Abstract
Simple Summary
The transfer of a normal intestinal microbial community from healthy donors by way of their fecal material into patients with various diseases is an emerging therapeutic approach, particularly to treat patients with recurrent or refractory C. difficile infections (CDI). This approach, called fecal microbiota transplant (FMT), is increasingly being applied to patients with hematologic and oncologic diseases to treat recurrent CDI, modulate treatment-related complications, and improve cancer treatment outcome. In this review paper, we discussed the principles and methods of FMT. We examined the results obtained thus far from its use in hematologic and oncologic patients. We also propose novel uses for the therapeutic approach and appraised the challenges associated with its use, especially in this group of patients.
Abstract
Understanding of the importance of the normal intestinal microbial community in regulating microbial homeostasis, host metabolism, adaptive immune responses, and gut barrier functions has opened up the possibility of manipulating the microbial composition to modulate the activity of various intestinal and systemic diseases using fecal microbiota transplant (FMT). It is therefore not surprising that use of FMT, especially for treating relapsed/refractory Clostridioides difficile infections (CDI), has increased over the last decade. Due to the complexity associated with and treatment for these diseases, patients with hematologic and oncologic diseases are particularly susceptible to complications related to altered intestinal microbial composition. Therefore, they are an ideal population for exploring FMT as a therapeutic approach. However, there are inherent factors presenting as obstacles for the use of FMT in these patients. In this review paper, we discussed the principles and biologic effects of FMT, examined the factors rendering patients with hematologic and oncologic conditions to increased risks for relapsed/refractory CDI, explored ongoing FMT studies, and proposed novel uses for FMT in these groups of patients. Finally, we also addressed the challenges of applying FMT to these groups of patients and proposed ways to overcome these challenges.
Keywords: fecal microbiota transplant, hematologic diseases, oncologic diseases, outcome, challenges
1. Introduction
The human intestinal tract is colonized by thousands of different microbial species. In the last two decades, various studies have established the importance of these microbial organisms in maintaining and facilitating human health and well-being. These commensal microbial communities play vital roles in regulating host metabolism, maintaining intestinal microbial homeostasis, and influencing the host’s adaptive immunity [1]. Consequently, it is not surprising that alterations in the normal microbial composition result in disease states. Therefore, it follows that restoring the intestinal microbial composition may treat disease states and ameliorate symptoms.
Many factors affect normal intestinal microbial composition [2]. The most common factor by far is medication, especially broad-spectrum antibiotics. In addition to removing the causative factors and waiting for the spontaneous normalization of the normal intestinal microbial community, probiotics and prebiotics may help with the recovery. However, the most rapid and effective method through which to restore the intestinal microbiome is through a fecal microbiota transplant (FMT) from donors with a normal intestinal microbial composition. FMT involves the instillation of stool that has been collected from a healthy donor and processed according to different institution-specific protocols into the intestinal tract of a patient with an altered intestinal microbiome. The term fecal microbiota refers to the complex array of microorganisms that live symbiotically within the intestinal tract of the host.
The concept of FMT is not new. It was first used in China in the form of a “yellow soup” in the fourth century to treat diarrhea [3]. There were also some reports about the consumption of fresh, warm camel feces by the Bedouins as a remedy for bacterial dysentery [4]. The first documented successful use of FMT was in 1958, when it was used to treat four patients affected by pseudomembranous colitis [5]. However, it was not until 1983 when the next case of successful use of FMT in a patient with Clostridioides difficile (C. difficile) infection (CDI) was reported [6].
FMT has since primarily been applied to patients with relapsed/refractory CDI. However, there has been increasing use of this therapeutic approach for other intestinal and systemic diseases, albeit on a research basis. Surveys in the United States and in Europe have indicated that the number of procedures being performed has climbed rapidly over the last few years [7,8]. Therefore, FMT is an emerging therapeutic approach with very broad potential applicability. Due to the complexity of the diseases and their treatment, patients with hematologic and oncologic diseases may be particularly suitable candidates for FMT. In this paper, we will discuss the principles and biologic effects of FMT, examine the factors rendering patients with hematologic and oncologic conditions to increased risks of relapsed/refractory CDI, explore the ongoing FMT studies, and propose novel uses of FMT in these groups of patients. Finally, we will address the challenges of applying FMT to these groups of patients and propose ways to overcome these challenges.
2. Steps and Biologic Effects of FMT
2.1. The Steps of FMT
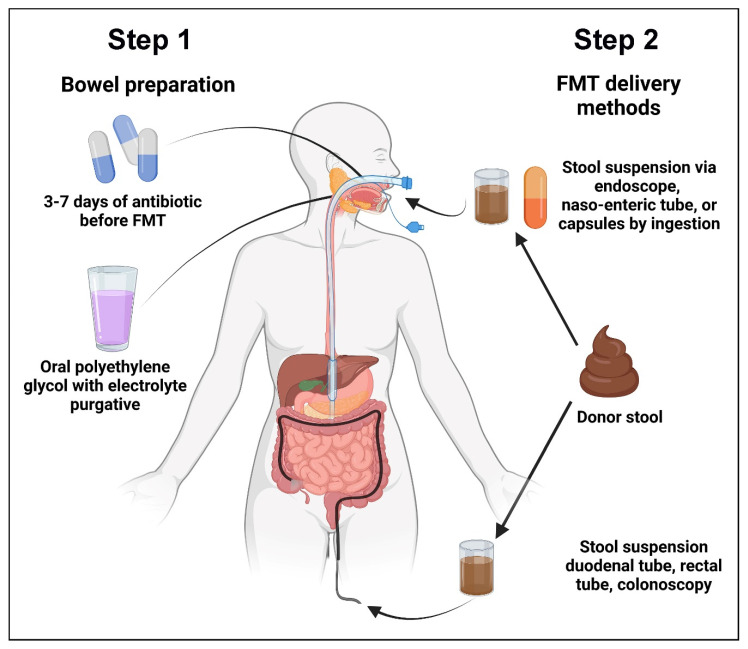
FMT can be divided into two steps (Figure 1): (1). bowel preparation and (2). decal material delivery. Step 1 of FMT involves bowel preparation using antibiotics to create the spatial niche for the transplanted microbes to populate and proliferate. The importance of this has been clearly demonstrated in a mouse model of FMT, in which pre-transplant antibiotic treatment facilitated more efficient engraftment compared to no bowel preparation or bowel preparation using a laxative [9]. Unlike in patients with CDI who usually have very restricted intestinal microbial diversity, bowel preparation with antibiotics may be even more important for successful FMT for non-CDI purposes. Based on these considerations, the European Consensus Conference on FMT recommends that patients with recurrent CDI should receive three days of either vancomycin or fidaxomicin before the FMT procedure [10], although we typically administer oral vancomycin for seven days prior to FMT in patients with active colitis, with the last dose being given 24 h before the procedure. The aim of the antibiotics is to decrease the abundance of C. difficile load and to create space for the establishment of the transplanted donor microbes. Routine administration of oral antibiotics in the absence of active colitis is generally not recommended due to concerns of diminished efficacy, especially in patients with diarrhea-predominant irritable bowel disease, in which antibiotic pretreatment has been shown to significantly reduce bacterial engraftment [11]. Bowel preparation with two to three liters of oral polyethylene glycol with electrolyte purgative is carried out on the day prior to FMT. Typically, 200–300 g of donor stool suspended in 200 to 300 mL of sterile normal saline is administered within ten minutes of the preparation of the stool mixture. The patients resume regular diet and medications two hours after the procedure. There is currently no consensus on the optimal protocol for FMT administration, and the protocol varies at each institution.
