Abstract
Simple Summary
Dysbiosis, which is an imbalance of gut microbial composition and function, can be caused by several external as well as internal factors, contributing to the onset of human and animal disorders, not limited to the gastrointestinal tract. Accordingly, the mechanisms leading to disease development involve a crucial interaction between the gut microbiota, their metabolic products, and the host. The expanded endocannabinoid system, also known as the “endocannabinoidome”, includes endocannabinoids (e.g., anandamide) and endocannabinoid-like mediators (e.g., palmitoylethanolamide), their receptors and metabolic enzymes. Dysregulation of this newly recognized endogenous system is also involved in several diseases. It is becoming increasingly apparent that a link between the endocannabinoidome and the gut microbiome exists. Here, we review some of the latest discoveries related to the functional link between these two complex systems and the disorders emerging from the malfunctioning of such a mutual interaction: for example, idiopathic inflammation, chronic enteropathies, metabolic disease and certain neuroinflammatory disorders. It is expected that in the near future new nutritional tools will emerge based on the expanding knowledge in this cutting-edge field.
Abstract
There is growing evidence that perturbation of the gut microbiome, known as “dysbiosis”, is associated with the pathogenesis of human and veterinary diseases that are not restricted to the gastrointestinal tract. In this regard, recent studies have demonstrated that dysbiosis is linked to the pathogenesis of central neuroinflammatory disorders, supporting the existence of the so-called microbiome-gut-brain axis. The endocannabinoid system is a recently recognized lipid signaling system and termed endocannabinoidome monitoring a variety of body responses. Accumulating evidence demonstrates that a profound link exists between the gut microbiome and the endocannabinoidome, with mutual interactions controlling intestinal homeostasis, energy metabolism and neuroinflammatory responses during physiological conditions. In the present review, we summarize the latest data on the microbiome-endocannabinoidome mutual link in health and disease, focalizing the attention on gut dysbiosis and/or altered endocannabinoidome tone that may distort the bidirectional crosstalk between these two complex systems, thus leading to gastrointestinal and metabolic diseases (e.g., idiopathic inflammation, chronic enteropathies and obesity) as well as neuroinflammatory disorders (e.g., neuropathic pain and depression). We also briefly discuss the novel possible dietary interventions based not only on probiotics and/or prebiotics, but also, and most importantly, on endocannabinoid-like modulators (e.g., palmitoylethanolamide) for intestinal health and beyond.
Keywords: chronic enteropathies, dysbiosis, endocannabinoidome, endocannabinoids, idiopathic inflammation, metabolic disorders, microbiome, neuroinflammation, obesity, oleoylethanolamide, palmitoylethanolamide
1. The Gut Microbiota and Microbiome
The gastrointestinal (GI) tract of mammals is inhabited by a complex ecosystem of microorganisms, collectively indicated as the gut microbiota, including bacteria, archaea, fungi, viruses and protozoa, whose collective genome (and ensuing proteome and metabolome) is indicated as the gut microbiome [1,2]. In recent years, research involving high-throughput DNA sequencing and shotgun metagenome, proteomics and metabolomics has experienced a true technological revolution and our knowledge about the gut microbiota as well as its role in animal health and disease has grown exponentially [2,3]. Indeed these molecular technologies have allowed the identification of both human and pet unculturable gut bacteria (by far the most abundant microbiota population), currently estimated to range between 1012 and 1014, outnumbering host cells by several times [4]. Moreover, although feline and canine gut microbiomes remain poorly characterized, the new molecular tools shed some light on phylogenetic and functional similarities between pet and human gut microbiomes [5].
The gut microbiome plays an important role in physiology, metabolism and nutrition of the host, both in humans and animals. In particular, it provides the enzymatic apparatus necessary for the fermentation of non-digestible fibers from plant foods, producing bioactive metabolites, including short-chain fatty acids (SCFAs) such as acetate, propionate and butyrate [6,7,8,9]. The SCFAs play important roles by promoting colonic homeostasis, protecting against colitis [10] as well as stimulating the secretion of glucagon-like peptide-1, thereby overseeing glucose homeostasis [11]. Butyrate is the primary energy source for colonocytes [12] and able to exert anti-inflammatory activity through the inhibition of the nuclear factor NF-κB activation [13], while acetate and propionate are used in the liver for lipogenesis and gluconeogenesis [14]. Finally, butyrate and propionate are also able to promote regulatory T-cell generation in the periphery, in a way dependent, among others, on their histone deacetylase inhibitory activity [15].
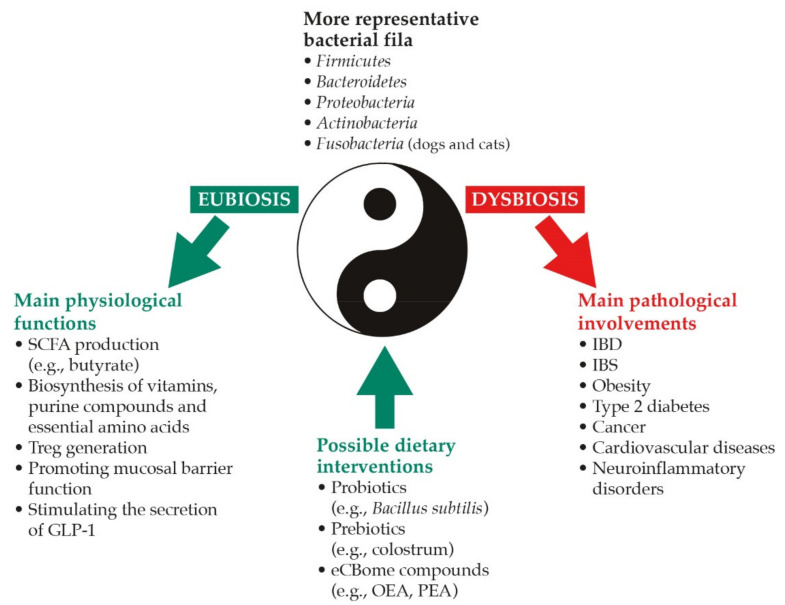
Moreover, the gut microbiome is involved in the biosynthesis of vitamins (such as vitamin K and most of the water-soluble B vitamins), essential amino acids [16,17] and purine compounds that are used for nucleotide biogenesis by the gut mucosa and promote the mucosal barrier function [18] (Figure 1, left side).
Figure 1.
The yin and yang of gut microbiome in intestinal health and beyond. See text for detailed explanation. Abbreviations: eCBome, endocannabinoidome; GLP-1, glucagon-like peptide-1; IBD, inflammatory bowel disease; IBS, irritable bowel disease; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; SCFA, short-chain fatty acid; Treg, regulatory T-cell.
It was formerly believed that all mammals are sterile before birth, but recent studies have suggested the presence of microorganisms in the placenta, amniotic fluid and umbilical cord [19,20]. However, it is during birth that newborns are exposed to an extensive number of bacterial microbes through the environment and contact with the mother’s vaginal and fecal microbiomes [21]. In humans, the gut microbial communities change in composition during the first years of life, with variability from baby to baby and depending on infant diet (breast or formula milk) [22]. In the following years (i.e., within the three-year period after birth) the gut microbiome converges in an ‘adult-like’ profile [23,24]. Although studies on the gut microbiome in neonatal dogs and cats are scarce, a similar trend was observed in puppies and kittens, with substantial inter-individual and temporal microbial variability during the early postnatal period [25,26,27]. Similar to humans, early microbial colonization, biodiversity as well as composition of the new-born gut microbiota in puppies and kittens is mainly influenced by vertical transmission from the mother as well as mode of delivery, feeding type and human-pet interaction [7].
In adult humans, the predominant bacterial phyla of the normal gut flora are Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, with the first two prevailing in healthy adults [28]; whereas the predominant bacterial genera are anaerobic genera, such as Bacteroides, Eubacterium, Clostridium, Ruminococcus and Faecalibacterium [23]. The core composition of the gut microbiota tends to remain stable in adulthood and change in old age. In fact, in a study carried out on 178 elderly individuals, Claesson and colleagues found a correlation between diet, microbiota composition and health status, indicating a role for diet-driven microbiota alterations in varying rates of health decline upon ageing [29]. The predominant bacterial phyla found in healthy feline and canine gastrointestinal (GI) tract and faecal samples parallel quite well those in humans, with Firmicutes, Bacteroidetes and Proteobacteria being among the co-dominant phyla [5] (Figure 1, top). Bacteroides, Fusobacterium and Prevotella 9 are the dominant genera of canine and feline gut microbiota [30]. The main observed difference is relative to Fusobacteria, which usually inhabits—albeit in low proportions—the canine and feline, but not human, healthy gut [5]. Interestingly, a trend toward age-related changes in microbiota is also observed in dogs [31], with lower acetate levels and decreased total SCFAs in faeces compared to adults [32], as well as lower alpha diversity (i.e., species abundance diversity in a given sample) [33]. In dogs, a significant decline in Fusobacteria with age was also found by some authors [34]. Of note, age-related gut microbiome composition has been related to short-term memory decline, with better memory performance being associated with a lower proportion of Actinobacteria [34]. Finally, although in a different way compared to human beings and dogs, the composition of the feline intestinal microbiota also changes with age [35].
2. Dysbiosis and Possible Dietary Interventions
Pathological perturbations of the gut microbial ecosystem balance (the latter known as eubiosis) are defined as dysbiosis [36]. Specifically, dysbiosis can result from the (i) loss of beneficial microorganisms, (ii) excessive growth of potentially harmful organisms, and (iii) reduced overall microbial diversity, with these changes being almost never mutually exclusive [37]. As a result, dysbiosis is able to induce and sustain an inflammatory condition through the predominance of pro-inflammatory microorganisms and the reduction of commensals, promoting immune tolerance mechanisms. The imbalance in the gut microbiota composition has been repeatedly shown to be associated with chronic GI disorders [38,39] and metabolic diseases [40]. In humans, for example, the two main subtypes of human inflammatory bowel disease (IBD), i.e., Crohn’s disease and ulcerative colitis, are associated with dysbiosis characterized by decreased biodiversity and reduced abundance of several types of bacteria belonging to phyla Firmicutes and Bacteroidetes [41]. This is far from the original hypothesis “one-microbe-one-disease”, while supporting the modern view of an imbalance between the entire gut microbiota and the host as the foundation of several GI (and extra-GI) disorders [37] (Figure 1, right side).
