Abstract
Simple Summary
The extent of axillary surgery has been reduced in recent years to minimize side effects. However, a negative impact of reduced surgery on outcome must be avoided. We investigated for whom the extent of surgery can be safely reduced by examining early-stage breast cancer patients converting from lymph node (LN)-positive to LN-negative disease after neoadjuvant systemic treatment (NAST). Of 242 initially LN-positive patients treated within the GeparOcto trial, 54.5% were classified as LN-negative after NAST, 31.8% as LN-positive, and for 13.6% data were missing. Overall, 92.1% of patients underwent complete axillary LN dissection, with 6.6% undergoing sentinel LN dissection only. At surgery, 55.4% of patients had no signs of cancer in the LN, 45.0% had no signs of cancer in the breast (of those 8.3% had involved LN), and 41.3% had no signs of cancer at all. Patients with involved LN still had a bad prognosis. Conversion from LN-positive to LN-negative after NAST is of highest prognostic value. Surgical axillary staging after NAST is essential in these patients to offer tailored treatment.
Abstract
Background: The conversion of initially histologically confirmed axillary lymph node-positive (pN+) to ypN0 after neoadjuvant systemic treatment (NAST) is an important prognostic factor in breast cancer (BC) patients and may influence surgical de-escalation strategies. We aimed to determine pCR rates in lymph nodes (pCR-LN), the breast (pCR-B), and both (tpCR) in women who present with pN+ BC, to assess predictors for response and the impact of pCR-LN, pCR-B, and tpCR on invasive disease-free survival (iDFS). Methods: Retrospective, exploratory analysis of 242 patients with pN+ at diagnosis from the multicentric, randomized GeparOcto trial. Results: Of 242 patients with initially pN+ disease, 134 (55.4%) had a pCR-LN, and 109 (45.0%) a pCR-B. Of the 109 pCR-B patients, 9 (8.3%) patients had involved LN, and 100 (41.3%) patients had tpCR. Those with involved LN still had a bad prognosis. As expected, pCR-B and intrinsic subtypes (TNBC and HER2+) were identified as independent predictors of pCR-LN. pCR-LN (ypN0; hazard ratio 0.42; 95%, CI 0.23–0.75; p = 0.0028 for iDFS) was the strongest independent prognostic factor. Conclusions: In initially pN+ patients undergoing NAST, the conversion to ypN0 is of high prognostic value. Surgical axillary staging after NAST is still essential in these patients to offer tailored treatment.
Keywords: breast cancer, neoadjuvant therapy, axillary surgery, pathological complete response, lymph node, prognosis
1. Introduction
The extent of axillary surgery is constantly decreasing in breast cancer surgery [1]. Since adjuvant decision making is increasingly based on biological and molecular factors, clinical trials are ongoing to investigate whether axillary staging (sentinel lymph node biopsy, SLNB) can be avoided completely in patients with early breast cancer and clinically unsuspicious nodes who undergo primary surgery [2].
Neoadjuvant systemic treatment (NAST) is becoming increasingly popular to de-escalate the surgical extent and provide prognostic information to tailor systemic treatment after surgery, especially in triple-negative (TNBC) and HER2-positive breast cancer [3,4].
Meta-analyses have shown that pathological complete response (pCR) after NAST is related to outcome and prognosis [5,6,7,8]. There are no data regarding pCR in breast and initially histologically proven metastatic involved lymph nodes and its impact on outcome.
The management of axillary lymph nodes in the context of NAST has been discussed intensively in recent years [9,10]. While SLNB has been accepted as an axillary staging procedure after NAST for cN0 patients, the surgical approach in patients who convert from histologically confirmed lymph node involvement (pN+) before NAST to clinically unsuspicious lymph nodes (ycN0) thereafter remains a matter of debate [11,12]. SLNB as a minimal invasive staging procedure is associated with a false-negative rate (FNR) of >14% in this group of patients, which is considered unacceptable by most surgeons [13]. New procedures that include the use of markers to locate a biopsy-proven positive lymph node and its targeted removal after NAST along with the SLN (targeted axillary dissection, TAD) were introduced recently and have shown false-negative rates consistently below 10% [12]. The risk for missing positive lymph nodes is, however, not only related to the FNR but also to risk of nodal involvement. The higher the pCR rates in node-positive patients, the lower the individual failure risk. Thus far, it is unclear if the FNRs associated with different staging procedures translate into oncologic outcome.
GeparOcto was a randomized phase III trial (NCT02125344) in high-risk early breast cancer that has shown similar pCR rates following NAST with intense dose-dense EPC or weekly PM(Cb) as previously published [14].
Here, we investigate the pCR rates in axillary lymph nodes (pCR-LN), breast (pCR-B), and both (tPCR) in patients with biopsy-proven initial axillary lymph node involvement (pN+). In addition, we evaluate predictors of pCR and assess the relation between pCR-LN, pCR-B, and tpCR and their relevance on outcome. The impact of these results on a risk-adapted surgical strategy for axillary management is discussed.
2. Materials and Methods
2.1. Study Design and Participants
GeparOcto was a neoadjuvant, randomized trial for patients with previously untreated, high-risk early breast cancer with an indication for chemotherapy [14]. From December 2014 through June 2016, 945 patients with high-risk early breast cancer were included and started treatment in 57 centers in Germany.
