Abstract
A variety of innate defense factors in saliva such as lysozyme and lactoferrin contribute to mucosal protection and modulate Candida populations in the oral cavity. It is also known that in human immunodeficiency virus (HIV)-infected individuals significant variations in the concentrations of lysozyme and lactoferrin in saliva occur during disease progression. Therefore, the aim of this study was to determine the in vitro susceptibility to human lactoferrin and hen egg white lysozyme of genotypically similar oral Candida albicans isolates obtained from six HIV-infected ethnic Chinese during sequential visits over a 12-month period. The similarity of the genotypes (50 in total) was evaluated using a randomly amplified polymorphic DNA assay. A blastospore viability assay was performed to evaluate the sensitivity of the organisms to lysozyme and lactoferrin. Exposure to physiological concentrations of either lysozyme (30 μg/ml) or lactoferrin (20 μg/ml) caused a rapid loss of viability among all isolates to a varying extent. None of the sequential C. albicans isolates demonstrated significant differences in sensitivity to either protein from one visit to the next; similar results were noted when the different genotypes from the same individual were compared. On Spearman correlation analysis of two genotypes that were sequentially isolated from a single patient, a significant negative correlation between lysozyme (r = −0.88; P < 0.02) (but not lactoferrin) resistance and the duration of HIV disease was seen. These results imply that a minority of C. albicans isolates that persist intraorally in individuals with HIV disease develop progressive resistance to innate salivary antifungal defenses such as lysozyme, possibly as an adaptive response. However, the vast majority of the Candida isolates appear to succumb to these nonspecific host immune mediators abundantly present in the oral environment.
Candida albicans is the main cause of oral candidiasis in patients with human immunodeficiency virus (HIV) infection and AIDS (13, 38). Almost 90% of AIDS patients suffer from oropharyngeal or esophageal candidiasis at some stage of their disease (38). As HIV infection progresses, so does the oral colonization by Candida, and it eventually becomes a permanent oral resident despite prophylactic antifungal therapy (1, 36, 45). With the development of DNA fingerprinting methods, it is now possible to investigate strain relatedness and emergence of novel strains of C. albicans by sequentially sampling a cohort of individuals either with or without symptomatic oral candidiasis. Several authors have shown that AIDS patients are frequently infected with the same C. albicans strains over recurrent episodes of oral thrush (5, 34, 47, 60), and others have found, for instance in Candida vaginitis, that the same yeast strain may persist through successive episodes of infection (48, 49). Although these and other studies have traced the yeast genotypes over multiple infection episodes (29, 36, 45), not many have investigated the phenotypic attributes of these genetically similar strains that persist intraorally (43).
Lysozyme (also called muramidase) and lactoferrin are two major nonimmunological antimicrobial proteins in saliva and are thought to modulate Candida populations in the oral cavity (39). A number of researchers including our group have investigated the in vitro fungicidal activity of lysozyme against several Candida species (14, 25, 41, 42, 44, 55). These studies have demonstrated a significant dose-, time-, and strain-dependent killing effect when Candida species are exposed to lysozyme.
Lactoferrin, an iron-binding, acute-phase protein in saliva (11, 54) has a demonstrable microbicidal or microbistatic effect in vitro (8, 18, 59). Recently, in a series of studies we demonstrated the anticandidal effect of iron-free apolactoferrin, obtained from human colostrum (31, 44).
It is now known that HIV-infected individuals demonstrate a significant reduction in salivary gland secretions (2, 3, 12, 15, 30, 46, 62). In one study, Muller et al. (30) noted that a decreased output of parotid lactoferrin in parallel with markedly reduced secretory immunoglobulin A (IgA) contributed to the frequent oral infections observed in a group of HIV-seropositive subjects (30). Nonetheless, others were unable to detect significant differences in lactoferrin concentrations in stimulated parotid saliva in HIV-infected subjects and healthy controls (3, 24).
With regard to salivary lysozyme, many have reported elevated lysozyme concentrations in HIV-infected individuals with clinically detectable oral candidiasis (2, 3, 15, 23, 56, 62). Although the quality and the quantity of these nonimmune defense proteins in HIV-infected patients have been investigated, their contribution to the antifungal defenses of the oral cavity during disease progression is virtually unknown.
We hypothesized that the high prevalence of C. albicans and/or the incidence of oral candidiasis in HIV infection may be due to the emergence of virulent strains of the yeast during disease progression which may have acquired resistance to the salivary defenses such as lactoferrin and lysozyme. Hence, the main objective of this study was to evaluate and compare in vitro the susceptibilities of genotypically similar sequential isolates of C. albicans to two nonimmune defense factors of the oral mucosal immune system, i.e., lysozyme and lactoferrin.
MATERIALS AND METHODS
Candida isolates and growth conditions.
A total of 165 C. albicans isolates were obtained from a cohort of HIV-infected individuals attending an outpatient AIDS clinic during sequential therapy sessions over 1 year. The demographic data for this patient cohort are shown in Table 1. The organisms were recovered using the oral rinse technique of Samaranayake et al. (40). In brief, the patients were requested to rinse the mouth for 60 s with 10 ml of phosphate-buffered saline (pH 7.3, 0.1 M) supplied in a sterile universal container. The sample was expectorated into the container and immediately transferred to the laboratory, where the oral rinse was concentrated by spinning at 1,700 × g for 10 min, resuspended in 2 ml of sterile phosphate-buffered saline, and vortex mixed for 30 s. The concentrated oral rinse was then dispensed onto a Sabouraud dextrose agar (SDA) plate in an archimedean spiral using a spiral plater (model DU; Spiral Systems Inc., Cincinnati, Ohio). The plates were incubated for 48 h at 37°C, and up to five yeast colonies per sample were randomly chosen by a single investigator (Y.H.S.) and subcultured onto SDA plates. The pure yeast cultures were then harvested, suspended in water in sterile vials, and stored at −20°C. The organisms were identified by the germ tube test, growth at 45°C, chlamydospore formation, and API 20C AUX (Bio-Merieux, Marcy l'Etoile, France) assimilation tests, and the phenotype was further defined using CHROMagar Candida plates (CHROMagar, Paris, France) (33). Their identities were reconfirmed using the new improved APILAB Plus (Bio-Merieux) assay to exclude Candida dubliniensis. The yeasts were then stored in vials with multiple glass beads (Microbank; Pro-Lab Diagnostics, Ontario, Canada) at −70°C, subcultured monthly on SDA (Gibco Ltd., Paisley, United Kingdom), and maintained at 4°C during the experimental period. The purity of the cultures was confirmed periodically by visualization of Gram-stained organisms and the germ tube test.