Although human and pet IBD (the latter better referred to as idiopathic inflammation) differ in certain respects [42], dysbiosis characterizes a variety of GI disorders in pets, too [43,44,45,46,47]. The recently developed fecal canine dysbiosis index has been found to specifically separate healthy dogs from dogs with chronic enteropathies [48]. Interestingly, dysbiosis networks in cats with chronic enteropathies resemble those found in people with IBD [47] more closely than those in dogs [49].
Indeed, dysbiosis has been implicated in a wide range of diseases far beyond IBD. Obesity, diabetes mellitus, cancer, cardiovascular diseases and neuroinflammatory disorders (like, for example, neuropathic pain and depression) are just a few examples of human and pet diseases with clear differences in fecal bacterial composition compared to healthy conditions [37,50,51,52,53,54,55]. Not to mention that aside from the gut microbiome, the skin and oral microbiome are becoming increasingly involved in other human and pet disorders, such as atopic dermatitis [56,57] and periodontal diseases [58,59].
Fortunately, the microbiome can be reshaped through different interventions, including nutrition, in order to reverse or attenuate dysbiosis-mediated disorders [37,60]. In this regard, probiotics and prebiotics are widely and successfully used in human and veterinary medicine [61,62,63]. Probiotics are specific live microorganisms that, orally administered in adequate amounts, confer beneficial effects to the host, while prebiotics are selective dietary substrates able to induce changes in the gut microbiota composition and/or activity, with the same aim to benefit the host. Despite the huge variety of different beneficial probiotics, very few are approved for animals. Probiotics, in fact, are feed additives under Regulation (EC) No. 1831/2003 with restrictions on their use. Up to few years ago, only one probiotic strain (i.e., Enterococcus faecium) could be used for cats and dogs. Currently, the endospore-forming Bacillus subtilis is also admitted and is used in animal feeds, besides being available for humans either as an over-the-counter prophylactic for mild GI disorders or as a health food or nutritional supplement. The advantage of endospore-forming probiotics over probiotics given as vegetative cells is that spore formation provides long-term survival even in extreme environmental conditions. B. subtilis, for example, was shown to survive at temperatures ranging from 4 °C to 60 °C and pH from 3 to 11 [64]. B. subtilis is a normal constituent of human and canine microbiota [5,9,65], decreases during enteropathies [43,66] and improves intestinal permeability while restoring gut microbiota homeostasis upon supplementation [67,68,69].
On the prebiotic side, studies have shown that dietary supplementation of several different prebiotics exert beneficial effects in IBD through changes in the intestinal microflora composition, i.e., increasing beneficial bacteria such as Bifidobacteria and Lactobacilli and improving the intestinal epithelial barrier [70]. Bovine colostrum, for example, has repeatedly been shown to (i) inhibit gut pathogens while stimulating the growth of a healthy microbiota (the so-called eubiotic effect) [71,72,73], (ii) reduce iatrogenic injury to the gut [74], (iii) prevent and limit diarrhoea of different origins [74,75].
As it will be discussed below, an extensive amount of research is now linking the endocannabinoidome to gut health and microbiome homeostasis was recently reviewed [76]. Dietary interventions with endocannabinoid-like compounds may thus represent a breakthrough in the management of dysbiosis-driven disorders (Figure 1, bottom).
3. The Endocannabinoidome: A Brief Introduction with Special Reference to Energy Metabolism and Gastrointestinal Homeostasis
The endocannabinoid (eCB) system is a lipid-derived signaling apparatus composed of: (i) G protein-coupled cannabinoid receptors type 1 and type 2, CB1 and CB2; (ii) two main endogenous ligands of such receptors (the so-called endocannabinoids), derived from arachidonic acid, i.e., N-arachidonoyl-ethanolamine (AEA or anandamide) and 2-arachidonoyl-glycerol (2-AG), the latter being first isolated from the canine gut [77]; and (iii) the anabolic and catabolic enzymes for the endocannabinoids [78]. AEA and 2-AG are biosynthesized “on demand” through the action of lipases that are stimulated by elevation of intracellular calcium or activation of G proteins [79]. Although the aforementioned molecules are the main and historical components of the eCB system, a great number of other members have also been recognized. In fact, besides AEA and 2-AG, other putative endogenous ligands of CB1 and CB2 receptors have been discovered, such as O-arachidonoyl-ethanolamine (virodhamine), 2-arachidonoyl-glyceryl ether (or noladin ether), oleamide and N-arachidonoyl-dopamine [78].
N-oleoyl-ethanolamine (OEA), N-palmitoyl-ethanolamine (PEA), N-stearoyl-ethanolamine (SEA) and N-linoleoyl-ethanolamine (LEA) are examples of so-called endocannabinoid-like compounds. Although lacking strong affinity for either CB1 or CB2 receptors, they indeed share with AEA a similar chemical structure (i.e., they are all N-acylethanolamines, NAEs), as well as the enzymes for the biosynthesis and degradation [78,80,81]. Moreover, together with CB1 and CB2 receptors, other targets have been identified for the endocannabinoids and endocannabinoid-like molecules, such as the transient receptor potential vanilloid type-1 (TRPV1) channel, the G protein-coupled receptor 55 (GPR55) or 119 (GPR119) and peroxisome proliferator activated receptors (PPAR)α and γ [80,81,82]. Consequently, the combination of all these players, including proteins and lipids, led to the expansion of the eCB system into the endocannabinoidome (eCBome), which is currently considered to include about one hundred endocannabinoid-like mediators, more than 20 anabolic and catabolic enzymes and more than 12 receptors [83,84].
With regard to the GI tract, cannabinoid and cannabinoid-related receptors showed wide distribution in several mammals, including mice, pigs, ferrets, dogs, cats, horses and human beings [85,86,87,88,89,90,91,92,93,94,95,96,97,98]. In canine and feline species, CB1 receptor immunolabeling is mainly observed on enteric neurons, nerve fibers, gastric parietal cells, epithelial cells (including goblet cells and enteroendocrine cells) [90,96,97,98]. On the contrary, immunoreactivity for CB2 is generally scanty in epithelial cells, while preferentially observed in perivascular immune cells, e.g., macrophages, B cells and mast cells [90,96,97,98]. GPR55 localizes in smooth muscle cells as well as in lamina propria macrophages, plasma cells, and mast cells [90]. PPARα immunoreactivity is mainly expressed in enteric glia and enteroglial cells [90,96,97], while there is no evidence of PPARγ expression in the canine and feline GI tract (Table 1). It is worth noting that recent studies revealed changes in the expression of some eCBome receptors, i.e., GPR55, CB1 and CB2, during intestinal inflammation and chronic colitis, thus suggesting that eCBome signaling is involved in gut homeostasis [98,99]. Some main aspects are briefly discussed below.
Table 1.
Main distribution of the investigated eCBome receptors in the gastrointestinal (GI) tract of either dogs (grey), cats (yellow) or both species (green). Modified from [98,100].
| Cell Type | CB1 | CB2 | GPR55 | PPARα | PPARγ |
|---|---|---|---|---|---|
| Lamina propria cells | |||||
| Enterocytes/Colonocytes | |||||
| Mast cells | |||||
| Immunocytes | |||||
| Smooth muscle cells | |||||
| Macrophages | |||||
| Goblet cells | |||||
| Submucosal plexus neurons and glia | |||||
| Myenteric plexus glia | |||||
| Myenteric plexus neurons | |||||
| Enteroendocrine cells | |||||
| Enteric neurons | |||||
| Enteroglial cells |
3.1. The Endocannabinoidome, Food Intake and Energy Metabolism
The eCBome has a key role in food intake and energy metabolism. The identification of OEA and other NAEs in the GI tract of reptiles and changes in the levels of these lipid compounds during fed compared to fasted conditions confirmed that the eCBome may represent an evolutionarily ancient system in the regulation of energy metabolism [101], as had been suggested by the findings of the role in food intake in invertebrate species (see [102] for review) and in fish [103]. According to several lines of evidence, the eCBome is involved in peripheral glucose and lipid metabolism by controlling the metabolic function of the adipose tissue, liver, endocrine pancreas and GI tract [1]. A dysregulation of the eCB system in these tissues promotes obesity and metabolic syndrome [104,105]. Accordingly, specific correlations between different eCBome players and markers of obesity as well as insulin and glucose homeostasis have been described [106]. For example, food deprivation and re-feeding affect peripheral levels of several eCBome ligands not only in reptiles and fish, but also in mammals, and today the eCBome is confirmed to regulate food intake and energy processing [107]. In human volunteers, plasma levels of NAEs and 2-monoacylglycerols (e.g., 2-AG) correlated with body fat mass and visceral adipose tissue [108]. In particular, NAE plasma levels were found to increase with increased fat mass, whereas circulating 2-AG levels increased with increased visceral fat mass [108]. Self-reported dietary intakes of fatty acids also correlated with plasma levels of 2-AG, omega-3-fatty acid-derived NAEs and 2-monoacylglycerol, irrespective of the body fat distribution [108]. Interestingly, it has been found that a 2-day Mediterranean diet intervention enhances plasma levels of NAEs and 2-monoacylglycerols derived from oleic acid and from omega-3-fatty acids [108].
Moreover, in abdominally obese people it was found that plasma levels of 2-AG positively correlated with accumulation of visceral adipose tissue and high triacylglycerol plasma levels [109]. Correspondingly, fasting salivary AEA directly correlated with body mass index, waist circumference and fasting insulin [110] and its plasma levels significantly associated with adiposity [111,112]. On the contrary, body weight loss decreased salivary AEA [110] as well as 2-AG and triacylglycerol plasma levels, visceral adiposity, and insulin resistance [109].
Of note, animals with genetic deletion of the NAE biosynthetic enzyme (specifically at the intestinal epithelial level) resulted in obesity and steatosis upon high-fat diet exposure [113]. In these animals, the endogenous levels of NAEs declined—in particular AEA and PEA—and levels of 2-AG were also decreased [113]. Incidentally, the latter result was unexpected (2-AG is not biosynthesized by the deleted pathway) and could have been due to decreased PEA levels [113], given that PEA increased 2-AG in different experimental conditions [114,115]. Insulin resistance, i.e., a hallmark of type 2 diabetes mellitus, was also found to be associated with an imbalanced NAE profile (i.e., reduced PEA/AEA and OEA/AEA ratios) [116]. Moreover, increased levels of AEA (with higher mRNA expression of AEA biosynthetic enzyme and lower expression of the degrading one) as well as decreased 2-AG levels (with increased MAGL expression) were also found in the adipose tissues of diabetic mice, together with increased mRNA expression of CB1 [117,118].