High-risk breast cancer was defined as TNBC or HER2-positive, independent of lymph node status or node-positive, luminal HER2-negative disease. Diagnosis of breast cancer was histologically confirmed by core biopsy. HER2-positive was defined as immunohistochemistry (IHC) 3+ or in situ hybridization (ISH) according to ASCO-CAP guidelines of 2013. HER2 and hormone receptor expression (negative if estrogen and progesterone receptor <1% by IHC) was assessed by central pathology. Lymphocyte-predominant breast cancer (LPBC) was defined as tumors having high tumor-infiltrating lymphocyte (TIL) levels (≥60%). All patients underwent disease staging by examination (breast and axilla), mammography, and ultrasound (breast und axillary lymph nodes). Distant metastases were excluded before study inclusion. Histologic confirmation of clinically suspicious (palpation and/or sonography) lymph nodes was highly recommended. Clipping of axillary lymph nodes was left to the discretion of the physicians.
The study was approved by the institutional review board and authorities and patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients were randomized in a 1:1 ratio to two different dose-intensified, dose-dense approaches: iddEPC (epirubicin 150 mg/m2 every 2 weeks for 3 cycles, followed by paclitaxel 225 mg/m2 every 2 weeks for 3 cycles, followed by cyclophosphamide 2000 mg/m2 every 2 weeks for 3 cycles) or PM(Cb) (paclitaxel 80 mg/m2 18 times weekly, administered concurrent with NPLD (Myocet® Transpharm Logistic GmbH, (Distributor on behalf of TEVA), Einstein Str. 2, 89179 Baimerstetten, Germany) 20 mg/m2 18 times weekly, and in TNBC, administered concurrently with weekly carboplatin (AUC 1.5) 18 times). Dual HER2-blockade with trastuzumab and pertuzumab was given in HER2-positive disease.
2.2. Objectives
The primary aim of this analysis was the assessment of the pCR rate of axillary lymph nodes, of the primary tumor in the breast, and both after NAST in patients with histologically confirmed metastatic involved axillary lymph nodes at initial presentation. The second aim was to evaluate predictors for pCR-LN, pCR-B, and tpCR. As a third objective, we compared the impact of pCR rates in the 3 groups on invasive disease-free survival (iDFS). The overall objective was a risk assessment for surgical de-escalation strategies after NAST in pN+/ycN0 patients.
2.3. Assessment of Endpoints
After completion of NAST, all patients underwent standard breast surgery. The axillary approach was left to the discretion of the surgeons and to patient preference. After NAST, axillary lymph nodes were evaluated clinically (palpation and/or ultrasound) as ycN0, ycN+, or ycNx (missing data). Ultrasound of axillary lymph nodes was not recommended due to its low sensitivity. pCR was defined as no invasive residuals in the lymph nodes (ypN0), the breast (ypT0/is), or both tpCR (ypT0/is ypN0) after NAST. All removed axillary lymph nodes were examined by hematoxylin-eosin staining only. IHC of lymph nodes for cytokeratin was not performed. Micrometastases (ypN1mi) were defined as ypN1. All histopathological reports were centrally reviewed for pCR assessment. Invasive disease-free survival was defined as no ipsilateral regional invasive breast cancer recurrence, distant recurrence, contralateral invasive breast cancer, or death.
2.4. Statistical Analysis
Cross-tables with 2-sided Fisher’s exact test (for binary parameters) or χ2-test were used to report and compare rates. Median and range was reported for continuous parameters. Multivariate logistic regressions were performed for different pCR definitions to compare rates according to histological nodal status at baseline. Those were adjusted for other baseline parameters and treatment arm and odds ratios (OR) and their 95% confidence intervals (CI) were reported. For iDFS, Kaplan–Meier curves were plotted and compared with log-rank test; Cox proportional hazard model was used to report hazard ratio (HR) with 95% CI. All statistical analyses were performed with the use of SAS software, version 9.4.
3. Results
3.1. Patient and Tumor Baseline Characteristics
In total, 945 patients with a high-risk early breast cancer were included in the GeparOcto trial and started treatment. The majority of the patients presented with TNBC (N = 403, 42.6%) or HER2-positive disease (N = 382, 40.4%). Four hundred and thirty-one (46.2%) of the 945 patients had clinically suspicious or positive lymph nodes at the time of presentation. Initially nodal involvement by core biopsy (pN+) was confirmed in 242 (25.6%) patients (Figure S1). The baseline characteristics of these 242 patients before NAST are shown in Table S1. The median age was 47 years (range 21–74) and the median tumor size assessed by ultrasound was 27 mm (range 8–147). Sixty-three patients (26.0%) had TNBC, while 69 women (28.5%) presented with HER2-positive and 110 (45.5%) with luminal HER2-negative disease.
3.2. Management of Axillary Lymph Nodes after Neoadjuvant Systemic Treatment
After NAST, 132 of 242 (54.5%) patients were staged as ycN0, 77 (31.8%) as ycN+, and 33 (13.6%) as ycNx (missing data). Overall, 223 (92.1%) patients (ycN0: 94.6%, ycN+: 90.9%, and ycNx: 93.9%) underwent complete ALND. A regular SLNB with single tracer alone was performed in 16 (6.6%) of 242 patients (Table S2).
3.3. Pathological Response after NAST
Seventy-three out of 132 patients were staged as ycN0 (55.4%) and had a pCR-LN, while in ycN+ patients, the ypN0 rate was as low as 57.1% and for ypNx 51.5%. An overall pCR-LN was observed in 134/242 (55.4%) of initially pN+ patients. Micrometastases were found in eight patients with ypN1mi and two with ypN0 i+, respectively. The ypN0 rate was highly related to pCR-B (92.0%) and tumor subtype (81.6% for HER2-positive, 75.0% for TNBC, and 25.9% for luminal HER2-negative disease) (Table 1). A pCR-B irrespective of nodal status was confirmed in 109 (45.0%) patients with significant differences between subtypes (71.0% in HER2-positive, 65.1% in TNBC, and 17.3% in luminal HER2-negative). A tpCR was observed in 100 patients (41.3%), with comparable rates in HER2-positive (66.7%) and TNBC (60.3%) and a significantly lower rate in luminal HER2-negative (14.6%; p < 0.001) patients (Table S3).