TABLE 1.
Demographic data for patients examined
| Patient group and designation | Sexa | Age (yr) | CDCb classification of HIV infection | Antifungal agent(s) given | No. of visits | Total no. of C. albicans isolates tested |
|---|---|---|---|---|---|---|
| History of symptomatic oral candidiasis | ||||||
| HK2 | M | 47 | B3 | Nystatin | 6 | 16d |
| HK10 | M | 36 | C3 | Ketoconazole; FZc | 7 | 26 |
| HK39 | M | 34 | C3 | FZ | 6 | 28 |
| Without symptomatic oral candidiasis | ||||||
| HK1 | F | 29 | A3 | 8 | 26 | |
| HK4 | M | 34 | B3 | 7 | 34 | |
| HK5 | M | 41 | A3 | 5 | 35 |
M, male; F, female.
CDC, Centers for Disease Control and Prevention.
FZ, fluconazole.
From each patient on each visit, 1 to 5 CFU were selected.
Genotypic characterization. (i) Preparation of DNA for randomly amplified polymorphic DNA (RAPD) analysis.
Yeast obtained from stock cultures stored at −70°C was subcultured on yeast-peptone-dextrose medium (1% peptone yeast extract, 2% glucose, 1.5% agar) at 37°C for 24 h, and single colonies were transferred to 20 ml of yeast-peptone-dextrose broth (1% peptone, 1% yeast extract, 2% glucose) and incubated at 30°C under aerobic conditions to the stationary phase (as assessed by the measurement of the optical density of the culture at 600 nm). Following incubation, yeasts were harvested by centrifugation at 4,000 × g for 5 min and washed in 1 M sorbitol (dissolved in deionized water). The yeast pellet was resuspended in 1.5 ml of SE buffer (1.2 M sorbitol, 0.1 M EDTA [pH 8.0]) containing 3 μl of β-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.) and 0.5 mg of yeast lytic enzyme (Lyticase; Sigma), incubated at 37°C for at least 1 h until formation of spheroplasts, and harvested by centrifugation at 2,500 × g for 5 min. These spheroplasts were washed twice in SE buffer, resuspended in 1.5 ml of 0.15 M NaCl–0.1 M EDTA [pH 8.0], lysed by addition of proteinase K (final concentration, 500 μg/ml) and sodium dodecyl sulfate (1% [wt/vol] final concentration) followed by the addition of RNase (500 μg/ml), and incubated at 55°C for 1 h. The resulting supernatant obtained following centrifugation at 13,000 × g was extracted twice with phenol and once with phenol-chloroform prior to precipitation of DNA by addition of an equal volume of 2-propanol. The DNA precipitated was dissolved in 100 μl of TE buffer (10 mM Tris, 0.1 mM EDTA [pH 8.0]) (6).
(ii) RAPD analysis.
Thermocycling was performed in a model PTC-150-16 and 25 minicycler machine (MJ Research, Watertown, Mass.). Fifty microliters of the PCR master mix containing approximately 200 ng of yeast DNA as template, 5 μl of 10× PCR buffer (200 mM Tris-HCl [pH 8.4] and 500 mM KCl), 200 μM deoxynucleoside triphosphates, 25 mM MgCl2, 1 μM primer, and 1.5 U of Taq polymerase (Life Technologies, Gaithersburg, Md.) was used for PCR. The first five cycles included 30 s of denaturation at 94°C, 2 min of annealing at 52°C (primer RSD12; 5′CCGCAGCCA3′) (Life Technologies), and 2 min of primer extension, followed by 45 cycles of 30 s of denaturation at 94°C, 2 min of annealing at 57°C (primer RSD12), and 2 min of primer extension at 72°C. The reaction mixture was held at 72°C for 15 min. Control tubes without template DNA were included in each run, and reproducibility was checked for each reaction (19, 51). The PCR products were electrophoresed in an agarose gel (1.2%) for approximately 2 h at room temperature in TBE buffer (89 mM Tris, 89 mM boric acid, 2.5 mM EDTA [pH 8.0]), stained with ethidium bromide, and visualized with UV light.
Preparation of Candida inoculum for protein sensitivity tests.
The stock culture of the test yeast isolate was grown on SDA for 18 to 24 h at 37°C. A loopful of the fresh isolate was inoculated into brain heart infusion broth (Oxoid Ltd., Basingstoke, United Kingdom) and grown aerobically at 37°C. After 18 h of incubation, at the stationary phase of growth, the yeasts were harvested by centrifugation at 3,500 × g for 5 min. The yeast pellet thus obtained was washed twice by suspension in ice-cold 0.05 mM KCl (which was buffered to pH 7.0 with KOH) and harvested by centrifugation at 3,500 × g for 5 min (50). The yeasts were resuspended in the buffered KCl to yield a final concentration equivalent to an optical density of 0.63 to 0.65 at 520 nm (approximately 7 × 106 cells/ml) using a UV spectrophotometer (Ultrospec III; Pharmacia LKB, Biochrom Ltd., Cambridge, England).
Human lactoferrin.
A stock solution of human apolactoferrin (Sigma) was used for all susceptibility assays. This solution at a concentration of 2,000 μg of lactoferrin per ml was prepared with sterile distilled water, stored at 4°C, and used within a week.
Hen egg white lysozyme.
Hen egg white lysozyme (Sigma Chemical Co., Poole, United Kingdom) was used for all experiments. A stock solution of lysozyme (3,000 μg/ml) was prepared with sterile distilled water, stored at 4°C, and used within a week. The unitary activity of hen egg white lysozyme is half as much as that of human lysozyme derived from human milk (Sigma Chemical Co., St. Louis, Mo.).
