength, a sunscreen or garment that blocks 90% of incident UV radiation has an absorbance of 1 [−log (0.1)], while one that blocks 99% of incident UV has an absorbance of 2 [−log (0.01)]. The transmittance of a sunscreen or textile is measured with a spectrophotometer equipped with an integrating sphere, which allows capture of all radiation that is diffusely scattered and transmitted through the sample. It is important to note that absorbance, as used in the quantitative expressions described here, represents more than just absorbed light; it also includes alternate means of radiation attenuation including the reflection and/or scattering of incident light.
The critical wavelength is the wavelength below which 90% of the total absorbance (A) of a sunscreen in the atmosphere-penetrating UVR region is contained (Figure 1B):
| (4) |
In other words, the absorbance of a particular sunscreen is measured as a function of wavelength between 290–400 nm (UVB from 290–315 and UVA from 315–400). UVB-specific sunscreen ingredients are not effective at preventing transmission in the UVA range, producing absorbance curves that peak at the shorter end of the wavelength spectrum. In contrast, UVA filters shift the absorbance curves to the right at the longer UVA wavelengths. Sunscreens that block both UVA and UVB radiation have absorbance curves that extend across the majority of the spectral region (Figure 1B). The area under the curve is summed (integrated) from 290 nm to 400 nm. The critical wavelength is the wavelength at which 90% of the total area under the absorbance curve is reached. Therefore, a higher critical wavelength indicates that the absorbance of a material is proportionally greater at longer wavelengths and offers more relative protection from UVA radiation. However, a UVA-specific agent with little absorbance in the UVB range could have a very high critical wavelength yet inadequate protection against UVB or sunburn. Therefore, the critical wavelength should never be interpreted in isolation and is only meaningful when SPF is also taken into consideration. The United States Food and Drug Administration (FDA) requires sunscreen to have a CW greater than 370 nm to be labeled as broad-spectrum [46].
% UV-Blocking. The critical-wavelength requirement does not exist for garments. Instead, some countries require sun-protective garments to have less than 5% transmittance in the UVA region (T(UVA), Equation (5)) [47].
| (5) |
These values are often reported as % UVA blocking = 100% − T(UVA), where T(UVA) is expressed as a percentage. Similarly, transmittance in the UVB region, T(UVB), is determined using an analogous equation but evaluating wavelengths from 280 to 315 nm, and % UVB blocking = 100% − T(UVB) where T(UVB) is expressed as a percentage.
In this paper, we compare the SPF, UPF, CW, and % UV-Blocking of commercial sunscreens to those of modern sun-protective clothing. We follow this with a discussion of the consequences of exposure of human skin to UVA radiation and summarize new data on the mutagenic properties of visible light. We highlight the potential of sun-protective clothing to offer superior protection from the underappreciated risks posed by these wavelengths of solar radiation.
2. Materials and Methods
2.1. Fabrics
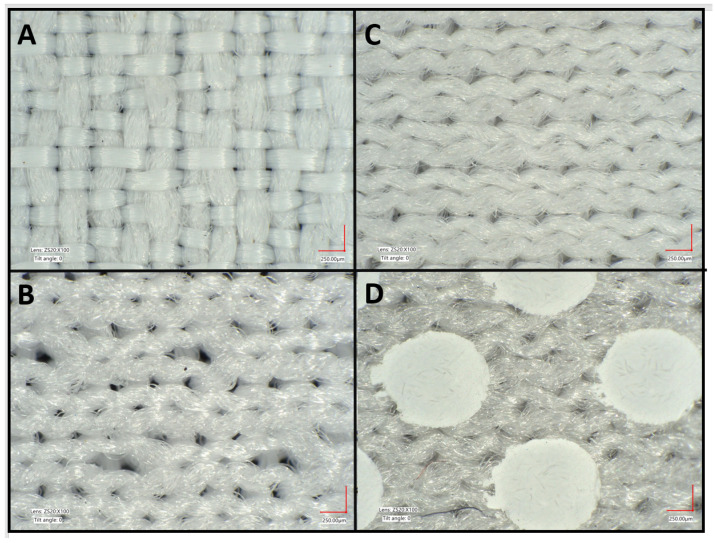
Four fabric samples (Table 1, Figure 2) used in commercial sun-protective apparel (Columbia Sportswear, Portland, OR, USA) were chosen for the study because they are representative of modern sun-protective materials: one nylon woven and three polyester knit fabrics. The three knit fabrics include two different common knit structures, a pique and an interlock. Of the two interlock knit fabrics, one includes a TiO2 dot print covering about 30% of its surface. These white dots are present for heat mitigation, and work by reflecting more solar radiation and emitting more thermal radiation than the underlying polyester fabric [49]. These four fabrics are generally thinner and lighter than the ones for which UPF and SPF data have been previously reported in the literature [24]. All fabrics were dyed to be off-white.
Table 1.
Specifications of tested fabrics (A) and commercial sunscreens (B). FD = fully drawn, DTY = drawn textured yarn, D = denier *, f = filaments. All knit fabrics are 28 gauge.
| (A) Fabrics | |||
| Fabric | Yarn(s) |
Areal Density
(g/m2) |
Thickness (mm) |
| Nylon Woven | 70D(48f) × 160D(144f) | 107 | 0.36 |
| Polyester Pique Knit | 75D(72f) × 75D(36f) FD | 180 | 0.74 |
| Polyester Interlock Knit | 50D/72f FD DTY | 90 | 0.48 |
| Polyester Interlock Knit w/TiO2 dot print at 30% surface coverage | 50D/72f FD DTY | 95 | 0.46 |
| (B) Commercial Sunscreens | |||
| Sunscreen | Labeled SPF | Active Ingredients | |
| Sunscreen A | 30 Broad-spectrum |
avobenzone 3%, homosalate 8%, octisalate 4.5%, octocrylene 6% | |
| Sunscreen B | 50 Broad-spectrum |
avobenzone 3%, homosalate 10%, octisalate 4.5%, octocrylene 8% |
|
* Denier is a measure of linear density, an indicator of yarn or filament size. More specifically, denier is the weight in grams of 9000 m of yarn or filament.
Figure 2.
Four commercial fabrics were selected for study: (A) 107-gsm (g/m2) nylon woven, (B) 180-gsm polyester pique knit, (C) 90-gsm polyester interlock knit, and (D) 95-gsm polyester interlock knit with TiO2 dot print at 30% surface coverage. These fabrics are currently used in sun-protective apparel. Each fabric is constructed of multifilament synthetic yarns from 50 to 160 denier. Fabric images were taken using a Keyence VHX-7000 Digital Microscope (Itasca, IL, USA). Scale bar is 250 µm.
2.2. Sunscreens
Two organic, broad-spectrum sunscreens (SPF 30 and SPF 50, Table 1) were selected for comparison with the four fabrics. The sunscreens were the same brand and contained identical active ingredients: avobenzone, homosalate, octisalate, and octocrylene. The SPF 50 sunscreen had a higher percentage of homosalate (10% vs. 8%) and octocrylene (8% vs. 6%). These active ingredients are four of the sixteen UV filters listed in the FDA Code of Federal Regulations [50] and are a common combination used in multiple brands of U.S. sunscreens. Tests were performed with newly opened, unexpired products.
2.3. In Vitro UPF Testing
2.3.1. Fabric UPF
UPF measurements of the textile fabrics were conducted as described in the American Association of Textile Chemists and Colorists (AATCC) Test Method 183 [48]. Briefly, each dry, unstretched fabric was placed in a UV-2000S Ultraviolet Transmittance Analyzer (Labsphere Inc., North Sutton, NH, USA) to capture diffuse transmittance from 280 to 400 nm. Five unique measurements were taken at different sample orientations rotated by 45° between each measurement. UPF was calculated for the transmission spectrum of each sample using the formula in Equation (2), and then an average UPF and standard deviation were calculated from the individual UPF values [48].