In this scenario, CB1 agonism seems to be the most involved pathway in eCBome regulation of feeding and energy metabolism. In particular, it has been repeatedly reported that CB1 activation increases (i) food intake, adipogenesis and lipogenesis, as well as insulin and leptin resistance in the adipose tissue [105,119,120]; (ii) the expression of enzymes involved in de novo lipogenesis, as well as insulin resistance and dyslipidemia in the liver [121]; and (iii) insulin secretion and trafficking of insulin granules in the endocrine pancreas [105]. On the other hand, CB1 activation reduces gut motility by several mechanisms, including the inhibition of acetylcholine release from cholinergic neurons [122].
It is noteworthy that the activation eCBome targets different from CB1 can conversely play beneficial roles during metabolic disorders. In particular, it has been demonstrated that (i) TRPV1 inhibits food intake, improves insulin sensitivity and stimulates thermogenesis [123], (ii) PPARα stimulates fatty acid β-oxidation [124], (iii) GPR55 enhances insulin sensitivity and reduces obesity [125], (iv) CB2 reduces insulin resistance and contributes to the management of diabetes due to its anti-oxidant and anti-inflammatory properties [126], and (v) PPARγ stimulates insulin sensitivity [82]. Accordingly, direct and indirect agonists of these receptors exert modulatory and protective effects on energy metabolism and related disorders. For example, OEA signaling is considered a biosensor for dietary fat [127]. Its production in enterocytes and mobilization in small intestine are stimulated by food intake [128] and in particular, by the release of oleic acid during fat digestion [127]. Newly formed OEA activates PPARα, which leads to satiety possibly through the vagus nerve [127] and the so-called gut-brain axis responsible for controlling food intake [129]. Although less potent than OEA, also LEA was shown to decrease food intake through the same receptor target following oral administration in rodents [130].
Interestingly, two recent clinical trials in obese patients as well as patients with non-alcoholic fatty liver disease showed that OEA supplementation decreased anthropometric measures including body mass index and waist circumference [131,132]. Moreover, dietary supplementation with SEA restores pancreas lipid composition under obesity-induced insulin resistant conditions [133]. Finally, PEA in a bioavailable micronized form has been recently shown to play an important protective role against a hallmark of metabolic disease, i.e., non-alcoholic steatohepatitis [134]. Obesity-related pro-inflammatory states also benefited from PEA administration [135]. The results have been suggested to depend on the anti-inflammatory effects of PEA which have been repeatedly reported not only on immune cells, such as lymphocytes and mast cells [136,137,138], but also specifically on adipocytes [139].
Generally speaking, one may conclude that increased levels of AEA and decreased levels of OEA, PEA and SEA are associated with increased feeding behavior and reduced thermogenesis as well as increased markers of inflammation in adipose tissue and insulin resistance, as recently and extensively reviewed [140,141].
3.2. The Endocannabinoidome and Intestinal Permeability Barrier
Accumulating evidence suggests the pivotal role exerted by the eCBome in the pathophysiology of GI disorders [142]. In particular, several ligands and receptors of the eCBome are involved in the regulation of GI motility and secretion, intestinal inflammation and mucosal barrier permeability, as recently reviewed elsewhere [142,143,144]. In this paragraph, description will be limited to the involvement of the eCBome in the so-called “leaky gut”, i.e., the impairment of the gut barrier which is associated not only to different enteropathies (including IBD), but also some of the metabolic disorders we have focused on in the above paragraph, e.g., obesity and diabetes mellitus.
First, it is interesting to note that mucosal and plasma levels of eCBome mediators change during gut inflammatory conditions. For example, dogs with chronic enteropathies have recently been found to have increased or decreased plasma levels of PEA, 2-AG and AEA, depending on the single compound and the specific enteropathy, i.e., whether it was food-, antibiotic- or immunosuppressive-responsive or belonged to the protein-losing subtype [145]. Moreover, levels of PEA are increased in colon biopsies from patients with coeliac disease and dogs with IBD as well as experimentally induced gut inflammation [100,146,147,148], while markedly decreased in animals fed unbalanced diets [149,150,151]. Interestingly, in mice with genetic deletion of NAE biosynthetic enzyme, a marked inflammatory tone was observed in the basal state, which was believed to result from the observed decline in the levels of PEA [152]. On the contrary, the inhibition of PEA degradative enzyme—which results in the increase of PEA levels in the colon—was found to reduce colon inflammation in two models of IBD [151].
Similar findings were observed following PEA oral administration, using either the same [151] or different models of colon inflammation [148,153], with the compound being also able to normalize post-inflammatory increase in intestinal motility [154]. Using labeled dextran it was also shown that oral administration of ultramicronized PEA (i.e., the highly bioavailable and most effective PEA formulation [80,155,156]) significantly counteracted the increased gut permeability in a mouse model of IBD, through either CB2-, GPR55-, or PPARα-mediated mechanisms [153].
Moreover, OEA and PEA were effective in preventing the cytokine-induced increased permeability in CaCo-2 cells compared to vehicle, the effect being dependent on TRPV1 and PPARα, respectively [157]. Actually, PEA not only prevented but also reversed the increase in permeability, since it was effective even when applied 72 h after the induction of inflammation [157]. Similar findings were reported with hypoxia-induced permeability in CaCo-2 cells following treatment with OEA and PEA, while treatment with AEA and 2-AG further increased permeability (via CB1 receptors) [158]. In this regard, it is also interesting to note that plasma AEA concentrations in obese subjects were negatively related to duodenal expression of tight junction proteins, suggesting that increased AEA may contribute to altered intestinal permeability in human obesity [111].
Summarizing, AEA—like bacterial lipopolysaccharides and inflammatory cytokines—is considered a “gate opener” with regard to gut barrier function, while PEA exerts a beneficial effect on the permeability barrier and is considered a “gate keeper” [140,159].
4. The Endocannabinoidome-Gut Microbiome Axis in Intestinal Health and beyond
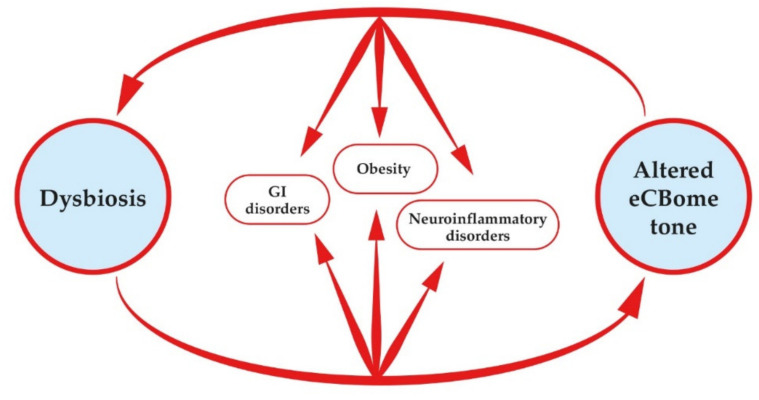
Currently, it is becoming clear that the eCBome and gut microbiota mutually affect each other [1,117,140]. Several lines of evidence are recently suggesting that the altered eCBome tone featuring obesity and diabetes is correlated with gut dysbiosis [127]. Likewise, eCBome is increasingly being considered an important link between the gut microbiome and certain neuroinflammatory disorders [160]. A summary overview is given in Figure 2 and the available evidence is summarized below.
Figure 2.
Crosstalk between the gut microbiota and the eCBome. Dysbiosis causes an altered tone of the eCBome, which in turn can feedback on dysbiosis. Disrupted cross talk between these two complex systems is involved in the pathogenesis of gut inflammatory diseases, obesity and neuroinflammatory and mood disorders (e.g., neuropathic pain and depression).
One of the most interesting studies found that sub-chronic administration of the eCBome mediator OEA changed the faecal microbiota profile of mice fed a normal diet towards a “lean-like phenotype”, shifting the Firmicutes/Bacteroidetes ratio in favor of the latter [161]. In different experimental models associated with dysbiosis (e.g., antibiotic treatment, high fat diet-induced obesity), Muccioli and colleagues also highlighted a strict relationship between the colonic eCBome tone and gut dysbiosis [162]. In particular, perturbations of gut microbiota or genetic disruptions of the gut barrier reduced colonic mRNA expression for CB1 and changed the mRNA expression of eCB hydrolases (i.e., eCB degrading enzymes), with the main result being the increase of AEA levels [162]. Prebiotic supplementation reduced gut permeability in high-fat-diet-induced obese mice, a similar finding being observed when a selective antagonist of CB1 was used [162]. Prebiotic administration to obese mice also increased mRNA expression of AEA hydrolase in the adipose tissue, correspondingly decreasing local levels of AEA [162]. Interestingly, these changes were associated with reduced adipocyte differentiation and lipogenesis, as well as less fat mass development in obese mice [162]. Again, similar findings were observed when a selective CB1 antagonist was used [162]. In line with these findings, decreased mRNA expression of CB1 and CB2 in enterocolic biopsies from privately-owned dogs with colonic dysmotility disturbances was significantly counteracted by a 90 day-probiotic treatment [98].
Furthermore, some interesting data come from studies on bacteria genetically engineered to biosynthesize the precursor for NAE biosynthesis. Once incorporated into the gut microbiota they exerted a protective function against obesity, provided that a sufficient active anabolic enzyme was present in either the host or engineered bacteria [163,164].
In addition, a bidirectional relationship between eCBome tone and gut dysbiosis has been suggested in atherosclerosis [162,165] and diabetes [117,118]. In particular, in the adipose tissues of diabetic mice, specific changes in the composition of the gut microbiota were observed concurrently with changes in the eCBome tone (mainly increased AEA and decreased 2-AG levels) [117,118].
Some interesting evidence on the role played by NAEs on gut microbiota also comes from studies on specific genetic deletion of eCB anabolic pathways. In particular, under a control diet, animals with an adipocyte-specific deletion of the NAE biosynthetic enzyme developed a shift in the composition of the gut microbiota together with an obese phenotype (i.e., deranged adipose and whole-body lipid metabolism, altered browning process, insulin resistance and glucose intolerance) [152]. Long-term antibiotic treatment significantly reversed all the alterations, whereas transferring gut microbiota to germ-free mice partially replicated them [152]. Similar results were observed in animals with genetic intestinal deletion of the NAE biosynthetic enzyme [113]. Upon high-fat diet exposure, these animals not only developed obesity and steatosis, but dysbiosis was also observed [113]. When a protective bacterium, i.e., A. muciniphila [166,167], was administered, it maintained its efficacy against obesity and metabolic syndrome, suggesting that intestinal NAEs did not affect the probiotic efficacy in this condition [113].