Fungicidal assays.
The fungicidal effects of human lactoferrin and lysozyme on test C. albicans isolates were determined by the method of Soukka et al. (50) with minor modifications. Test suspensions of 100 μl of 200-μg/ml human lactoferrin or of 300-μg/ml hen egg white lysozyme and 100 μl of the yeast suspension were dispensed into sterile incubation tubes containing 800 μl of 0.05 mM phosphate-buffered KCl (0.05 mM; pH 7.0) to yield a yeast cell concentration of 5 × 105/ml. Thus, the final concentrations of human lactoferrin and lysozyme in the test suspensions were 20 and 30 μg/ml, respectively. These concentrations were chosen because they were physiologically similar to natural levels in saliva (52, 53). In the control sample, 100 μl of sterile distilled water was substituted for the protein. Both test and control tubes were then incubated at 37°C for 1 h with gentle shaking. After incubation, the test and control tubes were carefully vortexed, 100-μl samples were diluted 1:50 and plated on SDA using a spiral plater (Autoplate 4000; Spiral Biotech, Inc., Bethesda, Md.), and the resultant CFU were quantified after 48 h of incubation at 37°C. All experiments described above were conducted on two occasions with quadruplicate samples on each occasion.
Computing the fungicidal value of human lactoferrin (FLF) or hen egg white lysozyme (FLZ).
The fungicidal activity of human lactoferrin (FLF) or lysozyme (FLZ) was computed using the formula FLF or FLZ = (CFU per milliliter of control suspension − CFU per milliliter of test suspension)/(CFU per milliliter of control suspension). Thus, the higher the FLF or FLZ value for a particular C. albicans isolate, the higher the sensitivity of the yeast to the protein.
Statistical analysis.
Statistical analysis was conducted by the Kruskal-Wallis test (Statistical Package for Social Sciences, version 9; SPSS, Chicago, Ill.) to determine significant differences in sensitivity to either human lactoferrin or hen egg white lysozyme between sequential C. albicans isolates from six HIV-infected patients and the sensitivity of similar or dissimilar genotypes obtained from individual patients. Spearman correlation analysis was conducted to investigate the progressive sensitivity of the proteins to different, sequentially isolated genotypes of the same individual.
RESULTS
Patients.
The demographic data for the HIV-infected patient cohort from which the yeasts were isolated are shown in Table 1. Three of six patients studied had a history of symptomatic oral candidiasis and were managed using nystatin, ketoconazole, and fluconazole during the 12-month study period. The remainder of the cohort did not present with symptomatic oral candidiasis and were not on antifungals during the study period.
Genotypes of sequential C. albicans isolates.
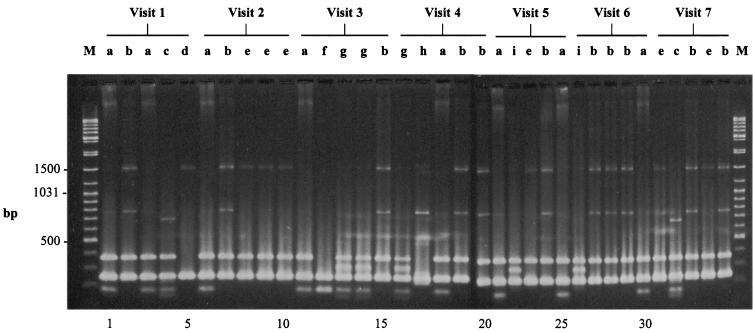
The RAPD genotypic profiles of the successive yeast isolates from each patient were categorized according to different band positions by visual comparison. For instance, the genotypic profiles of the 35 sequential C. albicans isolates of patient HK5 are shown in Fig. 1. Similar differences in genetic profiles were also seen in the sequential Candida isolates from the remaining five patients. The isolates from patients HK5 and HK10 elicited the greatest diversity, with nine genotypes each, while the isolates from patients HK1 and HK2 elicited the least, with two and three genotypes, respectively. In general, the identical genotype could be consistently isolated from the same patient during the 12-month period, while some patients had more than one. For example, in the case of HK1 a single identical genotype was found on five of six sequential visits. Similarly identical genotypes were recovered on all five visits from HK2 and on four of seven visits from HK4, and one genotype was recovered on six of seven visits and another was recovered on all seven visits from HK5. Overall, the 50 C. albicans isolates from the six HIV-infected patients demonstrated remarkable genetic variation, with multiple RAPD profiles.
FIG. 1.
RAPD fingerprints of 35 sequential oral C. albicans isolates (obtained during visits 1 to 7) from HIV-infected patient HK5, after electrophoretic separation generated by amplifying genomic DNA with primer RSD12. Genotypes (a to i) are indicated above the lanes. Sizes of bands indicate the numbers of base pairs. Lanes M, PCR markers (Sigma).
Fungicidal effect of hen egg white lysozyme on sequential oral isolates of C. albicans.
Exposure to a standard concentration of hen egg white lysozyme (30 μg/ml) indicated that all C. albicans isolates, irrespective of the genotype, were inhibited by the protein as indicated by the reduction in CFU in the test compared with the control cultures. The results of this lysozyme-mediated growth inhibition expressed in terms of the FLZ value (fungicidal effect of lysozyme) for all 50 sequential Candida isolates are shown in Table 2. The yeasts demonstrated a wide range of sensitivity to this enzyme, with an FLZ range of 0.17 to 0.93. An isolate from patient HK4 (genotype II; visit 3) was the most susceptible (FLZ = 0.93) to lysozyme, while another from patient HK5 (genotype II; visit 3) was the least susceptible (FLZ = 0.17). On statistical analysis (Kruskal-Wallis test), there were no significant differences in the sensitivities to lysozyme between C. albicans isolates of (i) similar genotypes obtained during sequential visits of individual patients or (ii) different genotypes obtained from the same patient on successive visits. However, when the mean FLZ values of Candida isolates from different individuals were compared, significant differences were noted (P < 0.05).
TABLE 2.