2.3.2. Sunscreen UPF
UPF measurements of two commercial broad-spectrum sunscreens were conducted using AATCC Test Method 183 [48]. Of note, many studies refer to spectrophotometric testing of sunscreens as “in vitro SPF” and to erythema measurements of textiles on human skin as “in vivo UPF.” To eliminate confusion, we will use the terms “sunscreen UPF” and “fabric SPF” throughout. The two sunscreens were applied in accordance with International Organization for Standardization (ISO) 2444 to UVR-transparent quartz slides at a density of 2 mg/cm2. Additional testing was performed at a density of 1 mg/cm2 to simulate real-world consumer application [51]. Transmittance was measured using a Thermo Fisher Evolution Bio260 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). To account for non-uniformity in coating thickness, three slides were prepared for each sunscreen SPF level and coating density. Ten measurements were taken in random positions on each slide. UPF was calculated for each measurement, and the UPF is reported as the mean and standard deviation of these measurements.
2.4. In Vivo SPF Testing
2.4.1. SPF of Fabrics
The fabric SPF on human skin of the four textile samples were measured by a third party (AMA Laboratories, New City, NY, USA) using the standard procedure described by the International SPF Test Method of COLlPA, CTFA, JCIA and CTFA-SA [52]. The study was approved by the AMA Laboratories Internal Review Board. Briefly, the dose of UVR was supplied by either a 150 or 300 watt Xenon Arc Solar Simulator (Solar Light Co., Philadelphia, PA, USA), each with a continuous emission spectrum from 290 to 400 nm. Both were equipped with dichroic mirrors and a 1-mm Schott WG-320 filter to produce a simulated solar UVA-UVB emission spectrum. These were also equipped with a 1-mm UG 11 filter to remove reflected heat as well as visible and infrared radiation.
A total of five healthy adult volunteers (ages 36 to 64 years) were selected by AMA laboratories. Three volunteers (one from each Fitzpatrick Skin Type I, II, and III to maximize the human variation studied) underwent SPF testing for each fabric [53] (Tables S1 and S2). All individuals provided written informed consent. The bilateral infrascapular area of each subject’s back was used as the test site. Test sites were cleaned with a dry cotton pad, and rectangular areas of at least 30 cm2 were demarcated. A minimum of five progressive UVR doses were administered within this site to determine a subject’s minimal erythemal dose on unprotected skin (MEDunprotected). Each subject’s MEDunprotected was defined as the shortest time of exposure (or lowest UVR dose required) that produced minimally perceptible erythema at 16 to 24 h post exposure. A control sunscreen with standard SPF 15 or 16 was used as a verification technical control.
Once the MEDunprotected had been determined, subjects returned to the lab for SPF testing of the fabrics. The test fabric was secured closely to each subject’s skin without stretching and using a thin layer of adhesive tape on the sample periphery to cover a minimum area of 30 cm2. Based upon each subject’s previously determine MEDunprotected, the test areas were irradiated with a series of progressively higher UVR doses (minimum of five). The subjects returned to the testing facility 16–24 h after UVR exposure for determination of the MED of protected skin (MEDprotected) by a blinded evaluator. Each fabric was tested on three subjects and the mean SPF was determined for each individual fabric as the ratio of MEDprotected/MEDunprotected (Equation (1)).
2.4.2. SPF of Sunscreens
For the purposes of this study, the package label SPFs of the commercial sunscreens were used. SPFs of commercial sunscreens in the U.S. are determined in accordance with FDA regulations as described in the Code of Federal Regulations 21 [46].
3. Results
3.1. In Vivo SPF Values Are Higher for Fabrics Than Sunscreens
In vivo SPF measurements of the fabrics ranged from 60 to 80, with the nylon woven fabric having the lowest SPF and the polyester interlock knit having the highest SPF (Table 2). All fabrics had a higher SPF than the SPFs on the sunscreen package labels.
Table 2.
Measured UPF, SPF, Critical Wavelength, UVA and UVB blocking capabilities of studied fabrics and sunscreens. ** Each fabric SPF is the average of measurements across three subjects of Fitzpatrick skin types I, II, and III to maximize the human variation studied. Each UPF quantity is an average of 5 unique measurements per textile and 10 unique measurements per sunscreen at each of the two concentrations.
| Photoprotective Modality | SPF | UPF | Critical Wavelength (nm) |
UVA- Blocking |
UVB-Blocking |
|---|---|---|---|---|---|
| Polyester Pique Knit | 77 ± 6 | 214 ± 21 | 383 | 98.49 ± 0.25% | 99.76 ± 0.03% |
| Nylon Woven | 60 ± 5 | 356 ± 41 | 370 | 96.14 ± 0.08% | 99.93 ± 0.02% |
| Polyester Interlock Knit | 80 | 492 ± 45 | 371 | 97.03 ± 0.27% | 99.95% |
| Polyester Interlock Knit w/TiO2 dot print at 30% surface coverage | 73 ± 6 | 649 ± 107 | 379 | 98.48 ± 0.28% | 99.95% |
| Sunscreen A (2 mg/cm2) | 30 # | 16 ± 12 | 371 | 74.05 ± 10.17% | 89.35 ± 8.28% |
| Sunscreen A (1 mg/cm2) | 30 # | 5.3 ± 3.2 | 365 | 54.00 ± 11.33% | 76.45 ± 13.46% |
| Sunscreen B (2 mg/cm2) | 50 # | 31 ± 19 | 373 | 82.13 ± 8.71% | 94.23 ± 7.16% |
| Sunscreen B (1 mg/cm2) | 50 # | 14 ± 17 | 368 | 65.03 ± 14.64% | 84.79 ± 14.42% |
** Error indicated as standard deviations; when not indicated, the standard deviation of the measurements is 0. For critical wavelength, the minimum value is reported. # Commercial sunscreen SPFs were taken from the package label.
3.2. In Vitro UPF Values Are Higher for Fabrics Than Sunscreens
In vitro UPF measurements for all four fabrics exceeded 200 (Table 2). UPF values of sunscreens demonstrated dose- and SPF-dependence, as expected. Sunscreen B (SPF 50) applied at an areal density of 2 mg/cm2 showed the greatest UPF (31 ± 19), while Sunscreen A (SPF 30) applied at 1 mg/cm2 had the lowest UPF (5.3 ± 3.2).
3.3. All Fabrics Have CWs That Meet the Criteria for Broad-Spectrum Labeling
All four fabrics had critical wavelengths (CWs) greater than or equal to 370 nm, the minimum value that the FDA requires for a sunscreen to be considered broad-spectrum (Table 2). Both sunscreens exhibited a broad-spectrum CW of at least 370 nm at an areal density of 2 mg/cm2, but not at 1 mg/cm2.