Figure 1.
The two steps of fecal microbiota transplant. In Step 1, patients undergo bowel preparation with oral antibiotics followed by laxative. At least 24 h after the last dose of oral antibiotics, the patient will receive the donor fecal material via capsule, naso-enteral tubes, or upper or lower gastrointestinal endoscopy.
Up until 1989, fecal material was delivered by retention enemas. However, alternative methods were subsequently developed, including fecal infusion via duodenal tubes, rectal tubes, colonoscopy, and colonic transendoscopic enteral tubing [12,13]. Nowadays, enteral routes include the use of an endoscope, a naso-enteric tube, or capsules by ingestion. FMT for recurrent CDI is equally successful whether given via colonoscopy, nasogastric tube, or enemas administered at home [14]. A meta-analysis of four studies on the relative rate of CDI cure following oral FMT capsules compared to FMT delivered through colonoscopy was performed and did not find any differences in efficacy between the two methods. There were no reports of serious adverse effects that could be attributed to oral FMT capsules other than those associated with treatment failure. Oral FMT capsules are becoming more accessible and should be administered as per the protocol of the capsule manufacturer. One possible barrier to their use is that the number of capsules that has to be ingested for a full dose is frequently large and may lead to the gastrointestinal symptoms of nausea, vomiting, and bloating [15]. However, a larger meta-analysis involving 24 studies reported that FMT by lower gastrointestinal endoscopy was superior to all other delivery methods [16].
2.2. Biologic Consequences of FMT
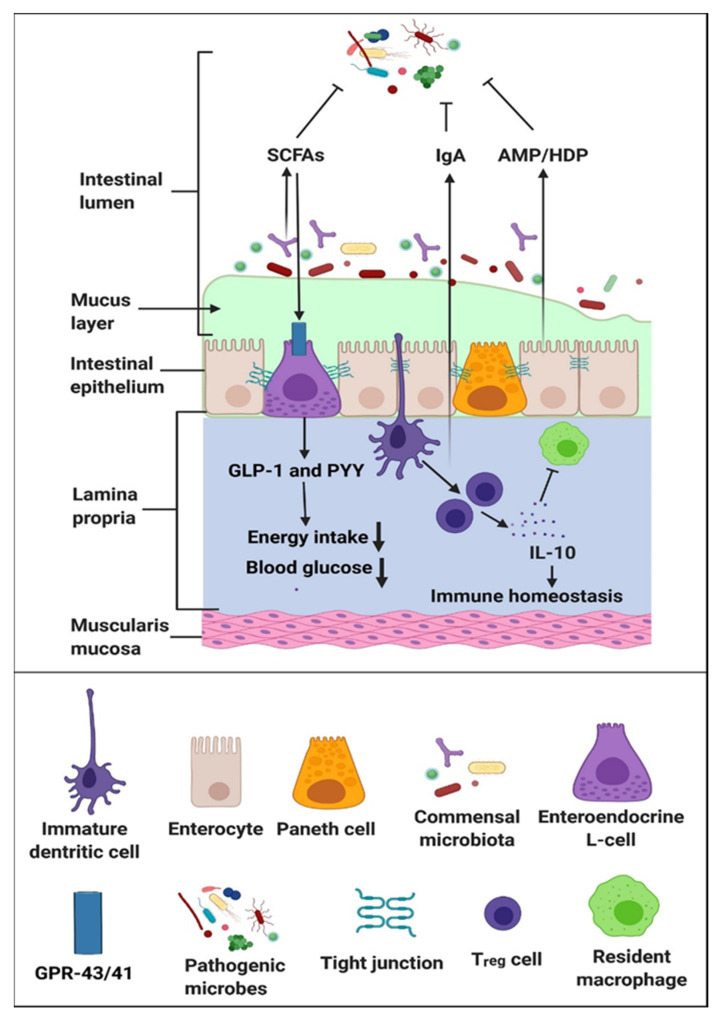
Intestinal microbial communities regulate host metabolism, maintain intestinal microbial homeostasis, and modulate the host immune response (Figure 2) [17]. As a result, FMT re-equilibrates these functions in patients with the disease state due to intestinal dysbiosis. Unlike CDI in which the intestinal dysbiosis is clearly characterized by an overgrowth of toxigenic C. difficile [13], it remains unclear if the intestinal dysbiosis observed in other pathologic conditions is just an association rather than causation. If the relationship between the disease state and the intestinal microbial composition is merely one of association, restoring the normal intestinal microbial profile will not result in the improvement of the disease state and the amelioration of symptoms.
Figure 2.
Fecal microbiota transplant restores intestinal microbial composition to modulate the adaptive immune responses, re-establish intestinal microbial homeostasis, and alter the host metabolism. Short chain fatty acids such as butyrate and propionate interact with G-protein coupled receptors GPR-43/41 on L cells to produce glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), which contributes to reducing food intake and improving glucose metabolism [17].
Outside the context of hematologic and oncologic conditions, FMT has been used to regulate host metabolism in both animal models of obesity [18,19] and in obese humans [20,21]. FMT from lean donors resulted in variable improvements in the insulin-sensitivity in obese recipients [22,23] and in patients with metabolic syndromes [22,23]. Improvement was associated with an increased abundance of butyrate-producing intestinal microbes.
FMT has also been used to re-establish normal intestinal microbial homeostasis. Currently, the most common indication for FMT is for relapsed/refractory CDI. FMT restores the diversity of the intestinal microbial compositions to create an ecologic competition between organisms to overcome and treat C. difficile overgrowth. Success rates of nearly 90% have been reported in most studies in patients with recurrent/refractory CDI (Table 1) [24,25,26,27,28]. Restoration of the normal intestinal microbial composition may also successfully eradicate colonization by multidrug resistant organisms such as extended-spectrum beta-lactamase-producing (ESBL) Escherichia coli (E. coli) [29], vancomycin-resistant Enterococcus (VRE) [30], and carbapenem-resistant Enterobacteriaceae (CRE) [30].
Table 1.