Fungicidal effects of 30 μg of hen egg white lysozyme per ml (FLZ) against 50 sequential oral isolates of C. albicans obtained from six HIV-infected individualsa
| Patient designation | Genotype | Expt no. | Susceptibility to lysozyme (FLZ)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient visit no.
|
Mean | SEM | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| HK1 | I | 1 | 0.30 | 0.29 | 0.54 | 0.30 | 0.26 | 0.34 | 0.11 | ||
| 2 | 0.27 | 0.29 | 0.54 | 0.31 | 0.25 | 0.33 | 0.12 | ||||
| HK2 | I | 1 | 0.68 | 0.53 | 0.78 | 0.78 | 0.80 | 0.71 | 0.11 | ||
| 2 | 0.70 | 0.53 | 0.78 | 0.78 | 0.80 | 0.72 | 0.11 | ||||
| HK4 | I | 1 | 0.94 | 0.73 | 0.90 | 0.26 | 0.71 | 0.31 | |||
| 2 | 0.94 | 0.74 | 0.89 | 0.28 | 0.71 | 0.30 | |||||
| II | 1 | 0.75 | 0.69 | 0.93 | 0.34 | 0.68 | 0.25 | ||||
| 2 | 0.76 | 0.71 | 0.94 | 0.35 | 0.69 | 0.25 | |||||
| HK5 | I | 1 | 0.64 | 0.65 | 0.56 | 0.40 | 0.48 | 0.33 | 0.51 | 0.13 | |
| 2 | 0.63 | 0.64 | 0.59 | 0.39 | 0.48 | 0.35 | 0.51 | 0.13 | |||
| II | 1 | 0.57 | 0.51 | 0.17 | 0.55 | 0.20 | 0.32 | 0.24 | 0.37 | 0.17 | |
| 2 | 0.55 | 0.45 | 0.17 | 0.56 | 0.25 | 0.30 | 0.28 | 0.37 | 0.15 | ||
| HK10 | I | 1 | 0.43 | 0.47 | 0.55 | 0.57 | 0.51 | 0.07 | |||
| 2 | 0.42 | 0.47 | 0.54 | 0.58 | 0.50 | 0.07 | |||||
| II | 1 | 0.62 | 0.44 | 0.42 | 0.49 | 0.11 | |||||
| 2 | 0.63 | 0.43 | 0.40 | 0.49 | 0.13 | ||||||
| III | 1 | 0.67 | 0.63 | 0.65 | 0.03 | ||||||
| 2 | 0.66 | 0.68 | 0.67 | 0.01 | |||||||
| IV | 1 | 0.71 | 0.58 | 0.65 | 0.09 | ||||||
| 2 | 0.70 | 0.60 | 0.65 | 0.07 | |||||||
| HK39 | I | 1 | 0.51 | 0.55 | 0.57 | 0.90 | 0.84 | 0.67 | 0.18 | ||
| 2 | 0.51 | 0.55 | 0.58 | 0.90 | 0.84 | 0.68 | 0.18 | ||||
| II | 1 | 0.62 | 0.83 | 0.87 | 0.77 | 0.13 | |||||
| 2 | 0.64 | 0.83 | 0.87 | 0.78 | 0.12 | ||||||
| Total | 0.59 | 0.14 | |||||||||
Roman numerals designate different genotypes isolated from individual patients. Strains with identical roman numerals from different patients are unrelated.
In addition, we performed Spearman correlation analysis of the FLZ values of similar genotypes over successive visits. In the event, genotypes I (six isolates) and II (seven isolates) of a single patient, HK5, showed a highly significant negative correlation (r = −0.88; P < 0.02) between sensitivity to lysozyme and sequential patient visits, implying progressive development of resistance to the enzyme over the study period.
Fungicidal effect of human lactoferrin on sequential oral isolates of C. albicans.
The results of the fungicidal effect of human lactoferrin expressed in terms of FLF value (fungicidal effect of lactoferrin) for the 50 C. albicans isolates obtained during successive visits of six HIV-infected patients are shown in Table 3. The isolates demonstrated a wide range of sensitivity (0.09 to 0.85) to human lactoferrin. Of the 50 Candida isolates tested, the most susceptible (FLF = 0.85) was a yeast isolate from patient HK4 (genotype I; visit 1) and the least susceptible (FLF = 0.09) organisms were isolated from patients HK5 (genotype II; visit 3) and HK10 (genotype II; visit 3). On statistical analysis (Kruskal-Wallis test), no significant differences in the fungicidal values for lactoferrin (FLF) were noted between (i) C. albicans isolates of similar genotypes obtained during sequential visits of individual patients or (ii) different genotypes obtained from the same patient on successive visits. However, when the mean FLF values of Candida isolates from different individuals were compared, significant differences were noted (P < 0.05).
TABLE 3.
Fungicidal effects of 20 μg of human lactoferrin per ml (FLF) against 50 sequential oral isolates of C. albicans obtained from six HIV-infected individualsa
| Patient designation | Genotype | Expt no. | Susceptibility to lactoferrin (FLF)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient visit no.