3.4. % UVB-Blocking Is Greater in Fabrics Relative to Sunscreens
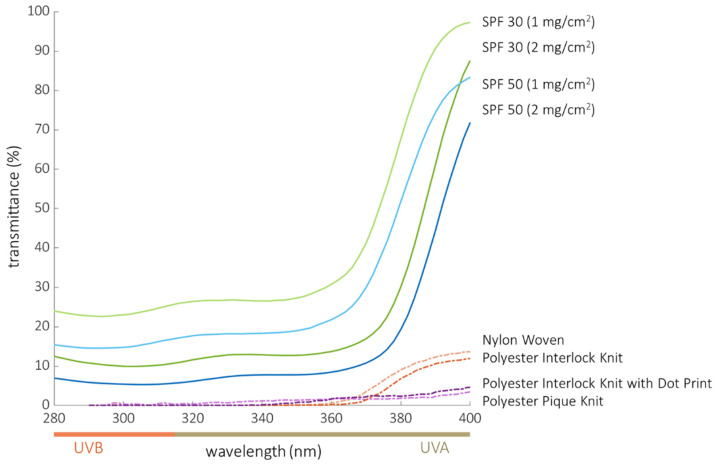
All four textiles blocked > 99% of UVB (which equates to a transmittance < 1% in the UVB region) with little difference detected between the four fabric types (Table 2). Both sunscreens blocked UVB in a dose- and SPF-dependent manner (range of sunscreen protection = 76–94%), with neither providing as much protection as the fabrics. The fabrics exhibited consistently higher and less variable UVB blocking compared to the two commercial sunscreens at both areal densities. The UVR spectra of the textiles and sunscreens (Figure 3) show that all textiles had lower transmittance than the sunscreens over the UVB wavelengths.
Figure 3.
Transmittance curves of the two commercial sunscreens applied at 2 mg/cm2 and 1 mg/cm2 compared to curves of the four fabrics tested. Each curve is an average of multiple measurements.
3.5. % UVA-Blocking Is Greater in Fabrics Relative to Sunscreens
UVA blocking by the fabrics ranged from 96% to 98%, with the polyester fabrics having slightly better performance than the nylon fabric (transmittance < 4% in the UVA region, Table 2). As with UVB, sunscreen UVA blocking increased with areal density and SPF, ranging from 54% for the SPF 30 formulation applied at 1 mg/cm2 to 82% for the SPF 50 sunscreen applied at 2 mg/cm2. The best sunscreen UVA blocker, SPF 50 applied at 2 mg/cm2, did not block UVA as well as the lowest performing fabric (nylon, at 96%). All four textiles had much lower UVA transmittance than the sunscreens. The transmittance of all tested sunscreens increased rapidly in the UVA region from 380 to 400 nm (Figure 3).
4. Discussion
The four textiles in our study provided superior UVR protection when compared to the two commercial sunscreens tested (Table 2, Figure 3). All four fabrics successfully blocked erythemogenic UVR at a level better than the sunscreens, as measured by UVB transmittance, SPF, and UPF metrics. All four fabrics were superior to the sunscreens with respect to blocking full spectrum UVR, as measured by UVA transmittance and CW metrics. There was substantial disparity between the SPF and UPF values determined for both fabric and sunscreen. Despite previous reports to the contrary [40], this is not surprising given the fact that they are measured in different ways, using transmittance of UVR to calculate UPF in contrast to induction of a downstream cutaneous reaction (erythema) to calculate SPF. UPF is indirectly calculated using a mathematic weighting to simulate the erythemogenic potential of the UVR, whereas SPF directly measures development of erythema in response to UVR. In addition to the absolute performance of fabrics and sunscreens, there was substantial variability of protection by sunscreens in our study, despite great efforts made to assure consistency (Table 2). This variability is likely due to the difficulty in uniformly applying sunscreen and highlights an inherent weakness in sunscreens with respect to inconsistent application and the propensity of sunscreens to wear or wash off. Despite these challenges, sunscreens remain an important tool of our armamentarium against skin cancer when protective clothing is not an option.
4.1. Tailored Use of Fabrics and Sunscreens
In general, utilization of both photoprotective clothing and sunscreen offers improved UV protection. However, some activities and climates make wearing photoprotective clothing difficult or undesirable, and it is impractical to cover the entire body with clothing. Similarly, sunscreens can be messy and difficult to apply and re-apply in proper amounts, especially after vigorous exercise or water-related activities. Ideally, these two photoprotective methods can be combined and tailored for each person and for the outdoor activities in which they are engaged. However, there are additional factors that should be considered with respect to the use of clothing and sunscreen.
4.2. Limitations in the Use of Sunscreen for Photoprotection
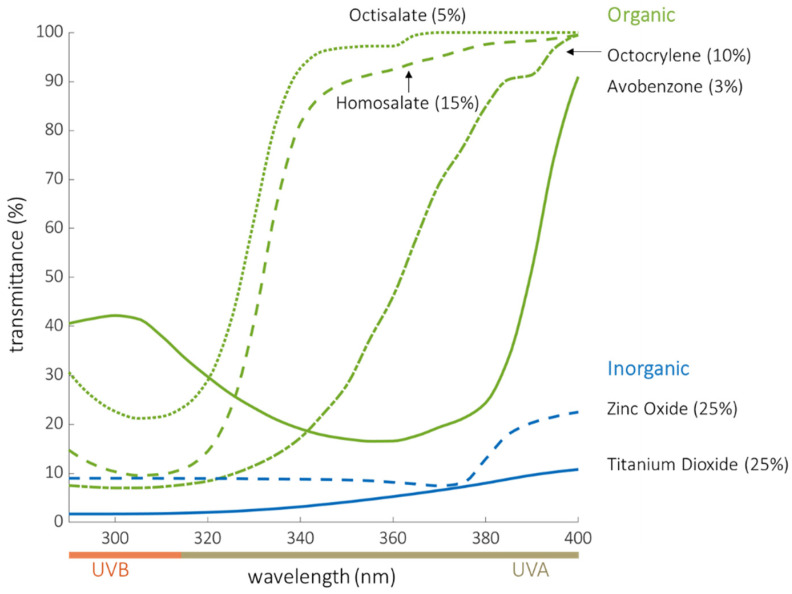
We now know that radiation across the entire UVR spectrum damages DNA. A review of the published absorbance spectra of active organic and inorganic sunscreens shows that nearly all U.S. sunscreen ingredients provide little or limited protection at wavelengths longer than 380 nm [54,55,56,57,58,59] (Figure 4). The curves in Figure 4 reflect the transmission of sunscreen ingredients at the maximum concentration allowed by the FDA. When applied at high concentrations (25%), the inorganic compounds (ZnO and TiO2) do provide superior protection across the UV spectrum compared to organic filters, but still show up to 20% transmittance beyond 380 nm. However, very few sunscreens contain inorganic filters at these high concentrations as they can be difficult to apply and have a chalky, cosmetically less acceptable appearance. Most inorganic sunscreens on the market contain 10–20% ZnO and 2–14% TiO2 [60]. It is also worth noting that eight broad-spectrum, organic UV filters commonly used in other countries but not yet approved by the FDA also show an increase in transmittance beyond 380 nm [61,62].
Figure 4.
Transmittance of organic sunscreen filters (tested in this study) and inorganic sunscreen filters (ZnO and TiO2). Data were obtained from the BASF sunscreen simulator [59] where the maximum concentration allowable by the FDA [46] was used to generate each curve.
This gap in UVR protection is significant since long-wavelength UVA is capable of producing reactive oxygen species and CPDs that can lead to skin cancer [9,10,11]. Although the carcinogenic potential of UVB and UVC were first to be shown to produce mutagenic CPDs and 6-4 photoproducts in DNA, a robust body of literature now exists supporting the carcinogenic potential of UVA as well [9,10,11,63]. Recent studies also show that longer UVA wavelengths (UVA1, 340–400 nm) induce more solar UV-signature mutations than shorter UV wavelengths [64]. Lawrence and colleagues demonstrated that human skin irradiated at 385 nm generated CPDs, which increased for 2 h and persisted for 24 h without evidence of repair [65]. Additionally, irradiation of human skin with UVA1 and visible light produces biomarkers of DNA damage in the form of increased TP53 and BCL-2 expression, eliciting a DNA damage response without producing visible erythema [63]. Further, data from Runger et al. suggest that UVA-induced CPDs may be more mutagenic than those produced by UVB due to a lower and shorter-lived activation of protective cell cycle arrest pathways [66].