Results of selected studies on the use of FMT for relapsed/refractory CDI.
| Reference | Data Source | Number of Patients (n) | Outcome | Adverse Events |
|---|---|---|---|---|
| Youngster et al. [24] | A prospective study | 180 using oral frozen capsules | CDI resolved in 82% of patients after a single treatment, rising to a 91% cure rate with two treatments. | Three cases of Grade 2 or above adverse reactions deemed related to the FMT were reported: One transient high fever, two new endoscopic diagnoses of ulcerative colitis. |
| Furuya-Kanamori et al. [25] | A collaborative analysis of patient data from 14 studies | 305 (207 by lower and 98 by upper gastrointestinal route) | Risk of clinical failure was 5.6% and 17.9% in those treated by upper gastrointestinal route, and 4.9% and 8.5% in those treated by lower gastrointestinal route at Day 30 and 90, respectively. | Not reported. |
| Liu et al. [26] | Single center retrospective data | 25 procedures (via feeding tube (n = 11), upper gastrointestinal endoscopy (n = 8), or colonoscopy (n = 6) in 24 patients) | Symptoms resolved in 21 of 24 patients (87.5%). Three patients who did not respond underwent a second FMT and all three responded | No serious adverse reactions were attributed to FMT. |
| Ponte et al. [27] | Single center retrospective study | 34 (via upper gastrointestinal endoscopy (n = 30) or colonoscopy (n = 4) | Cure after one FMT in 22/25 (88%) and after two or more FMT in another 2/25 (8%). | No serious adverse reactions were reported. |
| Kelly et al. [28] | FMT National Registry Data | 222 had follow-up at 1 month and 123 at 6 months. | 90% cure rate at 1 month and 96% cure rate at 6 months. | At 1 month, 1% had hospitalization for diarrhea and severe abdominal pain, felt probably related to FMT; at 6 months, 1% developed irritable bowel syndrome and 1% inflammatory bowel disease. |
Since the intestinal microbial community modulates host immune responses, FMT has been applied to patients with inflammatory bowel disease. Randomized studies and non-randomized studies with a control arm have found higher clinical remission at eight weeks in patients with ulcerative colitis who were treated with FMT compared to the groups treated with placebo colonoscopic infusion [31]. To date, there has not been any published randomized clinical trial of FMT in Crohn’s disease. However, a meta-analysis of 11 case series and uncontrolled observational cohort studies found that slightly more than 50% of the patients achieved clinical remission [32]. Administration of a second FMT within 4 months of the initial FMT treatment maintained the clinical benefits of the first FMT treatment [33].
FMT has also been tried in other conditions, such as in human irritable bowel syndrome [34,35] and autism spectrum disorder [36], and in mice and humans for multiple sclerosis [37,38] and in mice for Parkinson’s disease [39]. In all of these disease states, the target for FMT is the gut–brain axis, which may be related to the breakdown of the gut barrier functions due to changes in intestinal metabolomics, such as the decrease in the production of short chain fatty acids caused by alterations in the normal intestinal microbial composition.
3. CDI in Patients with Hematologic and Oncologic Diseases
Patients with hematologic malignancies are particularly at risk for the development of CDI. CDI occurs in 7–14% of cases [40], and recurrent CDI (rCDI) occurs in 11–31% [41,42] of patients with hematologic malignancies such as acute leukemias, multiple myeloma, and Hodgkin’s and non-Hodgkin’s lymphoma. The incidence of CDI in patients with acute myeloid leukemia has been reported to be between 4.8 and 9%, and in those who undergo autologous hematopoietic stem cell transplantation (HSCT), a rate between 4.9 and 7.5% is observed; in those who undergo allogeneic HSCT, between a 14–30.4% incidence is observed in allogenic HSCT recipients [43,44]. The cumulative risks for developing peri-transplant CDI for those patients undergoing allogeneic HSCT who had CDI within 9 months of the transplant was reported to be nearly 40% [45]. Similarly, the incidence of CDI among those with solid tumors was also very high, reported to be between 10–20% [46]. CDI in these patients adds to the morbidities of the already debilitated physical state due to the underlying malignancies and may contribute to treatment-related mortality. CDI-related mortality in these patients is approximately 20% [47]. CDI is, therefore, a significant complication in patients who are receiving chemotherapy for malignant diseases.
3.1. Factors Predisposing Patients to CDI
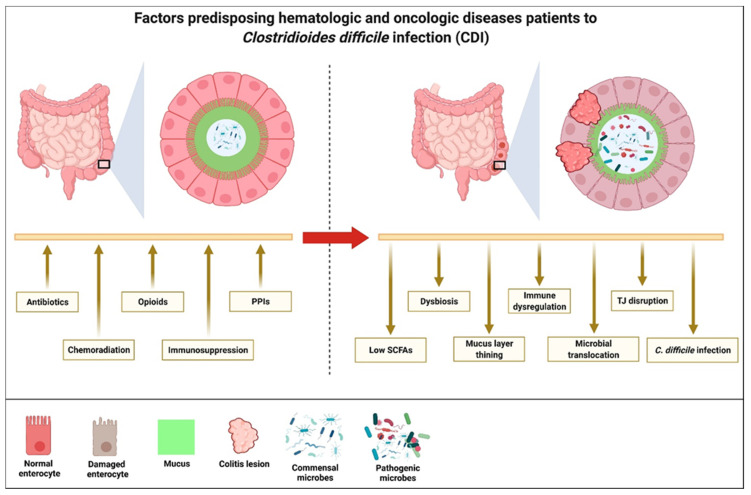
The mechanisms that are responsible for CDI pathogenesis in these groups of patients are multifactorial. In general, CDI risks are increased if there are changes to the normal commensal microbiota community (intestinal dysbiosis), innate intestinal immunity, or disruption to the integrity of the intestinal epithelial lining (Figure 3). By far, the biggest culprit contributing to the risks for CDI in these patients is the liberal use of broad-spectrum antibiotics that alter the intestinal microbial diversity and density, providing the opportunity for the colonization and proliferation of C. difficile, which is resistant to these antibiotics. Although the early initiation of broad-spectrum antibiotics reduces morbidity and mortality in patients who develop fever in the presence of chemoradiation-induced neutropenia [48], a retrospective study of 251 adult cancer patients found that despite patients having an absolute neutrophil count of more than 500/µL and 75% of the patients testing positive for a respiratory virus, 32% were still prescribed broad-spectrum antibiotics [49].
Figure 3.
Patients with hematologic and oncologic diseases are more likely to develop Clostridioides difficile infection due to the frequent use of antibiotics, opioid analgesia and proton pump inhibitors, chemoradiation, and immunosuppressive agents. As a result, a change in the intestinal microbial composition and integrity of the epithelium results in the reduced production of short chain fatty acids, intestinal dysbiosis, thinning of the mucin layers, immune dysregulation, interruption to tight-junction formation, increased translocation of luminal microbes into the systemic circulation, and the development of Clostridioides difficile infections.
One of the first deterrence to C. difficile colonization in the intestine is the acidity of the gastric secretion. Both C. difficile spores and vegetative forms are inhibited by low gastric pH. It is therefore not surprising that the use of proton pump inhibitors (PPIs) is associated with an increased risk of CDI. A meta-analysis of 23 observational studies involving more than 300,000 patients found that PPI use was associated with a 65% increase in the incidence of CDI [50]. PPIs are often prescribed to hematologic and oncologic patients with severe thrombocytopenia and mucositis following chemoradiation therapy to reduce the risk of gastrointestinal bleeding. Therefore, PPIs increase the susceptibility of these patients to CDI.
The primary bile acids, chenodeoxycholic acid (CDCA) and cholic acid (CA), which make up 95% of the primary bile acids within the intestine foster C. difficile spore gemination to the vegetative cells within the ileum [51]. Medications that affect the transit time of these primary bile acids will favor the germination of the C. difficile spore to promote the colonization, proliferation, and induction of CDI. Opioids induce intestinal hypomotility that will increase the bile acid transit time. Opioids have also been found to induce intestinal dysbiosis [52]. The incidence of hospital-onset CDI among chronic opioid users is two times higher than that of the general hospital population [53]. Chronic opioid use to treat cancer-related pain therefore increases the risk of CDI in these patients by not only inducing intestinal dysbiosis but by also creating a condition that promotes the proliferation of C. difficile.