The role of 2-AG on the gut microbiota has also been recently investigated. Mice with monoglyceride lipase deletion not only presented with higher intestinal levels of 2-AG and congeners [168], but were also resistant to diet-induced obesity and metabolic disturbances, and most importantly they exhibited changes in gut microbiota [169]. Conversely, ablation of gut microbiome in germ-free mice resulted in intestinal eCBome changes at either the level of receptors (e.g., CB1, PPARα and GPR55), enzymes or NAEs, regardless of age and gender [170]. These changes could be entirely of partially reversed following fecal microbiome transfer from conventionally raised mice [170].
Finally, a recent ex vivo study has reported that bacterial monocultures treated with a specific and highly concentrated NAE cocktail including AEA, LEA, OEA and PEA, promoted the growth of microbial species found to be over-presented in IBD, while reducing the growth of those depleted in IBD [171]. The study thus suggested that NAEs strongly affect bacterial growth and reflect altered bacterial abundances associated with IBD pathogenesis [171]. Although very interesting, these findings are apparently inconsistent with the protective effects played by NAEs in gut disorders (see above); the difference could partly depend on the fact that in vivo complexity was not sufficiently recapitulated by the ex vivo setting. Even more so if one considers that a remarkable switch in the NAE precursors was found after administration of engineered NAE-producing bacteria as a function of the type of dietary fatty acids [172]. The results of this study also appear to be in contrast with a recent report, where, using data from a 6-week exercise intervention and a cross sectional validation cohort of obese/overweight individuals, baseline serum levels of AEA and OEA were positively associated with alpha diversity as well as SCFA producing bacteria such as Bifidobacterium, Coprococcus 3 and Faecalibacterium. Additionally, AEA was positively associated with butyrate. Serum AEA, OEA and PEA all increased significantly with exercise and changes in AEA correlated with butyrate, whereas increases in AEA and PEA correlated with decreases in TNF-α and IL-6. It was calculated that these two NAEs mediated one third of the effect of SCFAs on these cytokines [173]. Whilst very interesting, because carried out in obese/overweight volunteers, this study seems to contrast with the concept, discussed above, that while PEA counteracts inflammation, AEA may worsen it. Clearly, the overall pro-inflammatory or anti-inflammatory action of AEA depends on the receptors it modulates (respectively, CB1 and GPR55, on the one hand, or TRPV1 and CB2 on the other hand), which in turn might depend on the baseline context under study (such as exercise or the presence or lack of obesity/overweight).
Taken together, the findings discussed above suggest the eCBome-gut microbiome axis plays a key role in intestinal and metabolic health [169], with gut microbiome controlling the eCBome tone and vice versa [170].
4.1. Diet, Microbiome and Endocannabinoidome Tone
Currently, only few studies have investigated the possible link(s) between diet-induced perturbations of gut microbiota profile and changes in the eCBome tone. Lacroix and colleagues demonstrated that high fat-high sucrose diet not only lead to glucose intolerance, obesity and hyperinsulinemia in mice, but also altered the gut microbiota profile as well as the intestinal and serum eCBome tone [174]. In particular, they found that during high fat-high sucrose diet low abundance of metabolically beneficial genera correlated with increased ileal levels of AEA and plasma levels of both AEA and 2-AG [174]. Accordingly, ileal mRNA expression of AEA and 2-AG degrading enzymes was decreased, whereas the expression of 2-AG biosynthesizing enzyme was increased [174]. Moreover, decreased mRNA expression for either PPARα or CB2 was found [174]. Correlation analyses suggested that interactions between gut microbiome and eCBome not only exist but could also affect the development of dysbiosis as well as diet-induced metabolic disturbances [174].
In addition, Castonguay-Paradis and colleagues have recently demonstrated that the abundance of some gut microbiota taxa in human subjects is associated with increased plasma levels of NAEs and 2-monoacylglycerols, which, and particularly those derived from omega-3 fatty acids, in turn correlated positively with the dietary intake of the respective fatty acids, irrespective of fat mass [108]. According to the authors, the finding suggests that dietary interventions aimed at properly manipulating the eCBome tone may counteract metabolic disturbances linked to gut dysbiosis [108].
4.2. Microbiome-Gut-Brain Axis and Endocannabinoidome: “Omics” Interactions go Central
Accumulating evidence is suggestive of the eCBome linking the gut microbiome to central nervous system pathophysiology. One excellent example is pain perception in vitamin D deficient mice [175]. It has recently been found that these animals concurrently present marked dysbiosis, with lower microbial diversity, together with tactile allodynia and neuronal hyperexcitability [175]. Most notably, vitamin D deficient mice also showed changes in the eCBome at both spinal and colon level (e.g., increased AEA levels) [175]. Interestingly, treatment with ultramicronized PEA reversed chronic pain and neuronal excitability normalized spinal eCB changes and increased some specific commensal gut bacteria, in particular A. muciniphila [175], known to exert intestinal protective effects [113,166,167]. More importantly, the results suggested that, at least in part, the analgesic effects of ultramicronized PEA were peripheral in nature and dependent on gut microbiota [175].
A further example of the involvement of gut microbiome-eCBome interactions in central neuroinflammatory disorders is depression. Among other psychiatric disorders, depression is indeed associated with dysbiosis, intestinal inflammation and loss of gut integrity [176,177]. In particular, in a mouse model of antimicrobial cocktail-induced dysbiosis, depressive behaviors and reduced social recognition memory as well as increased of gut inflammation were observed [178]. Notably, these changes were also accompanied by increased biochemical and functional changes at hippocampus level, including activation of astrocytes and microglia; moreover, hippocampal and gut alteration of some eCBome members were evident [178]. In fact, increased TRPV1 phophosphorylation/sensitization was observed in the hippocampus, whereas decreased levels of N-acyl-serotonins (i.e., TRPV1 antagonists and inhibitors of NAE degradation) were found in the small intestine [178]. Importantly, probiotic supplementation counteracted the depressive-like behavior, normalized social activity and reduced gut inflammation as well as biochemical and functional hippocampal alterations, while reverting the decrease of gut N-acyl-serotonin levels [178].
From a large cohort study on nearly 800 volunteer twins it has recently emerged that the eCBome mediates the relationship between gut microbiome and anhedonia/amotivation [179]. In particular, the authors tested the hypothesis that either reduced serum levels of PEA or increased stool levels of PEA would mediate the association between microbial diversity and anhedonia/amotivation. Indeed, the association was found to be mediated by faecal, but not serum, levels of PEA [179].
Additionally, many studies have reported gut dysbiosis to be associated with autism spectrum disorders [180,181]. Again, eCBome-microbiome interactions seem to play a role, as recently suggested by a study in BTBR mice, a strain displaying autistic-like features, including social deficits and repetitive behavior [182]. Administration of ultramicronized PEA improved the altered behavioral phenotype, the effect being dependent on PPARα activation [182]. Ultramicronized PEA also restored hippocampal mitochondrial function and decreased the expression of pro-inflammatory cytokines at hippocampal, serum, and colonic level [182]. Importantly, gut permeability and faecal microbiota profile showed improvements following PEA administration, with the main finding being the rise of Firmicutes/Bacteroidetes ratio, mainly due to the increase of butyrate-producing Clostridiales [182]. Taken together, the results suggested that PEA (i) controlled neuroinflammation, (ii) exerted anti-inflammatory effects at colonic and systemic level, (iii) restored gut homeostasis by improving gut integrity and remodeling gut microbiota composition [182].
It is finally worth mentioning the mutual nature of eCBome-microbiome interactions in central nervous system (CNS) pathophysiology, i.e., not only that the eCBome controls microbiome, but that the reverse also holds true. Manca and collaborators have indeed recently shown that the overall impaired eCBome signaling observed in the brain of germ-free mice was attenuated by fecal microbiome transfer from conventionally raised mice [183].
The eCBome tone may thus play unexpected roles not only in gut homeostasis and energy metabolism, but also in the CNS consequences of dysbiosis.
4.3. Microbiota as a Potential Source of Endocannabinoidome Mediators
An expanding theme is the potential capability of commensal microorganisms to affect eCBome signaling by directly producing NAE-like molecules able to bind the host G-protein–coupled receptors. N-acyl-3-hydroxypalmitoyl-glycine, called Commendamide, is the first of these molecules to be identified [184,185]. N-oleoyl serinol is a further member of microbiota-encoded NAE family. In particular, it is produced by commensal bacteria and acts as GPR119 agonist, sharing a similar structure as well as mechanism with OEA which actually activates GPR119 [186]. N-oleoyl serinol has been found to regulate metabolic hormones and glucose homeostasis as efficiently as OEA [186]. Additionally, it has been discovered that some microbiota-derived molecules may also act through the host TRPV1 (the 2021 Nobel Prize-winning receptor). In particular, a linoleic acid metabolite produced by gut lactic acid bacteria, i.e., 10-oxo-12(Z)-octadecenoic acid also referred to as KetoA, was able to augment energy metabolism through the activation of TRPV1 channels, thus protecting mice from diet-induced obesity and ameliorating obesity-associated metabolic disorders [187]. Finally, gut Clostridia were very recently shown to conjugate some neurotransmitters or neurotransmitter-like molecules, such as dopamine, tyramine and tryptamine, with diet- and human-derived fatty acids to produce long chain fatty acid amides that modulate the activities of host GPCRs, including some eCBome receptors [188].
5. Conclusions
In summary, the data reviewed in this article clearly point to the existence of an eCBome-gut microbiome axis. The malfunctioning of this axis may be involved in a variety of disorders wherein intestinal dysfunction plays a role, such as obesity, chronic inflammatory enteropathies as well as neuroinflammatory disorders. Mediators of the eCBome and their receptors appear to influence the complex and still largely unexplored communication between the host and its gut microbiome. The design and development of eCBome receptor agonists, antagonists and allosteric modulators as well as anabolic/catabolic enzyme inhibitors may thus represent future therapeutic interventions for gut dysbiosis-driven diseases. Likewise, targeting gut microbiome with dietary interventions (e.g., prebiotics, probiotics) may be of potential use for the prevention and treatment of disorders related to eCBome dysfunction. In this scenario, food for special medical purposes and dietetic complementary feeds (for human and veterinary use, respectively) containing ultramicronized PEA—alone or in combination with probiotics and prebiotics—may be readily available nutritional tools to keep pathological alterations of the eCBome-gut microbiome axis under control. It is expected that in the near future new nutritional tools will emerge based on the expanding knowledge in this cutting-edge field. In particular, balanced diets containing amounts of fatty acid precursors for eCBome mediators with different biological activities (such as PEA and AEA, for example), could be designed, based on the increasingly accepted concept that dietary fatty acids are a strong determinant of plasma and tissue levels of such mediators [108,189]. These options will hopefully represent valuable and safer alternatives to current treatments, such as antibiotics for chronic enteropathies, which not only cause long-term negative alterations of gut microbiota but are also a global concern (i.e., because of the development of antibiotic resistance), both in human and veterinary medicine [190,191,192].