Table 1.
Pathological lymph node status after NAST according to clinical nodal status after NAST and biological subtype.
| Biological Subtype | ypN0 N (%) |
ypN+ N (%) |
p-Value a | |
|---|---|---|---|---|
| ycN0 | luminal HER2- (N = 58) | 15 (25.9) | 43 (74.1) | <0.001 |
| TNBC (N = 36) | 27 (75.0) | 9 (25.0) | ||
| HER2+ (N = 38) | 31 (81.6) | 7 (18.4) | ||
| Overall (N = 132) | 73 (55.3) | 59 (44.7) | ||
| ycN+ | luminal HER2- (N = 35) | 14 (40.0) | 21 (60.0) | 0.010 |
| TNBC (N = 21) | 13 (61.9) | 8 (38.1) | ||
| HER2+ (N = 21) | 17 (81.0) | 4 (19.0) | ||
| Overall (N = 77) | 44 (57.1) | 33 (42.9) | ||
| ycNx * | luminal HER2- (N = 17) | 6 (35.3) | 11 (64.7) | 0.080 |
| TNBC (N = 6) | 3 (50.0) | 3 (50.0) | ||
| HER2+ (N = 10) | 8 (80.0) | 2 (20.0) | ||
| Overall (N = 33) | 17 (51.5) | 16 (48.5) |
Abbreviations: TNBC, triple-negative breast cancer; NAST, neoadjuvant systemic treatment; a comparing ypN0 rate between subtypes; * missing data.
Multivariate logistic regression analysis revealed pCR-B as the strongest predictor for pCR-LN (OR 31.7, 95% CI 12.0–84.0). Pre-NAST tumor stage (cT4 vs. cT1-3, OR 0.15, 95% CI 0.03–0.63; p = 0.01), grading (G3 vs. G1–2, OR 2.19, 95% CI 1.12–4.28), tumor biology (HER2-positive vs. luminal HER2-negative, OR 8.94, 95% CI 4.02–19.89; p < 0.001; TNBC vs. luminal HER2-negative, OR 3.55, 95% CI 1.71–7.37, p < 0.001), and LPBC (yes vs. no, OR 3.39, 95% CI 1.29–8.96, p = 0.014) were further identified as independent predictors of pCR-LN.
For pCR-B (ypT0/is) and tpCR only, the biological subtype (TNBC and HER2-positive versus luminal HER2-negative) and LPBC were identified as independent predictors. There were no significant pCR differences between both arms (Table S4).
3.4. Invasive Disease-Free Survival in Patients with Initially Lymph Node-Positive Breast Cancer
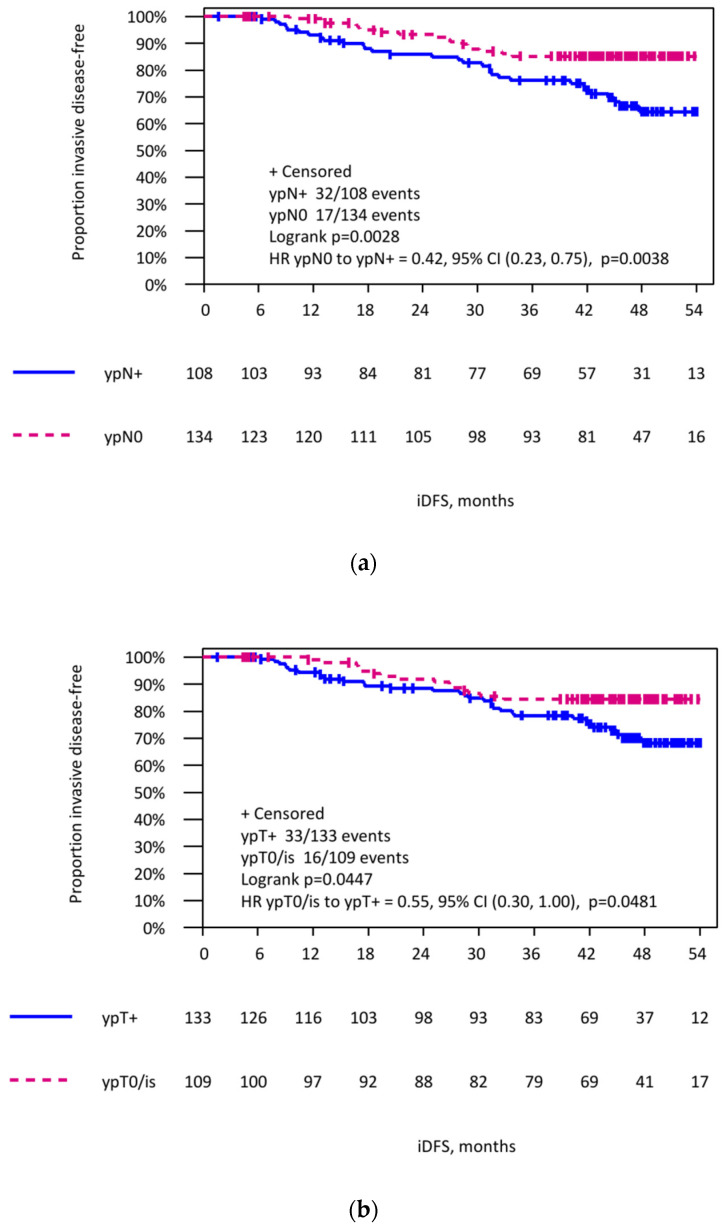
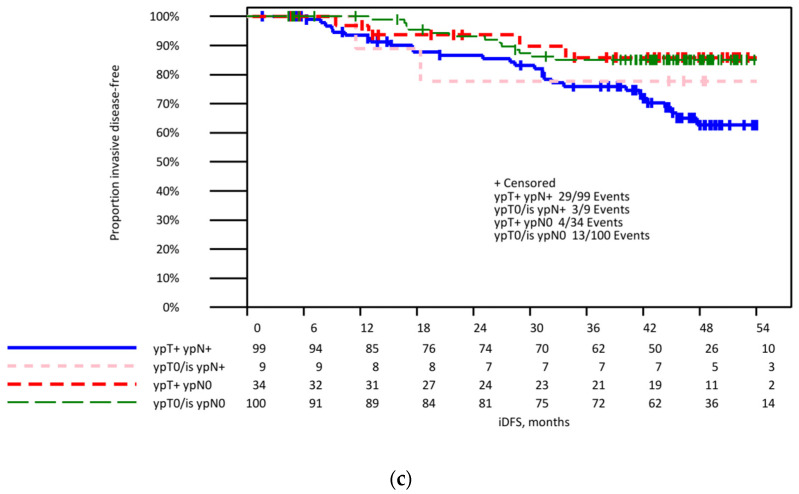
After a median follow-up period of 46.5 months, patients with pCR-LN had a significantly better iDFS compared to patients with residual tumor in the lymph nodes (HR 0.42; 95% CI 0.23–0.75; log-rank p = 0.0028). A smaller difference in iDFS was observed between patients with a pCR-B alone compared to patients with a non-pCR-B (HR 0.55; 95% CI 0.30–1.00; log-rank p = 0.045). Irrespective of the tumor residuals in the breast, the persistent lymph node involvement was the strongest predictor for iDFS (Figure 1; ypT0/is ypN0 vs. ypT+ ypN+ HR 0.42, 95% CI 0.22–0.80, p = 0.009; ypT+ ypN0 vs. ypT+ ypN+ HR 0.40, 95% CI 0.14–1.14, p = 0.087; ypT0/is ypN+ vs. ypT+ ypN+ HR 0.88, 95% CI 0.27–2.93, p = 0.84).
Figure 1.
Invasive disease-free survival in patients with initially lymph node-positive breast cancer. (a) In patients with pcR (ypN0), respectively, non-pCR (ypN+) in lymph nodes after neoadjuvant systemic treatment. (b) According to the pCR in breast and nodes (ypT0/is ypN0), after neoadjuvant systemic treatment. (c) Invasive disease-free survival in patients with initially lymph node-positive breast cancer according to the pCR in breast (ypT0/is) and nodes (ypN0) after neoadjuvant systemic treatment.
4. Discussion