Patients with hematologic and oncologic diseases are rendered more susceptible to CDI because their innate host immunity is suppressed due to treatment [54]. This occurs due to the primary disease process or the one that is induced by the chemotherapeutic agents used to treat the diseases. Chemotherapeutic agents affect host immunity by their direct cytotoxic effects on the lymphocytes and by inducing neutropenia. In the setting of allogeneic HSCT, the use of immunosuppressive agents to prevent or treat graft-versus-host disease (GVHD) has also been found to increase host susceptibility to CDI [54].
Intestinal epithelial injury in the form of mucositis interacts bidirectionally with CDI. On the one hand, CDI induces mucosal damage, on the other hand, the presence of mucosal injury places the host an increased risk of CDI. Normal intestinal epithelium not only consists of enterocytes but also of supportive cells that include the goblet cells that are responsible for the production of mucin and Paneth cells that produce the antimicrobial peptide (AMP) [55]. Both intestinal mucin and AMP regulate the intestinal microbial community and density. Changes in the intestinal microbial community and density may not only result in alterations in the intestinal microbial metabolites such as in the short chain fatty acids (SCFAs) that play a major role in enterocyte health [56], but they may also create a niche favoring the colonization and proliferation of C. difficile. Damage to the normal intestinal epithelium, by chemotherapy or GVHD, will affect the integrity and functions of the goblet cells and Paneth cells and alter the production of mucin and AMP, respectively. Injury to the intestinal epithelium can also result in the release of damage-associated molecular patterns (DAMPs) that will also affect the intestinal microbial composition and density [57]. Cancer patients receiving chemotherapy that induces mucositis and patients with GVHD are there for at a higher risk for the development of CDI.
3.2. Use of FMT in Hematologic and Oncologic Patients outside Treatment of CDI
The immune regulatory effects of the intestinal microbial community have been exploited for treating acute GVHD following allogeneic HSCT. In total, the efficacy of FMT has been reported in 72 patients with corticosteroid-refractory acute GVHD (Table 2) [58,59,60,61,62,63,64,65]. Responses were observed in more than 50% of these groups of patients. More importantly, the procedures were all well tolerated, except for the development of lower gastrointestinal bleeding and hypoxemia in one patient and of bacteremia in two patients, although it was deemed unrelated to the FMT in all three cases. However, fatal donor-derived ESBL septicemia was reported in two patients who received FMT, one patient with hepatitis C infection in a clinical study of FMT for refractory hepatic encephalopathy, and another patient with therapy-related myelodysplastic syndrome in a study on the use of pre-emptive FMT following allogeneic HSCT [66]. The risks for such complications will be reduced as more stringent screen for the microbial composition in donors become more stringent.
Table 2.
Reported results of FMT for corticosteroid-refractory GVHD.
| Reference | Data Source | Number of Patients (n) | Outcome | Adverse Events |
|---|---|---|---|---|
| Kakihana et al. [58] | Single center prospective study | 4 (received a total of 7 FMT by nasogastric administration) | 3 CR and 1 PR | 1 case of lower gastrointestinal bleed and hypoxemia, may not be related to FMT |
| Spindelboeck et al. [59] | Retrospective case series | 3 (received a total of 9 FMT by colonoscopy) | 2 CR and 1 PR | None reported |
| Qi et al. [60] | Single center prospective study | 8 (received a total of 12 FMT by nasogastric administration) | 5 CR and 1 PR | None reported |
| Shouval et al. [61] | Single center prospective study | 7 (received a total of 15 FMT by capsule administration) | 2 CR | 2 episodes of bacteremia, deemed unrelated to FMT |
| van Lier et al. [62] | Single center prospective study | 15 (received a total of 15 FMT by nasoduodenal tube administration) | 10 CR | None reported |
| Zhao et al. [63] | Single center open-label Phase I/II study | 41 (23 assigned to FMT and 18 to control. FMT administered by nasojejunal or gastric tube) | Overall response rate of 82.6% (52.2% CR and 30.4% PR) in the FMT group and 39% (all PR) in the control group on Day 14 after FMT, and an overall response rate of 69.5% (56.5% CR and 13% PR) in the FMT group and 50% (16% CR and 34% PR) in the control group on Day 21 after FMT | No difference in the adverse events between the FMT group and the control group. |
| Goeser et al. [64] | Two-center retrospective study | 11 (9 by capsule and 2 by nasojejunal tube administration) | Attenuation of stool volume and frequency was observed in all 11 patients | Abdominal pain occurred in 3 patients and vomiting in 1 patient |
| Mao et al. [65] | Case report | 1 (received two cycles of FMT administered by capsules) | CR | None reported |
Preclinical observations determined that the intestinal microbiota affected the response to immune checkpoint inhibitors (ICIs) [67]. Various retrospective studies also found that broad-spectrum antibiotics alter the intestinal microbial community and adversely impacted responses in cancer patients being treated with ICIs [68,69,70,71]. Based on these findings, two studies were performed on the use of FMT in a cohort of patients with immunotherapy-refractory malignant melanoma to determine whether the FMT could reverse the refractoriness to anti-Program Cell Death (PD) 1 immunotherapy. Three of the ten patients in one study restored the response to immunotherapy following FMT [72], and 6 of 15 in another study showed clinical benefits [73].
3.3. Ongoing FMT Studies in Patients with Hematologic and Oncologic Diseases
The initial successes observed with FMT in patients with hematologic and oncologic diseases have led to many clinical studies being currently ongoing in various institutions worldwide. Currently, there are nearly 40 studies registered with Clinicaltrials.gov. Table 3 shows the representative studies in the US and in Europe. These studies primarily evaluate the safety of FMT, the use of FMT to prevent and treat GVHD following allogeneic HSCT, improvement of ICI response, and the treatment of the complications that arise due to cancer therapy. It is expected that many of these studies will report their mature data on these outcomes within the next five years.
Table 3.
Clinical studies registered in Clinicaltrials.gov for hematologic and oncologic patients in the US and in Europe.