The high absorbance of textiles in the near UVA region raises the question of whether there are biological effects of visible light against which textiles might also be protective. UVR and visible light have been shown to generate reactive oxygen species (ROS) that damage sensitive biomolecules in the skin. ROS produced by solar radiation in the UVB, UVA, and visible wavelengths cause damage to DNA that is potentially mutagenic. Photons in the UV region are the most efficient at producing ROS, but studies of the action spectrum of sunlight indicate that more than 50% of free radicals, including ROS, arise from visible light with wavelengths in the range of 400 to 700 nm [67]. The free radicals produced by visible light have the same carcinogenic and ageing effects as their counterparts produced by UV. The principal differences in the effects of various wavelengths of light on generation of ROS in the skin are the chromophores that mediate radical production. Light-absorbing chromophores in the skin include nucleic acids, aromatic amino acids, urocanic acid, NADH and NADPH, cytochromes, riboflavins, porphyrins, melanin and its precursors, and β-carotene [68]. These chromophores can act as photosensitizers that catalyze the generation of ROS and reactive nitrogen species that, if not quenched by cellular antioxidant defenses, can damage sensitive biomolecules such as DNA, RNA, proteins and lipids.
Blue light (400–500 nm) is known to generate pigmentary changes in human skin, particularly visible in persons with darker skin (Fitzpatrick phototypes III and IV). Irradiation of the skin on the backs of healthy volunteers with 415 nm blue-violet light produced hyperpigmentation, independent of p53 activation, that persisted for months after exposure [69]. In subsequent mechanistic studies, the laboratory of Thierry Passeron showed evidence that this effect is mediated by a dedicated sensor, opsin-3 (OPN3) which is expressed in melanocytes [70]. Activation of melanogenesis downstream of OPN3 is calcium dependent, activates the protein kinase CAMKII, and leads to the phosphorylation of the transcription factor MITF and thus increased transcription of melanin synthesis enzymes tyrosinase and dopachrome tautomerase (DCT). Blue light also facilitates the formation of a protein complex that contains tyrosinase and DCT. This complex leads to sustained tyrosinase activity that likely mediates the long-lasting hyperpigmentation that is observed in skin type III and higher.
Taken together, these data suggest that available sunscreens fail to protect against wavelengths of radiation, both UVB and UVA, that produce CPD and 6-4 photoproducts, which can result in mutations that drive carcinogenesis. In addition, there are hyperpigmentation effects of visible light, also not blocked by current sunscreens, that are of significant cosmetic concern to many individuals with darker skin. This makes protection from the full spectrum of UV and visible radiation an important goal, but one that may be difficult to achieve because of the strong cosmetic preference for transparent sunscreens. Sunscreens that protect in the 380–700 nm region are opaque to the human eye, so most sunscreens lack protection in the long UVA and visible spectrum. Importantly, photoprotective clothing may represent a partial solution to this problem since opaque clothing effectively blocks both UV and visible radiation.
4.3. Limitations in the Use of Clothing for Photoprotection
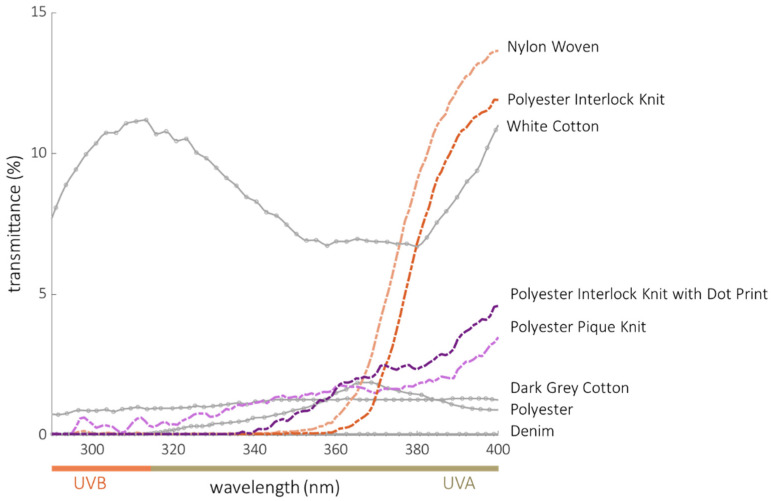
Few studies in the literature have directly compared the performance of UV-protective clothing to sunscreen. In 2018, Coyne et al. reported the broad-spectrum protection afforded by then-available commercial clothing [71]. Selected results from analysis of those data are shown in Figure 5 re-plotted as transmittance versus wavelength for comparison with the four fabrics that we tested. Because this figure only includes fabrics, the upper limit of the transmittance scale is 15%. In contrast, the transmittance scale in Figure 3, which includes both fabrics and sunscreens, must extend to 100% to display the increasing transmittance, and reduced protection, of the sunscreens beginning around 370 nm.
Figure 5.
Transmittance spectra of the four fabrics designed for UV protection tested in this study (orange and purple lines) compared to “normal” clothing items tested by Coyne, et al. (gray lines). The data from Coyne, et al. were digitized, converted to transmittance and represent the average of the 16 measurements as reported in the original study. White cotton and dark grey cotton were GAP, Inc. 100% cotton shirts. The denim was 69% cotton, 30% polyester, 1% spandex GAP jeans. Polyester refers to a 84% polyester, 16% spandex Coolibar rash guard [71].
The Coyne data plotted in Figure 5 are summarized in Table 3. The denim jeans, dark grey cotton shirt, and polyester/spandex photoprotective rash guard provided the most protection. With the exception of the white cotton shirt, all fabrics blocked > 99% of UVB, blocked > 96% of UVA, and met the minimum critical wavelength (370 nm) to be considered broad-spectrum protection. The fabrics in our study provide comparable broad-spectrum protection (Figure 3, Table 2) without requiring dark dyes. Since lighter colored fabrics (such as those tested in our study) generally provide the least protection for a given fabric structure, dyes and pigments added for deeper coloration would lend even more UVR protection than that indicated in Table 2. As shown in the Coyne data, a common white cotton shirt does not typically provide as much UVR protection (UPF = 9, Table 3) as a dark grey shirt made of the same material (UPF = 98, Table 3). On the other hand, the denim tested in the Coyne study provided even better UVR protection (UPF = 2000, Table 3). While fabric weights and thicknesses were not provided in the Coyne study, denim is typically heavier and darker than fabrics used for shirts. Thus, the Coyne data additionally demonstrate that sufficiently heavy and dark fabrics, such as denim, will effectively block UVR. The primary challenge for textile engineers is to provide outstanding UVR protection in garments using fabrics that are lightweight and air permeable (attributes that are linked to comfort in hot environments), thereby increasing the likelihood that a person would choose clothing for photoprotection.
Table 3.
UV-protective measurements of textiles from the literature [66] *.
| Textile Fabrics | UPF | Critical Wavelength (nm) | UVA Blocking | UVB Blocking |
|---|---|---|---|---|
| White Cotton | 9 | 389 | 91.7% | 89.9% |
| Dark Grey Cotton | 98 | 389 | 98.8% | 99.1% |
| Denim | 2000 | 389 | 100% | 100% |
| Polyester | 721 | 387 | 99.0% | 100% |
* UPF and critical wavelength shown are the values reported in Coyne et al., the UVA and UVB blocking is calculated from the digitized average of the measurements.