Author Contributions
Conceptualization, A.S.M. and S.P.; formal analysis, A.S.M. and S.P.; resources, A.S.M. and S.P.; writing—original draft preparation, A.S.M. and S.P.; writing—review and editing, A.S.M., V.D.M. and S.P.; visualization, A.S.M., V.D.M. and S.P.; supervision, V.D.M. and S.P.; project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero dello Sviluppo Economico, grant number F/200052/01/X45, CUP number B41B19000490008 and COR number 1705822.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.S.M. and S.P. are employees of Epitech Group SpA; S.P. and V.D.M. are co-inventors on patents on Adelmidrol and/or Palmitoylethanolamide, respectively, which are unrelated to the present study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iannotti F.A., Di Marzo V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021;248:R83–R97. doi: 10.1530/JOE-20-0444. [DOI] [PubMed] [Google Scholar]
- 2.Barko P.C., McMichael M.A., Swanson K.S., Williams D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018;32:9–25. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg G., Rybakova D., Fischer D., Cernava T., Vergès M.-C.C., Charles T., Chen X., Cocolin L., Eversole K., Corral G.H., et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilla R., Suchodolski J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2019;6:498. doi: 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng P., Swanson K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015;113((Suppl. S6)):17. doi: 10.1017/S0007114514002943. [DOI] [PubMed] [Google Scholar]
- 6.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 7.Alessandri G., Argentini C., Milani C., Turroni F., Ossiprandi M.C., van Sinderen D., Ventura M. Catching a glimpse of the bacterial gut community of companion animals: A canine and feline perspective. Microb. Biotechnol. 2020;13:1708–1732. doi: 10.1111/1751-7915.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilla R., Suchodolski J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. Small Anim. Pract. 2021;51:605–621. doi: 10.1016/j.cvsm.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Blake A., Suchodolski J. Importance of gut microbiota for the health and disease of dogs and cats. Anim. Front. 2016;6:37. doi: 10.2527/af.2016-0032. [DOI] [Google Scholar]
- 10.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segain J.P., Raingeard de la Blétière D., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., Blottière H.M., Galmiche J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte N., Fernández-Rilo A.C., Palomba L., Di Marzo V., Cristino L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020;21:1554. doi: 10.3390/ijms21051554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBlanc J.G., Milani C., de Giori G.S., Sesma F., van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.S., Wang R.X., Goldberg M.S., Clifford G.P., Kao D.J., Colgan S.P. Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. iScience. 2020;23:101226. doi: 10.1016/j.isci.2020.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cresci G.A., Bawden E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015;30:734–746. doi: 10.1177/0884533615609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado M.C., Rautava S., Aakko J., Isolauri E., Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. 1)):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blake A.B., Cigarroa A., Klein H.L., Khattab M.R., Keating T., Coevering P.V.D., Lidbury J.A., Steiner J.M., Suchodolski J.S. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J. Vet. Intern. Med. 2020;34:2345–2356. doi: 10.1111/jvim.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deusch O., O’Flynn C., Colyer A., Swanson K.S., Allaway D., Morris P. A Longitudinal Study of the Feline Faecal Microbiome Identifies Changes into Early Adulthood Irrespective of Sexual Development. PLoS ONE. 2015;10:e0144881. doi: 10.1371/journal.pone.0144881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal S., Tikhonov E., Aroch I., Hefetz L., Turjeman S., Koren O., Kuzi S. Developmental intestinal microbiome alterations in canine fading puppy syndrome: A prospective observational study. NPJ Biofilms Microbiomes. 2021;7:52. doi: 10.1038/s41522-021-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 29.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O’Connor E.M., Cusack S., Harris H.M.B., Coakley M., Lakshminarayanan B., O’Sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 30.Alessandri G., Milani C., Mancabelli L., Longhi G., Anzalone R., Lugli G.A., Duranti S., Turroni F., Ossiprandi M.C., van Sinderen D., et al. Deciphering the Bifidobacterial Populations within the Canine and Feline Gut Microbiota. Appl. Environ. Microbiol. 2020;86:e02875-19. doi: 10.1128/AEM.02875-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuoka H., Shimada K., Kiyosue-Yasuda T., Kiyosue M., Oishi Y., Kimura S., Yamada A., Hirayama K. Transition of the intestinal microbiota of dogs with age. Biosci. Microbiota Food Health. 2017;36:27–31. doi: 10.12938/bmfh.BMFH-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro É.d.M., Peixoto M.C., Putarov T.C., Monti M., Pacheco P.D.G., Loureiro B.A., Pereira G.T., Carciofi A.C. The effects of age and dietary resistant starch on digestibility, fermentation end products in faeces and postprandial glucose and insulin responses of dogs. Arch. Anim. Nutr. 2019;73:485–504. doi: 10.1080/1745039X.2019.1652516. [DOI] [PubMed] [Google Scholar]
- 33.Mizukami K., Uchiyama J., Igarashi H., Murakami H., Osumi T., Shima A., Ishiahra G., Nasukawa T., Une Y., Sakaguchi M. Age-related analysis of the gut microbiome in a purebred dog colony. FEMS Microbiol. Lett. 2019;366:fnz095. doi: 10.1093/femsle/fnz095. [DOI] [PubMed] [Google Scholar]
- 34.Kubinyi E., Bel Rhali S., Sándor S., Szabó A., Felföldi T. Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs. Animals. 2020;10:1488. doi: 10.3390/ani10091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuoka H., Shimada K., Kiyosue-Yasuda T., Kiyosue M., Oishi Y., Kimura S., Ohashi Y., Fujisawa T., Hotta K., Yamada A., et al. Transition of the intestinal microbiota of cats with age. PLoS ONE. 2017;12:e0181739. doi: 10.1371/journal.pone.0181739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkins L.J., Monga M., Miller A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019;9:12918. doi: 10.1038/s41598-019-49452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microbiol. Ecol. Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019;15:69–70. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Butcher J., Mack D., Stintzi A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2015;21:139–153. doi: 10.1097/MIB.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 42.Dandrieux J.R.S. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016;57:589–599. doi: 10.1111/jsap.12588. [DOI] [PubMed] [Google Scholar]
- 43.Suchodolski J.S., Dowd S.E., Wilke V., Steiner J.M., Jergens A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE. 2012;7:e39333. doi: 10.1371/journal.pone.0039333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blake A.B., Guard B.C., Honneffer J.B., Lidbury J.A., Steiner J.M., Suchodolski J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE. 2019;14:e0224454. doi: 10.1371/journal.pone.0224454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giaretta P.R., Suchodolski J.S., Jergens A.E., Steiner J.M., Lidbury J.A., Cook A.K., Hanifeh M., Spillmann T., Kilpinen S., Syrjä P., et al. Bacterial Biogeography of the Colon in Dogs With Chronic Inflammatory Enteropathy. Vet. Pathol. 2020;57:258–265. doi: 10.1177/0300985819891259. [DOI] [PubMed] [Google Scholar]
- 46.Mondo E., Marliani G., Accorsi P.A., Cocchi M., Di Leone A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019;9:253–258. doi: 10.4314/ovj.v9i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsilio S., Pilla R., Sarawichitr B., Chow B., Hill S.L., Ackermann M.R., Estep J.S., Lidbury J.A., Steiner J.M., Suchodolski J.S. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 2019;9:19208. doi: 10.1038/s41598-019-55691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.AlShawaqfeh M.K., Wajid B., Minamoto Y., Markel M., Lidbury J.A., Steiner J.M., Serpedin E., Suchodolski J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017;93:fix136. doi: 10.1093/femsec/fix136. [DOI] [PubMed] [Google Scholar]
- 49.Vázquez-Baeza Y., Hyde E.R., Suchodolski J.S., Knight R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016;1:16177. doi: 10.1038/nmicrobiol.2016.177. [DOI] [PubMed] [Google Scholar]
- 50.Jansen V.L., Gerdes V.E., Middeldorp S., van Mens T.E. Gut microbiota and their metabolites in cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2021;35:101492. doi: 10.1016/j.beem.2021.101492. [DOI] [PubMed] [Google Scholar]
- 51.Ojeda J., Ávila A., Vidal P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021;10:1299. doi: 10.3390/jcm10061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kieler I.N., Osto M., Hugentobler L., Puetz L., Gilbert M.T.P., Hansen T., Pedersen O., Reusch C.E., Zini E., Lutz T.A., et al. Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci. Rep. 2019;9:4822. doi: 10.1038/s41598-019-41195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cintio M., Scarsella E., Sgorlon S., Sandri M., Stefanon B. Gut Microbiome of Healthy and Arthritic Dogs. Vet. Sci. 2020;7:92. doi: 10.3390/vetsci7030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bermudez Sanchez S., Pilla R., Sarawichitr B., Gramenzi A., Marsilio F., Steiner J.M., Lidbury J.A., Woods G.R.T., Suchodolski J.S., German A.J. Untargeted fecal metabolome analysis in obese dogs after weight loss achieved by feeding a high-fiber-high-protein diet. Metabolomics. 2021;17:66. doi: 10.1007/s11306-021-01815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C.J., Sears C.L., Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020;1461:37–52. doi: 10.1111/nyas.14107. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi T., Imanishi I. Epithelial-immune crosstalk with the skin microbiota in homeostasis and atopic dermatitis—A mini review. Vet. Dermatol. 2021 doi: 10.1111/vde.13007. [DOI] [PubMed] [Google Scholar]
- 57.Hwang J., Thompson A., Jaros J., Blackcloud P., Hsiao J., Shi V.Y. Updated understanding of Staphylococcus aureus in atopic dermatitis: From virulence factors to commensals and clonal complexes. Exp. Dermatol. 2021;30:1532–1545. doi: 10.1111/exd.14435. [DOI] [PubMed] [Google Scholar]