Surgical de-escalation strategies in lymph node surgery after NAST are currently intensively debated. Sentinel lymph node biopsy has replaced ALND as treatment in cN0 patients even after NAST. A retrospective analysis of the National Cancer Data Base (NCDB) in cN0 patients who presented with HER2-positive or TNBC disease and had a pCR-B demonstrated a rate of nodal involvement after NAST below 2%. The authors concluded that any axilla surgery might be omitted in this subset of patients [15]. In view of these data, new prospective trials are ongoing to omit any lymph node surgery in these women (EUBREAST1-trial, NCT04101851). In a pooled analysis with 57,531 unique patients, pCR-LN rates were reported in 60% of hormone receptor-negative/HER2-positive, 48% of TNBC, 45% of hormone receptor-positive/HER2-positive, 35% of luminal B, 18% of hormone receptor-positive/HER2-negative, and 13% of luminal A breast cancer. The pCR rates were independent of clinically or pathologically proven node status [16].
In the light of those data and initiatives, we investigated in the pN+ subset of the patients enrolled in the neoadjuvant GeparOcto study the rate of ypN+ patients in patients with a breast pCR and its prognostic value. The rates for pCR-LN, pCR-B, and tpCR were 55.4%, 45.0%, and 41.3%, respectively. This shows that at least 8% of these high-risk patients with a breast pCR still had nodal involvement. These data also reveal that more than 50% of patients converted to a histologically negative lymph node status and would be overtreated with full ALND. Minimal invasive axillary surgery to reliably assess the lymph node status after NAST appears therefore crucial. This result is comparable to data reported from a prospective cohort study presented by Tadros et al., which showed a ypN0 stage of almost 90% for patients with biopsy proven pN+, who had a pCR-B [17]. These data were confirmed by another retrospective analysis from the NCDB on 30,821 patients with a ypN+ rate of 12.4% in this cohort of patients [15]. In contrast, Samiei et al. demonstrated a nodal involvement rate of 45% after NAST for cN+ patients with pCR-B [18]. The difference of clinically nodal involvement and histologically proven nodal involvement is important and must be considered.
In addition, as expected, breast pCR was identified as the most important predictor of pCR-LN.
For patients with initially node-positive disease, the optimal staging procedure to assess the ypN status is currently not clearly defined. The SLNB technique is associated with an FNR of around 14%, which is considered as unacceptably high by most surgeons. New techniques such as the TAD, that combines SLNB with the removal of a (pre-NAST) biopsy-proven lymph node (target lymph node), that may be located by a multitude of different markers, is becoming increasingly popular due to an FNR lower than 7% [19], respectively <9% [20,21]. Barrio et al. recently reported that in patients with cN1 disease converted to ycN0 and three or more negative SLN after SLNB alone, and nodal recurrence rates were low, without routine nodal clipping [22]. These findings would also support omitting ALND in these patients. Thus far, however, no data are available that provide prospective evidence on the oncologic outcome of different surgical staging procedures and provide information and the associated quality of life. The prospective AXSANA study (EUBREAST-03, NCT4373685) addresses these issues [20].