4.4. Limitations of Current Photoprotection Metrics
Our data and that of Coyne, et al. also demonstrate that CW alone does not provide a useful characterization of UVR protection. The white cotton fabric exhibits a critical wavelength of 389 nm (Table 3), which technically meets the FDA definition of broad-spectrum protection for a sunscreen. However, the same fabric has a UPF rating of 9 and the actual protection provided across wavelengths is the lowest of all of the fabrics in this comparison. The % UVA and UVB blocking values, 91.7% and 89.9%, respectively, are better indicators of the minimal level of broad-spectrum protection provided by this white cotton shirt.
Additionally, the specific shape of the UV transmittance curve can generate misleading results for measurements of SPF, UPF and CW. In the case of materials that exhibit UV transmission that increases rapidly through the UVA region, it is possible for a sunscreen or a garment to provide poor UV protection despite having a seemingly adequate SPF, UPF and CW. We illustrate this concept in Figure 6 and Table 4. The solid curve represents the measured transmittance of Sunscreen B applied at 2 mg/cm2, which has a labeled SPF of 50 but a measured UPF of 31. The dashed curve is a hypothetical sunscreen with a similar transmittance shape profile to Sunscreen B, but modified to achieve a UPF of 50. One can conceptualize this shift as a person applying sunscreen at a higher areal density than the recommended 2 mg/cm2 (which is much higher than that typically applied by consumers [51]). Although the hypothetical dashed curve now represents a UPF value of 50, and maintains a broad-spectrum CW of 374 nm, this “new” sunscreen still only blocks 87% of UVA, which is considered inadequate relative to the 95% UVA blocking criteria used by some countries for sun-protective clothing. As this example demonstrates, the combination of UPF and UVA blocking may provide more suitable measures for broad-spectrum protection. Numerous jurisdictions, including the European Union, require this combination as an indication of broad-spectrum protection for garments [47].
Figure 6.
Illustration of the limitations of current measures of sun protection. The solid curve shows the measured transmittance of Sunscreen B applied at 2 mg/cm2, which has a SPF rating of 50 but had a measured UPF of only 31. The dashed curve was generated using a shifting function to simulate a transmittance curve with an average UPF of 50. For both the measured and simulated curve, the rapid increase in transmittance in the UVA region represents reduced protection from UVA radiation.
Table 4.
Data from Shifting Sunscreen B Transmittance Curve to Simulate UPF = 50.
| Sunscreen | UPF | SPF | Critical Wavelength (nm) |
UVA Blocking |
UVB Blocking |
|---|---|---|---|---|---|
| Sunscreen B (2 mg/cm2) | 31 ± 19 | 50 # | 373 | 82.13 ± 8.71% | 94.23 ± 7.16% |
| Shifted Sunscreen B | 50 ± 30 | - | 374 | 86.70 ± 5.66% | 96.75 ± 4.03% |
# Commercial sunscreen SPFs were taken from the package label.
4.5. Limitations of This Study
Our small sample size and use of only one brand of commercial organic sunscreen may limit generalizability, although most brands select from the same ingredient list. Our study did not directly test inorganic UV filters, but the transmission curves generated using the BASF sunscreen simulator [59] (Figure 4) show that ZnO and TiO2 demonstrate up to 20% transmittance beyond 380 nm. The high concentrations of ZnO and TiO2 required to achieve adequate UVA protection may result in a sunscreen that can be difficult to apply and have a chalky, cosmetically less acceptable appearance. The method we used to determine the UPF of sunscreens had limitations including variability in sunscreen application on the slide and the slide’s differing properties and structure compared to human skin. Challenges posed by in vitro UPF testing of sunscreens have been demonstrated in prior studies, and to date, no in vitro UPF test for sunscreens has been approved due to difficulty in generating reproducible and reliable results from lab to lab [72,73].
The SPF and UPF measurements were made under controlled laboratory conditions. These do not account for the real-world photodegradation of sunscreen UV filters, or the changes in performance due to the known decrease in areal density of the sunscreens as a consequence of friction, sweating, or water exposure [74,75]. In contrast, real-world performance of textiles is likely superior in most situations. In our study, fabrics were taped directly to the skin instead of suspended above the skin. On-skin testing has been reported to drastically reduce SPF in fabrics due to the incident UVR passing directly through the open parts of the fabric structure [76,77]. Although the SPF of the fabrics tested may represent a “worst-case” scenario, all SPFs exceeded 60.
5. Conclusions
In our study, we demonstrate that the four tested fabrics provided superior UVB, UVA, and overall broad-spectrum protection when compared to two commercial sunscreens. These data, coupled with mounting concerns regarding sunscreen ingredients and excipients (including biological impacts [78], potential environmental harms [79], and difficulty to apply correctly [51]), underscore that clothing should be considered the cornerstone of UV protection. Nevertheless, sunscreen remains an important modality for UV protection, especially on areas of the body such as the face and hands were where clothing may be impractical. In the future, the most effective and widely adopted strategies will likely incorporate both photoprotective clothing and sunscreens with absorbances extending into the visible light range.
Photoprotection modalities, and their regulatory labeling, are imperfect and must evolve with our understanding of the mutagenic potential of solar radiation beyond that of UVB: erythema is not the only biologically relevant endpoint and may not be the endpoint most closely associated with carcinogenesis. To provide comprehensive protection from skin cancer, photo-ageing, and hyperpigmentation, strategies that impede the entire spectrum of potentially damaging solar light are necessary. A better future metric of photoprotection might simultaneously assess total transmittance across the entire spectrum of radiation and then mathematically adjust for the known rate of mutations generated at each wavelength.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14030542/s1, Table S1: Inclusion and exclusion criteria of subjects undergoing in vivo SPF testing of fabrics. Table S2: Characteristics of the individuals undergoing in vivo SPF testing of fabrics.
Author Contributions
Conceptualization, D.M.A. and H.W.B.; methodology, D.M.A. and H.W.B.; software, M.A. and D.M.A.; validation, M.A. and D.M.A.; formal analysis, M.A., D.M.A. and H.W.B.; investigation, D.M.A., H.W.B., R.A.D., T.C. and J.B. (Jennifer Beem); resources, H.W.B.; data curation, M.A., T.P.S., D.M.A. and R.A.D.; writing—original draft preparation, E.G.B., J.B. (Joshua Bezecny), D.M.A., H.W.B., J.B. (Jennifer Beem), D.E.B., P.B.C. and S.A.L.; writing—review and editing, E.G.B., J.B. (Joshua Bezecny), M.A., D.M.A., H.W.B., R.A.D., J.B. (Jennifer Beem), D.E.B., R.K., P.B.C. and S.A.L.; visualization, M.A. and D.M.A.; supervision, H.W.B.; project administration, D.M.A. and H.W.B.; funding acquisition, H.W.B. All authors have read and agreed to the published version of the manuscript.
Funding
The Columbia Sportswear Company provided funding for the data collection and portions of the analysis. The OHSU Department of Dermatology and War on MelanomaTM provided salary support for the OHSU investigators.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in this study are available in the text and supplementary figures. Figure 5 was generated using data available from Coyne et al. [71]. Figure 6 was generated using the publicly available BASF sunscreen simulator: https://sunscreensimulator.basf.com/Sunscreen_Simulator [59].