| NCT# | Study | Primary Outcome Measurements | Number of Patients (n) |
|---|---|---|---|
| 02928523 | PreventiOn of DYSbioSis Complications With Autologous FMT in AML Patients (ODYSSEE) | Evaluation of efficacy in dysbiosis correction and multidrug resistant bacteria based on bacterial culture | 20 |
| 03678493 | A Study of FMT in Patients With AML Allo HSCT in Recipients | Efficacy of FMT in AML patients and allo-HSCT recipients in reducing the incidence of infections | 120 |
| 04935684 | Faecal Microbiota Transplantation After Allogeneic Stem Cell Transplantation (TMF-Allo) | GVHD and relapse-free survival rate after allogeneic hematopoietic stem cell transplantation | 150 |
| 04269850 | Fecal Microbiota Transplantation With Ruxolitinib and Steroids as an Upfront Treatment of Severe Acute Intestinal GVHD (JAK-FMT) | Overall survival | 20 |
| 05094765 | Fecal Microbiota Transplant (FMT) Capsule for Improving the Efficacy of GI-aGVHD | Overall survival and Grade 3 or above adverse events | 15 |
| 02269150 | Autologous Fecal Microbiota Transplantation (Auto-FMT) for Prophylaxis of Clostridium Difficile Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation | CDI up to one year after entry into study | 59 |
| 03214289 | Fecal Microbiota Transplantation for Steroid Resistant and Steroid Dependent Gut Acute Graft Versus Host Disease | Serious adverse events | 4 |
| 02733744 | Fecal Microbiota Transplantation After HSCT | Feasibility on the number of participants able to ingest 15 FMT capsules over a 2-day period | 18 |
| 03359980 | Treatment of Steroid Refractory Gastro-intestinal Acute GVHD afteR AllogeneiC HSCT With fEcal Microbiota tranSfer (HERACLES) | Efficacy of FMT in steroid refractory -gastro-intestinal acute GVHD at Day 28 | 24 |
| 03819803 | Fecal Microbiota Transplantation in aGvHD After ASCT | Remission at Day 90 | 15 |
| 04038619 | Fecal Microbiota Transplantation in Treating Immune-Checkpoint Inhibitor Induced-Diarrhea or Colitis in Genitourinary Cancer Patients | Tolerability and response | 40 |
| 02770326 | Safety of Stool Transplant for Patients With Difficult to Treat C. Difficile Infection | Incidence of CDI | 10 |
| 04116775 | Fecal Microbiota Transplant and Pembrolizumab for Men With Metastatic Castration Resistant Prostate Cancer. | Anticancer effect of FMT from responders to pembrolizumab to non-responders. | 32 |
| 04040712 | Fecal Microbiota Transplantation in Diarrhea Induced by Tyrosine-kinase Inhibitors | Resolution of diarrhea four weeks after FMT | 20 |
| 03819296 | Role of Gut Microbiome and Fecal Transplant on Medication-Induced GI Complications in Patients With Cancer | Differences in stool microbiome pattern and adverse events | 800 |
| 04951583 | Fecal Microbial Transplantation Non-Small Cell Lung Cancer and Melanoma (FMT-LUMINATE) | Overall response rate | 70 |
| 04521075 | A Phase Ib Trial to Evaluate the Safety and Efficacy of FMT and Nivolumab in Subjects With Metastatic or Inoperable Melanoma, MSI-H, dMMR or NSCLC | Overall response rate and adverse events | 42 |
| 04163289 | Preventing Toxicity in Renal Cancer Patients Treated With Immunotherapy Using Fecal Microbiota Transplantation (PERFORM) | Rate of immune-related colitis associated with ipilimumab/nivolumab treatment | 20 |
| 04729322 | Fecal Microbiota Transplant and Re-introduction of Anti-PD-1 Therapy (Pembrolizumab or Nivolumab) for the Treatment of Metastatic Colorectal Cancer in Anti-PD-1 Non-responders | Overall response rate | 15 |
| 04924374 | Microbiota Transplant in Advanced Lung Cancer Treated With Immunotherapy | Measurements of safety | 20 |
| 03341143 | Fecal Microbiota Transplant (FMT) in Melanoma Patients | Overall response rate | 18 |
| 03353402 | Fecal Microbiota Transplantation (FMT) in Metastatic Melanoma Patients Who Failed Immunotherapy | Rate of adverse events and engraftment | 40 |
| 04988841 | Assessing the Tolerance and Clinical Benefit of feCAl tranSplantation in patientS With melanOma (PICASSO) | Safety and tolerability | 60 |
| 04577729 | The IRMI-FMT Trial | Progression-free survival | 60 |
3.4. Harnessing the Potentials of FMT for Future Studies in Hematologic and Oncologic Diseases
The potential range of functions of a balanced intestinal microbial composition is wide. This provides great opportunities to tap into these potentials. Thus far, FMT has primarily been employed to restore the normal microbial homeostasis to treat CDI and to exploit the immune regulatory effects to treat corticosteroid-refractory GVHD following allogeneic HSCT and to restore the treatment responsiveness in melanoma patients who developed refractoriness to immunotherapy. Based on the assumption that the host immune system may have already developed a tolerance to the intestinal microbiota, it may be possible to extend the immune regulatory mechanisms of FMT to induce immune tolerance and to reduce the risk of developing intestinal GVHD using a combined allogeneic HSCT and FMT from the same donor.
Various studies have implicated a breakdown in the intestinal barrier function being responsible for the pathology of certain diseases. The breakdown of the intestinal barrier occurs frequently in patients with hematologic and oncologic diseases due to the direct cytotoxic effects of chemotherapy on the enterocytes or indirect effects of chemotherapy in modifying the intestinal microbiome and interrupting with the formation of the paracellular tight junctions (TJs) [74]. This increases the risks for the translocation of luminal bacterial products into the systemic circulation to induce culture-negative fever and bacteria to elicit bacteremia and septicemia. Fortifying the gut barrier and restoring the mucosal integrity using keratinocyte growth factors resulted in a reduction in the incidence of culture-negative fever and documented bacteremia/septicemia following high-dose chemotherapy and HSCT [75]. The facet of a balanced intestinal microbial composition in maintaining the gut barrier function through the production of the SCFAs that fortify enterocyte health, and paracellular TJ development may therefore be tapped into for similar purposes.
Recent studies in sickle cell disease (SCD) in mice [76,77] and in humans [78,79] have highlighted the presence and the role of disrupted gut barrier functions in affecting the phenotypes of the disease. This has been associated with intestinal dysbiosis that is characterized by a lower abundance of Alistipes and Pseudobutyrivirio [80]. Manipulation of the intestinal microbial community with the antibiotic rifaximin that led to an increased abundance of Akkermansia [81] was associated with a reduced frequency of painful vaso-occlusive crisis [82], creating an opportunity to use FMT from non-sickle cell donors with or without ex vivo enrichment with Akkermansia or Alistipes, which may be explored in the future to change the disease course in SCD.
4. Challenges Facing FMT Use in Hematologic and Oncologic Patients
The risk of introducing new infections remains the biggest concern of applying FMT to patients with hematologic conditions and oncologic patients. This anxiety among treating physicians has been amplified following the report on the fatal ESBL E. coli septicemia in patients with myelodysplastic syndrome who received pre-emptive FMT [66]. The risks are obviously higher in these groups of patients who are often neutropenic and immunosuppressed and who have an intestinal barrier that is already compromised. Therefore, any use of FMT in this group of patients, even if being used for CDI treatment, should be carried out in tightly controlled well-designed clinical studies.
Another challenge that faces these patients is the risk for bowel perforation and gastrointestinal bleeding due to instrumentation during FMT in a background context of intestinal mucositis. The development of capsule-delivered FMT should reduce this risk.