The individual risk of missing tumor involvement with a minimal invasive staging procedure (with its associated specific FNR) depends on the rate of patients with a complete tumor response in the lymph nodes.
The prognostic significance of low-volume residual nodal disease after NAST (ypN0 i+/ypN1(mi)) is unclear. In an analysis of more than 36,000 patients from Dana-Farber/Brigham and Women’s Cancer Center (DFBWCC) and the NCDB, patients with ypN0(i+) and ypN1(mi) disease had a twofold higher risk of death compared to ypN0 patients (p < 0.001) [23]. In another series of 134 patients with initially node-positive disease, the incidence of breast cancer-related, loco-regional events and death from BC were similar between patients with unaffected SLNs and women with micrometastatic lymph node involvement (28.9% vs. 30.2%, p = 0.954; 21.6% vs. 13.4%, p = 0.840; 12.9% vs. 24.5%, p = 0.494). Overall survival and disease-free survival were lower for patients with macrometastatic disease in the SLN [24]. In 59% of patients with micrometastatic lymph node involvement after NACT, Moo et al. found one or more additional positive nodes at ALND. They concluded that in patients with low-volume SLN metastases after NAST completion ALND should be performed [25].
The ongoing ALLIANCE 11202 trial (NCT01901094) compared ALND with axillary radiation for patients with a positive LN after NAST. The results might reduce axillary surgery in this cohort.
Current diagnostic tools such as palpation, ultrasound, and even magnetic resonance imaging (MRI) for evaluation of the axillary lymph node status following NAST have an unacceptable low accuracy, even after inclusion of clinico-pathological criteria [25,26,27,28,29,30,31]. Therefore, in patients with initially affected axillary lymph nodes and ycN0 after NAST, an evaluation of lymph nodes by imaging alone cannot be recommended. Half of ycN0 patients still have histologically involved lymph nodes. Vice versa, we observed a pCR rate in 57.1% of the patients with clinically affected lymph nodes (ycN+) after NAST. This underscores the importance of assessing the histopathological axillary lymph node status after NAST.
In our study, we found a strong relation between tumor subtype and pCR-LN, pCR-B, and tpCR. While the pCR-LN rate was as high as 81.6% for HER2-positive and 75.0% for TNBC, it was only 25.9% in luminal HER2-negative tumors. For pCR-B, the rates were 71.0%, 65.1%, and 17.3%, respectively. A similar relation between response and tumor subtype was reported from Barron et al. with pCR-B rates of 43.3% in HER2-positive, 37.7% in TNBC, and 12.7% in luminal HER2-negative disease [15]. In a pooled analysis of 33 studies with 57,531 clinically node-positive patients before NAST axillary pCR according to subtypes were reported in 60% (hormone receptor-negative/HER2-positive), 59% (HER2-positive/hormone receptor-negative or positive), 48% TNBC, 45% (hormone receptor-positive/HER2-positive), 35% (luminal B), 18% (hormone receptor-positive/HER2-negative), and 13% (luminal A) breast cancer [16]. The difference in magnitude of the effect of NAST on pCR rates may be explained by a modern dose-dense intensified schedule as used in the GeparOcto trial. To our knowledge, this is the first study to analyze the effect of pCR-LN, pCR-B, and tpCR on iDFS in patients with initially pN+ separately. We found that pCR-LN, pCR-B, and tpCR are associated with improved iDFS. However, pCR-LN is the strongest predictor for iDFS. These data indicate that irrespective of tumor residuals in the breast, the persistent lymph node involvement drives the prognosis.
A strength of our multicenter trial is the initial histological confirmation of lymph node status by core biopsy in all patients with suspicious lymph nodes. Patients who received an SLNB for cN+ were excluded. Patients were treated in a prospectively randomized trial with a specific systemic treatment and data on axillary diagnostic and treatment were captured predefined. Most patients received axillary lymph node dissection according to the historical guidelines offering robust data on pCR-LN. A limitation is the unplanned and retrospective design of this analysis and the small patient number.
5. Conclusions
In conclusion, lymph node involvement is the major driver of prognosis after NAST. Surgical staging of the axilla in pN+ patients who convert to ycN0 remains important because 50% overall and 8.3% of the breast pCR patients still had involved lymph nodes. Patients with a good response in the breast have a low likelihood of residual axillary disease. Axillary dissection to stage the axilla should be avoided as an overtreatment. For patients who convert from pN+ to ycN0, minimal invasive staging procedures (SLNB, TAD) should be recommended. Prospective studies to define outcome and quality of life of different techniques are warranted.
Acknowledgments
We would like to thank all patients and their families participating in the trial, the network of investigators and study personnel, the team at the GBG Headquarters, and Bianca Lederer for editorial assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14030521/s1, Figure S1: Consort diagram, Table S1: Baseline characteristics of included patients before neoadjuvant systemic treatment, Table S2: Management of axillary lymph nodes after neoadjuvant systemic treatment dependent on clinical lymph node status, Table S3: pCR rates in lymph nodes and breast according to different definitions and according to biological tumor type in patients with pN+ at baseline, Table S4: Multivariate logistic regression for pCR-LN (with and without pCR-B as a covariate), pCR-B and for tpCR in all 242 patients with pN+ by biopsy.