|
Mean | SEM | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| HK1 | I | 1 | 0.12 | 0.22 | 0.35 | 0.10 | 0.13 | 0.18 | 0.10 | ||
| 2 | 0.14 | 0.22 | 0.35 | 0.10 | 0.14 | 0.19 | 0.10 | ||||
| HK2 | I | 1 | 0.23 | 0.14 | 0.38 | 0.38 | 0.63 | 0.35 | 0.19 | ||
| 2 | 0.25 | 0.15 | 0.40 | 0.36 | 0.61 | 0.35 | 0.17 | ||||
| HK4 | I | 1 | 0.86 | 0.67 | 0.65 | 0.12 | 0.58 | 0.32 | |||
| 2 | 0.85 | 0.67 | 0.63 | 0.12 | 0.57 | 0.31 | |||||
| II | 1 | 0.39 | 0.34 | 0.85 | 0.16 | 0.44 | 0.29 | ||||
| 2 | 0.39 | 0.37 | 0.84 | 0.14 | 0.44 | 0.29 | |||||
| HK5 | I | 1 | 0.31 | 0.31 | 0.20 | 0.17 | 0.11 | 0.23 | 0.22 | 0.08 | |
| 2 | 0.30 | 0.18 | 0.24 | 0.12 | 0.10 | 0.22 | 0.19 | 0.08 | |||
| II | 1 | 0.46 | 0.38 | 0.10 | 0.32 | 0.08 | 0.16 | 0.11 | 0.23 | 0.15 | |
| 2 | 0.45 | 0.28 | 0.08 | 0.36 | 0.14 | 0.12 | 0.14 | 0.22 | 0.14 | ||
| HK10 | I | 1 | 0.31 | 0.16 | 0.23 | 0.49 | 0.30 | 0.14 | |||
| 2 | 0.29 | 0.19 | 0.28 | 0.45 | 0.30 | 0.11 | |||||
| II | 1 | 0.38 | 0.29 | 0.07 | 0.25 | 0.16 | |||||
| 2 | 0.38 | 0.27 | 0.11 | 0.25 | 0.14 | ||||||
| III | 1 | 0.12 | 0.38 | 0.25 | 0.18 | ||||||
| 2 | 0.13 | 0.39 | 0.26 | 0.18 | |||||||
| IV | 1 | 0.13 | 0.25 | 0.19 | 0.08 | ||||||
| 2 | 0.11 | 0.22 | 0.17 | 0.08 | |||||||
| HK39 | I | 1 | 0.36 | 0.44 | 0.15 | 0.13 | 0.30 | 0.28 | 0.13 | ||
| 2 | 0.38 | 0.43 | 0.15 | 0.14 | 0.31 | 0.28 | 0.13 | ||||
| II | 1 | 0.20 | 0.12 | 0.23 | 0.18 | 0.06 | |||||
| 2 | 0.18 | 0.11 | 0.22 | 0.17 | 0.06 | ||||||
| Total | 0.29 | 0.12 | |||||||||
Roman numerals designate different genotypes isolated from individual patients. Strains with identical roman numerals from different patients are unrelated.
Spearman correlation analysis of the FLF values of two different genotypes over successive visits, i.e., genotypes I and II of a single patient, HK5 (obtained from six and seven sequential visits, respectively), tended to demonstrate decreasing susceptibility to human lactoferrin between visits during a 1-year period of observation. Although this was not statistically significant (r = −0.78; P > 0.05), it was notable that the identical strains showed a highly significant negative correlation between sensitivity to lysozyme and disease duration (P < 0.02; r = −0.88).
DISCUSSION
A number of investigators have reported that Candida species from HIV-infected patients with recurrent episodes of oral thrush are significantly less diverse genetically than commensal strains from healthy individuals (47, 60). This implies that strains derived from the same parental stock may persist through recurrent infections in these immunocompromised patients. Others have reported similar findings on comparison of serotypes from immunologically compromised patients (including patients with AIDS) and healthy individuals (7).
Although a variety of different DNA typing procedures such as pulsed-field gel electrophoresis, restriction fragment length polymorphism, and RAPD analysis have been employed for deciphering the genetic profiles of individual Candida isolates by previous workers (34), it appears that the last method is equally as sensitive as others for this purpose (6, 19). The RAPD technique requires only a minute quantity of yeast DNA and is fast and reliable for strain delineation (17, 19). Hence, in the present investigation we used the RAPD technique to characterize the genotypic relatedness among the sequential oral C. albicans isolates from the six HIV-infected individuals. Prior to choosing the primer RSD12 for the study, we evaluated others such as RSD6, RSD8, RSD10, and RSD12 (58), and it was observed that the first demonstrated the highest discriminatory power in differentiating our collection of Candida strains into different genotypes. As in similar previous studies (21), we noted up to five different genotypes from a single visit. Further, sequential yeast isolates derived from the same patient yielded similar as well as dissimilar genotypes, confirming the usefulness of this technique for isotype analyses.
The antifungal effects of both human lactoferrin and hen egg white lysozyme on Candida were examined according to the methods described previously by Nikawa et al. (32) and Tobgi et al. (55), respectively. Our group and others have used these blastospore susceptibility assays previously (31, 32, 44, 61), and in the present investigation they once again proved reliable and sensitive. Using the identical method, Samaranayake et al. (44) noted the mean (range) FLZ and FLF values of 0.32 (0.27 to 0.36) and 0.34 (0.21 to 0.45), respectively, for five Candida isolates from healthy individuals. This compares with 0.59 (0.33 to 0.78) and 0.29 (0.17 to 0.58) for lysozyme and lactoferrin, respectively, for the negative cohort of the present study. Results indicate a heightened sensitivity of C. albicans to lysozyme, but not lactoferrin, in patients with HIV infection. However, further studies are warranted to confirm or refute these findings, due to the small number of isolates tested (five in total) from the healthy cohort.
In general, the results of the lysozyme assay indicate that sequential C. albicans isolates from individuals with HIV infection are broadly similar with no significant variability in the susceptibility of isolates derived possibly from the same parental stock. Lysozyme is a constituent of saliva with a concentration range of 1.5 to 57 μg of human lysozyme equivalents ml−1 (35, 52). The enzyme is present in higher concentrations in plaque fluid than in whole saliva (9), and activated polymorphonuclear leukocytes also release this enzyme extracellularly (20). The antifungal properties of lysozyme are thought to be mediated through the enzymatic hydrolysis of N-glycosidic linkages in the microbial cell wall and injury to the cytoplasmic membrane following direct cationic-protein binding (25). Studies of the interaction between lysozyme and Candida species have shown significant inter- and intraspecies variations in susceptibility to this enzyme (41, 44, 55), and this should be borne in mind when the results from sequential isolates are considered.
With regard to lactoferrin, none of the sequential strains in general demonstrated either increased or decreased susceptibility to the protein to a significant extent during the 12-month study period. Lactoferrin is found in saliva and other external secretions such as tears and bronchial secretions (26) and is also a constituent of the polymorphonuclear leukocytes (4, 27). The concentration of lactoferrin in unstimulated parotid saliva is about 7 to 20 μg/ml (10, 37) but decreases upon stimulation. The fungicidal nature of lactoferrin is thought to be due to (i) sequestration of ferrous ions, leading to deprivation of elemental iron needed for yeast metabolism (28); (ii) structural changes induced on yeast cell walls (32); or (iii) the activation of intracellular autolytic enzyme systems consequential to lactoferrin adsorption (16). As with lysozyme, significant inter- and intraspecies variations in candidal sensitivity to lactoferrin have been observed previously (32, 44, 50, 61).