Conflicts of Interest
E.G.B. has no relevant conflicts to declare; J.B. (Joshua Bezecny) has no relevant conflicts to declare; M.A. works at Exponent Inc., a consulting firm contracted by Columbia Sportswear Company to assist with the measurements and data analysis; T.P.S. works at Exponent Inc., a consulting firm contracted by Columbia Sportswear Company to assist with the measurements and the data analysis; D.M.A. works at Exponent Inc., a consulting firm contracted by Columbia Sportswear Company to assist with the measurements and the data analysis; H.W.B. is an employee of Columbia Sportswear Company; R.A.D. was employed by Columbia Sportswear Company at the time of this project; T.C. is an employee of Columbia Sportswear Company; J.B. (Jennifer Beem) is an employee of Columbia Sportswear Company; D.B. has no relevant conflicts to declare; R.K. has no relevant conflicts to declare; P.B.C. has no relevant conflicts to declare; S.A.L. has no relevant conflicts to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnold M., de Vries E., Whiteman D.C., Jemal A., Bray F., Parkin D.M., Soerjomataram I. Global Burden of Cutaneous Melanoma Attributable to Ultraviolet Radiation in 2012. Int. J. Cancer. 2018;143:1305–1314. doi: 10.1002/ijc.31527. [DOI] [PubMed] [Google Scholar]
- 2.Hulur I., Skol A.D., Gamazon E.R., Cox N.J., Onel K. Integrative Genetic Analysis Suggests That Skin Color Modifies the Genetic Architecture of Melanoma. PLoS ONE. 2017;12:e0185730. doi: 10.1371/journal.pone.0185730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guy G.P., Thomas C.C., Thompson T., Watson M., Massetti G.M., Richardson L.C. Vital Signs: Melanoma Incidence and Mortality Trends and Projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015;64:591–596. [PMC free article] [PubMed] [Google Scholar]
- 4.Skin Cancer. [(accessed on 1 April 2021)]. Available online: https://www.aad.org/media/stats-skin-cancer.
- 5.Aitken J.F., Youlden D.R., Baade P.D., Soyer H.P., Green A.C., Smithers B.M. Generational Shift in Melanoma Incidence and Mortality in Queensland, Australia, 1995–2014. Int. J. Cancer. 2018;142:1528–1535. doi: 10.1002/ijc.31141. [DOI] [PubMed] [Google Scholar]
- 6.Iannacone M.R., Green A.C. Towards Skin Cancer Prevention and Early Detection: Evolution of Skin Cancer Awareness Campaigns in Australia. Melanoma Manag. 2014;1:75–84. doi: 10.2217/mmt.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sample A., He Y.-Y. Mechanisms and Prevention of UV-Induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018;34:13–24. doi: 10.1111/phpp.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battie C., Jitsukawa S., Bernerd F., Del Bino S., Marionnet C., Verschoore M. New Insights in Photoaging, UVA Induced Damage and Skin Types. Exp. Dermatol. 2014;23((Suppl. S1)):7–12. doi: 10.1111/exd.12388. [DOI] [PubMed] [Google Scholar]
- 9.Marionnet C., Pierrard C., Golebiewski C., Bernerd F. Diversity of Biological Effects Induced by Longwave UVA Rays (UVA1) in Reconstructed Skin. PLoS ONE. 2014;9:e105263. doi: 10.1371/journal.pone.0105263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan A.Q., Travers J.B., Kemp M.G. Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environ. Mol. Mutagenes. 2018;59:438–460. doi: 10.1002/em.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brash D.E. UV-Induced Melanin Chemiexcitation: A New Mode of Melanoma Pathogenesis. Toxicol. Pathol. 2016;44:552–554. doi: 10.1177/0192623316632072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premi S., Wallisch S., Mano C.M., Weiner A.B., Bacchiocchi A., Wakamatsu K., Bechara E.J.H., Halaban R., Douki T., Brash D.E. Photochemistry. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S., Jin S.-G., Pfeifer G.P. Formation of Cyclobutane Pyrimidine Dimers at Dipyrimidines Containing 5-Hydroxymethylcytosine. Photochem. Photobiol. Sci. 2013;12:1409–1415. doi: 10.1039/c3pp50037c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannistraro V.J., Pondugula S., Song Q., Taylor J.-S. Rapid Deamination of Cyclobutane Pyrimidine Dimer Photoproducts at TCG Sites in a Translationally and Rotationally Positioned Nucleosome In Vivo. J. Biol. Chem. 2015;290:26597–26609. doi: 10.1074/jbc.M115.673301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jans J., Schul W., Sert Y.-G., Rijksen Y., Rebel H., Eker A.P.M., Nakajima S., van Steeg H., de Gruijl F.R., Yasui A., et al. Powerful Skin Cancer Protection by a CPD-Photolyase Transgene. Curr. Biol. 2005;15:105–115. doi: 10.1016/j.cub.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Cole C., Shyr T., Ou-Yang H. Metal Oxide Sunscreens Protect Skin by Absorption, Not by Reflection or Scattering. Photodermatol. Photoimmunol. Photomed. 2016;32:5–10. doi: 10.1111/phpp.12214. [DOI] [PubMed] [Google Scholar]
- 17.Sander M., Sander M., Burbidge T., Beecker J. The Efficacy and Safety of Sunscreen Use for the Prevention of Skin Cancer. CMAJ. 2020;192:E1802–E1808. doi: 10.1503/cmaj.201085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldman R.A., Grant-Kels J.M. The Role of Sunscreen in the Prevention of Cutaneous Melanoma and Nonmelanoma Skin Cancer. J. Am. Acad. Dermatol. 2019;80:574–576.e1. doi: 10.1016/j.jaad.2018.06.069. [DOI] [PubMed] [Google Scholar]
- 19.Suozzi K., Turban J., Girardi M. Cutaneous Photoprotection: A Review of the Current Status and Evolving Strategies. Yale J. Biol. Med. 2020;93:55–67. [PMC free article] [PubMed] [Google Scholar]
- 20.Green A., Williams G., Nèale R., Hart V., Leslie D., Parsons P., Marks G.C., Gaffney P., Battistutta D., Frost C., et al. Daily Sunscreen Application and Betacarotene Supplementation in Prevention of Basal-Cell and Squamous-Cell Carcinomas of the Skin: A Randomised Controlled Trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 21.van der Pols J.C., Williams G.M., Pandeya N., Logan V., Green A.C. Prolonged Prevention of Squamous Cell Carcinoma of the Skin by Regular Sunscreen Use. Cancer Epidemiol. Biomark. Prev. 2006;15:2546–2548. doi: 10.1158/1055-9965.EPI-06-0352. [DOI] [PubMed] [Google Scholar]