Author Contributions
B.G. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design, B.G., V.N., S.L. and T.K.; acquisition, analysis, or interpretation of data, B.G., A.S., V.M., M.G., H.T., D.K., C.H., C.D., K.L., J.H. (Jörg Heil), J.H. (Jens Huober), B.A., P.K., M.H., M.U., K.K., C.J., J.T., F.S., J.-U.B., K.R., P.A.F., V.N., S.L. and T.K.; drafting of the manuscript, B.G., V.N., S.L. and T.K.; critical revision of the manuscript for important intellectual content, B.G., A.S., V.M., M.G., H.T., D.K., C.H., C.D., K.L., J.H. (Jörg Heil), J.H. (Jens Huober), B.A., P.K., M.H., M.U., K.K., C.J., J.T., F.S., J.-U.B., K.R., P.A.F., V.N., S.L. and T.K.; statistical analysis, V.N. and F.S.; supervision, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The GeparOcto trial was financially supported by Amgen GmbH, Germany; funding number n.a.; Roche Pharma AG, Germany; funding number n.a.; TEVA GmbH, Germany; funding number n.a.; Vifor Pharma GmbH Germany; funding number n.a.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Medizinische Fakultät Heidelberg (approval number, 291/2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request for researchers who provide translational research proposals. Proposals should be directed to http://www.gbg.de/de/forschung/translationale-forschung.php, (accessed on 28 November 2021); to gain access, data requestors will need to sign a data transfer agreement.
Conflicts of Interest
The authors declare the following conflicts of interest: Bernd Gerber reports speaker honoraria received from AstraZeneca, Novartis, Roche, Pfizer, Pierre Fabre, and personal fees from AstraZeneca, Celgene, Roche, Pfizer, Novartis, Lilly, outside the submitted work; Andreas Schneeweiss reports grants from Celgene, grants from Roche, grants from AbbVie, personal fees from Roche, personal fees from AstraZeneca, personal fees from Celgene, personal fees from Pfizer, personal fees from AstraZeneca, personal fees from Novartis, personal fees from MSD, personal fees from Tesaro, personal fees from Lilly, other from Roche, outside the submitted work; Volker Möbus reports speaker honoraria received from Amgen, AstraZeneca, Celgene, Roche, Teva. Consultancy honoraria from Roche, Amgen, Tesaro, and Myelo Therapeutics. Hans Tesch reports other from Pierre Fabre, other from Pfizer Pharma, other from Mundipharma, other from ClinSol, other from Novartis, other from Lilly, other from AMGEN, other from Grünenthal, other from Vifor, other from AstraZeneca, other from Mylan, other from BMS, during the conduct of the study; David Krug reports personal fees from MSD Sharp & Dome, outside the submitted work; Claus Hanusch reports personal fees from Roche, Lilly, Novartis, Pfizer, AstraZeneca, outside the submitted work; Carsten Denkert reports grants from European Commission H2020, grants from German Cancer Aid Translational Oncology, during the conduct of the study; personal fees from Novartis, personal fees from Roche, personal fees from MSD Oncology, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, from Molecular Health, grants from Myriad, personal fees from Merck, other from Sividon diagnostics, outside the submitted work. In addition, Denkert has a patent VMScope digital pathology software with royalties paid, a patent WO2020109570 A1-cancer immunotherapy pending, and a patent WO2015114146A1 and WO2010076322A1-therapy response issued. Kristina Lübbe reports personal fees and non-financial support from Roche, personal fees from AstraZeneca, personal fees and non-financial support from Lilly, personal fees and non-financial support from Novartis, personal fees from MSD, personal fees from Esai, personal fees from Genomic Health, outside the submitted work; Jens Huober reports personal fees from Lilly, personal fees from Novartis, personal fees from Pfizer, personal fees from Abbvie, personal fees from AstraZeneca, personal fees from MSD, personal fees from Celgene, personal fees from Roche, other from Daichii, other from Roche, other from Pfizer, grants from Novartis, grants from Hexal, outside the submitted work; Beyhan Ataseven reports personal fees and non-financial support from Roche, personal fees from AstraZeneca, personal fees from Clovis, non-financial support from PharmaMar, personal fees and non-financial support from Tesaro/GSK, personal fees from MSD, personal fees from Celgene, personal fees from Amgen, outside the submitted work; Michael Untch reports personal fees and non-financial support from Abbvie, personal fees and non-financial support from Amgen GmbH, personal fees and non-financial support from AstraZeneca, personal fees from BMS, personal fees and non-financial support from Celgene GmbH, personal fees and non-financial support from Daiji Sankyo, personal fees and non-financial support from Eisai GmbH, personal fees from Lilly Deutschland, personal fees and non-financial support from Lilly Int., personal fees and non-financial support from MSD Merck, personal fees and non-financial support from Mundipharma, personal fees and non-financial support from Myriad Genetics, personal fees and non-financial support from Odonate, personal fees and non-financial support from Pfizer GmbH, personal fees from PUMA Biotechnology, personal fees and non-financial support from Roche Pharma AG, personal fees and non-financial support from Sanofi Aventis Deutschland GmbH, personal fees and non-financial support from TEVA Pharmaceuticals Ind Ltd., personal fees and non-financial support from Novartis, personal fees from Pierre Fabre, personal fees and non-financial support from Clovis Oncology, personal fees from Seattle Genetics, outside the submitted work; Karin Kast reports other from MSD Sharpe and Dome GmBH, personal fees from Roche, personal fees from Pfizer, personal fees from AstraZeneca, outside the submitted work; Christian Jackisch reports personal fees from Roche, personal fees