Although there were no significant differences in the FLZ and FLF values of the majority of the sequential isolates, we noted that in two groups of isolates from patient HK5 belonging to genotypes I (six isolates) and II (seven isolates) the fungicidal effect of a standard dose of lysozyme, but not lactoferrin, significantly and progressively decreased over the 12-month study period. One explanation for this may be that the successive generations of Candida in this individual, whose disease was kept under control by antiretroviral agents, progressively developed resistance to the nonspecific salivary immune factor lysozyme (r = −0.88; P < 0.02) during the 12-month study period. As only 13 of 50 (26%) C. albicans isolates studied showed the emergence of such resistance to lysozyme, our data should be interpreted with caution. Nonetheless the results reported here support the contention that the emergence of these resistant C. albicans strains may perpetuate the recurrence of oral yeast colonization and infection, a hallmark of HIV disease. Unfortunately, we were unable to monitor the temporal variations in salivary lysozyme or lactoferrin concentrations in our cohort, although this might have shed further light on the emergence of resistance. For instance, there are reports to indicate alterations in major (parotid and submandibular) salivary gland function following HIV type 1 infection with effects on both the salivary composition and output (flow rate) (12, 30). Elevated concentrations of lysozyme have been observed in stimulated as well as unstimulated submandibular or sublingual saliva (3, 62) and in stimulated parotid saliva (23, 30) from HIV-infected individuals. Kirstila et al. (15) have reported that all innate, nonimmune salivary defense factors were equally abundant and present possibly at higher concentrations in a group of patients with common variable immunodeficiency when compared with age- and sex-matched immunologically competent healthy subjects (15). We previously reported elevated salivary lysozyme concentrations in mixed saliva in a Hong Kong cohort of HIV-infected ethnic Chinese (23% higher than the HIV-free group [P < 0.0001] [56]). In addition, Schiodt et al. (46) demonstrated that patients with HIV-associated salivary gland disease had increased levels of lysozyme and salivary IgA and decreased levels of salivary proteins compared with the HIV-negative controls. Thus, it is tempting to speculate that the emergence of lysozyme resistance in the small number of Candida isolates reported herein could be due to an innate rise in salivary lysozyme levels during HIV disease progression.
With regard to lactoferrin, Muller et al. (30) reported that the lactoferrin output significantly decreased in a group of 44 subjects with HIV infection in parallel with a markedly reduced parotid secretory IgA output. Analysis of a group of Centers for Disease Control and Prevention stage IV AIDS patients also showed a decrease in lactoferrin in comparison with HIV-negative controls (22). Interestingly, Van Der Strate et al. (57) have observed that, in a group of 15 HIV-infected subjects, the titers of Candida present in the oral cavity were unaffected by salivary lactoferrin concentration. Whereas lysozyme levels appear to increase during HIV disease, the reverse seems to be the case for lactoferrin, and our results match these observations, as none of the sequential isotypes tested showed either a significant increase or a significant decrease in sensitivity to lactoferrin over a 12-month period.
To conclude, the present data give us a tantalizing glimpse of the adaptive responses of oral Candida species to innate antimicrobial defenses in saliva, such as lysozyme, in HIV infection. To our knowledge, the present study is the first to report this phenomenon, and further work is warranted to clarify the reported findings and elucidate the true role of these and other oral secretions in chronic oral candidal colonization in patients with HIV disease.
REFERENCES
- 1.Alexander B D, Perfect J R. Antifungal resistance towards the year 2000. Drugs. 1997;54:657–678. doi: 10.2165/00003495-199754050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson J C, Yeh C-K, Bermudez D, Fox P C, Baum B J. Longitudinal evaluation of major salivary gland function in HIV-1 infected patients. J Oral Pathol Med. 1989;18:469–470. doi: 10.1111/j.1600-0714.1989.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson J C, Yeh C-K, Oppenheim F G, Bermudez D, Baum B J, Fox P C. Elevation of salivary antimicrobial proteins following HIV-1 infection. J Acquir Immune Defic Syndr. 1990;3:41–48. [PubMed] [Google Scholar]
- 4.Baggiolini M, De Duve C, Masson P L, Heremans J F. Association of lactoferrin with specific granules in rabbit heterophilic leukocytes. J Exp Med. 1970;131:559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bart-Delabesse E, Boiron P, Carlotti A, Dupont B. Candida albicans genotyping in studies with patients with AIDS developing resistance to fluconazole. J Clin Microbiol. 1993;31:2933–2937. doi: 10.1128/jcm.31.11.2933-2937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bostock A, Khattak M N, Matthews R, Burnie J. Comparison of PCR fingerprinting, by random amplification of polymorphic DNA, with other molecular typing methods for Candida albicans. J Gen Microbiol. 1993;139:2179–2184. doi: 10.1099/00221287-139-9-2179. [DOI] [PubMed] [Google Scholar]
- 7.Brawner D L, Cutler J E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984;43:966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole M F, Arnold R, Mestecky J, Kulhavy R, McGhee J R. Studies with human lactoferrin and Streptococcus mutans. In: Stiles H, Loesche W, O'Brien T, editors. Microbial aspects of dental caries II—1976. Washington, D.C.: Information Retrieval; 1976. pp. 274–359. [Google Scholar]