- 22.Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced Melanoma after Regular Sunscreen Use: Randomized Trial Follow-Up. J. Clin. Oncol. 2010;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 23.Menter J.M., Hollins T.D., Sayre R.M., Etemadi A.A., Willis I., Hughes S.N. Protection against UV Photocarcinogenesis by Fabric Materials. J. Am. Acad. Dermatol. 1994;31:711–716. doi: 10.1016/S0190-9622(94)70230-6. [DOI] [PubMed] [Google Scholar]
- 24.Louris E., Sfiroera E., Priniotakis G., Makris R., Siemos H., Efthymiou C., Assimakopoulos M.N. Evaluating the Ultraviolet Protection Factor (UPF) of Various Knit Fabric Structures. IOP Conf. Ser. Mater. Sci. Eng. 2018;459:012051. doi: 10.1088/1757-899X/459/1/012051. [DOI] [Google Scholar]
- 25.Robson J., Diffey B.L. Textiles and Sun Protection. Photodermatol. Photoimmunol. Photomed. 1990;7:32–34. [PubMed] [Google Scholar]
- 26.Algaba I., Riva A., Crews R.C. Influence of fiber type and fabric porosity on the UPF of summer fabrics. AATCC Rev. 2004;4:26–31. [Google Scholar]
- 27.Welsh C., Diffey B. The Protection against Solar Actinic Radiation Afforded by Common Clothing Fabrics. Clin. Exp. Dermatol. 1981;6:577–582. doi: 10.1111/j.1365-2230.1981.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 28.Aguilera J., de Gálvez M.V., Sánchez-Roldán C., Herrera-Ceballos E. New Advances in Protection Against Solar Ultraviolet Radiation in Textiles for Summer Clothing. Photochem. Photobiol. 2014;90:1199–1206. doi: 10.1111/php.12292. [DOI] [PubMed] [Google Scholar]
- 29.Ghazi S., Couteau C., Coiffard L.J.M. What Level of Protection Can Be Obtained Using Sun Protective Clothing? Determining Effectiveness Using an In Vitro Method. Int. J. Pharm. 2010;397:144–146. doi: 10.1016/j.ijpharm.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Davis S., Capjack L., Kerr N., Fedosejcvs R. Clothing as Protection from Ultraviolet Radiation: Which Fabric Is Most Effective? Int. J. Dermatol. 1997;36:374–379. doi: 10.1046/j.1365-4362.1997.00046.x. [DOI] [PubMed] [Google Scholar]
- 31.Dubrovski P.D., Golob D. Effects of Woven Fabric Construction and Color on Ultraviolet Protection. Text. Res. J. 2009;79:351–359. doi: 10.1177/0040517508090490. [DOI] [Google Scholar]
- 32.Riva A., Algaba I., Pepió M., Prieto R. Modeling the Effects of Color on the UV Protection Provided by Cotton Woven Fabrics Dyed with Azo Dyestuffs. Ind. Eng. Chem. Res. 2009;48:9817–9822. doi: 10.1021/ie9006694. [DOI] [Google Scholar]
- 33.Sarkar A.K. An Evaluation of UV Protection Imparted by Cotton Fabrics Dyed with Natural Colorants. BMC Dermatol. 2004;4:15. doi: 10.1186/1471-5945-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan C., Au C. In-Vitro Analysis of the Effect of Constructional Parameters and Dye Class on the UV Protection Property of Cotton Knitted Fabrics. PLoS ONE. 2015;10:e0133416. doi: 10.1371/journal.pone.0133416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S.Q., Kopf A.W., Marx J., Bogdan A., Polsky D., Bart R.S. Reduction of Ultraviolet Transmission through Cotton T-Shirt Fabrics with Low Ultraviolet Protection by Various Laundering Methods and Dyeing: Clinical Implications. J. Am. Acad. Dermatol. 2001;44:767–774. doi: 10.1067/mjd.2001.112384. [DOI] [PubMed] [Google Scholar]
- 36.Gambichler T., Hatch K.L., Avermaete A., Altmeyer P., Hoffmann K. Influence of Wetness on the Ultraviolet Protection Factor (UPF) of Textiles: In Vitro and in Vivo Measurements. Photodermatol. Photoimmunol. Photomed. 2002;18:29–35. doi: 10.1034/j.1600-0781.2002.180105.x. [DOI] [PubMed] [Google Scholar]
- 37.Gies P.H., Roy C.R., Toomey S., McLennan A. Protection against Solar Ultraviolet Radiation. Mutat. Res. 1998;422:15–22. doi: 10.1016/S0027-5107(98)00181-X. [DOI] [PubMed] [Google Scholar]
- 38.Bech-Thomsen N., Wulf H.C., Ullman S. Xeroderma Pigmentosum Lesions Related to Ultraviolet Transmittance by Clothes. J. Am. Acad. Dermatol. 1991;24:365–368. doi: 10.1016/0190-9622(91)70054-6. [DOI] [PubMed] [Google Scholar]
- 39.Crews P., Hustvedt G. The Ultraviolet Protection Factor of Naturally-Pigmented Cotton. J. Cotton Sci. 2005;9:47–55. [Google Scholar]
- 40.Gies H.P., Roy C.R., Holmes G. Ultraviolet Radiation Protection by Clothing: Comparison of In Vivo and In Vitro Measurements. Radiat. Prot. Dosim. 2000;91:247–250. doi: 10.1093/oxfordjournals.rpd.a033210. [DOI] [Google Scholar]
- 41.A Reference Action Spectrum for Ultraviolet Induced Erythema in Human Skin. International Commission on Illumination; Vienna, Austria: 1993. CIE Publication 106; 106/4. [Google Scholar]
- 42.Sayre R.M., Cole C., Billhimer W., Stanfield J., Ley R.D. Spectral Comparison of Solar Simulators and Sunlight. Photodermatol. Photoimmunol. Photomed. 1990;7:159–165. [PubMed] [Google Scholar]
- 43.Kennedy C., Bajdik C.D., Willemze R., De Gruijl F.R., Bouwes Bavinck J.N., Leiden Skin Cancer Study The Influence of Painful Sunburns and Lifetime Sun Exposure on the Risk of Actinic Keratoses, Seborrheic Warts, Melanocytic Nevi, Atypical Nevi, and Skin Cancer. J. Investig. Dermatol. 2003;120:1087–1093. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 44.Lew R.A., Sober A.J., Cook N., Marvell R., Fitzpatrick T.B. Sun Exposure Habits in Patients with Cutaneous Melanoma: A Case Control Study. J. Dermatol. Surg. Oncol. 1983;9:981–986. doi: 10.1111/j.1524-4725.1983.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 45.Diffey B.L., Tanner P.R., Matts P.J., Nash J.F. In Vitro Assessment of the Broad-Spectrum Ultraviolet Protection of Sunscreen Products. J. Am. Acad. Dermatol. 2000;43:1024–1035. doi: 10.1067/mjd.2000.109291. [DOI] [PubMed] [Google Scholar]
- 46.CFR—Code of Federal Regulations Title 21. [(accessed on 26 November 2021)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=201.327.
- 47.Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on personal protective equipment and repealing Council Directive 89/686/EEC. Off. J. Eur. Union. 2016;59:51–98. [Google Scholar]
- 48.AATCC Committee RA106 . Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation through Fabrics. American Association of Textile Chemists and Colorists; Research Triangle, NC, USA: 2020. AATCC TM183-2020. [Google Scholar]
- 49.Blackford M.E., Mergy J.T., Beckham H. Multispectral Cooling Fabric. 10,709,186 B2. U.S. Patent. 2020 July 14;
- 50.21 CFR 352.10—Sunscreen Active Ingredients. [(accessed on 26 November 2021)]; Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-352/subpart-B/section-352.10.