- 58.Krumbeck J.A., Reiter A.M., Pohl J.C., Tang S., Kim Y.J., Linde A., Prem A., Melgarejo T. Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens. 2021;10:904. doi: 10.3390/pathogens10070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diao J., Yuan C., Tong P., Ma Z., Sun X., Zheng S. Potential Roles of the Free Salivary Microbiome Dysbiosis in Periodontal Diseases. Front. Cell Infect. Microbiol. 2021;11:711282. doi: 10.3389/fcimb.2021.711282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badri D.V., Jackson M.I., Jewell D.E. Dietary Protein and Carbohydrate Levels Affect the Gut Microbiota and Clinical Assessment in Healthy Adult Cats. J. Nutr. 2021;151:3637–3650. doi: 10.1093/jn/nxab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khor B., Snow M., Herrman E., Ray N., Mansukhani K., Patel K.A., Said-Al-Naief N., Maier T., Machida C.A. Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease. Microorganisms. 2021;9:496. doi: 10.3390/microorganisms9030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H., Zhao F., Hou Q., Huang W., Liu Y., Zhang H., Sun Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019;10:2618–2629. doi: 10.1039/C9FO00087A. [DOI] [PubMed] [Google Scholar]
- 63.Rossi G., Cerquetella M., Gavazza A., Galosi L., Berardi S., Mangiaterra S., Mari S., Suchodolski J.S., Lidbury J.A., Steiner J.M., et al. Rapid Resolution of Large Bowel Diarrhea after the Administration of a Combination of a High-Fiber Diet and a Probiotic Mixture in 30 Dogs. Vet. Sci. 2020;7:21. doi: 10.3390/vetsci7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silley P. Do bacteria need to be regulated? J. Appl. Microbiol. 2006;101:607–615. doi: 10.1111/j.1365-2672.2006.02849.x. [DOI] [PubMed] [Google Scholar]
- 65.Honneffer J.B., Minamoto Y., Suchodolski J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014;20:16489–16497. doi: 10.3748/wjg.v20.i44.16489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minamoto Y., Otoni C.C., Steelman S.M., Büyükleblebici O., Steiner J.M., Jergens A.E., Suchodolski J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015;6:33–47. doi: 10.1080/19490976.2014.997612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao M., Chen C., Yuan Z., Li W., Zhang M., Cui N., Duan Y., Zhang X., Zhang P. Dietary Bacillus subtilis supplementation alleviates alcohol-induced liver injury by maintaining intestinal integrity and gut microbiota homeostasis in mice. Exp. Ther. Med. 2021;22:1312. doi: 10.3892/etm.2021.10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herstad H.K., Nesheim B.B., L’Abée-Lund T., Larsen S., Skancke E. Effects of a probiotic intervention in acute canine gastroenteritis—A controlled clinical trial. J. Small Anim. Pract. 2010;51:34–38. doi: 10.1111/j.1748-5827.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 69.Horosheva T.V., Vodyanoy V., Sorokulova I. Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: A randomized, double-blind, placebo-controlled clinical trial. JMM Case Rep. 2014;1:e004036. doi: 10.1099/jmmcr.0.004036. [DOI] [Google Scholar]
- 70.Lee J.S., Wang R.X., Alexeev E.E., Colgan S.P. Intestinal Inflammation as a Dysbiosis of Energy Procurement: New Insights into an Old Topic. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2021.1880241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satyaraj E., Reynolds A., Pelker R., Labuda J., Zhang P., Sun P. Supplementation of diets with bovine colostrum influences immune function in dogs. Br. J. Nutr. 2013;110:2216–2221. doi: 10.1017/S000711451300175X. [DOI] [PubMed] [Google Scholar]
- 72.Gore A.M., Satyaraj E., Labuda J., Engler R., Sun P., Kerr W., Conboy-Schmidt L. Supplementation of Diets With Bovine Colostrum Influences Immune and Gut Function in Kittens. Front. Vet. Sci. 2021;8:675712. doi: 10.3389/fvets.2021.675712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandwe K., Kelly P. Colostrum Therapy for Human Gastrointestinal Health and Disease. Nutrients. 2021;13:1956. doi: 10.3390/nu13061956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menchetti L., Traina G., Tomasello G., Casagrande-Proietti P., Leonardi L., Barbato O., Brecchia G. Potential benefits of colostrum in gastrointestinal diseases. Front. Biosci. (Schol. Ed.) 2016;8:331–351. doi: 10.2741/s467. [DOI] [PubMed] [Google Scholar]
- 75.Bodammer P., Zirzow E., Klammt S., Maletzki C., Kerkhoff C. Alteration of DSS-mediated immune cell redistribution in murine colitis by oral colostral immunoglobulin. BMC Immunol. 2013;14:10. doi: 10.1186/1471-2172-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meletis C. The Important Role of the Endocannabinoid System and the Endocannabinoidome in Gut Health. Altern. Ther. Health Med. 2019;25:24–28. [PubMed] [Google Scholar]
- 77.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharm. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 78.Iannotti F.A., Di Marzo V., Petrosino S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016;62:107–128. doi: 10.1016/j.plipres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Di Marzo V., De Petrocellis L., Bisogno T., Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: An historical overview and some recent developments. Lipids. 1999;34((Suppl. S319—S325)) doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- 80.Petrosino S., Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017;174:1349–1365. doi: 10.1111/bph.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrosino S., Schiano Moriello A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci. 2020;21:9526. doi: 10.3390/ijms21249526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iannotti F.A., Vitale R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells. 2021;10:586. doi: 10.3390/cells10030586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018;17:623–639. doi: 10.1038/nrd.2018.115. [DOI] [PubMed] [Google Scholar]
- 84.Di Marzo V., Silvestri C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients. 2019;11:1956. doi: 10.3390/nu11081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulkarni-Narla A., Brown D.R. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- 86.Van Sickle M.D., Oland L.D., Ho W., Hillard C.J., Mackie K., Davison J.S., Sharkey K.A. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- 87.Coutts A.A., Irving A.J., Mackie K., Pertwee R.G., Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J. Comp. Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- 88.Storr M., Sibaev A., Marsicano G., Lutz B., Schusdziarra V., Timmermans J.-P., Allescher H.D. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am. J. Physiol. Gastrointest Liver Physiol. 2004;286:G110–G117. doi: 10.1152/ajpgi.00148.2003. [DOI] [PubMed] [Google Scholar]
- 89.Duncan M., Davison J.S., Sharkey K.A. Review article: Endocannabinoids and their receptors in the enteric nervous system. Aliment. Pharmacol. Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 90.Galiazzo G., Giancola F., Stanzani A., Fracassi F., Bernardini C., Forni M., Pietra M., Chiocchetti R. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem. Cell Biol. 2018;150:187–205. doi: 10.1007/s00418-018-1684-7. [DOI] [PubMed] [Google Scholar]
- 91.Marquéz L., Suárez J., Iglesias M., Bermudez-Silva F.J., Rodríguez de Fonseca F., Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright K., Rooney N., Feeney M., Tate J., Robertson D., Welham M., Ward S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 93.Pazos M.R., Tolón R.M., Benito C., Rodríguez C.F., Gorgojo J.J., Nevado M., Álvarez M., Arias F., Almodóvar F., Fernández M.T.P., et al. Cannabinoid CB1 Receptors Are Expressed by Parietal Cells of the Human Gastric Mucosa. J. Histochem. Cytochem. 2008;56:511–516. doi: 10.1369/jhc.2008.950741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ligresti A., De Petrocellis L., Di Marzo V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiological. Rev. 2016;96:1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 95.Toschi A., Galiazzo G., Piva A., Tagliavia C., Mazzuoli-Weber G., Chiocchetti R., Grilli E. Cannabinoid and Cannabinoid-Related Receptors in the Myenteric Plexus of the Porcine Ileum. Animals. 2021;11:263. doi: 10.3390/ani11020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stanzani A., Galiazzo G., Giancola F., Tagliavia C., De Silva M., Pietra M., Fracassi F., Chiocchetti R. Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract. Histochem. Cell Biol. 2020;153:339–356. doi: 10.1007/s00418-020-01854-0. [DOI] [PubMed] [Google Scholar]
- 97.Galiazzo G., Tagliavia C., Giancola F., Rinnovati R., Sadeghinezhad J., Bombardi C., Grandis A., Pietra M., Chiocchetti R. Localisation of Cannabinoid and Cannabinoid-Related Receptors in the Horse Ileum. J. Equine Vet. Sci. 2021;104:103688. doi: 10.1016/j.jevs.2021.103688. [DOI] [PubMed] [Google Scholar]
- 98.Rossi G., Gioacchini G., Pengo G., Suchodolski J.S., Jergens A.E., Allenspach K., Gavazza A., Scarpona S., Berardi S., Galosi L., et al. Enterocolic increase of cannabinoid receptor type 1 and type 2 and clinical improvement after probiotic administration in dogs with chronic signs of colonic dysmotility without mucosal inflammatory changes. Neurogastroenterol. Motil. 2020;32:e13717. doi: 10.1111/nmo.13717. [DOI] [PubMed] [Google Scholar]
- 99.Grill M., Hasenoehrl C., Kienzl M., Kargl J., Schicho R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem. Cell Biol. 2019;151:5–20. doi: 10.1007/s00418-018-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gugliandolo E., Peritore A.F., Piras C., Cuzzocrea S., Crupi R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Vet. Sci. 2020;7:78. doi: 10.3390/vetsci7020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Astarita G., Rourke B.C., Andersen J.B., Fu J., Kim J.H., Bennett A.F., Hicks J.W., Piomelli D. Postprandial increase of oleoylethanolamide mobilization in small intestine of the Burmese python (Python molurus) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1407–R1412. doi: 10.1152/ajpregu.00664.2005. [DOI] [PubMed] [Google Scholar]
- 102.De Petrocellis L., Di Marzo V. Cell signalling: Why fasting worms age slowly. Nature. 2011;473:161–163. doi: 10.1038/473161a. [DOI] [PubMed] [Google Scholar]
- 103.Valenti M., Cottone E., Martinez R., De Pedro N., Rubio M., Viveros M.P., Franzoni M.F., Delgado M.J., Di Marzo V. The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J. Neurochem. 2005;95:662–672. doi: 10.1111/j.1471-4159.2005.03406.x. [DOI] [PubMed] [Google Scholar]