from Celgene, personal fees from AstraZeneca, during the conduct of the study; Jens-Uwe Blohmer reports personal fees from Amgen, personal fees from AstraZeneca, personal fees from Lilly, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, grants and personal fees from Sysmex, personal fees from Roche, personal fees from Pierre Fabre, outside the submitted work; Kerstin Rhiem reports personal fees from AstraZeneca, personal fees from Pfizer, personal fees from MSD, outside the submitted work; Peter A. Fasching reports personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi-Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from Seattle Genetics, personal fees from Roche, personal fees from Hexal, during the conduct of the study; Sibylle Loibl reports grants and other from Abbvie, grants, non-financial support, and other from Amgen, grants, non-financial support, and other from AstraZeneca, other from Bayer, non-financial support and other from BMS, grants, non-financial support, and other from Celgene, personal fees from Chugai, grants, non-financial support, and other from Daiichi-Sankyo, other from Eirgenix, other from GSK, grants from Immunomedics/Gilead, other from Ipsen, other from Lilly, other from Merck, grants, non-financial support, and other from Novartis, grants, non-financial support, and other from Pfizer, other from Pierre Fabre, other from Prime/Medscape, other from Puma, grants, non-financial support, and other from Roche, other from Samsung, other from Seagen, grants and non-financial support from Vifor, outside the submitted work. In addition, Loibl has a patent EP14153692.0 pending, a patent EP21152186.9 pending, a patent EP15702464.7 with royalties paid, and a patent null with royalties paid. Thorsten Kühn reports personal fees from Celgene, Roche, Endomagnetics, Mammotome, Pfizer. No other potential conflict of interest relevant to this article was reported.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Kelemen P.R., Ollila D.W., Hansen N.M., Whitworth P.W., Blumencranz P.W., et al. Effect of axillary dissection vs. no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The acosog z0011 (alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimer T., Stachs A., Nekljudova V., Loibl S., Hartmann S., Wolter K., Hildenrandt G., Gerber B. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (c/i t1-2) in the context of breast concerving therapy: First results following commencement of the intergroup-sentinel-mamma (insema) trial. Geburtsh Frauenheilk. 2017;77:149–157. doi: 10.1055/s-0042-122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gion M., Pérez-García J.M., Llombart-Cussac A., Sampayo-Cordero M., Cortés J., Malfettone A. Surrogate endpoints for early-stage breast cancer: A review of the state of the art, controversies, and future prospects. Ther. Adv. Med. Oncol. 2021;13:17588359211059587. doi: 10.1177/17588359211059587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiec L., Shah A.N. Risk-based Approaches for Optimizing Treatment in HER2-Positive Early Stage Breast Cancer. Semin. Oncol. 2020;47:249–258. doi: 10.1053/j.seminoncol.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Cortazar P., Geyer C.E., Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann. Surg. Oncol. 2015;22:1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirier J., Body G., Jourdan M.L., Bedouet L., Fleurier C., Pilloy J., Arbion F., Ouldamer L. Impact of pathological complete response to neoadjuvant chemotherapy in invasive breast cancer according to molecular subtype. Gynecol. Obstet. Fertil. Senol. 2017;45:535–544. doi: 10.1016/j.gofs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Gustavo Werutsky G., Untch M., Hanusch C., Fasching P.A., Blohmer J.U., Seiler S., Denkert C., Tesch H., Jackisch C., Gerber B., et al. Locoregional recurrence risk after neoadjuvant chemotherapy: A pooled analysis of nine prospective neoadjuvant breast cancer trials. Eur. J. Cancer. 2020;130:92–101. doi: 10.1016/j.ejca.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Kuehn T., Bauerfeind I., Fehm T., Fleige B., Hausschild M., Helms G., Lebeau A., Liedtke C., von Minckwitz G., Nekljudova V., et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (sentina): A prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 10.Boughey J.C., Suman V.J., Mittendorf E.A., Ahrendt G.M., Wilke L.G., Taback B., Leitch A.M., Kuerer H.M., Bowling M., Flippo-Morton T.S., et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The acosog z1071 (alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter S., Neuman H., Mamounas E.P., Bedrosian I., Moulder S., Montero A.J., Jagsi R. Debating the optimal approach to nodal management after pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:42–48. doi: 10.1200/EDBK_237701. [DOI] [PubMed] [Google Scholar]
- 12.Simons J.M., van Nijnatten T.J.A., van der Pol C.C., Luiten E.J.T., Koppert L.B., Smidt M.L. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: A systematic review and meta-analysis. Ann. Surg. 2019;269:432–442. doi: 10.1097/SLA.0000000000003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tee S.R., Devane L.A., Evoy D., Rothwell J., Geraghty J., Prichard R.S., McDermott E.W. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br. J. Surg. 2018;105:1541–1552. doi: 10.1002/bjs.10986. [DOI] [PubMed] [Google Scholar]