The biggest challenge affecting the successful use of FMT in patients with hematologic and oncologic diseases is the persistence of the factors predisposing these patients to the conditions that need FMT. Patients being treated for CDI will likely still require the frequent use of broad-spectrum antibiotics throughout the course of their cancer treatments. The continued use of systemic antibiotics has been found to predict FMT failure [83]. Even after completing the courses of chemotherapy, these patients also remain in an immunosuppressed state that predisposes them to further risks for CDI. In patients treated for GVHD, the intestinal microbiome likely reverses back to a dysbiotic state a few months after FMT since the alloreactivity persists in the background, and the use of immunosuppressive agents continues. Therefore, intermittent repeat FMT will be needed to maintain the restored intestinal microbial composition. The availability of capsule-delivered FMT may provide the solution, although it is still associated with the adverse events of diarrhea and abdominal discomfort/pain/cramping [84]. One could envisage the initial restoration of the intestinal microbial composition using a full FMT followed by daily/weekly maintenance of the microbiome using FMT capsules. Interestingly, a recent systematic review of the procedures performed over the last two decades found that FMT-related adverse events were the lowest when the colonic transendoscopic tubing method was used (6.33%) and the highest with gastroscopy (31.92%). The incidence of FMT-related adverse events was unexpectedly high with capsules (28.97%) [84], arguing against the safety of the capsule method in the treated patients, although how the capsule method compares with the other methods in patients with hematologic and oncologic diseases remains to be determined.
The problems associated with re-infection have been investigated in various studies. The incidence of failure and re-infection has been estimated to be around 14% [83]. Repeat FMT significantly reduces the failure rate in patients treated for CDI [16]. Patients who experience recurrence can, however, still be salvaged with bezlotoxumab [85].
5. Concluding Remarks
FMT is an emerging therapeutic approach that has an enormous number of potential applications. However, concerns remain among treating physicians on its use in patients with hematologic and oncologic diseases due to concerns related to introducing infections. A further barrier preventing the successful use of FMT in these groups of patients is the persistence of the factors predisposing the patients to the conditions needing FMT. Future work will focus on methods to overcome these obstacles. Until the indications are well-established, FMT in patients with hematologic and oncologic diseases should only be performed in closely monitored clinical trials.
Acknowledgments
Figures created with BioRender.com.
Author Contributions
Conceptualization, S.H.L. and S.J.; methodology, S.H.L. and D.D.; validation, D.D.; formal analysis, S.H.L.; investigation, M.B.Z., S.N. (Stephanie Niforatos)., D.N., S.N. (Sandy Nasr) and M.O.; data curation, M.B.Z., S.N. (Stephanie Niforatos), D.N., S.N. (Sandy Nasr) and M.O.; writing—original draft preparation, M.B.Z., S.N. (Stephanie Niforatos), D.N., S.N. (Sandy Nasr) and M.O.; writing—review and editing, S.H.L.; visualization, D.D.; supervision, S.H.L. and S.J.; project administration, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., et al. The gut microbiota and host health: A new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen L., Duffy A. Factors influencing the gut microbiota, inflammation, and Type 2 Diabetes. J. Nutr. 2017;147:1468S–1475S. doi: 10.3945/jn.116.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt L.J. Fecal microbiota transplant: Respice, Adspice, Prospice. J. Clin. Gastroenterol. 2015;49:S65–S68. doi: 10.1097/MCG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 4.Lewin R.A. More on merde. Perspect. Biol. Med. 2001;44:594–607. doi: 10.1353/pbm.2001.0067. [DOI] [PubMed] [Google Scholar]
- 5.Eiseman B., Silen W., Bascom G.S., Kauvar A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 6.Schwan S., Sjolin U., Trottestam B., Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. doi: 10.1016/S0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- 7.Mamoon L., Olesen S.W. Fecal microbiota transplant annually and their positive clinical impact. Clin. Transl. Gastroenterol. 2020;11:e00247. doi: 10.14309/ctg.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baunwall S.M.D., Terveer E.M., Dahlerup J.F., Erikstrup C., Arkkila P., Vehreschild M.J., Ianiro G., Gasbarrini A., Sokol H., Kump P.K., et al. The use of Faecal Microbiota Transplantation (FMT) in Europe: A Europe-wide survey. Lancet Reg. Health Eur. 2021;9:100181. doi: 10.1016/j.lanepe.2021.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji S.K., Yan H., Jiang T., Guo C.Y., Liu J.J., Dong S.Z., Yang K.L., Wang Y.J., Cao Z.J., Li S.L. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting Xenomicrobiota colonization. Front. Microbiol. 2017;8:1208. doi: 10.3389/fmicb.2017.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cammarota G., Ianiro G., Tilg H., Rajilić-Stojanović M., Kump P., Satokari R., Sokol H., Arkkila P., Pintus C., Hart A., et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P., Alm E.J., Kelley J.M., Cheng V., Smith M., Kassam Z., Nee J., Iturrino J., Lembo A. Effects of antibiotic pretreatment on bacterial engraftment after fecal microbiota transplant (FMT) in IBS-D. Gut Microbes. 2022;14:e2020067. doi: 10.1080/19490976.2021.2020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fecal Microbiota Transplantation-Standardization Study Group Nanjing consensus on methodology of washed microbiota transplantation. Chin. Med. J. 2020;133:2330–2332. doi: 10.1097/CM9.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Z., Xiang J., He Z., Zhang T., Xu L., Cui B., Li P., Huang G., Ji G., Nie Y., et al. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc. Int. Open. 2016;4:E610–E613. doi: 10.1055/s-0042-105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakken J.S., Borody T., Brandt L.J., Brill J.V., Demarco D.C., Franzos M.A., Kelly C., Khoruts A., Louie T., Martinelli L.P., et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du C., Luo Y., Walsh S., Grinspan A. Oral fecal microbiota transplant capsules are safe and effective for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2021;55:300–308. doi: 10.1097/MCG.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 16.Baunwall S.M.D., Lee M.M., Eriksen M.K., Mullish B.H., Marchesi J.R., Dahlerup J.F., Hvas C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;29–30:100642. doi: 10.1016/j.eclinm.2020.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spreckley E., Murphy K.G. The L-cell in nutritional sensing and the regulation of appetite. Front. Nutr. 2015;2:23. doi: 10.3389/fnut.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagdasarian N., Rao K., Malani P.N. Diagnosis and treatment of Clostridium difficile in adults: A systematic review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigorescu I., Dumitrascu D.L. Implication of gut microbiota in diabetes mellitus and obesity. Acta Endocrinol. 2016;12:206–214. doi: 10.4183/aeb.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alang N., Kelly C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitano M., Covasa M. Microbiota transplant in the treatment of obesity and diabetes: Current and future perspectives. Front. Microbiol. 