- 104.Balsevich G., Petrie G.N., Hill M.N. Endocannabinoids: Effectors of glucocorticoid signaling. Front. Neuroendocrinol. 2017;47:86–108. doi: 10.1016/j.yfrne.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Ruiz de Azua I., Lutz B. Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues. Cell Mol. Life Sci. 2019;76:1341–1363. doi: 10.1007/s00018-018-2994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mallipedhi A., Prior S.L., Dunseath G., Bracken R.M., Barry J., Caplin S., Eyre N., Morgan J., Baxter J.N., O’Sullivan S.E., et al. Changes in plasma levels of N-arachidonoyl ethanolamine and N-palmitoylethanolamine following bariatric surgery in morbidly obese females with impaired glucose homeostasis. J. Diabetes Res. 2015;2015:680867. doi: 10.1155/2015/680867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Izzo A.A., Piscitelli F., Capasso R., Aviello G., Romano B., Borrelli F., Petrosino S., Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: Relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br. J. Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castonguay-Paradis S., Lacroix S., Rochefort G., Parent L., Perron J., Martin C., Lamarche B., Raymond F., Flamand N., Di Marzo V., et al. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci. Rep. 2020;10:15975. doi: 10.1038/s41598-020-72861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Marzo V., Côté M., Matias I., Lemieux I., Arsenault B.J., Cartier A., Piscitelli F., Petrosino S., Alméras N., Després J.-P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: Associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- 110.Matias I., Gatta-Cherifi B., Tabarin A., Clark S., Leste-Lasserre T., Marsicano G., Piazza P.V., Cota D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS ONE. 2012;7:e42399. doi: 10.1371/journal.pone.0042399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Little T.J., Cvijanovic N., DiPatrizio N.V., Argueta D.A., Rayner C.K., Feinle-Bisset C., Young R.L. Plasma endocannabinoid levels in lean, overweight, and obese humans: Relationships to intestinal permeability markers, inflammation, and incretin secretion. Am. J. Physiol. Endocrinol. Metab. 2018;315:E489–E495. doi: 10.1152/ajpendo.00355.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jumpertz R., Guijarro A., Pratley R.E., Piomelli D., Krakoff J. Central and Peripheral Endocannabinoids and Cognate Acylethanolamides in Humans: Association with Race, Adiposity, and Energy Expenditure. J. Clin. Endocrinol. Metab. 2011;96:787–791. doi: 10.1210/jc.2010-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Everard A., Plovier H., Rastelli M., Van Hul M., de Wouters d’Oplinter A., Geurts L., Druart C., Robine S., Delzenne N.M., Muccioli G.G., et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019;10:457. doi: 10.1038/s41467-018-08051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petrosino S., Schiano Moriello A., Cerrato S., Fusco M., Puigdemont A., De Petrocellis L., Di Marzo V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharm. 2016;173:1154–1162. doi: 10.1111/bph.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musella A., Fresegna D., Rizzo F.R., Gentile A., Bullitta S., De Vito F., Guadalupi L., Centonze D., Mandolesi G. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci. Rep. 2017;7:7363. doi: 10.1038/s41598-017-07519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fanelli F., Mezzullo M., Repaci A., Belluomo I., Ibarra Gasparini D., Di Dalmazi G., Mastroroberto M., Vicennati V., Gambineri A., Morselli-Labate A.M., et al. Profiling plasma N-Acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol. Metab. 2018;14:82–94. doi: 10.1016/j.molmet.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geurts L., Neyrinck A.M., Delzenne N.M., Knauf C., Cani P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 118.Geurts L., Lazarevic V., Derrien M., Everard A., Van Roye M., Knauf C., Valet P., Girard M., Muccioli G.G., François P., et al. Altered Gut Microbiota and Endocannabinoid System Tone in Obese and Diabetic Leptin-Resistant Mice: Impact on Apelin Regulation in Adipose Tissue. Front. Microbiol. 2011;2:149. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirkham T.C., Williams C.M., Fezza F., Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 121.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005;115:1298–1305. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coutts A.A., Pertwee R.G. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharm. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Christie S., Wittert G.A., Li H., Page A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. 2018;9:420. doi: 10.3389/fendo.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong F., Pan S., Guo Y., Xu P., Zhai Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules. 2019;24:2545. doi: 10.3390/molecules24142545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lipina C., Walsh S.K., Mitchell S.E., Speakman J.R., Wainwright C.L., Hundal H.S. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J. 2019;33:1299–1312. doi: 10.1096/fj.201800171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumawat V.S., Kaur G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019;862:172628. doi: 10.1016/j.ejphar.2019.172628. [DOI] [PubMed] [Google Scholar]
- 127.Piomelli D. A fatty gut feeling. Trends Endocrinol. Metab. 2013;24:332–341. doi: 10.1016/j.tem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu J., Astarita G., Gaetani S., Kim J., Cravatt B.F., Mackie K., Piomelli D. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J. Biol. Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DiPatrizio N.V. Endocannabinoids and the Gut-Brain Control of Food Intake and Obesity. Nutrients. 2021;13:1214. doi: 10.3390/nu13041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen H.S. Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharm. Res. 2014;86:18–25. doi: 10.1016/j.phrs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 131.Laleh P., Yaser K., Abolfazl B., Shahriar A., Mohammad A.J., Nazila F., Alireza O. Oleoylethanolamide increases the expression of PPAR-A and reduces appetite and body weight in obese people: A clinical trial. Appetite. 2018;128:44–49. doi: 10.1016/j.appet.2018.05.129. [DOI] [PubMed] [Google Scholar]
- 132.Tutunchi H., Ostadrahimi A., Saghafi-Asl M., Hosseinzadeh-Attar M.-J., Shakeri A., Asghari-Jafarabadi M., Roshanravan N., Farrin N., Naemi M., Hasankhani M. Oleoylethanolamide supplementation in obese patients newly diagnosed with non-alcoholic fatty liver disease: Effects on metabolic parameters, anthropometric indices, and expression of PPAR-α, UCP1, and UCP2 genes. Pharmacol. Res. 2020;156:104770. doi: 10.1016/j.phrs.2020.104770. [DOI] [PubMed] [Google Scholar]
- 133.Onopchenko O.V., Kosiakova G.V., Oz M., Klimashevsky V.M., Gula N.M. N-stearoylethanolamine restores pancreas lipid composition in obesity-induced insulin resistant rats. Lipids. 2015;50:13–21. doi: 10.1007/s11745-014-3960-1. [DOI] [PubMed] [Google Scholar]
- 134.Hu J., Ying H., Yao J., Yang L., Jin W., Ma H., Li L., Zhao Y. Micronized Palmitoylethanolamide Ameliorates Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis via Inhibiting Inflammation and Restoring Autophagy. Front. Pharmacol. 2021;12:744483. doi: 10.3389/fphar.2021.744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matias I., Gonthier M.-P., Petrosino S., Docimo L., Capasso R., Hoareau L., Monteleone P., Roche R., Izzo A.A., Di Marzo V. Role and regulation of acylethanolamides in energy balance: Focus on adipocytes and β-cells. Br. J. Pharmacol. 2007;152:676–690. doi: 10.1038/sj.bjp.0707424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chiurchiù V., Battistini L., Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–364. doi: 10.1111/imm.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chiurchiù V., Leuti A., Smoum R., Mechoulam R., Maccarrone M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018;32:5716–5723. doi: 10.1096/fj.201800107R. [DOI] [PubMed] [Google Scholar]
- 138.Petrosino S., Schiano Moriello A., Verde R., Allarà M., Imperatore R., Ligresti A., Mahmoud A.M., Peritore A.F., Iannotti F.A., Di Marzo V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflamm. 2019;16:274. doi: 10.1186/s12974-019-1671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoareau L., Buyse M., Festy F., Ravanan P., Gonthier M.-P., Matias I., Petrosino S., Tallet F., d’Hellencourt C.L., Cesari M., et al. Anti-inflammatory effect of palmitoylethanolamide on human adipocytes. Obesity (Silver Spring) 2009;17:431–438. doi: 10.1038/oby.2008.591. [DOI] [PubMed] [Google Scholar]
- 140.Cani P.D., Plovier H., Van Hul M., Geurts L., Delzenne N.M., Druart C., Everard A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016;12:133–143. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 141.Rahman S.M.K., Uyama T., Hussain Z., Ueda N. Roles of Endocannabinoids and Endocannabinoid-Like Molecules in Energy Homeostasis and Metabolic Regulation: A Nutritional Perspective. Ann. Rev. Nutr. 2021;41:177–202. doi: 10.1146/annurev-nutr-043020-090216. [DOI] [PubMed] [Google Scholar]
- 142.DiPatrizio N.V. Endocannabinoids in the Gut. Cannabis Cannabinoid Res. 2016;1:67–77. doi: 10.1089/can.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pesce M., D’Alessandro A., Borrelli O., Gigli S., Seguella L., Cuomo R., Esposito G., Sarnelli G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell Mol. Med. 2018;22:706–715. doi: 10.1111/jcmm.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jansma J., Brinkman F., van Hemert S., El Aidy S. Targeting the endocannabinoid system with microbial interventions to improve gut integrity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;106:110169. doi: 10.1016/j.pnpbp.2020.110169. [DOI] [PubMed] [Google Scholar]
- 145.Febo E., Crisi P.E., Oddi S., Pietra M., Galiazzo G., Piscitelli F., Gramenzi A., Prinzio R.D., Di Tommaso M., Bernabò N., et al. Circulating Endocannabinoids as Diagnostic Markers of Canine Chronic Enteropathies: A Pilot Study. Front. Vet. Sci. 2021;8:655311. doi: 10.3389/fvets.2021.655311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D’Argenio G., Petrosino S., Gianfrani C., Valenti M., Scaglione G., Grandone I., Nigam S., Sorrentini I., Mazzarella G., Di Marzo V. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J. Mol. Med. 2007;85:523–530. doi: 10.1007/s00109-007-0192-3. [DOI] [PubMed] [Google Scholar]
- 147.Darmani N.A., Izzo A.A., Degenhardt B., Valenti M., Scaglione G., Capasso R., Sorrentini I., Di Marzo V. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: Review of the available pre-clinical data, and first human studies. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 148.Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 149.Carta G., Murru E., Vargiu R., Collu M., Carta M., Banni S., Stancampiano R. Essential fatty acids deficient diet modulates N-Acylethanolamide profile in rat’s tissues. Prostaglandins Leukot Essent Fat. Acids. 2020;153:102053. doi: 10.1016/j.plefa.2020.102053. [DOI] [PubMed] [Google Scholar]