- 9.Cole M F, Hsu S D, Baum B J, Bowen W H, Sierra L I, Aquirre M, Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981;31:998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Paola C, Mandel I D. Lactoferrin concentration in human parotid saliva as measured by an enzyme-linked immunosorbent assay (ELISA) J Dent Res. 1980;59:1463–1465. doi: 10.1177/00220345800590090101. [DOI] [PubMed] [Google Scholar]
- 11.Ellison R T, Laforce F M, Giehl T J, Boose D S, Dunn B E. Lactoferrin and transferrin damage of the Gram-negative outer membrane is modulated by Ca2+ and Mg2+ J Gen Microbiol. 1990;136:1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- 12.Fox P C. Saliva and salivary gland alterations in HIV infection. J Am Dent Assoc. 1991;122:46–48. doi: 10.14219/jada.archive.1991.0331. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan D, Greenspan J S. HIV-related oral disease. Lancet. 1996;348:729–733. doi: 10.1016/S0140-6736(96)02308-2. [DOI] [PubMed] [Google Scholar]
- 14.Kamaya T. Lytic action of lysozyme on Candida albicans. Mycopathol Mycol Appl. 1970;42:197–207. doi: 10.1007/BF02051947. [DOI] [PubMed] [Google Scholar]
- 15.Kirstila V, Tenovuo J, Ruuskanen O, Nikoskelainen J, Irjala K, Vilja P. Salivary defense factors and oral health in patients with common variable immunodeficiency. J Clin Immunol. 1994;14:229–236. doi: 10.1007/BF01552309. [DOI] [PubMed] [Google Scholar]
- 16.Laible N, Germaine G R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect Immun. 1985;48:720–728. doi: 10.1128/iai.48.3.720-728.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasker B A, Carle G F, Kobayashi G S, Medoff G. Comparison of the separation of Candida albicans chromosome-size DNA by pulsed-field gel electrophoresis techniques. Nucleic Acids Res. 1989;17:3783–3793. doi: 10.1093/nar/17.10.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lassiter M O, Newsome A L, Sams L D, Arnold R R. Characterization of lactoferrin interaction with Streptococcus mutans. J Dent Res. 1987;66:480–485. doi: 10.1177/00220345870660021601. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann P L, Lin D, Lasker B A. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J Clin Microbiol. 1992;30:3249–3254. doi: 10.1128/jcm.30.12.3249-3254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerche A, Bisgaard H, Christensen J D, Venge P, Dahl R, Snedergaard J. Lactoferrin, myeloperoxidase, lysozyme, and eosinophilic cationic protein in exudate in delayed type hypersensitivity. Allergy. 1988;43:139–145. doi: 10.1111/j.1398-9995.1988.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 21.Leung W K, Dassanayake R S, Yau J Y Y, Jin L J, Yam W C, Samaranayake L P. Oral colonization, phenotypic, and genotypic profiles of Candida species in irradiated, dentate, xerostomic nasopharyngeal carcinoma survivors. J Clin Microbiol. 2000;38:2219–2226. doi: 10.1128/jcm.38.6.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X S, Delfraissy J F, Grangeot-Keros L, Rannou M T, Pillot J. Rapid and constant detection of HIV antibody response in saliva of HIV-infected patients; selective distribution of anti-HIV activity in the IgG isotype. Res Virol. 1994;145:369–377. doi: 10.1016/s0923-2516(07)80042-2. [DOI] [PubMed] [Google Scholar]
- 23.Mandel I D, Barr C E, Turgeon L. Longitudinal study of parotid saliva in HIV-1 infection. J Oral Pathol Med. 1992;21:209–213. doi: 10.1111/j.1600-0714.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 24.Marder M Z, Barr C E, Mandel I D. Cytomegalovirus presence and salivary composition in acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1985;60:373–376. doi: 10.1016/0030-4220(85)90258-0. [DOI] [PubMed] [Google Scholar]
- 25.Marquis G, Montplaisir S, Garzon S, Strykowski H, Auger P. Fungitoxicity of muramidase, ultrastructural damage to Candida albicans. Lab Investig. 1982;46:627–636. [PubMed] [Google Scholar]
- 26.Massons P, Heremans J. Studies of lactoferrin, the iron binding protein of secretions. Protides Biol Fluids. 1966;14:115–124. [Google Scholar]
- 27.Massons P, Heremans J, Schonne E. Lactoferrin and iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazurier J, Spik G. Comparative studies of the iron-binding properties of human transferrins. Biochim Biophys Acta. 1980;718:643–658. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- 29.McCullough M, Ross B, Reade P C. Oral Candida albicans from patients infected with the human immunodeficiency virus and characterization of a genetically distinct subgroup of Candida albicans. Aust Dent J. 1995;40:91–97. doi: 10.1111/j.1834-7819.1995.tb03122.x. [DOI] [PubMed] [Google Scholar]
- 30.Muller F, Holberg-Petersen M, Rollag H, Degre M, Brandtzaeg P, Froland S S. Nonspecific oral immunity in individuals with HIV infection. J Acquir Immune Defic Syndr. 1992;5:46–51. [PubMed] [Google Scholar]
- 31.Nikawa H, Samaranayake L P, Tenovuo J, Hamada T. The effect of antifungal agents on the in vitro susceptibility of Candida albicans to apo-lactoferrin. Arch Oral Biol. 1994;39:921–923. doi: 10.1016/0003-9969(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 32.Nikawa H, Samaranayake L P, Tenovuo J, Pang K M, Hamada T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch Oral Biol. 1993;38:1057–1063. doi: 10.1016/0003-9969(93)90167-k. [DOI] [PubMed] [Google Scholar]
- 33.Odds F C, Bermaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller M A, Rhine-Chalberg J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raeste A M, Touompo A. Lysozyme activity and flow rates of mixed saliva in children, adolescents and adults. Scand J Dent Res. 1976;84:418–422. doi: 10.1111/j.1600-0722.1976.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 36.Redding S W, Pfaller M A, Messer S A, Smith J A, Prows J, Bradley L L, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and DNA subtyping of multiple Candida albicans colonies from patients with AIDS and oral candidiasis suffering one or more episodes of infection. J Clin Microbiol. 1997;35:1761–1765. doi: 10.1128/jcm.35.7.1761-1765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudney J D, Kajander K C, Smith Q T. Correlations between human salivary levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A with different stimulatory states and over time. Arch Oral Biol. 1985;30:765–771. doi: 10.1016/0003-9969(85)90129-3. [DOI] [PubMed] [Google Scholar]