2020;11:590370. doi: 10.3389/fmicb.2020.590370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F., Dallinga-Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Kootte R.S., Levin E., Salojärvi J., Smits L.P., Hartstra A.V., Udayappan S.D., Hermes G., Bouter K.E., Koopen A.M., Holst J.J., et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26:611–619.e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Youngster I., Mahabamunuge J., Systrom H.K., Sauk J., Khalili H., Levin J., Kaplan J.L., Hohmann E.L. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. doi: 10.1186/s12916-016-0680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya-Kanamori L., Doi S.A., Paterson D.L., Helms S.K., Yakob L., McKenzie S.J., Garborg K., Emanuelsson F., Stollman N., Kronman M.P., et al. Upper versus lower gastrointestinal delivery for transplantation of fecal microbiota in recurrent or refractory Clostridium difficile infection: A collaborative analysis of individual patient data from 14 studies. J. Clin. Gastroenterol. 2017;51:145–150. doi: 10.1097/MCG.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 27.Lui R.N., Wong S.H., Lau L.H.S., Chan T.T., Cheung K.C.Y., Li A., Chin M.L., Tang W., Ching J.Y.L., Lam K.L.Y., et al. Faecal microbiota transplantation for treatment of recurrent or refractory Clostridioides difficile infection in Hong Kong. Hong Kong Med. J. 2019;25:178–182. doi: 10.12809/hkmj197855. [DOI] [PubMed] [Google Scholar]
- 28.Ponte A., Pinho R., Mota M., Silva J., Vieira N., Oliveira R., Rodrigues J., Sousa M., Sousa I., Carvalho J. Fecal microbiota transplantation in refractory or recurrent Clostridium difficile infection: A real-life experience in a non-academic center. Rev. Esp. Enferm. Dig. 2018;110:311–315. doi: 10.17235/reed.2018.5099/2017. [DOI] [PubMed] [Google Scholar]
- 29.Kelly C.R., Yen E.F., Grinspan A.M., Kahn S.A., Atreja A., Lewis J.D., Moore T.A., Rubin D.T., Kim A.M., Serra S., et al. Fecal microbiota transplantation is highly effective in real-world practice: Initial results from the FMT National Registry. Gastroenterology. 2021;160:183–192.e3. doi: 10.1053/j.gastro.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh R., van Nood E., Nieuwdorp M., van Dam B., ten Berge I.J., Geerlings S.E., Bemelman F.J. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin. Microbiol. Infect. 2014;20:O977–O978. doi: 10.1111/1469-0691.12683. [DOI] [PubMed] [Google Scholar]
- 31.Dinh A., Fessi H., Duran C., Batista R., Michelon H., Bouchand F., Lepeule R., Vittecoq D., Escaut L., Sobhani I., et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: A prospective comparative study. J. Hosp. Infect. 2018;99:481–486. doi: 10.1016/j.jhin.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Imdad A., Nicholson M.R., Tanner-Smith E.E., Zackular J.P., Gomez-Duarte O.G., Beaulieu D.B., Acra S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2018;11:CD012774. doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paramsothy S., Paramsothy R., Rubin D.T., Kamm M.A., Kaakoush N.O., Mitchell H.M., Castaño-Rodríguez N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis. 2017;11:1180–1199. doi: 10.1093/ecco-jcc/jjx063. [DOI] [PubMed] [Google Scholar]
- 34.Li P., Zhang T., Xiao Y., Tian L., Cui B., Ji G., Liu Y.Y., Zhang F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl. Microbiol. Biotechnol. 2019;103:349–360. doi: 10.1007/s00253-018-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzawi T., Lied G.A., Sangnes D.A., El-Salhy M., Hov J.R., Gilja O.H., Hatlebakk J.G., Hausken T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE. 2018;13:e0194904. doi: 10.1371/journal.pone.0194904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodiño-Janeiro B.K., Vicario M., Alonso-Cotoner C., Pascua-García R., Santos J. A review of microbiota and irritable bowel syndrome: Future in therapies. Adv. Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang D.W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A., Khoruts A., Geis E., Maldonado J., McDonough-Means S., et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S.E., et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borody T., Leis S., Campbell J., Torres M., Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) Am. J. Gastroenterol. 2011;106:S352. doi: 10.14309/00000434-201110002-00942. [DOI] [Google Scholar]
- 40.Sun M.F., Zhu Y.L., Zhou Z.L., Jia X.B., Xu Y.D., Yang Q., Cui C., Shen Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 41.McGlone S.M., Bailey R.R., Zimmer S.M., Popovich M.J., Tian Y., Ufberg P., Muder R.R., Lee B.Y. The economic burden of Clostridium difficile. Clin. Microbiol. Infect. 2012;18:282–289. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willems L., Porcher R., Lafaurie M., Casin I., Robin M., Xhaard A., Andreoli A.L., Rodriguez-Otero P., Dhedin N., Socié G., et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors, and outcome. Biol. Blood Marrow Transplant. 2012;18:1295–1301. doi: 10.1016/j.bbmt.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Kamboj M., Xiao K., Kaltsas A., Huang Y.T., Sun J., Chung D., Wu S., Sheahan A., Sepkowitz K., Jakubowski A.A., et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: Strain diversity and outcomes associated with NAP1/027. Biol. Blood Marrow Transplant. 2014;20:1626–1633. doi: 10.1016/j.bbmt.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anand A., Glatt A.E. Clostridium difficile infection associated with antineoplastic chemotherapy: A review. Clin. Infect. Dis. 1993;17:109–113. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 45.Kamthan A.G., Bruckner H.W., Hirschman S.Z., Agus S.G. Clostridium difficile diarrhea induced by cancer chemotherapy. Arch. Intern. Med. 1992;152:1715–1717. doi: 10.1001/archinte.1992.00400200139025. [DOI] [PubMed] [Google Scholar]
- 46.Agha A., Sehgal A., Lim M.J., Weber D., Hou J.Z., Farah R., Raptis A., Im A., Dorritie K., Marks S., et al. Peri-transplant Clostridium difficile infections in patients undergoing allogeneic hematopoietic progenitor cell transplant. Am. J. Hematol. 2016;91:291–294. doi: 10.1002/ajh.24263. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt-Hieber M., Bierwirth J., Buchheidt D., Cornely O.A., Hentrich M., Maschmeyer G., Schalk E., Vehreschild J.J., Vehreschild M.J.G.T., AGIHO Working Group Diagnosis and management of gastrointestinal complications in adult cancer patients: 2017 updated evidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann. Hematol. 2018;97:31–49. doi: 10.1007/s00277-017-3183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon Y.K., Kim M.J., Sohn J.W., Kim H.S., Choi Y.J., Kim J.S., Kim S.T., Park K.H., Kim S.J., Kim B.S., et al. Predictors of mortality attributable to Clostridium difficile infection in patients with underlying malignancy. Support. Care Cancer. 2014;22:2039–2048. doi: 10.1007/s00520-014-2174-7. [DOI] [PubMed] [Google Scholar]