- 14.Schneeweiss A., Mobus V., Tesch H., Hanusch C., Denkert C., Lubbe K., Huober J., Klare P., Kummel S., Untch M., et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (geparocto-gbg 84): A randomised phase iii trial. Eur. J. Cancer. 2019;106:181–192. doi: 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Barron A.U., Hoskin T.L., Day C.N., Hwang E.S., Kuerer H.M., Boughey J.C. Association of low nodal positivity rate among patients with erbb2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. 2018;153:1120–1126. doi: 10.1001/jamasurg.2018.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samiei S., Simons J.M., Engelen S.M.E., Beets-Tan R.G.H., Classe J.M., Smidt M.L. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: A systematic review and meta-analysis. JAMA Surg. 2021;156:e210891. doi: 10.1001/jamasurg.2021.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadros A.B., Yang W.T., Krishnamurthy S., Rauch G.M., Smith B.D., Valero V., Black D.M., Lucci A., Jr., Caudle A.S., DeSnyder S.M., et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152:665–670. doi: 10.1001/jamasurg.2017.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samiei S., van Nijnatten T.J.A., de Munck L., Keymeulen K., Simons J.M., Kooreman L.F.S., Siesling S., Lobbes M.B.I., Smidt M.L. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann. Surg. 2018;271:574–580. doi: 10.1097/SLA.0000000000003126. [DOI] [PubMed] [Google Scholar]
- 19.Kuemmel S., Heil J., Rueland A., Seiberling C., Harrach H., Schindowski D., Lubitz J., Hellerhoff K., Ankel C., Graßhoff S.T., et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (tad) in node-positive breast cancer patients. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000004572. online ahead of print . [DOI] [PubMed] [Google Scholar]
- 20.Banys-Paluchowski M., Gasparri M.L., de Boniface J., Gentilini O., Stickeler E., Hartmann S., Thill M., Rubio I.T., Di Micco R., Bonci E.A., et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: Current status, knowledge gaps, and rationale for the eubreast-03 axsana study. Cancers. 2021;13:1565. doi: 10.3390/cancers13071565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann S., Kühn T., de Boniface J., Stachs A., Winckelmann A., Frisell J., Wiklander-Bråkenhielm I., Stubert J., Gerber B., Reimer T. Carbon tattooing for targeted lymph node biopsy after primary systemic therapy in breast cancer: Prospective multicentre tattoo trial. Br. J. Surg. 2021;108:302–307. doi: 10.1093/bjs/znaa083. [DOI] [PubMed] [Google Scholar]
- 22.Barrio A.V., Montagna G., Mamtani A., Sevilimedu V., Edelweiss M., Capko D., Cody H.S., 3rd, El-Tamer M., Gemignani M.L., Heerdt A., et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy-a rare event. JAMA Oncol. 2021;7:1851–1855. doi: 10.1001/jamaoncol.2021.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong S.M., Almana N., Choi J., Hu J., Gagnon H., Natsuhara K., Shen A.H., DeSantis S., Dominici L., Golshan M., et al. Prognostic Significance of Residual Axillary Nodal Micrometastases and Isolated Tumor Cells After Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. Oncol. 2019;26:3502–3509. doi: 10.1245/s10434-019-07517-2. [DOI] [PubMed] [Google Scholar]
- 24.Canavese G., Tinterri C., Carli F., Garrone E., Spinaci S., Della Valle A., Barbieri E., Marrazzo E., Bruzzi P., Dozin B. Correlation between outcome and extent of residual disease in the sentinel node after neoadjuvant chemotherapy in clinically fine-needle proven node-positive breast cancer patients. Eur. J. Surg. Oncol. 2021;47:1920–1927. doi: 10.1016/j.ejso.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Moo T.A., Jochelson M.S., Zabor E.C., Stempel M., Raiss M., Mamtani A., Tadros A.B., El-Tamer M., Morrow M. Is clinical exam of the axilla sufficient to select node-positive patients who downstage after nac for slnb? A comparison of the accuracy of clinical exam versus mri. Ann. Surg. Oncol. 2019;26:4238–4243. doi: 10.1245/s10434-019-07867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boughey J.C., Ballman K.V., Hunt K.K., McCall L.M., Mittendorf E.A., Ahrendt G.M., Wilke L.G., Le-Petross H.T. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: Results from the american college of surgeons oncology group z1071 trial (alliance) J. Clin. Oncol. 2015;33:3386–3393. doi: 10.1200/JCO.2014.57.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwentner L., Helms G., Nekljudova V., Ataseven B., Bauerfeind I., Ditsch N., Fehm T., Fleige B., Hauschild M., Heil J., et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy—Results from the multi-center sentina trial. Breast. 2017;31:202–207. doi: 10.1016/j.breast.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Le-Petross H.T., McCall L.M., Hunt K.K., Mittendorf E.A., Ahrendt G.M., Wilke L.G., Ballman K.V., Boughey J.C. Axillary ultrasound identifies residual nodal disease after chemotherapy: Results from the american college of surgeons oncology group z1071 trial (alliance) AJR Am. J. Roentgenol. 2018;210:669–676. doi: 10.2214/AJR.17.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim W.H., Kim H.J., Park H.Y., Park J.Y., Chae Y.S., Lee S.M., Cho S.H., Shin K.M., Lee S.Y. Axillary pathologic complete response to neoadjuvant chemotherapy in clinically node-positive breast cancer patients: A predictive model integrating the imaging characteristics of ultrasound restaging with known clinicopathologic characteristics. Ultrasound Med. Biol. 2019;45:702–709. doi: 10.1016/j.ultrasmedbio.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Liedtke C., Gorlich D., Bauerfeind I., Fehm T., Fleige B., Helms G., Lebeau A., Staebler A., Ataseven B., Denkert C., et al. Validation of a nomogram predicting non-sentinel lymph node metastases among patients with breast cancer after primary systemic therapy—A transsentina substudy. Breast Care. 2018;13:440–446. doi: 10.1159/000489565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantor O., Sipsy L.M., Yao K., James T.A. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann. Surg. Oncol. 2018;25:1304–1311. doi: 10.1245/s10434-018-6345-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request for researchers who provide translational research proposals. Proposals should be directed to http://www.gbg.de/de/forschung/translationale-forschung.php, (accessed on 28 November 2021); to gain access, data requestors will need to sign a data transfer agreement.