- 38.Samaranayake L P. Oral mycoses in human immunodeficiency virus infection: a review. Oral Surg Oral Med Oral Pathol. 1992;73:171–180. doi: 10.1016/0030-4220(92)90191-r. [DOI] [PubMed] [Google Scholar]
- 39.Samaranayake L P, MacFarlane T W. Oral candidosis. London, United Kingdom: Wright-Butterworth; 1990. [Google Scholar]
- 40.Samaranayake L P, MacFarlane T W, Lamey P J, Ferguson M M. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J Oral Pathol. 1986;15:386–388. doi: 10.1111/j.1600-0714.1986.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 41.Samaranayake Y H, MacFarlane T W, Samaranayake L P, Aitchison T C. The in vitro lysozyme susceptibility of Candida species cultured in sucrose supplemented media. Microbios. 1993;74:23–28. [PubMed] [Google Scholar]
- 42.Samaranayake Y H, MacFarlane T W, Aitchison T C, Samaranayake L P. The in vitro lysozyme susceptibility of Candida albicans cultured in carbohydrate-supplemented media. Oral Microbiol Immunol. 1993;8:177–181. doi: 10.1111/j.1399-302x.1993.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 43.Samaranayake, Y. H., L. P. Samaranayake, P. C. Tsang, K. H. Wong, and K. W. S. Yeung. J. Oral Pathol. Med., in press. [DOI] [PubMed]
- 44.Samaranayake Y H, Samaranayake L P, Wu P C, So M. The antifungal effect of lactoferrin and lysozyme on Candida krusei and Candida albicans. APMIS. 1997;105:875–883. [PubMed] [Google Scholar]
- 45.Sangeorzan J A, Bradley S F, He X, Zairns L T, Ridenour G L, Tiballi R N, Kauffman C A. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 46.Schiodt M, Atkinson J C, Greenspan D, Fox P C, Dodd C L, Daniels T E, Greenspan J S. Sialochemistry in human immunodeficiency virus associated salivary gland disease. J Rheumatol. 1992;19:26–29. [PubMed] [Google Scholar]
- 47.Schmid J, Odds F C, Wiselka M J, Nicholson K G, Soll D R. Genetic similarity and maintenance of Candida albicans strains from a group of AIDS patients, demonstrated by DNA fingerprinting. J Clin Microbiol. 1992;30:935–941. doi: 10.1128/jcm.30.4.935-941.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schröppel K, Rotman M, Galask R, Mac K, Soll D R. Evolution and replacement of Candida albicans strains during recurrent vaginitis demonstrated by DNA fingerprinting. J Clin Microbiol. 1994;32:2646–2654. doi: 10.1128/jcm.32.11.2646-2654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soll D R, Galask R, Isley S, Gopala Rao T V, Stone D, Hicks J, Schmid J, Mac K, Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989;27:681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soukka T, Tenouvo J, Lenander-Lumikari M. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol Lett. 1992;90:223–228. doi: 10.1016/0378-1097(92)90650-d. [DOI] [PubMed] [Google Scholar]
- 51.Steffan P, Vazquez J A, Boikov D, Xu C, Sobel J D, Akins R A. Identification of Candida species by randomly amplified polymorphic DNA fingerprinting of colony lysates. J Clin Microbiol. 1997;8:2031–2039. doi: 10.1128/jcm.35.8.2031-2039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuchell R N, Mandel I D. A comparative study of salivary lysozyme in caries-resistant and caries-susceptible adults. J Dent Res. 1983;62:552–554. doi: 10.1177/00220345830620050701. [DOI] [PubMed] [Google Scholar]
- 53.Tenovuo J. Nonimmunoglobulin defense factors in human saliva. In: Tenovuo J, editor. Human saliva: clinical chemistry and microbiology. II. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 55–91. [Google Scholar]
- 54.Tenovuo J, Lumikari M, Soukka T. Salivary lysozyme, lactoferrin and peroxidases. Antibacterial effect on cariogenic bacteria and clinical application in preventive dentistry. Proc Finn Dent Soc. 1991;87:197–208. [PubMed] [Google Scholar]
- 55.Tobgi R S, Samaranayake L P, MacFarlane T W. The in vitro susceptibility of Candida species to lysozyme. Oral Microbiol Immunol. 1988;2:1–4. doi: 10.1111/j.1399-302x.1988.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsang C S P, Samaranayake L P. Salivary lysozyme and related parameters of a predominantly Chinese, HIV-infected cohort in Hong Kong. Oral Dis. 1999;5:241–246. doi: 10.1111/j.1601-0825.1999.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 57.Van Der Strate B W A, Harmsen M C, The T H, Sprenger H G, De Vries H, Eikelboom M C, Kuipers M E, Meijer D K F, Swart P J. Plasma lactoferrin levels are decreased in end-stage AIDS patients. Viral Immunol. 1999;12:197–203. doi: 10.1089/vim.1999.12.197. [DOI] [PubMed] [Google Scholar]
- 58.Waltimo T M, Dassanayake R S, Orstavik D, Haapasalo M P P, Samaranayake L P. Phenotypes and randomly amplified polymorphic DNA profiles of Candida albicans isolates from root canal infections in a Finnish population. Oral Microbiol Immunol. 2001;16:106–112. doi: 10.1034/j.1399-302x.2001.016002106.x. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whelan W L, Kirsch D R, Kwon-Chung K J, Wahl S M, Smith P D. Candida albicans in patients with the acquired immunodeficiency syndrome: absence of a novel or hypervirulent strain. J Infect Dis. 1990;162:513–518. doi: 10.1093/infdis/162.2.513. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y Y, Samaranayake Y H, Samaranayake L P, Nikawa H. In vitro susceptibility of Candida species to lactoferrin. Med Mycol. 1999;37:35–41. doi: 10.1046/j.1365-280x.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 62.Yeh C-K, Fox P C, Ship J A, Busch K A, Bermudez D K, Wilder A-M, Katz R W, Wolff A, Tylenda C A, Atkinson J C, Baum B J. Oral defense mechanisms are impaired early in HIV-1 infected patients. J Acquir Immune Defic Syndr. 1988;1:361–366. [PubMed] [Google Scholar]