- 49.Viscoli C., on behalf of the EORTC International Antimicrobial Therapy Group Management of infection in cancer patients: Studies of the EORTC International Antimicrobial Therapy Group (IATG) Eur. J. Cancer. 2002;38((Suppl. 4)):82–87. doi: 10.1016/S0959-8049(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 50.Krantz E.M., Zier J., Stohs E., Ogimi C., Sweet A., Marquis S., Klaassen J., Pergam S.A., Liu C. Antibiotic prescribing and respiratory viral testing for acute upper respiratory infections among adult patients at an ambulatory cancer center. Clin. Infect. Dis. 2020;70:1421–1428. doi: 10.1093/cid/ciz409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janarthanan S., Ditah I., Adler D.G., Ehrinpreis M.N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am. J. Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 52.Staley C., Weingarden A.R., Khoruts A., Sadowsky M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017;101:47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F., Meng J., Zhang L., Johnson T., Chen C., Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018;8:3596. doi: 10.1038/s41598-018-21915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichtbroun M., Jafri F., Chaudhary R.S., Batool S., Ahmed J., Lim S.H. High incidence of healthcare facility-acquired Clostridium difficile infections in chronic opioid users. J. Intern. Med. 2021;289:129–130. doi: 10.1111/joim.13124. [DOI] [PubMed] [Google Scholar]
- 55.Eze P., Balsells E., Kyaw M.H., Nair H. Risk factors for Clostridium difficile infections—An overview of the evidence base and challenges in data synthesis. J. Glob. Health. 2017;7:010417. doi: 10.7189/jogh.07.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouellette A.J. Antimicrobial Peptides and Human Disease. Volume 306. Springer; Berlin, Germany: 2006. Paneth cell α-defensin synthesis and function; pp. 1–25. Current Topics in Microbiology and Immunology. [DOI] [PubMed] [Google Scholar]
- 57.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 58.Seong S.Y., Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 59.Kakihana K., Fujioka Y., Suda W., Najima Y., Kuwata G., Sasajima S., Mimura I., Morita H., Sugiyama D., Nishikawa H., et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spindelboeck W., Schulz E., Uhl B., Kashofer K., Aigelsreiter A., Zinke-Cerwenka W., Mulabecirovic A., Kump P.K., Halwachs B., Gorkiewicz G., et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017;102:e210–e213. doi: 10.3324/haematol.2016.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi X., Li X., Zhao Y., Wu X., Chen F., Ma X., Zhang F., Wu D. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: A pilot study. Front. Immunol. 2018;9:2195. doi: 10.3389/fimmu.2018.02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shouval R., Geva M., Nagler A., Youngster I. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin. Hematol. Int. 2019;1:28–35. doi: 10.2991/chi.d.190316.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Lier Y.F., Davids M., Haverkate N.J.E., de Groot P.F., Donker M.L., Meijer E., Heubel-Moenen F.C.J.I., Nur E., Zeerleder S.S., Nieuwdorp M., et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020;12:eaaz8926. doi: 10.1126/scitranslmed.aaz8926. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y., Li X., Zhou Y., Gao J., Jiao Y., Zhu B., Wu D., Qi X. Safety and efficacy of fecal microbiota transplantation for Grade IV steroid refractory GI-GvHD patients: Interim results from FMT2017002 Trial. Front. Immunol. 2021;12:678476. doi: 10.3389/fimmu.2021.678476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goeser F., Sifft B., Stein-Thoeringer C., Farowski F., Strassburg C.P., Brossart P., Higgins P.G., Scheid C., Wolf D., Holderried T.A.W., et al. Fecal microbiota transfer for refractory intestinal graft-versus-host disease—Experience from two German tertiary centers. Eur. J. Haematol. 2021;107:229–245. doi: 10.1111/ejh.13642. [DOI] [PubMed] [Google Scholar]
- 66.Mao D., Jiang Q., Sun Y., Mao Y., Guo L., Zhang Y., Man M., Ouyang G., Sheng L. Treatment of intestinal graft-versus-host disease with unrelated donor fecal microbiota transplantation capsules. Medicine. 2020;99:e22129. doi: 10.1097/MD.0000000000022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFilipp Z., Bloom P.P., Torres Soto M., Mansour M.K., Sater M.R.A., Huntley M.H., Turbett S., Chung R.T., Chen Y.B., Hohmann E.L. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 68.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 69.Derosa L., Hellmann M.D., Spaziano M., Halpenny D., Fidelle M., Rizvi H., Long N., Plodkowski A.J., Arbour K.C., Chaft J.E., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinato D.J., Howlett S., Ottaviani D., Urus H., Patel A., Mineo T., Brock C., Power D., Hatcher O., Falconer A., et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed J., Kumar A., Parikh K., Anwar A., Knoll B.M., Puccio C., Chun H., Fanucchi M., Lim S.H. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7:e1507670. doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 73.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wardill H.R., Bowen J.M., Al-Dasooqi N., Sultani M., Bateman E., Stansborough R., Shirren J., Gibson R.J. Irinotecan disrupts tight junction proteins within the gut: Implications for chemotherapy-induced gut toxicity. Cancer Biol. Ther. 2014;15:236–244. doi: 10.4161/cbt.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsirigotis P., Triantafyllou K., Girkas K., Giannopoulou V., Ioannidou E., Chondropoulos S., Kalli T., Papaxoinis G., Pappa V., Papageorgiou E., et al. Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: A video-capsule endoscopy study. Bone Marrow Transplant. 2008;42:337–343. doi: 10.1038/bmt.2008.168. [DOI] [PubMed] [Google Scholar]
- 76.Poplawska M., Dutta D., Jayaram M., Salifu M., Chong N.S., Lim S.H. Intestinal pathophysiological abnormalities in steady state and after vaso-occlusive crisis in murine sickle cell disease. Br. J. Haematol. 2021 doi: 10.1111/bjh.17889. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 77.Xu C., Lee S.K., Zhang D., Frenette P.S. The gut microbiome regulates psychological-stress-induced inflammation. Immunity. 2020;53:417–428.e4. doi: 10.1016/j.immuni.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutta D., Methe B.A., Amar S., Morris A., Lim S.H. Intestinal injury and gut permeability in sickle cell disease. J. Transl. Med. 2019;17:183. doi: 10.1186/s12967-019-1938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim S.H., Dutta D. Clinicopathologic consequences following discontinuation of rifaximin in patients with sickle cell disease. Am. J. Hematol. 2020;95:E151–E153. doi: 10.1002/ajh.25798. [DOI] [PubMed] [Google Scholar]
- 80.Lim S.H., Morris A., Li K., Fitch A.C., Fast L., Goldberg L., Quesenberry M., Sprinz P., Methé B. Intestinal microbiome analysis revealed dysbiosis in sickle cell disease. Am. J. Hematol. 2018;93:E91–E93. doi: 10.1002/ajh.25019. [DOI] [PubMed] [Google Scholar]
- 81.Dutta D., Li K., Methe B., Lim S.H. Rifaximin on intestinally-related pathologic changes in sickle cell disease. Am. J. Hematol. 2020;95:E83–E86. doi: 10.1002/ajh.25722. [DOI] [PubMed] [Google Scholar]
- 82.Lim S.H., Dutta D., Moore J. Rifaximin in sickle cell disease. Am. J. Hematol. 2019;94:E325–E328. doi: 10.1002/ajh.25637. [DOI] [PubMed] [Google Scholar]
- 83.Tariq R., Saha S., Solanky D., Pardi D.S., Khanna S. Predictors and management of failed fecal microbiota transplantation for recurrent Clostridioides difficile infection. J. Clin. Gastroenterol. 2021;55:542–547. doi: 10.1097/MCG.0000000000001398. [DOI] [PubMed] [Google Scholar]
- 84.Marcella C., Cui B., Kelly C.R., Ianiro G., Cammarota G., Zhang F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021;53:33–42. doi: 10.1111/apt.16148. [DOI] [PubMed] [Google Scholar]
- 85.Hengel R.L., Ritter T.E., Nathan R.V., Van Anglen L.J., Schroeder C.P., Dillon R.J., Marcella S.W., Garey K.W. Real-world Experience of Bezlotoxumab for Prevention of Clostridioides difficile Infection: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2020;7:ofaa097. doi: 10.1093/ofid/ofaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]