- 150.Borrelli F., Izzo A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23:33–49. doi: 10.1016/j.beem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 151.Alhouayek M., Bottemanne P., Subramanian K.V., Lambert D.M., Makriyannis A., Cani P.D., Muccioli G.G. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015;29:650–661. doi: 10.1096/fj.14-255208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geurts L., Everard A., Van Hul M., Essaghir A., Duparc T., Matamoros S., Plovier H., Castel J., Denis R.G.P., Bergiers M., et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat. Commun. 2015;6:6495. doi: 10.1038/ncomms7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borrelli F., Romano B., Petrosino S., Pagano E., Capasso R., Coppola D., Battista G., Orlando P., Di Marzo V., Izzo A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015;172:142–158. doi: 10.1111/bph.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Capasso R., Orlando P., Pagano E., Aveta T., Buono L., Borrelli F., Di Marzo V., Izzo A.A. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: Involvement of CB1 receptors and TRPV1 channels. Br. J. Pharmacol. 2014;171:4026–4037. doi: 10.1111/bph.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Petrosino S., Cordaro M., Verde R., Schiano Moriello A., Marcolongo G., Schievano C., Siracusa R., Piscitelli F., Peritore A.F., Crupi R., et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018;9:249. doi: 10.3389/fphar.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Impellizzeri D., Bruschetta G., Cordaro M., Crupi R., Siracusa R., Esposito E., Cuzzocrea S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014;11:136. doi: 10.1186/s12974-014-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karwad M.A., Macpherson T., Wang B., Theophilidou E., Sarmad S., Barrett D.A., Larvin M., Wright K.L., Lund J.N., O’Sullivan S.E. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARα. FASEB J. 2017;31:469–481. doi: 10.1096/fj.201500132. [DOI] [PubMed] [Google Scholar]
- 158.Karwad M.A., Couch D.G., Wright K.L., Tufarelli C., Larvin M., Lund J., O’Sullivan S.E. Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα. Biochem. Pharmacol. 2019;168:465–472. doi: 10.1016/j.bcp.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 159.Clayton P., Hill M., Bogoda N., Subah S., Venkatesh R. Palmitoylethanolamide: A Natural Compound for Health Management. Int. J. Mol. Sci. 2021;22:5305. doi: 10.3390/ijms22105305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bisogno T., Lauritano A., Piscitelli F. The Endocannabinoid System: A Bridge between Alzheimer’s Disease and Gut Microbiota. Life. 2021;11:934. doi: 10.3390/life11090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Paola M., Bonechi E., Provensi G., Costa A., Clarke G., Ballerini C., De Filippo C., Passani M.B. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in Peyer’s patches of mice. Sci. Rep. 2018;8:14881. doi: 10.1038/s41598-018-32925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Z., Zhang Y., Guo L., Dosoky N., de Ferra L., Peters S., Niswender K.D., Davies S.S. Leptogenic effects of NAPE require activity of NAPE-hydrolyzing phospholipase D. J. Lipid Res. 2017;58:1624–1635. doi: 10.1194/jlr.M076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Z., Guo L., Zhang Y., Walzem R.L., Pendergast J.S., Printz R.L., Morris L.C., Matafonova E., Stien X., Kang L., et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 2014;124:3391–3406. doi: 10.1172/JCI72517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moludi J., Alizadeh M., Lotfi Yagin N., Pasdar Y., Nachvak S.M., Abdollahzad H., Sadeghpour Tabaei A. New insights on atherosclerosis: A cross-talk between endocannabinoid systems with gut microbiota. J. Cardiovasc. Thorac. Res. 2018;10:129–137. doi: 10.15171/jcvtr.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 168.Yoshida K., Kita Y., Tokuoka S.M., Hamano F., Yamazaki M., Sakimura K., Kano M., Shimizu T. Monoacylglycerol lipase deficiency affects diet-induced obesity, fat absorption, and feeding behavior in CB1 cannabinoid receptor-deficient mice. FASEB J. 2019;33:2484–2497. doi: 10.1096/fj.201801203R. [DOI] [PubMed] [Google Scholar]
- 169.Dione N., Lacroix S., Taschler U., Deschênes T., Abolghasemi A., Leblanc N., Di Marzo V., Silvestri C. Mgll Knockout Mouse Resistance to Diet-Induced Dysmetabolism Is Associated with Altered Gut Microbiota. Cells. 2020;9:2705. doi: 10.3390/cells9122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Manca C., Boubertakh B., Leblanc N., Deschênes T., Lacroix S., Martin C., Houde A., Veilleux A., Flamand N., Muccioli G.G., et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 2020;61:70–85. doi: 10.1194/jlr.RA119000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fornelos N., Franzosa E.A., Bishai J., Annand J.W., Oka A., Lloyd-Price J., Arthur T.D., Garner A., Avila-Pacheco J., Haiser H.J., et al. Growth effects of N-acylethanolamines on gut bacteria reflect altered bacterial abundances in inflammatory bowel disease. Nat. Microbiol. 2020;5:486–497. doi: 10.1038/s41564-019-0655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dosoky N.S., Guo L., Chen Z., Feigley A.V., Davies S.S. Dietary Fatty Acids Control the Species of N-Acyl-Phosphatidylethanolamines Synthesized by Therapeutically Modified Bacteria in the Intestinal Tract. ACS Infect. Dis. 2018;4:3–13. doi: 10.1021/acsinfecdis.7b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vijay A., Kouraki A., Gohir S., Turnbull J., Kelly A., Chapman V., Barrett D.A., Bulsiewicz W.J., Valdes A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. 2021;13:1997559. doi: 10.1080/19490976.2021.1997559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lacroix S., Pechereau F., Leblanc N., Boubertakh B., Houde A., Martin C., Flamand N., Silvestri C., Raymond F., Di Marzo V., et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems. 2019;4:e00407-19. doi: 10.1128/mSystems.00407-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guida F., Boccella S., Belardo C., Iannotta M., Piscitelli F., De Filippis F., Paino S., Ricciardi F., Siniscalco D., Marabese I., et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav. Immune. 2020;85:128–141. doi: 10.1016/j.bbi.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 176.Collins S.M., Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 177.Fond G., Boukouaci W., Chevalier G., Regnault A., Eberl G., Hamdani N., Dickerson F., Macgregor A., Boyer L., Dargel A., et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. 2015;63:35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 178.Guida F., Turco F., Iannotta M., De Gregorio D., Palumbo I., Sarnelli G., Furiano A., Napolitano F., Boccella S., Luongo L., et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immune. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 179.Minichino A., Jackson M.A., Francesconi M., Steves C.J., Menni C., Burnet P.W.J., Lennox B.R. Endocannabinoid system mediates the association between gut-microbial diversity and anhedonia/amotivation in a general population cohort. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Russo R., Cristiano C., Avagliano C., De Caro C., La Rana G., Raso G.M., Canani R.B., Meli R., Calignano A. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2018;25:3930–3952. doi: 10.2174/0929867324666170216113756. [DOI] [PubMed] [Google Scholar]
- 181.Cristiano C., Lama A., Lembo F., Mollica M.P., Calignano A., Mattace Raso G. Interplay Between Peripheral and Central Inflammation in Autism Spectrum Disorders: Possible Nutritional and Therapeutic Strategies. Front. Physiol. 2018;9:184. doi: 10.3389/fphys.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cristiano C., Pirozzi C., Coretti L., Cavaliere G., Lama A., Russo R., Lembo F., Mollica M.P., Meli R., Calignano A., et al. Palmitoylethanolamide counteracts autistic-like behaviours in BTBR T+tf/J mice: Contribution of central and peripheral mechanisms. Brain Behav. Immune. 2018;74:166–175. doi: 10.1016/j.bbi.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 183.Manca C., Shen M., Boubertakh B., Martin C., Flamand N., Silvestri C., Di Marzo V. Alterations of brain endocannabinoidome signaling in germ-free mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158786. doi: 10.1016/j.bbalip.2020.158786. [DOI] [PubMed] [Google Scholar]
- 184.Cohen L.J., Kang H.-S., Chu J., Huang Y.-H., Gordon E.A., Reddy B.V.B., Ternei M.A., Craig J.W., Brady S.F. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc. Natl. Acad. Sci. USA. 2015;112:E4825–E4834. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Foster J.R., Ueno S., Chen M.X., Harvey J., Dowell S.J., Irving A.J., Brown A.J. N-Palmitoylglycine and other N-acylamides activate the lipid receptor G2A/GPR132. Pharmacol. Res. Perspect. 2019;7:e00542. doi: 10.1002/prp2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cohen L.J., Esterhazy D., Kim S.-H., Lemetre C., Aguilar R.R., Gordon E.A., Pickard A.J., Cross J.R., Emiliano A.B., Han S.M., et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim M., Furuzono T., Yamakuni K., Li Y., Kim Y.-I., Takahashi H., Ohue-Kitano R., Jheng H.-F., Takahashi N., Kano Y., et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017;31:5036–5048. doi: 10.1096/fj.201700151R. [DOI] [PubMed] [Google Scholar]
- 188.Chang F.-Y., Siuti P., Laurent S., Williams T., Glassey E., Sailer A.W., Gordon D.B., Hemmerle H., Voigt C.A. Gut-inhabiting Clostridia build human GPCR ligands by conjugating neurotransmitters with diet- and human-derived fatty acids. Nat. Microbiol. 2021;6:792–805. doi: 10.1038/s41564-021-00887-y. [DOI] [PubMed] [Google Scholar]
- 189.Sihag J., Di Marzo V. (Wh)olistic (E)ndocannabinoidome-Microbiome-Axis Modulation through (N)utrition (WHEN) to Curb Obesity and Related Disorders. Lipids Health Dis. 2022;21:9. doi: 10.1186/s12944-021-01609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cerquetella M., Rossi G., Suchodolski J.S., Schmitz S.S., Allenspach K., Rodríguez-Franco F., Furlanello T., Gavazza A., Marchegiani A., Unterer S., et al. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 2020;61:211–215. doi: 10.1111/jsap.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Charani E., McKee M., Ahmad R., Balasegaram M., Bonaconsa C., Merrett G.B., Busse R., Carter V., Castro-Sanchez E., Franklin B.D., et al. Optimising antimicrobial use in humans—review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg. Health Eur. 2021;7:100161. doi: 10.1016/j.lanepe.2021.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Werner M., Suchodolski J.S., Straubinger R.K., Wolf G., Steiner J.M., Lidbury J.A., Neuerer F., Hartmann K., Unterer S. Effect of amoxicillin-clavulanic acid on clinical scores, intestinal microbiome, and amoxicillin-resistant Escherichia coli in dogs with uncomplicated acute diarrhea. J. Vet. Intern. Med. 2020;34:1166–1176. doi: 10.1111/jvim.15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.