- 51.Schalka S., Reis V.M.S.D., Cucé L.C. The Influence of the Amount of Sunscreen Applied and Its Sun Protection Factor (SPF): Evaluation of Two Sunscreens Including the Same Ingredients at Different Concentrations. Photodermatol. Photoimmunol. Photomed. 2009;25:175–180. doi: 10.1111/j.1600-0781.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 52.CTFA South Africa. COLIPA. JCIA . International Sun Protection Factor (SPF) Test Method. COLIPA; Bruxelles, Belgium: 2006. [Google Scholar]
- 53.AMA Laboratories . Evaluation of Sun Protection by SPF Determination (International)—Static. AMA Laboratories; New City, NY, USA: 2021. AMA Reference No. MS19.Textile.SPF.P5700.CSPC.INT.ST3. [Google Scholar]
- 54.Abid A.R., Marciniak B., Pędziński T., Shahid M. Photo-Stability and Photo-Sensitizing Characterization of Selected Sunscreens’ Ingredients. J. Photochem. Photobiol. A Chem. 2017;332:241–250. doi: 10.1016/j.jphotochem.2016.08.036. [DOI] [Google Scholar]
- 55.DeLeo V.A., Clark S., Fowler J., Poncet M., Loesche C., Soto P. A New Ecamsule-Containing SPF 40 Sunscreen Cream for the Prevention of Polymorphous Light Eruption: A Double-Blind, Randomized, Controlled Study in Maximized Outdoor Conditions. Cutis. 2009;83:95–103. [PubMed] [Google Scholar]
- 56.Holt E.L., Krokidi K.M., Turner M.A.P., Mishra P., Zwier T.S., Rodrigues N.D.N., Stavros V.G. Insights into the Photoprotection Mechanism of the UV Filter Homosalate. Phys. Chem. Chem. Phys. 2020;22:15509–15519. doi: 10.1039/D0CP02610G. [DOI] [PubMed] [Google Scholar]
- 57.Popov A.P., Zvyagin A.V., Lademann J., Roberts M.S., Sanchez W., Priezzhev A.V., Myllylä R. Designing Inorganic Light-Protective Skin Nanotechnology Products. J. Biomed. Nanotechnol. 2010;6:432–451. doi: 10.1166/jbn.2010.1144. [DOI] [PubMed] [Google Scholar]
- 58.Sohn M. UV Booster and Photoprotection. In: Wang S.Q., Lim H.W., editors. Principles and Practice of Photoprotection. Springer International Publishing AG; Cham, Switzerland: 2016. pp. 227–246. [Google Scholar]
- 59.BASF Sunscreen Simulator, BASF SE, Ludwigshafen. 2010. [(accessed on 26 November 2021)]. Available online: https://sunscreensimulator.basf.com/Sunscreen_Simulator/
- 60.DailyMed . U.S. National Library of Medicine; Bethesda, MD, USA: [(accessed on 26 November 2021)]. Available online: Https://Dailymed.Nlm.Nih.Gov/Dailymed. [Google Scholar]
- 61.Osterwalder U., Sohn M., Herzog B. Global State of Sunscreens. Photodermatol. Photoimmunol. Photomed. 2014;30:62–80. doi: 10.1111/phpp.12112. [DOI] [PubMed] [Google Scholar]
- 62.Diffey B. New Sunscreens and the Precautionary Principle. JAMA Dermatol. 2016;152:511–512. doi: 10.1001/jamadermatol.2015.6069. [DOI] [PubMed] [Google Scholar]
- 63.Edström D.W., Porwit A., Ros A.M. Effects on Human Skin of Repetitive Ultraviolet-A1 (UVA1) Irradiation and Visible Light. Photodermatol. Photoimmunol. Photomed. 2001;17:66–70. doi: 10.1034/j.1600-0781.2001.017002066.x. [DOI] [PubMed] [Google Scholar]
- 64.Ikehata H., Kawai K., Komura J., Sakatsume K., Wang L., Imai M., Higashi S., Nikaido O., Yamamoto K., Hieda K., et al. UVA1 Genotoxicity Is Mediated Not by Oxidative Damage but by Cyclobutane Pyrimidine Dimers in Normal Mouse Skin. J. Investig. Dermatol. 2008;128:2289–2296. doi: 10.1038/jid.2008.61. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence K.P., Douki T., Sarkany R.P.E., Acker S., Herzog B., Young A.R. The UV/Visible Radiation Boundary Region (385–405 nm) Damages Skin Cells and Induces “Dark” Cyclobutane Pyrimidine Dimers in Human Skin In Vivo. Sci. Rep. 2018;8:12722. doi: 10.1038/s41598-018-30738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rünger T.M., Farahvash B., Hatvani Z., Rees A. Comparison of DNA Damage Responses Following Equimutagenic Doses of UVA and UVB: A Less Effective Cell Cycle Arrest with UVA May Render UVA-Induced Pyrimidine Dimers More Mutagenic than UVB-Induced Ones. Photochem. Photobiol. Sci. 2012;11:207–215. doi: 10.1039/C1PP05232B. [DOI] [PubMed] [Google Scholar]
- 67.Zastrow L., Meinke M.C., Albrecht S., Patzelt A., Lademann J. From UV Protection to Protection in the Whole Spectral Range of the Solar Radiation: New Aspects of Sunscreen Development. Adv. Exp. Med. Biol. 2017;996:311–318. doi: 10.1007/978-3-319-56017-5_26. [DOI] [PubMed] [Google Scholar]
- 68.Sadowska M., Narbutt J., Lesiak A. Blue Light in Dermatology. Life. 2021;11:670. doi: 10.3390/life11070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duteil L., Cardot-Leccia N., Queille-Roussel C., Maubert Y., Harmelin Y., Boukari F., Ambrosetti D., Lacour J.-P., Passeron T. Differences in Visible Light-Induced Pigmentation According to Wavelengths: A Clinical and Histological Study in Comparison with UVB Exposure. Pigment Cell Melanoma Res. 2014;27:822–826. doi: 10.1111/pcmr.12273. [DOI] [PubMed] [Google Scholar]
- 70.Regazzetti C., Sormani L., Debayle D., Bernerd F., Tulic M.K., De Donatis G.M., Chignon-Sicard B., Rocchi S., Passeron T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018;138:171–178. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- 71.Coyne E.Q., Lichtman M.K., Simons J., Sarkar A.K., Rünger T.M. In Vitro Assessment of the Broad-Spectrum Ultraviolet Protection of Clothing. J. Am. Acad. Dermatol. 2018;79:373–375. doi: 10.1016/j.jaad.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 72.Cvetkovska A.D., Manfredini S., Ziosi P., Molesini S., Dissette V., Magri I., Scapoli C., Carrieri A., Durini E., Vertuani S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017;39:310–319. doi: 10.1111/ics.12377. [DOI] [PubMed] [Google Scholar]
- 73.Cole C. Sunscreens—What Is the Ideal Testing Model? Photodermatol. Photoimmunol. Photomed. 2014;30:81–87. doi: 10.1111/phpp.12095. [DOI] [PubMed] [Google Scholar]
- 74.Stokes R.P., Diffey B.L. The Water Resistance of Sunscreen and Day-Care Products. Br. J. Dermatol. 1999;140:259–263. doi: 10.1046/j.1365-2133.1999.02659.x. [DOI] [PubMed] [Google Scholar]
- 75.Stokes R.P., Diffey B.L. A Novel Ex Vivo Technique to Assess the Sand/Rub Resistance of Sunscreen Products. Int. J. Cosmet. Sci. 2000;22:329–334. doi: 10.1046/j.1467-2494.2000.00027.x. [DOI] [PubMed] [Google Scholar]
- 76.Gambichler T., Hatch K.L., Avermaete A., Bader A., Herde M., Altmeyer P., Hoffmann K. Ultraviolet Protection Factor of Fabrics: Comparison of Laboratory and Field-Based Measurements. Photodermatol. Photoimmunol. Photomed. 2002;18:135–140. doi: 10.1034/j.1600-0781.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- 77.Menzies S.W., Lukins P.B., Greenoak G.E., Walker P.J., Pailthorpe M.T., Martin J.M., David S.K., Georgouras K.E. A Comparative Study of Fabric Protection against Ultraviolet-Induced Erythema Determined by Spectrophotometric and Human Skin Measurements. Photodermatol. Photoimmunol. Photomed. 1991;8:157–163. [PubMed] [Google Scholar]
- 78.Matta M.K., Florian J., Zusterzeel R., Pilli N.R., Patel V., Volpe D.A., Yang Y., Oh L., Bashaw E., Zineh I., et al. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA. 2020;323:256–267. doi: 10.1001/jama.2019.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narla S., Lim H.W. Sunscreen: FDA Regulation, and Environmental and Health Impact. Photochem. Photobiol. Sci. 2020;19:66–70. doi: 10.1039/C9PP00366E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data presented in this study are available in the text and supplementary figures. Figure 5 was generated using data available from Coyne et al. [71]. Figure 6 was generated using the publicly available BASF sunscreen simulator: https://sunscreensimulator.basf.com/Sunscreen_Simulator [59].